Abstract
Sphingosine 1-phosphate (S1P), the natural sphingolipid ligand for a family of five G protein– coupled receptors (S1P1–S1P5Rs), regulates cell survival and lymphocyte circulation. We have shown that the pan-S1PR agonist, FTY720, attenuates kidney ischemia-reperfusion injury by directly activating S1P1 on proximal tubule (PT) cells, independent of the canonical lymphopenic effects of S1P1 activation on B and T cells. FTY720 also reduces cisplatin-induced AKI. Therefore, in this study, we used conditional PT-S1P1-null (PepckCreS1pr1fl/fl) and control (PepckCreS1pr1w/wt) mice to determine whether the protective effect of FTY720 in AKI is mediated by PT-S1P1. Cisplatin induced more renal injury in PT-S1P1-null mice than in controls. Although FTY720 produced lymphopenia in both control and PT-S1P1-null mice, it reduced injury only in control mice. Furthermore, the increase in proinflammatory cytokine (CXCL1, MCP-1, TNF-α, and IL-6) expression and infiltration of neutrophils and macrophages induced by cisplatin treatment was attenuated by FTY720 in control mice but not in PT-S1P1-null mice. Similarly, S1P1 deletion rendered cultured PT cells more susceptible to cisplatin-induced injury, whereas S1P1 overexpression protected PT cells from injury and preserved mitochondrial function. We conclude that S1P1 may have an important role in stabilizing mitochondrial function and that FTY720 administration represents a novel strategy in the prevention of cisplatin-induced AKI.
Keywords: ARF, proximal tubule, cisplatin, mitochondria
Cisplatin is a platinum-based chemotherapeutic drug used to treat various types of cancers.1 Nephrotoxicity, which occurs in about one-third of patients undergoing cisplatin treatment,2,3 often limits the use of cisplatin4; management of cisplatin-induced AKI rests primarily with decreasing the dose of cisplatin, thereby putting chemotherapeutic efficacy at risk.
Renal proximal tubule (PT) cells, a primary site of cisplatin toxicity, actively accumulate cisplatin through three transporters.5–8 Like other forms of AKI, cisplatin nephrotoxicity encompasses direct cellular8 as well as inflammatory and immune mechanisms, including elevated cytokines/chemokines and immune cell recruitment,9,10 that result from and may potentiate cell death. In vitro studies on renal epithelial cells11 and in vivo studies of kidney ischemia-reperfusion injury (IRI)12,13 demonstrated a role of sphingolipids in AKI. The similarity in pathophysiology between cisplatin-induced injury and other models of AKI led us to consider a potential protective role of sphingosine 1-phosphate (S1P) in cisplatin-induced AKI.
S1P, the ligand for five G protein–coupled receptors (S1P1R–S1P5R), evokes diverse cellular signaling responses.14–16 FTY720, through its active phosphorylated form, is a nonselective S1P agonist at S1P1 and SIP3–517,18 used in the treatment of multiple sclerosis.19 S1PR activation promotes cell survival.20,21 In various disease models, FTY720 causes reversible redistribution of lymphocytes from the circulation to secondary lymph tissue leading to its anti-inflammatory and tissue-protective effects.17,22 FTY720 protects kidneys from IRI through PT-S1P1 activation.12,13 S1P1 activation by various means, including S1P agonists SEW2871 and FTY720, decreases apoptosis and enhances cell survival in response to a variety of stressors23,24 by preventing mitochondrial dysfunction through mechanisms including decreased cytochrome c release,25 BAX translocation,26 and regulation of bcl-2 proteins.27
We hypothesized that FTY720 would reduce cisplatin-induced nephrotoxicity by directly targeting PT-S1P1. We demonstrate that S1P1 plays a critical role in improving mitochondrial function and rendering PT epithelial cells resistant to the apoptotic/necrotic effects of cisplatin.
Results
FTY720 Attenuates Inflammation and Cisplatin-Induced AKI
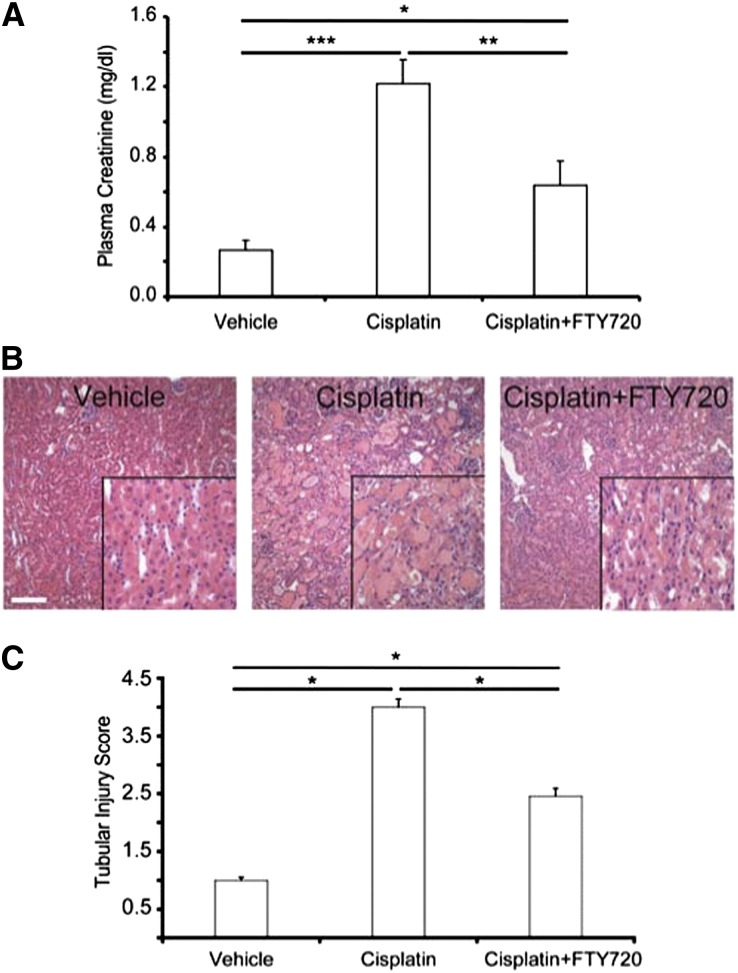
Treatment of wild-type (WT) mice with cisplatin caused a significant rise in plasma creatinine at 72 hours compared with vehicle-treated mice, and FTY720 attenuated this reduction in renal function (Figure 1A). Kidneys of cisplatin-treated mice had increased acute tubular necrosis (ATN) compared with vehicle-treated mice, and FTY720 reduced cisplatin-induced injury (Figure 1B). Histologic assessment of tubule injury (ATN score) paralleled functional data (Figure 1C).
Figure 1.
WT mice treated with FTY720 are protected from cisplatin-induced AKI. WT mice were treated with FTY720 (240 μg/kg) 1 hour before a single dose of cisplatin (27 mg/kg) and once each day on the next 2 days. (A) Plasma creatinine 72 hours after cisplatin. Vehicle, n=4; cisplatin, n=8; cisplatin+FTY720, n=11. (B) H&E staining of kidney sections from the same mice. Insets show a ×2.5 magnified image. (C) Semiquantitative analysis of tubular injury from H&E-stained kidney sections scored on a scale of 0–5. *P<0.05; **P<0.01; ***P<0.001. Values are the mean±SEM. H&E, hematoxylin and eosin. Bar, 100 μm.
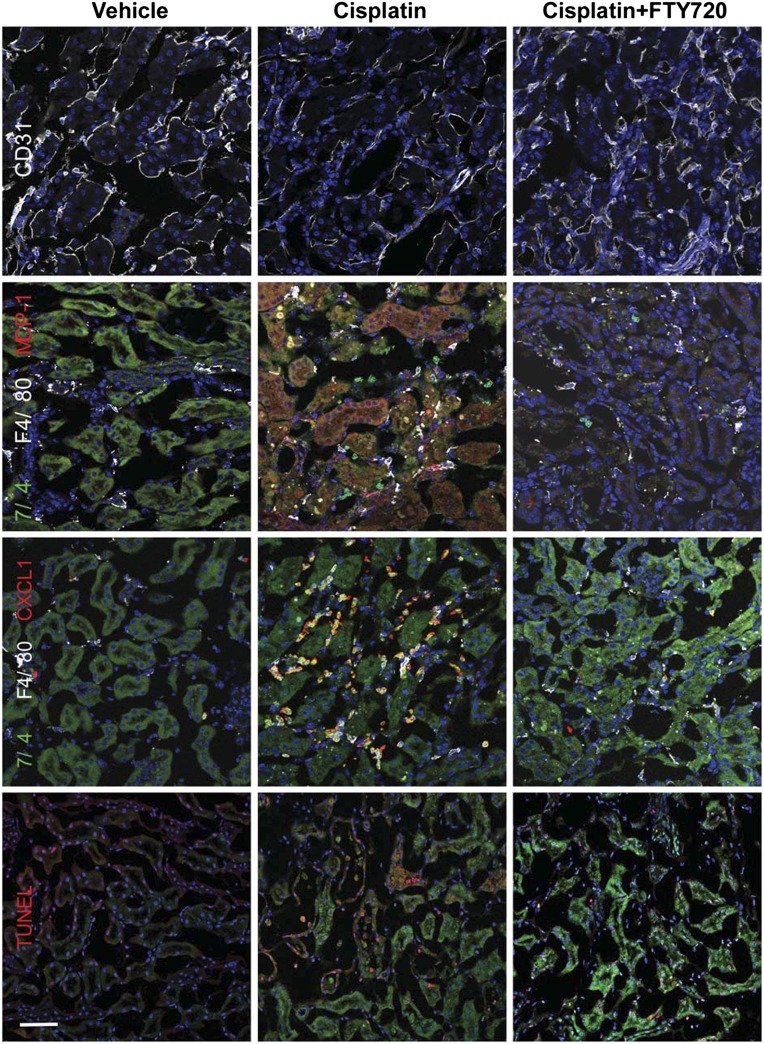
Compared with vehicle-treated mice, cisplatin treatment resulted in increased macrophages and neutrophils primarily in the outer medulla, and FTY720 reduced this cell infiltration compared with cisplatin alone (Supplemental Figure 1), as demonstrated by flow cytometry. Immunofluorescence labeling of kidney sections revealed more neutrophil (7/4) and monocyte (F4/80) infiltration and more CXCL1 and MCP-1 immunoreactivity (note MCP-1 immunofluorescence in PT consistent with increased mRNA levels; Table 1) after cisplatin treatment; FTY720 reduced these effects (Figure 2). As expected, some neutrophils in injured kidney were immunoreactive for CXCL1.28 Administration of FTY720 also preserved the endothelium (CD31) and reduced apoptosis (terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling [TUNEL]) compared with cisplatin treatment alone (Figure 2). Proinflammatory cytokine and chemokine mRNA levels increased significantly in kidney after cisplatin treatment compared with vehicle-treated mice, and this increased expression was reduced by FTY720 (Table 1).
