Abstract
Both fibroblast growth factor 23 (FGF-23) and asymmetric dimethylarginine (ADMA) are associated with progression of CKD. We tested the hypothesis that ADMA and FGF23 are interactive factors for CKD progression in a cohort of 758 patients with CKD in Southern Europe (mean eGFR±SD, 36±13 ml/min per 1.73 m2) and in a central European cohort of 173 patients with CKD (MMKD study, mean eGFR, 64±39 ml/min per 1.73 m2). In the first cohort, 214 patients had renal events (decrease in eGFR of >30%, dialysis, or kidney transplantation) during a 3-year follow-up. Both intact FGF-23 and ADMA predicted the incidence rate of renal events in unadjusted and adjusted analyses (P<0.001). There was a strong competitive interaction between FGF-23 and ADMA in the risk of renal events (P<0.01 in adjusted analyses); the risk associated with raised ADMA levels was highest in patients with low FGF-23 levels. These results were confirmed in the MMKD cohort, in which FGF-23 level was again an effect modifier of the relationship between plasma ADMA level and renal events (doubling of baseline serum creatinine, dialysis, or kidney transplantation) in the adjusted analyses (P<0.01). Furthermore, in the MMKD cohort there was a parallel, independent competitive interaction between symmetric dimethylarginine level and c-terminal FGF-23 level for the risk for renal events (P=0.001). These findings indicate that the association of ADMA level with the risk of CKD progression is modified by FGF-23 level and provide further evidence that dysregulation of the nitric oxide system is involved in CKD progression.
Keywords: chronic kidney disease, clinical epidemiology, Epidemiology and outcomes
Fibroblast growth factor 23 (FGF-23) is a pleiotropic hormone that is central to the regulation of bone mineral metabolism in health1,2 and disease states, including cardiovascular,3 metabolic,4 and renal diseases.5 In CKD, plasma FGF-23 increases at an early stage preceding the decline in 1,25-dihydroxyvitamin D [1,25(OH)2 D] and the rise in parathyroid hormone (PTH)6 and contributes to adaptive mechanisms that allow maintenance of phosphate balance in the face of nephron loss.7 However, kidney damage and progression of CKD may be the tradeoff of this mechanism, as indicated in experimental studies in animal models.8,9 In line with these experimental observations, two CKD cohort studies, the Mild and Moderate Kidney Disease (MMKD) study in central Europe,10 and four studies in the United States,11–14 as well as a large study in transplant recipients in Hungary,15 have coherently associated high plasma FGF-23 with CKD progression toward kidney failure.
Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide (NO) synthase and a modulator of cardiovascular16 and renal17 function, has been reported to be a risk factor for endothelial dysfunction18 and atherosclerosis19 associated with progression of CKD in several cohort studies.20–23 Experimental data show that ADMA administration in uninephrectomized mice induced glomerular and vascular fibrosis, with elevated deposits of collagen I, III, and fibronectin, as well as reduced peritubular capillaries.24
Interestingly, FGF-23 interferes with the NO system and is inversely associated with endothelium-dependent vasodilatation both in elderly people in the general population25 and in patients with CKD26 and represents a causal risk factor for left ventricular hypertrophy in experimental models.27 Furthermore, coherent evidence in CKD26,28 shows that FGF-23 is directly related to ADMA. The FGF-23–ADMA link may be mechanistically relevant because our previous study in patients with CKD found that the association between plasma FGF-23 and endothelial function (as measured by the forearm blood flow response to ischemia) was markedly attenuated after statistical adjustment for plasma ADMA concentration.26 This finding suggests that FGF-23 and ADMA are in the same causal pathway conducive to endothelial dysfunction. Integrity of the endothelium is fundamental for kidney health, and endothelial dysfunction has been associated with susceptibility to renal damage in experimental models29 and observational studies in CKD.30 Whether and how a putative FGF-23–ADMA interaction is implicated in progression of CKD are still unknown.
With this background in mind, we tested the hypothesis that elevated intact FGF-23 and ADMA levels may be interactive factors in the high risk for CKD progression in a large, well characterized cohort of patients with stage 2–5 CKD. To externally validate our findings, we tested whether the same interaction could be replicated in the MMKD cohort10 (i.e., in the cohort where for the first time high FGF-23 was associated with a high risk for CKD progression). Furthermore, limited to the MMKD cohort, we also tested the interaction between FGF-23 and another major methylarginine strictly related to ADMA, symmetric dimethylarginine (SDMA), which inhibits NO synthase by competing with l-arginine transport into endothelial cells.