Table 1.
Proinflammatory cytokine and chemokine expression in kidneys of WT mice treated with cisplatin or cisplatin+FTY720
| Cisplatin | Cisplatin+FTY720 | |
|---|---|---|
| CXCL1 | 60.9±26.4a | 13.7±6.9 |
| MCP-1 | 83.1±30.9a | 18.2±6.7 |
| IL1-β | 12.3±4.3a | 1.2±0.7b |
| IL-6 | 216.9±74.2a | 62.7±24.1b |
| TNF-α | 77.7±27.4a | 28.7±12.3 |
Doses of cisplatin and cisplatin+FTY720 as in Figure 1. mRNA expression (72 hours after cisplatin) relative to glyceraldehyde 3-phosphate dehydrogenase expressed as fold changes compared with vehicle. Values are the mean±SEM. n=6–8 in each group.
P<0.05 compared with vehicle.
P<0.05 compared with cisplatin.
Figure 2.
FTY720 treatment attenuates leukocyte infiltration and protects kidneys after cisplatin. Same treatments as in Figure 1. Immunofluorescence of kidney sections with endothelial cell (CD31; white), neutrophil (7/4; green, or yellow if merged with CXCL1 or MCP-1 labeling [red]), monocyte (F4/80; white), cytokine (MCP-1; red), and chemokine (CXCL1; red) labeling and apoptotic cell staining (TUNEL; red). Blue, nuclei stained with DAPI. TUNEL staining (red) in DAPI-labeled (blue) nuclei appears magenta in color-merged image. Bar, 50 μm.
PT-S1P1 Deficiency Leads to Enhanced Cisplatin-Induced Nephrotoxicity and Renders FTY720 Ineffective
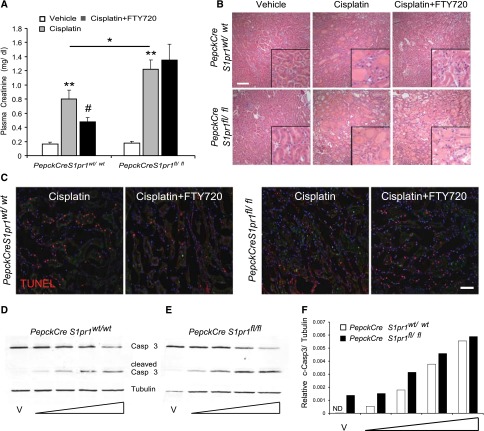
Our in vitro and in vivo deletion studies demonstrate that PT-S1P1 mediates protection from ischemia-reperfusion.13 To investigate the direct involvement of PT-S1P1 in cisplatin-induced nephrotoxicity, we used conditional deletion of S1pr1 in PT cells13 (see also tubular epithelial cell [TEC] characterization in Concise Methods). Cisplatin produced significantly more renal injury as measured by plasma creatinine in PepckCreS1pr1fl/fl than in PepckCreS1pr1wt/wt (control). FTY720 reduced cisplatin-induced nephrotoxicity in PepckCreS1pr1wt/wt but not in PepckCreS1pr1fl/fl (Figure 3A). Morphologic changes (Figure 3B) paralleled functional studies (ATN scores: PepckCreS1pr1wt/wt cisplatin 2.89±0.22 versus cisplatin+FTY720 1.66±0.21; PepckCreS1pr1fl/fl cisplatin 3.08±0.21 versus cisplatin+FTY720 3.56±0.28). As expected,12,13 FTY720 produced lymphopenia in PepckCreS1pr1fl/fl and PepckCreS1pr1wt/wt mice (data not shown). These data demonstrate that PT-S1P1 deficiency enhanced cisplatin-induced injury and that the protective effect of FTY720 in cisplatin-treated mice, as in mice exposed to IRI,12 is independent of lymphopenia but dependent on expression of S1P1 in PT cells.
Figure 3.
Deletion of PT S1P1R exacerbates injury and its presence is necessary for FTY720-mediated protection from cisplatin-induced AKI. Mice were treated with FTY720 1 hour before cisplatin and once each day on the next 2 days (as described in Figure 1). (A) Plasma creatinine levels of PepckCreS1pr1wt/wt (littermate controls) and PepckCreS1pr1fl/fl mice (deficient in S1P1 on PT epithelial cells) 72 hours after cisplatin. n=5–11. *P<0.05 compared with respective cisplatin; **P<0.01 compared with vehicle; #P<0.05 compared with cisplatin. (B) H&E staining of kidney sections. Insets show a ×2.5 magnified image. (C) Immunofluorescence labeling of apoptosis (TUNEL) in kidney sections from PepckCreS1pr1wt/wt and PepckCreS1pr1fl/fl mice. TUNEL labeling is barely detectable in kidney sections from vehicle-treated mice (Supplemental Figure 2). TUNEL staining (red) in DAPI-labeled (blue) nuclei appears magenta in color-merged image. (D and E) Representative quantitative fluorescence Western blot of caspase 3 (Casp 3) and cleaved-caspase 3 (c-Casp 3) (both Casp 3 and c-Casp 3 revealed with IRdye680RD secondary antibody) and tubulin (revealed with IRdye800CW secondary antibody) in homogenates of primary TEC cultures from PepckCreS1pr1wt/wt and PepckCreS1pr1fl/fl mice after incubations with vehicle (saline) or increasing concentrations of cisplatin for 24 hours. (F) Densitometric analysis of c-Casp 3 fluorescence (normalized to tubulin). Concentrations of cisplatin (indicated on blot and bar graph by widening triangle): 10, 20, 40, or 60 μM. n=2 wells in each of two replicate experiments. Values are the mean±SEM. H&E, hematoxylin and eosin; ND, not detectable; V, vehicle. Bar, 100 μm in B; 50 μm in C.
FTY720 reduced the cisplatin-induced increase in the number of macrophages and neutrophils in PepckCreS1pr1wt/wt kidneys but was ineffective in PepckCreS1pr1fl/fl mice (Supplemental Figure 2). Similarly, the cisplatin-induced increase in neutrophils, monocytes, CXCL1 expression (Supplemental Figure 2), and apoptosis (TUNEL) was reduced by FTY720 in PepckCreS1pr1wt/wt but not PepckCreS1pr1fl/fl mice (Figure 3C). Inflammatory markers (mRNA levels) increased significantly in PepckCreS1pr1wt/wt and PepckCreS1pr1fl/fl kidneys after cisplatin, but FTY720 attenuated the increased expression only in PepckCreS1pr1wt/wt and not in PepckCreS1pr1fl/fl mice (Table 2). Incubation with cisplatin ex vivo increased cleaved-caspase 3 in a concentration-dependent manner in primary TEC cultures isolated from PepckCreS1pr1wt/wt kidneys, and at lower cisplatin concentrations the cells from PepckCreS1pr1fl/fl, which lack S1P1, were more susceptible to injury (Figure 3, D–F).
Table 2.
Proinflammatory cytokine and chemokine expression in kidneys of PepckCreS1pr1wt/wt and PepckCreS1pr1fl/fl mice treated with cisplatin or cisplatin+FTY720
| PepckCreS1pr1wt/wt | PepckCreS1pr1fl/fl | |||
|---|---|---|---|---|
| Cisplatin | Cisplatin+FTY720 | Cisplatin | Cisplatin+FTY720 | |
| CXCL1 | 257.46±151.6a | 62.4±6.8 | 340.1±319.2a | 378.4±83.2 |
| MCP-1 | 188.2±114.1a | 22.7±5.1b | 40.7±25.8a | 87.1±16.3 |
| IL-1β | 21.7±13.1a | 3.7±1.5 | 18.6±11.4a | 175.9±146.6 |
| IL-6 | 503.7±252.1a | 88.3±30.6 | 116.6±67.2a | 192.2±48.8 |
| TNF-α | 96.4±41.0a | 35.5±9.9 | 69.1±38.5a | 51.1±16.5 |
Doses of cisplatin and cisplatin+FTY720 as in Figure 1. mRNA expression (72 hours after cisplatin) relative to glyceraldehyde 3-phosphate dehydrogenase expressed as fold changes compared with vehicle. Values are the mean±SEM. n=3–6 in each group.
P<0.05 compared with vehicle.
P<0.05 compared with cisplatin.
FTY720-Mediated Protection of Cisplatin-Induced PT Cell Injury In Vitro Is Attenuated by S1P1 Deficiency
The cisplatin-induced increase in chemokine and proinflammatory cytokine mRNA expression in TKPTS cells was reduced by phosphorylated FTY720 (FTY720-p; Table 3, left). Cisplatin disrupted the cytoskeletal structure and increased CXCL1 and MCP-1 immunoreactivity compared with TKPTS treated with vehicle or FTY720-p alone; pretreatment with FTY720-p attenuated these effects of cisplatin (Supplemental Figure 3). Cisplatin decreased cell viability (73.1%±6.3% of vehicle; P<0.05 relative to vehicle), whereas FTY720-p, which had no effect alone, preserved cell viability (90.9±3.3% of vehicle; P<0.05 compared with cisplatin alone) when cells were pretreated 1 hour before cisplatin.
Table 3.
Proinflammatory cytokine and chemokine expression in TKPTS cells (control and after siRNA S1P1 knockdown) treated with vehicle, FTY720-p, cisplatin, or cisplatin+FTY720-p
| Control | siRNA Knockdown | |||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | FTY720-p | Cisplatin | Cisplatin+FTY720-p | Vehicle | FTY720-p | Cisplatin | Cisplatin+FTY720-p | |
| CXCL1 | 1.0±0.40 | 1.8±0.13 | 21.9±3.20a | 8.2±1.5b | 1.0±0.01 | 1.1±0.08 | 16.8±0.88a | 17.8±1.06 |
| MCP-1 | 1.0±0.02 | 0.9±0.05 | 3.19±0.2 | 1.9±0.08 | 1.0±0.03 | 1.2±-0.05 | 2.7±0.24 | 2.8±0.34 |
| IL-1β | 1.0±0.6 | 14.5±5.9 | 186.8±98.4a | 68.7±9.9b | 1.0±0.20 | 1.6±0.2 | 6.9±1.9 | 12.4±1.17 |
| IL-6 | 1.0±0.5 | 1.8±0.64 | 21.3±8.8a | 16.5±0.8 | 1.0±0.21 | 1.9±0.10 | 126.5±13.4a | 153.3±15.2 |
| TNF-α | 1.0±0.7 | 1.3±0.5 | 13.2±10.2a | 1.69±1.03 | 1.0±0.03 | 1.1±0.11 | 21.6±0.92a | 20.7±0.877 |
The left columns show data for untreated TKPTS cells, whereas the right columns show data for cells transfected with S1pr1 siRNA 48 hours before the experiment. Control (scrambled siRNA) or knockdown cells were incubated with vehicle (saline) or cisplatin (20 μm) for an additional 24 hours. mRNA expression relative to glyceraldehyde 3-phosphate dehydrogenase expressed as fold changes compared with respective vehicle. Values are the mean±SEM. n=3 in each group.
P<0.05 compared with respective vehicle.
P<0.05 compared with respective cisplatin.
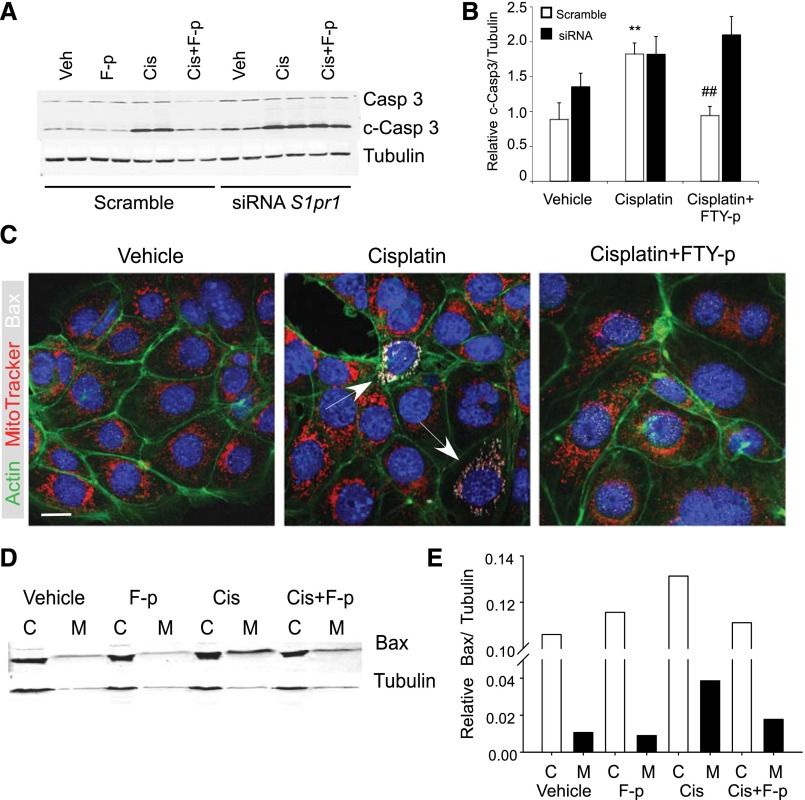
To complement our in vivo studies and demonstrate that PT-S1P1 mediates the protective effect of FTY720, we studied TKPTS cells transfected with S1p1r-specific small interfering RNA (siRNA); S1pr1 mRNA expression was reduced by 50%–80% (with no change in S1pr3 expression).13 The cisplatin-induced increase in cleaved-caspase 3 in TKPTS cells was prevented by FTY720-p. S1pr1 deficiency increased basal vehicle-treated and cisplatin-induced levels of cleaved-caspase 3, but FTY720-p was not protective (Figure 4, A and B); increased expression was dependent on the concentration of cisplatin (Supplemental Figure 4, A–C). FTY720-p pretreatment did not attenuate the cisplatin-induced increase in CXCL1, IL-6, and TNF-α expression in TKPTS cells transfected with S1pr1 siRNA (Table 3, right).
Figure 4.
Phosphorylated FTY720 (F-p)-–mediated protection of cisplatin-induced injury in TKPTS cells is attenuated by S1pr1 siRNA knockdown. (A) Mouse kidney PT (TKPTS) cells were transfected with scrambled or S1pr1 siRNA 48 hours before incubation with vehicle or FTY720-p (10 nM) for 1 hour and then cisplatin (20 μM) for an additional 24 hours. Western blot for full-length caspase 3, cleaved-caspase 3 (c-Casp 3), and tubulin from TKPTS cells transfected with scrambled or siRNA against S1pr1 (detection as in Figure 3). Western blot is from a representative experiment with three to four replicates of each treatment, and the results of three experiments (densitometric analysis) are shown in B. (C) Immunofluorescence labeling of actin cytoskeleton (FITC-phalloidin; Actin), apoptotic marker (Bcl-2–associated X protein; BAX White), and mitochondria (MitoTracker Red; Mito) in TKPTS cells treated with vehicle or cisplatin (20 μM for 24 hours) with or without prior incubation (1 hour) with FTY720-p (10 nM). Blue, nuclei stained with DAPI. Overlay of red MitoTracker and white BAX appears pink in merged images. Arrows, cells with increased BAX immunoreactivity. Images are from a representative experiment performed at least three times with two to three replicates of each treatment. (D and E) Western blot (D) and densitometric analysis (E) of BAX in cytoplasmic and mitochondrial fractions of TKPTS cells treated with vehicle or cisplatin (20 μM for 20 hours) with or without prior incubation (1 hour) with FTY720-p (10 nM). C, cytoplasmic; M, mitochondrial; Cis, cisplatin. Bar, 10 μm.
In examining the protective effect of S1P1 in cisplatin-induced apoptosis via the intrinsic mitochondrial pathway, we found that incubation of TKPTS cells with cisplatin increased expression of BAX and translocation from the cytoplasm (monomeric form) to mitochondria (polymerized form); as expected,26 these changes were prevented by FTY720-p pretreatment (Figure 4, C–E). Untreated primary cultures of PT epithelial cells from PepckCreS1pr1fl/fl showed marked disruption of cell structure compared with cells from PepckCreS1pr1wt/wt. Cell injury (disruption of normal cell structure and mitochondrial morphology; cell death) and mitochondrial BAX expression after cisplatin was greater in primary PT cell cultures from PepckCreS1pr1fl/fl kidneys than from PepckCreS1pr1wt/wt (Supplemental Figure 3).
Overexpression of S1pr1 Stabilizes Mitochondria and Protects TKPTS Cells from Cisplatin-Induced Injury
In contrast with S1P1-deficient cells, S1P1 overexpressing TKPTS cells were resistant to the effects of cisplatin. S1pr1-TKPTS cells (100%–110% viability relative to vehicle) were resistant to concentration-dependent (10–80 μM) cisplatin-induced decreases in cell viability observed in control pcDNA-TKPTS (80%–85% of vehicle). Cellular morphology and integrity of the cell culture monolayer were disrupted by cisplatin in a concentration-dependent manner in control cells (Figure 4, Supplemental Figure 3A), but were preserved in S1pr1-TKPTS (Figure 5, A, C, and D, Supplemental Figure 5D); cisplatin also increased cleaved-caspase 3 in control cells in a concentration-dependent manner (Supplemental Figure 4D). Therefore, 20 μM cisplatin (for 24 hours) was selected for additional experiments (Figure 5).
Figure 5.
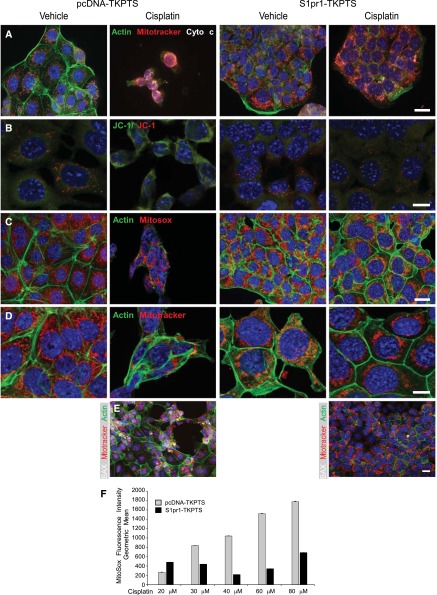
Overexpression of S1P1 protects TKPTS cells from cisplatin-induced cell death and mitochondrial injury. TKPTS cells were stably transfected (and maintained under G418 selection) with control pcDNA3.1 vector (pcDNA-TKPTS) or vector containing the sequence for S1p1r (S1pr1-TKPTS). Cells were incubated with vehicle (saline) or cisplatin (20 μM) for 24 hours and then were labeled with various combinations of cell and mitochondrial markers. (A) FITC-phalloidin to label actin (green), MitoTracker (red), cytochrome c (white). Overlay of red MitoTracker and white cytochrome c appears pink in merged images. (B) Membrane potential–sensitive red/green JC-1 is green in the cytoplasm and red within mitochondria with high membrane potential. (C) FITC-phalloidin (green) and MitoSox Red, which fluoresces more brightly with increasing mitochondrial superoxide. (D) FITC-phalloidin (green) and MitoTracker (red) showing mitochondrial morphology. (E) BAX labeling (white) appears white to pink upon translocation to and overlay with mitochondria (MitoTracker, red). Blue, nuclei stained with DAPI. (F) Mean fluorescence intensity of MitoSox Red in cells incubated with increasing concentrations of cisplatin for 24 hours and analyzed by flow cytometry. Flow histograms from a representative experiment are shown in Supplemental Figure 5. n=3 and the experiment was repeated two times. Bar, 20 μm in A, C, and E; 10 μm in B and D.