Results
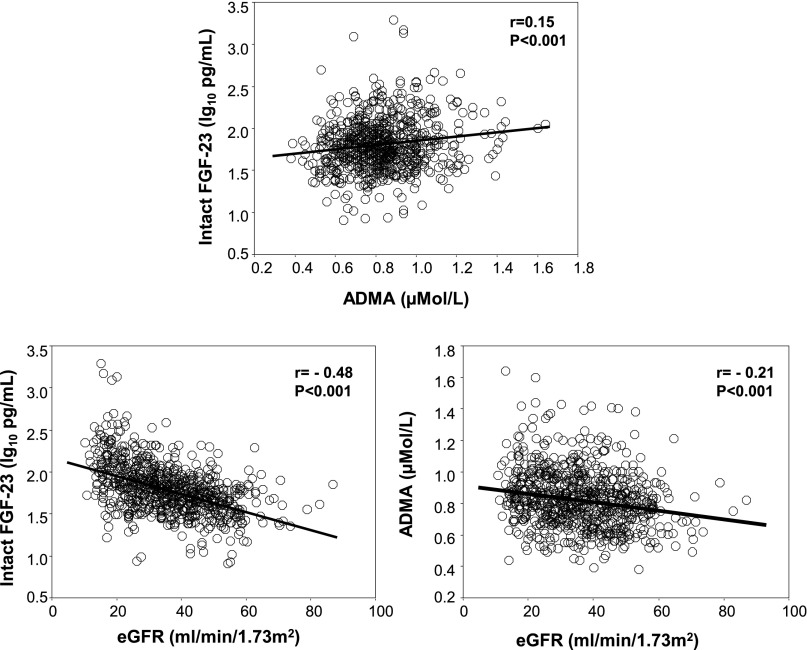
The Southern Italy cohort included 758 patients with stage 2–5 CKD (Table 1). Their mean age was 62±11 years, the average eGFR was 36±13 ml/min per 1.73 m2, and the median proteinuria was 0.6 g/24 hours (interquartile range [IQR], 0.2–1.5 g/24 hours). The average duration of follow-up was 29±11 months (range, 1.4–48 months). Sixty percent of patients were men, 50% were smokers, and 35% had diabetes. Overall, 237 patients (31%) had background cardiovascular comorbidities and 693 patients (91%) were receiving antihypertensive treatment. In this cohort, intact FGF-23 had a positively skewed distribution, with a median value of 61 pg/ml (IQR, 41–90 pg/ml). Plasma ADMA levels in the same cohort were normally distributed, with an average value of 0.83±0.18 µmol/L. On univariate analysis, circulating levels of intact FGF-23 and ADMA were significantly correlated (r=0.15; P<0.001), and both were inversely related to eGFR (Figure 1).
Table 1.
Main clinical and biochemical data of the Southern Italy study cohort
| Variable | Whole Group (n=758) | Nonprogressors (n=514) | Progressors (n=244) | P Value |
|---|---|---|---|---|
| Age (yr) | 62±11 | 62±11 | 61±11 | 0.10 |
| Men (%) | 60 | 60 | 59 | 0.72 |
| Smokers (%) | 50 | 48 | 53 | 0.20 |
| Diabetes (%) | 35 | 34 | 37 | 0.34 |
| With cardiovascular comorbidities (%) | 31 | 31 | 32 | 0.89 |
| Receiving oral hypoglycemic therapy (%) | 16 | 16 | 16 | 0.97 |
| Receiving insulin therapy (%) | 17 | 16 | 19 | 0.20 |
| Receiving antihypertensive treatment (%) | 97 | 96 | 98 | 0.13 |
| BMI (kg/m2) | 28.2±4.7 | 28.2±4.7 | 28.3±4.7 | 0.85 |
| Systolic BP (mmHg) | 134±18 | 132±17 | 137±19 | <0.001 |
| Diastolic BP (mmHg) | 78±11 | 77±10 | 79±11 | 0.003 |
| Heart rate (beats/min) | 72±10 | 72±9 | 73±10 | 0.20 |
| Creatinine (mg/dl) | 2.52±1.17 | 1.88±0.65 | 2.50±0.89 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 36±13 | 39±13 | 29±13 | <0.001 |
| Urinary protein (g/24 hr) | 0.60 (0.20–1.47) | 0.50 (0.20–1.10) | 1.10 (0.60–2.30) | <0.001 |
| Glucose (mg/dl) | 116±49 | 116±47 | 116±53 | 0.84 |
| Cholesterol (mg/dl) | 187±45 | 187±43 | 186±47 | 0.90 |
| HDL cholesterol (mg/dl) | 50±17 | 50±18 | 50±15 | 0.53 |
| LDL cholesterol (mg/dl) | 112±43 | 111±39 | 115±49 | 0.45 |
| Triglycerides (mg/dl) | 152±80 | 150±79 | 154±80 | 0.58 |
| Hemoglobin (g/dl) | 12.8±1.8 | 13.1±1.8 | 12.3±1.7 | <0.001 |
| Albumin (g/dl) | 4.2±0.5 | 4.2±0.5 | 4.0±0.5 | <0.001 |
| hs-C-reactive protein (mg/dl) | 2.35 (1.04–5.46) | 2.20 (1.02–5.41) | 2.61 (1.09–5.63) | 0.52 |