To examine mitochondrial function, cellular morphology, and susceptibility to apoptosis, we performed immunofluorescence labeling of vehicle- and cisplatin-treated pcDNA- and S1pr1-TKPTS (Figure 5). Consistent with BAX translocation to mitochondria after cisplatin-induced injury (Figure 4C; and in pcDNA-TKPTS, Figure 5E), where it disrupts mitochondrial integrity and allows leakage of cytochrome c, increased cytoplasmic cytochrome c was observed in cisplatin-treated control cells (indicative of apoptosis). By contrast, cytochrome c was retained in mitochondria of vehicle-treated pcDNA-TKPTS and vehicle- or cisplatin-treated S1pr1-TKPTS, where it colocalized with MitoTracker Red (Figure 5A). Mitochondrial BAX was observed in cisplatin-treated pcDNA-TKPTS but not S1pr1-TKPTS (Figure 5E, Supplemental Figure 5, E and F). Consistent with their resistance to cisplatin-induced cell death, S1pr1-TKPTS cells were less sensitive to the concentration-dependent cisplatin-induced increases in cleaved-caspase 3 than control cells (Supplemental Figure 4, D–F). Injured cisplatin-treated pcDNA-TKPTS but not S1pr1-TKPTS cells showed decreased accumulation of the mitochondrial membrane potential–sensitive JC-1 (Figure 5B). Concentration-dependent cisplatin-stimulated generation of mitochondrial superoxide, revealed by oxidation-dependent increased fluorescence of MitoSox Red indicator, was observed in control but not S1pr1-TKPTS cells (Figure 5, C and F, Supplemental Figure 5, G–J). In addition to the findings with JC-1 and MitoSox that S1P1 overexpression preserved mitochondrial health, labeling with MitoTrackerRed revealed a striking difference in mitochondrial morphology between the two populations of cells. MitoTracker-immunoreactive mitochondria in pcDNA-TKPTS showed normal morphology, but typical of apoptotic cells, appeared fragmented after cisplatin treatment. By contrast, MitoTracker immunoreactivity in S1pr1-TKPTS did not appear to change after cisplatin treatment (Figure 5D).
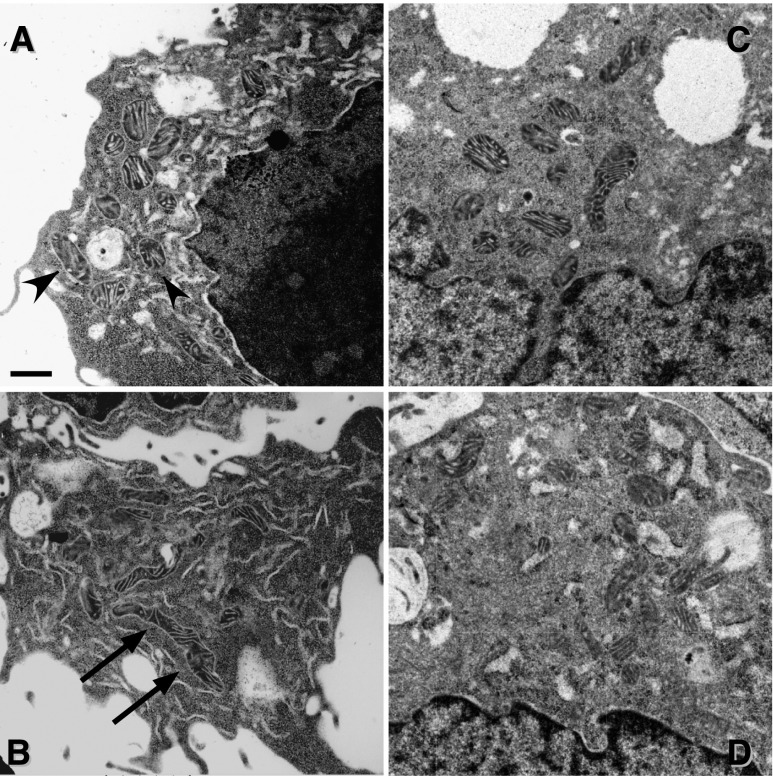
Ultrastructural analysis revealed that mitochondria in pcDNA-TKPTS were shorter and rounder (Figure 6A), whereas on average they were longer and thinner in S1pr1-TKPTS (Figure 6B), as confirmed by quantitative assessment of mitochondrial morphology. Analysis of mitochondrial contour measurements (Concise Methods) yielded values for compactness, feret maximum, and shape factor of 0.609±0.011, 0.506±0.022 and 4.036±0.036 for pcDNA-TKPTS (20 cells; 307 mitochondria) and 0.527±0.017*, 0.675±0.027*, and 4.400±0.070* for S1pr1-TKPTS, respectively (25 cells; 346 mitochondria; *P<0.001 versus respective value for pcDNA-TKPTS). In contrast with stable overexpression, transient S1pr1 knockdown with siRNA (Figure 6, C and D) or repeated treatment of TKPTS cells with FTY720 (which can induce receptor internalization and degradation in immune cells) did not alter mitochondrial contour measurements (Supplemental Table 1).
Figure 6.
Overexpression of S1P1 in TKPTS cells alters mitochondrial fragmentation state. (A and B) Electron micrographs demonstrating differences in mitochondrial morphology between control (A; control pcDNA-transfected; more compact mitochondria, arrowheads) and S1P1-overexpressing (B; more elongated mitochondria, arrows) TKPTS cells. (C and D) Mitochondrial morphology in TKPTS cells transfected with scrambled sequence (C) is not different from cells with siRNA knockdown of S1P1 (D). Bar, 0.5 μM.
S1P1 Overexpression Increases Mitochondrial Respiration in PT Cells
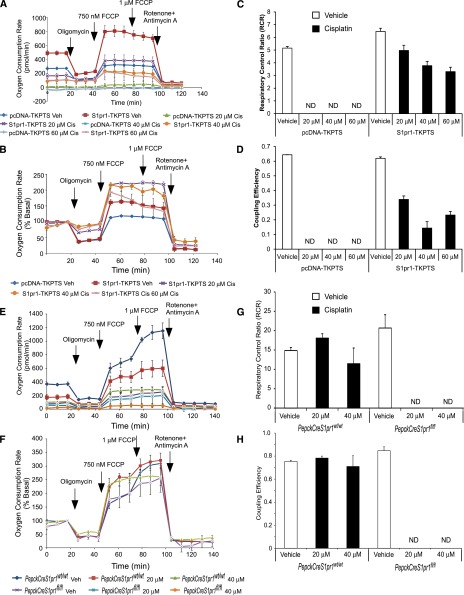
Analysis of mitochondrial bioenergetics in whole cells, by measuring oxygen consumption over time after the sequential addition of inhibitors of mitochondrial function,29 revealed marked differences between the two populations of cells (Figure 7). S1pr1-TKPTS had higher rates of basal mitochondrial respiration (2.5×) than pcDNA-TKPTS, usually indicative of either more mitochondria or increased mitochondrial activity (Figure 7, A and B). Vehicle-treated pcDNA- and S1pr1-TKPTS did not differ in their whole cell respiratory control ratio, the ratio between the maximal mitochondrial respiration rate induced by; carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (FCCP) and ATP-linked respiration (Figure 7C), or their coupling efficiency, the ratio of ATP-linked mitochondrial respiration to basal mitochondrial respiration (Figure 7D). Coupling efficiency is an indicator of the relative flux through the ATP synthase and proton leak pathways.29 Cisplatin caused a dose-dependent decrease in whole cell mitochondrial function (total basal respiration and ATP-linked respiration), whereas S1pr1-TKPTS cells were less susceptible to the effects of cisplatin than control cells (Figure 7, A–D). This indicates that S1P1 overexpression helps preserve the ability of mitochondria to synthesize ATP via ATP synthase after cisplatin treatment. This was further reflected in the marked decrease in oxygen consumption in cisplatin-treated control cells, to nearly undetectable levels, compared with more modest decreases in the two parameters in S1pr1-TKPTS cells (Figure 7, C and D). The reverse was found when comparing mitochondrial bioenergetics in primary TEC cultures isolated from PepckCreS1pr1fl/fl and PepckCreS1pr1wt/wt kidneys. The susceptibility to cisplatin effects on mitochondrial respiration was greater with PT-S1P1 deletion (therefore, only lower cisplatin concentrations were used) than in control cells (Figure 7, E–H).
Figure 7.
Overexpression of S1P1 in TKPTS cells stabilizes mitochondrial function. Control (pcDNA-TKPTS) and S1P1-overexpressing (S1pr1-TKPTS) cells were incubated with vehicle (saline) or cisplatin (20, 40, or 60 μM) for 24 hours, and OCR was measured in a mitochondrial stress test by using Seahorse XF extracellular flux technology. After measuring basal respiratory rate, cells were treated sequentially with the ATP synthase inhibitor oligomycin (0.75 μM), the mitochondrial uncoupler FCCP (0.75 then 1 μM), and the complex I and III inhibitors antimycin A (10 μM) plus rotenone (1 μM) as indicated. (A) OCR for control (control pcDNA3.1 vector-transfected; pcDNA-TKPTS) and S1pr1-overexpressing TKPTS (S1pr1-TKPTS) cells treated with vehicle (Veh) or cisplatin (Cis). (B) OCR expressed as a percentage of total basal respiration for vehicle-treated control cells and S1pr1-overexpressing TKPTS cells treated with vehicle or cisplatin. Cisplatin-treated control cells have extremely low OCR and are not shown in B. (C and D) Respiratory control ratio (C) and coupling efficiency (D) for vehicle- and cisplatin-treated control and S1pr1-overexpressing cells. (E–H) Same experiments as A–D except with primary TEC cultures from kidneys of PepckCreS1pr1wt/wt and PepckCreS1pr1fl/fl mice and with incubations of only the lower concentrations (20 or 40 μM) of cisplatin. n=3. Values are the mean±SEM. ND, not detectable.
Mitochondrial PCR Array Reveals Change in Expression of Genes Important in Mitochondrial Dynamics
We observed that altered mitochondrial dynamics in the presence or absence of S1P1 is associated with the response of cells to cisplatin and susceptibility to apoptosis. We next used PCR array profiling to examine changes in mitochondrial genes that may contribute to the observed changes in mitochondrial dynamics and stability in a cellular environment of S1P1 deletion (primary tubular cells isolated from PepckCreS1pr1wt/wt compared with PepckCreS1pr1fl/fl mice) or stable S1P1 overexpression (pcDNA- versus S1pr1-TKPTS). As demonstrated in the heat map of the 84 mitochondrial gene probe set (Supplemental Figure 6), there was increased expression in mitochondrial genes in S1P1 overexpressing TKPTS cells and decreased expression in S1P1-deficient primary tubule cells (Table 4). Although additional experiments are needed to explore the functional relevance of the regulation of specific genes by S1pr1 expression, there was a marked change in expression of genes important for fusion and fission (Table 4), consistent with our data on mitochondrial stability in the current studies.
Table 4.