| ADMA (µmol/L) | 0.83±0.18 | 0.81±0.18 | 0.87±0.19 | <0.001 |
| Calcium (mg/dl) | 9.4±0.7 | 9.5±0.6 | 9.2±0.7 | <0.001 |
| Phosphate (mg/dl) | 3.7±0.8 | 3.6±0.7 | 4.0±0.9 | <0.001 |
| Fractional phosphate excretion | 0.31 (0.20–0.45) | 0.31 (0.19–0.42) | 0.33 (0.22–0.51) | 0.01 |
| PTH (pg/ml) | 76 (52–124) | 68 (48–113) | 118 (75–184) | <0.001 |
| Intact FGF-23 (pg/ml) | 60.7 (41.4–91.2) | 55.3 (38.0–77.7) | 80.3 (52.3–138.1) | <0.001 |
| 1,25(OH)2D (pg/ml) | 29 (21–38) | 31 (24–40) | 25(18–33) | <0.001 |
| 25(OH)D (pg/ml) | 12.8 (9.6–17.2) | 13.6 (10.4–17.9) | 11.2 (7.7–14.4) | <0.001 |
Data are expressed as mean±SD, median and interquartile range, or percentage frequency, as appropriate. BMI, body mass index; hs, high-sensitivity.
Figure 1.
Associations between intact FGF-23, ADMA, and eGFR in the Southern Italy cohort. Data are correlation coefficient and P value.
Patients of the MMKD cohort were younger (mean age, 46±12 years) and had higher GFR (64±39 ml/min per 1.73 m2) than the other cohort. In these patients, the median value of intact FGF-23 was 34 pg/ml (IQR, 23–56 pg/ml) and that of c-terminal FGF-23 was 85 rU/ml (IQR, 49–183 rU/ml). The mean ADMA value was 0.47±0.12 µmol/L. The remaining characteristics of patients of the MMKD cohort are summarized in Supplemental Appendix 1.
Correlation Analyses in the Southern Italy Cohort
Intact FGF-23 correlated directly with serum PTH, serum phosphate, and fractional phosphate clearance; the use of antihypertensive drugs; 24-hour urinary protein; and serum triglycerides and correlated inversely with eGFR (Figure 1 and Supplemental Appendix 2), as well as with 1,25(OH)2D, and 25-hydroxyvitamin D (25[OH]D), hemoglobin and albumin. Plasma ADMA correlated directly with serum phosphate, 24-hour proteinuria, serum PTH, and use of insulin and correlated inversely with eGFR, 1,25(OH)2 D, 25(OH)D, hemoglobin, male sex, and serum calcium (Supplemental Appendix 2). In a multiple linear regression analysis of eGFR that included a series of potential confounders (hemoglobin, 1,25[OH]2D, 25[OH]D, PTH, phosphate, and 24-hour urinary protein), as well as all univariate correlates (P≤0.10) of intact FGF-23 and ADMA, intact FGF-23 was the first factor in rank (β=−0.29; P<0.001) explaining the variability in eGFR, followed by hemoglobin, 1,25(OH)2D, PTH, 24-hour urinary protein, phosphate, oral hypoglycemic therapy, systolic BP, ADMA, and C-reactive protein (Table 2). Introducing fractional phosphate excretion instead of serum phosphate into this model did not materially affect the results of the multivariate modeling (data not shown).
Table 2.