Identification by PCR array profiling of mitochondrial genes that are regulated by deletion or overexpression of S1pr1
| Symbol | Description | Related Function | S1pr1-TKPTS versus control | PepckCre-S1pr1fl/fl versus control |
|---|---|---|---|---|
| COX18 | COX18 cytochrome c oxidase assembly homolog | Mitochondrial fission and fusion | 4a | −13.66b |
| FIS1 | Fission 1 (mitochondrial outer membrane) homolog | 5.03a | −9.01b | |
| MFN1 | Mitofusin 1 | 6.36a | −7.37b | |
| DNM1L | Dynamin 1-like | 5.06a | −10.79b | |
| MFN2 | Mitofusin 2 | 4.32a | −12.92b | |
| OPA1 | Optic atrophy 1 (autosomal dominant) | 8.75a | 3.31a | |
| TIMM17B | Translocase of inner mitochondrial membrane 17, homolog B | Inner membrane translocation | 5.39a | 4.65a |
| FXC1 (TIMM10b) | Fracture callus 1 homolog | 3.14a | 6.44a | |
| IMMP2L | IMP2 inner mitochondrial membrane peptidase-like | 1.87 | 1.07 | |
| TAZ | Tafazzin | 3.32a | 4.43a | |
| TIMM50 | Translocase of inner mitochondrial membrane 50, homolog | 3.32a | 7.88a | |
| TIMM44 | Translocase of inner mitochondrial membrane 44, homolog | 2.55a | 4.11a | |
| TIMM10 | Translocase of inner mitochondrial membrane 10, homolog | 2.45a | −10.35b | |
| TOMM20 | Translocase of outer mitochondrial membrane 20 | Outer membrane translocation | 1.78 | −9.46b |
| TOMM22 | Translocase of outer mitochondrial membrane 22 | 3.1a | −10.21b | |
| TOMM34 | Translocase of outer mitochondrial membrane 34 | 2.3a | −9.27b | |
| TOMM40 | Translocase of outer mitochondrial membrane 40 | 1.75 | 3.34a | |
| TOMM40L | Translocase of outer mitochondrial membrane 40L | 2.69a | −11.98b | |
| TOMM70a | Translocase of outer mitochondrial membrane 70a | 3.32a | −9.46b | |
| AIP | Aryl hydrocarbon receptor interacting protein | Mitochondrion protein import | 4.5a | −9.79b |
| COX10 | COX10 homolog, cytochrome c oxidase assembly protein | 5.1a | −7.58b | |
| COX18 | COX18 homolog, cytochrome c oxidase assembly protein | 4a | −13.66b | |
| DNAJC19 | DnaJ (Hsp40) homolog, subfamily C, member 19 | 4.38a | −9.79b | |
| GRPEL1 | GrpE-like 1 | 3.66a | 9.7a | |
| HSPD1 | Heat shock 60 kD protein 1 (chaperonin) | 3.18a | 14.5a | |
| MIPEP | Mitochondrial intermediate peptidase | 5.31a | −7.02b | |
| SH3GLB1 | SH3-domain GRB2-like endophilin B1 | 3.34a | 18.74a | |
| AIP | Aryl hydrocarbon receptor interacting protein | Targeting proteins to mitochondria | 4.5a | −9.79b |
| DNAJC19 | DnaJ (Hsp40) homolog, subfamily C, member 19 | 4.38a | −9.79b | |
| FXC1(TIMM10B) | Fracture callus 1 homolog | 3.14a | 6.44a | |
| GRPEL1 | GrpE-like 1 | 3.66a | 9.7a | |
| HSPD1 | Heat shock 60 kD protein 1 (chaperonin) | 3.18a | 14.5a | |
| MIPEP | Mitochondrial intermediate peptidase | 5.31a | −7.02b | |
| IMMP2L | IMP2 inner mitochondrial membrane peptidase-like | 1.87 | 1.07 | |
| TSPO | Translocator protein (18 kD) | 5.78a | −9.46b | |
| Slc25a1 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 1 | Small molecule transport | 7.57a | −6.93b |
| Slc25a10 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 10 | 4.69a | −11.49b | |
| Slc25a12 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 12 | 6.32a | −12.48b | |
| Slc25a13 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 13 | 9.92a | −7.32b | |
| Slc25a14 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 14 | 4.32a | −13.66b | |
| Slc25a15 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 15 | 4.82a | 1.27 | |
| Slc25a16 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 16 | 8.17a | 1.04 | |
| Slc25a17 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 17 | 3.76a | 7.77a | |
| Slc25a19 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 19 | 3.76a | −1.6 | |
| Slc25a2 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 2 | 5.06a | −11.49b | |
| Slc25a20 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 20 | 3.97a | 2.6a | |
| Slc25a21 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 21 | 1.06 | −11.49b | |
| Slc25a22 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 22 | 3.48a | −15.05b | |
| Slc25a23 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 23 | 23.43a | −7.37b | |
| Slc25a24 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 24 | 2.62a | −9.33b | |
| Slc25a25 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 25 | 3.58a | 4.11a | |
| Slc25a27 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 27 | 3.76a | −12.4b | |
| Slc25a3 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 3 | 3.81a | 80.9a | |
| Slc25a30 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 30 | 5.39a | −7.17b | |
| Slc25a31 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 31 | 1 | −11.49b | |
| Slc25a37 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 37 | 3.2a | −11.65b | |
| Slc25a4 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 4 | 2.91a | 32.4 | |
| Slc25a5 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 5 | 3.43a | 32.4 | |
| AIP | Aryl hydrocarbon receptor interacting protein | Mitochondrial transport | 4.5a | −9.79b |
| BAK1 | BCL2-antagonist/killer 1 | 6.11a | 7.1a | |
| BCL2 | B cell CLL/lymphoma 2 | 1.32 | −13.01b | |
| BCL2L1 | BCL2-like 1 | 1.79 | −3.61b | |
| FXC1 | Fracture callus 1 homolog (rat) | 3.14a | 6.44a | |
| HSP90AA1 | Heat shock protein 90 kD α (cytosolic), class A member 1 | 1.89 | −8.95b | |
| HSPD1 | Heat shock 60 kD protein 1 (chaperonin) | 3.18a | 14.5a | |
| IMMP2L | IMP2 inner mitochondrial membrane peptidase-like | 1.87 | 1.07 | |
| MFN2 | Mitofusin 2 | 4.32a | −12.92b | |
| MIPEP | Mitochondrial intermediate peptidase | 5.31a | −7.02b | |
| MTX2 | Metaxin 2 | 3.58a | −9.93b | |
| STARD3 | StAR-related lipid transfer (START) domain containing 3 | 4.38a | 1.75 | |
| TRP53 | Tumor protein p53 | 3.97a | −10b | |
| TSPO | Translocator protein (18 kD) | 5.78a | −9.46b | |
| UCP1 | Uncoupling protein 1 (mitochondrial, proton carrier) | 1 | −11.49b | |
| UCP2 | Uncoupling protein 2 (mitochondrial, proton carrier) | 4.08a | 41.87a | |
| UCP3 | Uncoupling protein 3 (mitochondrial, proton carrier) | 5.78a | −11.49b | |
| BAK1 | BCL2-antagonist/killer 1 | Membrane polarization and potential | 6.11a | 7.1a |
| BCL2 | B cell CLL/lymphoma 2 | 1.32 | −13.01b | |
| BCL2L1 | BCL2-like 1 | 1.79 | −3.61b | |
| TRP53 | Tumor protein p53 | 3.97a | −10b | |
| SOD1 | SOD 1, soluble | 6.02a | −9.27b | |
| BNIP3 | BCL2/adenovirus E1B interacting protein 3 | 14.93a | −2.77b |
Pathway expression analysis of genes sorted and grouped by related mitochondrial functions. ΔC(t) was calculated for each gene of interest followed by the ΔΔC(t) between groups and then fold change in expression (2−ΔΔCt) relative to the appropriate control, shown here as upregulation (positive values for fold change) or downregulation (negative values for fold change). Only absolute values for fold change >2 are noted.
Positive value for fold change.
Negative value for fold change.
Discussion
S1P1 activation is important for maintaining cell viability; global deletion is embryonically lethal.30 We previously demonstrated that the protective effect of S1P1 agonists FTY720 or SEW2871 in IRI12 was mediated by activation of S1P1 expressed on PT cells, independent of lymphopenia.13 Cisplatin use in cancer therapy is often limited by nephrotoxicity, specifically, PT injury. Here, we demonstrated that FTY720, a drug that is approved for use in treating multiple sclerosis, protects kidneys from cisplatin-induced injury. Administration of FTY720 before cisplatin reduced immune cell infiltration, preserved endothelial integrity, reduced apoptosis, and attenuated the increase in cytokine/chemokine expression in kidney. Conditional knockouts (PepckCreS1pr1fl/fl) demonstrated the protective phenotype of PT-S1P1 in cisplatin nephrotoxicity; cisplatin-induced injury was greater in the absence of PT-S1P1 both in vivo and in TKPTS cells after siRNA knockdown. Furthermore, the protective effect of FTY720 required PT-S1P1 as demonstrated both in vivo (PepckCreS1pr1fl/fl) and in vitro (siRNA knockdown of S1pr1 in TKPTS cells). The loss of FTY720 effectiveness in knockdown experiments, even without 100% deletion, could be due to a threshold for receptor number. The possibility that receptor downregulation could account for the loss of FTY720 protection in these cells will require future experiments examining receptor internalization, desensitization, functional uncoupling, or phosphorylation state. We find in vivo that both homozygous knockouts and mice heterozygous for PT S1pr1 deletion are more susceptible to injury, and FTY720 does not provide protection from IRI.13 Overexpression of S1pr1 rendered TKPTS cells resistant to cisplatin and increased mitochondrial stability, which may contribute to the S1P1-mediated protective mechanism in these cells. These studies, while further emphasizing the protective role of S1P1 in AKI, demonstrate that by directly targeting S1P1 on PT epithelial cells, FTY720 could provide a novel mechanism for protection from cisplatin-induced nephrotoxicity.
In AKI,9,31,32 inflammation plays a key role in cisplatin nephrotoxicity.8 Although TNF-α contributes prominently to cisplatin nephrotoxicity by inducing cytokines/chemokines and increasing immune cell infiltration, the role of other mediators is not as well defined. We have shown that FTY720 has a direct effect on PT-S1P1 to protect kidneys from the cytotoxic effects of cisplatin. Any potential protective effect of lymphopenia on AKI33 may have been masked by the absence of PT-S1P1, as also seen in FTY720 protection from IRI.13
Our in vitro studies with short-term siRNA knockdown studies confirmed that the protective effect of FTY720 in vivo required PT-S1P1. In addition, our in vitro studies demonstrate that FTY720 acts directly on TKTPS cells without involvement of immune cells8 to protect PT cells from direct toxicity. FTY720 could have an immunosuppressant effect in vitro, absent of immune cells, by reducing expression and release of proinflammatory mediators from TKPTS cells (Table 3). In contrast with our receptor deletion experiments, overexpressing S1P1 in PT cells enhances cell viability by reduced sensitivity to cisplatin toxicity, suggesting that protection may derive from increased S1P1 signaling driven by endogenous S1P and in an experimental therapeutic setting by FTY720. In experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis, FTY720 may have a direct neuroprotective effect that is dependent on the S1pr1 expression in astrocytes.34 In our studies, stimulation of PT-S1P1 reduced cisplatin-induced apoptosis, perhaps by blocking the mitochondrial apoptotic pathway, but additional studies are needed to further elucidate the mechanisms of PT-S1P1 stimulation.
A relatively large number of effective experimental treatments have been identified for prevention of cisplatin nephrotoxicity, but none have yet seen clinical use. Although our studies did not investigate whether FTY720 altered cisplatin’s antitumor activity, FTY720 may itself be useful in treating cancer and could theoretically potentiate the antitumor effect of cisplatin. FTY720 can (1) induce G1 phase arrest and apoptosis leading to inhibition of gastric cancer cell proliferation,35 (2) produce caspase-independent cell death of acute lymphoblastic leukemia cells,36 and (3) mediate lung tumor suppression via activation of PP2A-RIPK1–dependent necrosis/apoptosis.37
Our analysis of mitochondrial structure and function was prompted both by the importance of mitochondria as a target in cisplatin toxicity and the role of mitochondrial fragmentation in cell injury in the kidney.35,38,39 PT cells do not rapidly divide; therefore, mechanisms other than nuclear DNA cross-linking may be important in cisplatin nephrotoxicity. Accumulation of positively charged cisplatin metabolites inside negatively charged mitochondria may render mitochondrial DNA more susceptible to cross-linking than nuclear DNA. Renal PTs may therefore be especially sensitive to cisplatin because of their high density of mitochondria and the potential for activation of apoptosis and necrosis through a variety of pathways, including the mitochondrial pathway. The dynamic balance of mitochondrial fission/fusion in normal physiologic states preserves mitochondrial and cellular homeostasis. These mitochondrial shape changes occur in healthy cells during processes such as cell division, to maintain mitochondrial number and function, but also in cell injury and death, when they are accompanied by alterations in mitochondrial respiration and by oxidative damage due to increased mitochondrial reactive oxygen species. Disruption of either fission or fusion can contribute to mitochondrial dysfunction.40 Hence, there has been considerable interest in targeting mitochondria to improve or maintain mitochondrial stability and prevent tissue damage.41
Our findings on mitochondrial respiratory function and ultrastructure in S1P1-overexpressing TKPTS cells suggest that S1P1 could be a suitable target for maintaining mitochondrial integrity in kidney tubule cells. Although they not conclusive, the morphometric findings that mitochondria are longer and thinner in S1P1 overexpressing TKPTS cells suggest that the dynamic balance of fission and fusion may have shifted. This is consistent with a protective phenotype, as similar ultrastructural analyses show that mitochondria are elongated in PTs of control mice but are fragmented after IRI26; mitochondrial fragmentation contributes to disruption of mitochondrial function and eventually cell death in AKI.42 The observed increases in mitochondrial gene expression, such as those important for fission and fusion, and in basal mitochondrial respiration rate in cells overexpressing S1P1 support the concept that S1P1 play a role in mitochondrial stabilization. In addition, increased PGC-1α expression in these cells (data not shown) is consistent with increased mitochondrial biogenesis and the removal of reactive oxygen species to preserve cell function.43 Conversely, increased susceptibility to injury in mice or cells deficient in S1P1 is consistent with the decreases in gene expression revealed in the mitochondrial array and with observed changes in indicators of disrupted mitochondrial health (increased apoptosis and cytochrome c, changes in mitochondrial membrane potential, BAX translocation). In contrast with stable S1P1-overexpressing cells, mitochondrial morphometrics did not change in the transient knockdown experiments, but response to this short-term change in receptor expression may only be revealed after injury. It remains to be determined whether the protective effect of S1P on mitochondria is mediated through intracellular cascades triggered by binding to its cell surface receptor (S1P1) or through a direct effect of S1P on mitochondria.44,45
In summary, we have demonstrated that PT-S1P1 is an important target to attenuate cisplatin-induced AKI by preserving mitochondrial function and morphology. We conclude that targeting PT-S1P1 may represent a novel strategy in the prevention of cisplatin-induced AKI.
Concise Methods
Materials
Cisplatin (Sigma-Aldrich, St. Louis, MO), requiring reconstitution in saline, was used for initial experiments; subsequently, a formulation that is available in solution (1 mg/ml; Teva Parenteral Medicines Inc., Irvine, CA) was used. Both formulations produced the same experimental effect. FTY720 and FTY720-p, the phosphorylated, active form of FTY720, were kindly provided by Novartis (Basel, Switzerland). In contrast with in vivo studies, where the prodrug, FTY720, is activated through phosphorylation by endogenous sphingosine kinase 2, the phosphorylated, active form of FTY720, FTY720-p, was used for in vitro studies.
Animals and Drug Administration
All experiments were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the University of Virginia Animal Care and Use Committee. Experiments were performed on 10- to 12-week-old male C57Bl/6 mice; WT mice for initial experiments were from the National Cancer Institute (Frederick, MD). Global S1pr1 deficiency is embryonically lethal.30 PepckCreS1P1fl/fl mice were generated by breeding S1pr1fl/fl (generously provided by Dr. Richard L. Proia, NIH) and PepckCre mice (a kind gift of Dr. Volker Haase, Vanderbilt University)46 as previously described13 and littermates with WT S1pr1 alleles were used as controls. Mice were maintained on a standard diet and water was freely available. Cisplatin was dissolved in saline at a concentration of 1 mg/ml. Mice were given a single intraperitoneal injection of either vehicle (saline) or cisplatin (27 mg/kg body wt). FTY720 was prepared in a 3% fatty acid–free BSA (Sigma-Aldrich)/1×PBS solution (BSA/PBS vehicle). FTY720 (240 μg/kg) or BSA/PBS vehicle was administered intraperitoneally 1 hour before cisplatin and again 1 and 2 days after cisplatin. Blood was collected for measurement of plasma creatinine, anticoagulated blood was analyzed for leukocyte counts (HEMAVET 850; CDC Technologies, Oxford, CT), and mice were euthanized 72 hours after cisplatin.
Assessment of Kidney Function and Histology
Plasma creatinine, as a measure of kidney function, was determined using a colorimetric assay according to the manufacturer's protocol (Sigma-Aldrich). For histology, kidneys were fixed overnight in 0.2% sodium periodate/1.4% dl-lysine/4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (4% PLP), and embedded in paraffin. Kidney sections were prepared for hematoxylin and eosin staining as previously described47 and viewed by light microscopy (Carl Zeiss AxioSkop) under ×200 magnification. Photographs were taken and brightness/contrast adjustment was made with a SPOT RT camera (software version 3.3; Spot Imaging Solutions/Diagnostic Instruments Inc., Sterling Heights, MI). The histologic change in the outer medulla was expressed as ATN, scored on a scale of 1–5, as previously described.12
Quantitative Real-Time RT-PCR
Total RNA was extracted, and single-stranded cDNA was synthesized as previously described.12,13 Primers were obtained from Integrated DNA Technologies (Coralville, IA); primer sequences for S1P1–5,12 CXCL1, IL-6, and TNF-α,28 and IL-1β and MCP-148 were as described previously. Quantitative PCR was performed using a MyIQ Single Color Real-Time PCR Detection System iCycler (Bio-Rad). Samples were calculated with normalization to glyceraldehyde-3-phosphate dehydrogenase.13
Cell Culture: TKPTS Cells
Mouse PT kidney cells (TKPTS cells; E. Bello-Reuss, Texas Tech University, Galveston, TX), cells from a mouse PT cell line,49 were maintained in complete DMEM/F12 medium (Invitrogen, Carlsbad, CA; supplemented with 10% FBS and 1% penicillin/streptomycin) at 37°C in a humidified 5% CO2 atmosphere. For siRNA experiments, TKPTS cells were seeded in 12-well plates in complete medium without antibiotics the day before the experiment. Two ready-to-use validated double-stranded 21-nucleotide siRNAs for S1p1r (D-051684-01 and D-051684-04; 25 nM) were transfected into TKPTS cells along with ON-TARGETplus Nontargeting siRNA (D-002810-01; Dharmacon Inc., Lafayette, CA) using oligofectamine (Invitrogen) diluted in Opti-MEM I (Invitrogen) overnight according to the manufacturer’s instructions. Transfected cells were then incubated with fresh complete DMEM/F12 medium for a total period of 48 hours before RNA extraction or before treatment with cisplatin (20 μM; 24 hours) or FTY720-p (10 nM; 1 hour before cisplatin). The phosphorylated, active form of FTY720, FTY720-p, is needed for in vitro studies. Incubations with cisplatin or FTY720-p were the same for untransfected cells, and cells were harvested after 24 hours. These concentrations and periods of incubation were selected from pilot experiments measuring cleaved-caspase 3 by Western blot, as an indicator of apoptosis, in control TKPTS cells treated with varying concentrations of cisplatin (0–100 μm) with and without FTY720-p (10 nM) for varying periods of time (1–24 hours). For electron microscopy, TKPTS cells were either transfected with siRNAs for S1p1r or scrambled sequence as above, or control cells were treated every day for 3 days with vehicle or FTY720 (10 nM) and harvested 24 hours after the last treatment.
Isolation and Primary Culture of Renal TECs from PepckCreS1P1wt/wt and PepckCreS1P1fl/fl Mice
Primary TECs were isolated and propagated as described50 but with minor modifications. Briefly, kidneys were removed from 12- to 16-week-old male mice and placed in HBSS (Gibco, Carlsbad, CA) at 4°C, with penicillin and streptomycin (1×; Gibco). The capsule was removed and the cortex was trimmed away from the medulla using small curved scissors. Cortical pieces were minced with a razor blade and suspended in 10 ml HBSS containing collagenase type II (200 U/ml; Worthington Chemical, Lakewood NJ) and an equal weight (relative to collagenase) of soybean trypsin inhibitor (Invitrogen) then placed on a rotator (70 rpm) in a 37°C incubator for 30 minutes. The suspension was then split into two 15-ml conical tubes and 5 ml heat-inactivated horse serum (Gibco) was added to each tube. The tubes were vortexed for 30 seconds and then left to sit upright, undisturbed for 1 minute. The top 7–8 ml was transferred to a 50-ml conical tube. An aliquot of the tubule suspension was used to determine the total weight of the tubules in each sample; the remaining tubules were pelleted by centrifugation at 200 relative centrifugal force for 7 minutes at 4°C and washed once in HBSS. The final tubule pellet was resuspended at 1 mg/ml in kidney culture medium: DMEM/F-12 culture media (Gibco) with insulin, transferrin, and selenium (5 μg/ml, 2.75 μg/ml, and 3.35 ng/ml, respectively; Gibco), hydrocortisone (40 ng/ml; Sigma-Aldrich), mouse EGF (25 ng/ml; BD Biosciences, San Jose, CA), and penicillin and streptomycin (Gibco). Tubules were plated in 24-well (1.5 ml), 12-well (2.25 ml), and 6-well (3 ml) tissue culture plates for experiments. Media were changed 24 hours after plating and every 48–72 hours thereafter. EGF was only included in the media for the first 24 hours after plating. TECs migrate out of the tubules and form a confluent monolayer by days 7–10. The epithelial origin of the TECs was confirmed by prominent E-cadherin immunoreactivity (Clone DECMA-1; eBioscience, San Diego, CA; Supplemental Figure 7). Cells were used between days 7 and 10 of culture. In addition, genotyping of samples of tail and isolated tubules from PepckCreS1P1wt/wt and PepckCreS1P1fl/fl mice using PCR and primers selective for the wt and floxed alleles (as previously described13) confirmed our prior characterization of the PepckCreS1P1fl/fl mice13 and demonstrated conclusively for the first time that S1pr1 is deleted from kidney tubules (Supplemental Figure 7C).
Stable Overexpression of S1pr1 in TKPTS Cells
Confluent monolayers of TKPTS cells were transfected either with pcDNA3.1/V5-His expression vector encoding the mouse S1P1 (S1pr1-TKPTS) or the control pcDNA3.1 vector (pcDNA-TKPTS) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were grown for 24 hours and washed with PBS before treatment with geneticin (G418 sulfate; 300–500 μg/ml; Gibco) in DMEM/F12. Individual colonies, obtained at 2–3 weeks, were expanded. Stably transfected pcDNA-TKPTS and S1pr1-TKPTS were maintained in complete medium supplemented with G418 for selection of cells with neomycin resistance. The level of expression (by RT-PCR) of S1P1 in S1pr1-TKPTS was 29-fold compared with pcDNA-TKPTS-transfected cells (Supplemental Figure 5, A and B), and there was no change in expression of S1P3–S1P5 or sphingosine kinases 1 and 2 (data not shown). Western blot showed a band of the correct predicted molecular weight for S1P1 (rabbit×S1P1, 1:250, clone H-60; Santa Cruz Biotechnology, Dallas, TX51,52) in cytoplasmic and membrane fractions of cell lysates of S1pr1-TKPTS that was not visible in the membrane fraction after treatment of cells with 10 nM FTY720-p for 24 hours (presumably due to internalization of the receptor). S1P1 was undetectable in untransfected TKPTS cells or pcDNA-TKPTS cells (Supplemental Figure 5C).
Western Blot Analyses
Cell lysates were prepared from TKPTS cells or primary TECs by sonicating in RIPA lysis buffer (Thermo Fisher Scientific) enriched with 1% protease and phosphatase inhibitor cocktail (formulation: sodium fluoride, sodium orthovanadate, β-glycerophosphate, sodium pyrophosphate, aprotinin, bestatin, E64, leupeptin, EDTA; Thermo Fisher Scientific). Kidney samples were prepared in the same buffer but tissues were first homogenized by shaking with metal beads (50 s−1, 10 minutes) using a TissueLyser (Qiagen) followed by sonication to improve protein extraction. Homogenates were centrifuged (12,000 rpm, 15 minutes, 4°C), and the protein concentration in the supernatant was measured by using the BCA protein assay kit (Pierce). Equal volumes of the remaining cell or tissue lysate supernatants were boiled with Leammli buffer and β-mercaptoethanol (10 minutes, 100°C); proteins were separated using 15% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore). The membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 hour at room temperature and then incubated with primary antibody (mouse anti–β-tubulin, 1:5000, 3F3-G2; Santa Cruz Biotechnology), anti-NaK-ATPase (1:500; Abcam, Inc. Cambridge, MA) or anti–glyceraldehyde-3-phosphate dehydrogenase (6C5, 1:10,000; Ambion, Austin, TX) to compensate for variations in protein loading, rabbit anti–caspase-3 (recognizes both full-length and cleaved-caspase 3, 1:1000; Cell Signaling Technology, Boston, MA), or rabbit anti-BAX (1:500; Cell Signaling Technology) diluted in Odyssey blocking buffer/0.1% Tween. The membranes were washed twice for 5 minutes each in 0.05% Tween/phosphate buffer solution, probed with goat anti-rabbit IRDye 680RD- and goat anti-mouse IRDye 800CW-labeled secondary antibody (1:20,000; LI-COR) in Odyssey blocking buffer/0.1% Tween/0.01% SDS for 1 hour at room temperature, and washed twice for 5 minutes in Tween/phosphate buffer solution. Air-dried membranes were imaged and densitometric analysis of bands was performed by using a LI-COR Odyssey Infrared Imaging System with Odyssey 3.0 analytical software (LI-COR) for multiplex detection of the two different antigens.
In some experiments, mitochondrial and membrane fractions of TKPTS cells were prepared using a commercial kit (Thermo Fisher Scientific), and 20 μg of protein from each fraction was loaded per lane.
FACS Analyses
Flow cytometry was used to analyze kidney leukocyte content 72 hours after cisplatin. Kidneys were extracted, minced, and digested as previously described.53 After blocking nonspecific Fc binding with anti-mouse CD16/32 (2.4G2), fresh kidney suspensions were incubated with fluorophore-labeled anti-mouse CD45 (30-F11). CD45-labeled samples were then incubated with different combinations of fluorophore-labeled mouse anti-F4/80 (BM8), GR-1 (Ly6G), or CD11b. 7-AAD (BD Biosciences) was added 15 minutes before analyzing the samples to separate live from dead cells. Subsequent flow cytometry data acquisition was performed on FACS Calibur (Becton Dickinson, San Jose, CA) with Cytek 8 color flow cytometry upgrade (Cytek Development, Inc., Fremont, CA). Data were analyzed by FlowJo software 9.0 (Treestar, Ashland, OR). Unless otherwise noted, all antibodies were purchased from eBioscience and were used at a concentration of 5 μg/ml.
Cell Viability Assay
Viable cells in TKPTS cell or primary TEC cultures were measured by using a colorimetric assay that is based on the detection of dehydrogenase activity (Cell Counting Kit-8; Dojindo Molecular Technologies Inc.). Cells were cultured in 96-well plates (25–30,000 cells in a volume of 100 μl media) the day before experiments. Cells were incubated with vehicle or cisplatin (20 μM) for 20 hours; 2 hours before the end of the incubation, WST-8, the kit’s tetrazolium salt, was added to wells according to the manufacturer’s directions, and cultures were protected from light. The resulting orange-colored formazan, formed by reduction of WST-8, was detected by measuring absorbance at 450 nm, and OD was corrected for background absorbance of media alone.
Immunofluorescence Labeling and Microscopy
TKPTS cells and primary tubule epithelial cells were grown on glass slides with flexiPERM silicon chambers (Sarstedt, Newton, NC). TKPTS cells and TECs were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 30 minutes. Kidneys were fixed in 1% PLP (1% paraformaldehyde, 1.4% dl-lysine, 0.2% sodium periodate in 0.1 M phosphate buffer, pH 7.4) overnight, incubated in 30% sucrose for 48 hours at 4°C, and embedded and frozen in optimal cutting temperature compound (Ted Pella Inc.). Frozen sections (7 μm) or fixed cells were permeabilized with 0.3% Triton X-100, and nonspecific binding was blocked with 10% horse serum (cells and kidney sections) and anti-mouse CD16/32 (10 μg/ml, kidney sections only; clone 2.4G2; StemCell Technologies Inc, Vancouver, BC, Canada). Sections or cells were labeled with FITC-labeled anti-neutrophil mAb (7 μg/ml, clone 7/4; Cedarlane, Burlington, NC), APC-labeled rat anti-CD31/PECAM-1 (5 μg/ml, clone MEC 13.3; BioLegend, San Diego, CA), or APC-labeled rat anti-F4/80 (7 μg/ml, clone BM8; Invitrogen) for 1 hour. Kidney sections or cells were stained with rabbit anti-mouse CXCL1 or MCP-1 (2.5 μg/ml; PeproTech, Rocky Hill, NJ) followed by Cy3-labeled donkey anti-rabbit IgG (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA). Apoptotic cells were detected by TUNEL assay (In Situ Cell Death Detection kit, TMR red; Roche) according to the manufacturer’s instructions. For mitochondrial studies, TKPTS cells or primary TECs were labeled with mouse anti-BAX (1:100 [Cell Signaling Technology]; followed by Alexa647-labeled donkey anti-mouse IgG, 1:500 [Molecular Probes]), MitoTrackerRed (25 nM, 30 minutes at 37°C; Invitrogen), mouse anti-cytochrome c (1:100 [Cell Signaling Technology]; followed by Alexa647-labeled donkey anti-mouse IgG, 1:500 [Molecular Probes]), MitoSox (5 μM, 10 minutes; Invitrogen), or JC-1 (10 μg/ml, 20 minutes at 37°C; Invitrogen). JC-1 is a mitochondrial membrane potential–sensitive carbocyanine probe; monomeric green fluorescent JC-1 is taken up into healthy higher membrane potential mitochondria, where it reversibly forms red-fluorescent aggregates, whereas with loss of mitochondrial membrane potential, JC-1 remains in the cytoplasm or diffuses out of mitochondria and is green. Actin was labeled with phalloidin-FITC (1 μg/ml; Sigma-Aldrich). Primary TECs were labeled with E-cadherin (see Supplemental Figure 7 for details). Nuclei were visualized using 4′,6-diamidino-2-phenylindole (DAPI). All specimens were mounted with ProLong Gold Antifade reagent with DAPI (Invitrogen). Images were acquired using the Carl Zeiss Axiovert 200 microscopy system with ApoTome imaging and Axiovision software (Carl Zeiss Microscopy, LLC, Thornwood, NY).
Electron Microscopy
Confluent cultures of TKPTS cells (stably expressing empty pcDNA vector or mS1P1 construct, or after transient S1pr1 siRNA knockdown or FTY720 treatment) were harvested, fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 M phosphate buffer for 45 minutes, washed with cold PBS and prepared for electron microscopy by incubating in 2% osmium (in 0.1 M phosphate buffer, pH 7.4) for 1 hour and then washing and dehydrating through a graded series of ethanol, and embedding in EmBed812 embedding resin (Electron Microscopy Sciences, Fort Washington, PA). Serial ultrathin sections (80–90 nm) of TKPTS cells were collected on copper mesh grids and stained with uranyl acetate and Reynolds lead citrate. Two to three grids from each sample were examined using a JEOL 1230 transmission electron microscope, and digital photographs of cells were captured by real-time digital imaging.
Ultrastructural Analyses
Electron micrographs were opened in StereoInvestigator software (MBF Bioscience, Williston, VT), which was calibrated to match the scale of the images, and mitochondrial contours within 20–25 cells/group (yielding >300 mitochondrial contours/group) were drawn to obtain an unbiased estimate of mitochondrial morphology. Contour measurements (feret minimum and maximum, area, perimeter, shape factor, compactness, roundness, and convexity) were automatically calculated using embedded equations within the software. Feret maximum (the longest projection of the minimal bounding box, i.e., parallel tangents apposing opposite sides of the profile), compactness (values are 0–1; a circle has a value of 1), and shape factor (defines a relationship of perimeter to area; a circle has a value of 3.54) are reported.
Measurements of Oxygen Consumption Rate and Mitochondrial Function in TKPTS Cells and Primary Cultures of Kidney PT Cells
The oxygen consumption rate (OCR), as an indicator of mitochondrial bioenergetics in whole cells, was measured using a Seahorse XF-24 Flux Analyzer (Seahorse Biosciences, Billerica, MA) as described.54 TKPTS cells were seeded in Seahorse 24-well tissue culture plates at a density of 2.5×104 cells/well and allowed to adhere for 24 hours. Primary cultures of TECs from kidney were grown to confluence on Seahorse 24-well tissue culture plates, as described above. Cells were treated with vehicle (saline) or cisplatin (20–60 μM for TKPTS cells; 20–40 μM for primary tubule cells) for 20 hours. Before the assay, the media was changed to unbuffered DMEM (12800-017, pH 7.4, 37°C; Gibco), and cells were equilibrated for 30 minutes at 37°C. After measuring basal respiratory rate, oligomycin (uncouples ATP-coupled respiration by inhibiting ATP synthase, 5 μM; Sigma-Aldrich), FCCP (750 nM and 1 μM, mitochondrial uncoupling agent that uncouples mitochondrial respiration from ATP and reveals maximal respiratory rate; Sigma-Aldrich), and rotenone (1 μM; Sigma-Aldrich) with antimycin A (electron transport chain [complex I and III) inhibitors, eliminates all mitochondrial respiration, 10 μM; Sigma-Aldrich) were injected sequentially during the assay. FCCP was used at two doses and monitored over time because it has a narrow dosing range in which it induces the maximal rate of mitochondrial respiration.54 OCR was measured in 3-minute periods of time (over a total period of 2 hours). Basal mitochondrial respiration, ATP-linked respiration, proton leak (non-ATP–linked oxygen consumption), maximal respiration, nonmitochondrial respiration, reserve respiratory capacity, respiratory control ratio, and coupling efficiency were determined in whole cells according to Brand et al.29 Three to four wells were used for each experimental group.
Parameters of mitochondrial function were calculated from the respiration rates measured at times before and after the addition of modulators of mitochondrial function. Basal mitochondrial respiration is total basal respiration rate minus nonmitochondrial respiration. ATP-linked respiration is revealed by the degree of decrease from basal respiration in the presence of oligomycin. Maximal mitochondrial respiration is measured after the addition of FCCP (with subtraction of nonmitochondrial respiration). Mitochondrial reserve capacity is the maximal minus basal mitochondrial respiration. Proton leak is the oligomycin-induced respiration minus nonmitochondrial respiration. Finally, nonmitochondrial respiration is the oxygen consumption remaining when the mitochondrial electron transport chain is inhibited by rotenone and antimycin A.
Mitochondrial PCR Array Profiling
Total RNA from TKPTS (pcDNA- and S1pr1-transfected; three culture plates of each, pooled) and primary epithelial cells (from PepckCreS1pr1wt/wt and PepckCreS1pr1fl/fl mice; cultures from three mice each, pooled) was extracted using TriReagent (Life Technologies, Grand Island, NY). Total RNA was treated with RNase-free DNase, and RT was performed with 1 μg of total RNA using an RT2 First-Strand Kit (SABioscience, Frederick, MD). Approximately 900 ng of cDNA was processed for quantitative real-time RT-PCR (RT-qPCR) of 84 genes involved in mitochondrial biogenesis and 12 housekeeping genes, including internal controls, by using an RT2 Profiler PCR Array Kit (RT2 Profiler PCR Array Mouse Mitochondria, PAMM-087Z; SABioscience) and Bio-Rad CFX96 real-time PCR system. PCR products were quantified by measuring SYBR Green fluorescent dye, and threshold cycle (Ct) values were calculated. An integrated web-based software package for the PCR Array System performed DDCt-based fold-change calculations from the uploaded raw threshold cycle data.
Statistical Analyses
GraphPad Instat 3 (GraphPad Software Inc., La Jolla, CA), SigmaPlot 11.0 (Systat Software Inc., Chicago, IL), and Canvas X (ACD Systems International Inc., Seattle, WA) were used to analyze and present the data. Data were analyzed, after transformation if needed to generate a normal distribution, by two-tailed t test or one- or two-way ANOVA with post hoc analysis as appropriate. P<0.05 was used to indicate significance.
Disclosures
M.D.O. received a grant from SphynKx Therapeutics.
Supplementary Material
Acknowledgments
We gratefully acknowledge the University of Virginia Advanced Microscopy Facility for ultrathin sectioning and staining and the University of Virginia Research Histology core, Dr. V. Brinkmann (Novartis, Basel, Switzerland) for providing FTY720-p, Dr. Elsa Bello-Reuss (Texas Tech University) for providing TKPTS cells, and all members of the Okusa laboratory.
This work was supported by grants from the American Diabetes Association (1-11-JF-17 to K.L.H.) and the National Institutes of Health (R01-DK085259, R01-DK062324, and T32-DK072922 to M.D.O.; R01-GM067958 to K.R.L.; AHA-11SDG7000007, K01-DK091444, and 2P30-DK079337 to A.B.; and K01-DK088967, R03-DK099489, 2P30-DK079337 to G.R.K.). M.D.O. received a grant from SphynKx Therapeutics.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121351/-/DCSupplemental.
References
- 1.Wang D, Lippard SJ: Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4: 307–320, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Beyer J, Rick O, Weinknecht S, Kingreen D, Lenz K, Siegert W: Nephrotoxicity after high-dose carboplatin, etoposide and ifosfamide in germ-cell tumors: Incidence and implications for hematologic recovery and clinical outcome. Bone Marrow Transplant 20: 813–819, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bulacio RP, Torres AM: Organic anion transporter 5 (Oat5) renal expression and urinary excretion in rats treated with cisplatin: A potential biomarker of cisplatin-induced nephrotoxicity. Arch Toxicol 87: 1953–1962, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E: Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol 167: 1477–1484, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabla N, Murphy RF, Liu K, Dong Z: The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol 296: F505–F511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Okusa MD: Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata M, Herrington J, Zager RA: Sphingosine: A mediator of acute renal tubular injury and subsequent cytoresistance. Proc Natl Acad Sci U S A 92: 8970–8974, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiegel S, Milstien S: Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta 1484: 107–116, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T: Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem 277: 6667–6675, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Spiegel S, Milstien S: Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 277: 25851–25854, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H: Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR: The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Pelletier D, Hafler DA: Fingolimod for multiple sclerosis. N Engl J Med 366: 339–347, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C: The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther 323: 626–635, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T: Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Yanagawa Y, Masubuchi Y, Chiba K: FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer’s patches by FTY720-induced lymphocyte homing. Immunology 95: 591–594, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkmann V: Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115: 84–105, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S: Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO: Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293: H3150–H3158, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Moon MH, Jeong JK, Lee YJ, Park SY: FTY720 protects neuronal cells from damage induced by human prion protein by inactivating the JNK pathway. Int J Mol Med 32: 1387–1393, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Li S, Niu X, Wang P, Wang J, Zhang M: Protective effect of FTY720 against sevoflurane-induced developmental neurotoxicity in rats. Cell Biochem Biophys 67: 591–598, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD: IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brand MD, Nicholls DG: Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL: Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106: 951–961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Eckle T: Ischemia and reperfusion—from mechanism to translation. Nat Med 17: 1391–1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosin DL, Okusa MD: Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, Rabb H: Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int 75: 526–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J: FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108: 751–756, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng T, Meng X, Wang J, Chen X, Yin D, Liang Y, Song X, Pan S, Jiang H, Liu L: PTEN- and p53-mediated apoptosis and cell cycle arrest by FTY720 in gastric cancer cells and nude mice. J Cell Biochem 111: 218–228, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ: FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy 7: 707–715, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP, Senkal CE, Garrett-Mayer E, De Palma RM, Fedarovich D, Liu A, Habib AA, Stahelin RV, Perrotti D, Ogretmen B: Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med 5: 105–121, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funk JA, Schnellmann RG: Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan M, Brooks C, Liu F, Sun L, Dong Z: Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83: 568–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreux PA, Houtkooper RH, Auwerx J: Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov 12: 465–483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP: Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest 85: 1599–1613, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM: PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S: Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J 25: 600–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez L, Paillard M, Price M, Chen Q, Teixeira G, Spiegel S, Lesnefsky EJ: A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol 106: 1341–1353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL, Lynch KR, Lobo PI, Li L, Okusa MD: Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J Immunol 189: 2584–2596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD: Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ernest S, Bello-Reuss E: Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Breggia AC, Himmelfarb J: Primary mouse renal tubular epithelial cells have variable injury tolerance to ischemic and chemical mediators of oxidative stress. Oxid Med Cell Longev 1: 33–38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H: STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 16: 1421–1428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omori K, Shikata Y, Sarai K, Watanabe N, Wada J, Goda N, Kataoka N, Shikata K, Makino H: Edaravone mimics sphingosine-1-phosphate-induced endothelial barrier enhancement in human microvascular endothelial cells. Am J Physiol Cell Physiol 293: C1523–C1531, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, Murrow BA, Calderone JA, Huang L, Divakaruni AS, Tomsig JL, Okabe K, Lo RH, Cameron Coleman G, Columbus L, Yan Z, Saucerman JJ, Smith JS, Holmes JW, Lynch KR, Ravichandran KS, Uchiyama S, Santos WL, Rogers GW, Okusa MD, Bayliss DA, Hoehn KL: Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metab 3: 114–123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.