Multiple linear regression analysis of eGFR in the Southern Italy study cohort
| Variable | β | P Value |
|---|---|---|
| FGF-23 | −0.29 | <0.001 |
| Hemoglobin | 0.21 | <0.001 |
| 1,25(OH)2D | 0.19 | <0.001 |
| PTH | −0.17 | <0.001 |
| 24-hr urinary protein | −0.11 | 0.001 |
| Phosphate | −0.11 | 0.001 |
| Oral hypoglycemic therapy | 0.10 | 0.001 |
| Systolic BP | −0.09 | <0.01 |
| ADMA | −0.07 | 0.01 |
| C-reactive protein | −0.07 | 0.02 |
| 25(OH)D | −0.08 | 0.06 |
| Sex | 0.05 | 0.11 |
| Calcium | −0.04 | 0.17 |
| Antihypertensive treatment | 0.04 | 0.22 |
| Triglycerides | −0.03 | 0.37 |
| Insulin therapy | 0.02 | 0.51 |
| Age | 0.01 | 0.75 |
| Body mass index | −0.005 | 0.87 |
| LDL cholesterol | −0.003 | 0.92 |
| Albumin | 0.00 | 1.00 |
Data are expressed as standardized regression coefficients and P values.
FGF-23, ADMA and Risk of CKD Progression in the Southern Italy Cohort
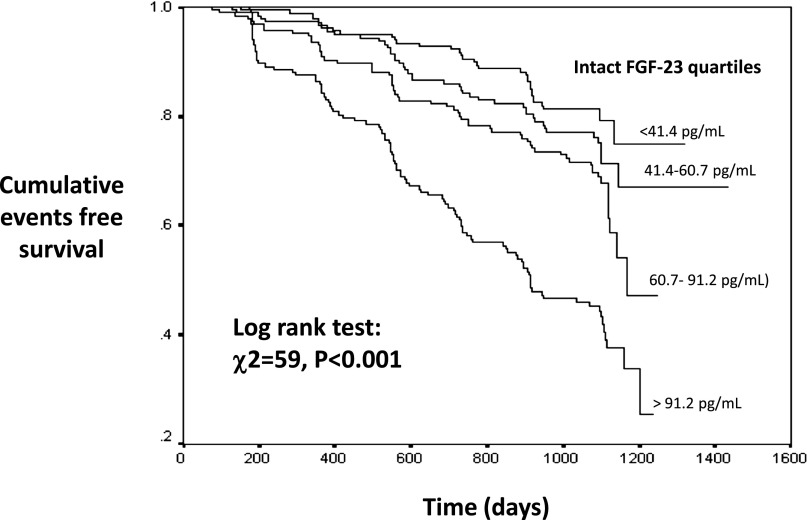
During the follow-up period, 244 patients had renal events (progressors)—i.e., a decrease in GFR of >30%, dialysis, or kidney transplantation—and 42 patients died. Progressors had higher circulating levels of intact FGF-23 and ADMA as well as higher systolic and diastolic BP, serum PTH, serum phosphate and fractional phosphate excretion, and proteinuria and lower hemoglobin, albumin, calcium, 1,25(OH)2D, 25(OH)D, and eGFR compared with nonprogressors (Table 1). In a Kaplan–Meier survival analysis, the risk of renal outcomes was higher in patients with increased levels of intact FGF-23; this risk was highest in patients in the fourth FGF-23 quartile (hazard ratio [HR], 4.0; 95% confidence interval [95% CI], 2.7 to 5.9) and intermediate in those in the third (HR, 1.9; 95% CI, 1.2 to 2.9) and the second (HR, 1.3; 95% CI, 0.9 to 2.1) quartiles compared with the lowest intact FGF-23 quartile (Figure 2). In a multivariate Cox regression analysis that include FGF-23 and ADMA expressed as a continuous variables, both FGF-23 and ADMA significantly predicted incident renal events (Supplemental Appendix 3A) in analyses adjusted for age, sex, albumin, phosphate, PTH, hemoglobin, 1,25(OH)2D, 25(OH)D, and 24-hour urinary protein, as well as for the full set of variables considered for the GFR model (Table 2). Introducing fractional phosphate excretion instead of serum phosphate into the model did not materially affect the strength of the relationship between FGF-23, ADMA, and renal events (data not shown). Similarly, in the MMKD cohort, Cox regression analysis adjusted for age, sex, proteinuria, and GFR (measured by iohexol clearance) showed that intact FGF-23 (HR for 20 pg/ml, 1.17; 95% CI, 1.09 to 1.26; P<0.001) and c-terminal FGF-23 (HR for 20 rU/ml, 1.04; 95% CI, 1.02 to 1.05; P<0.001) as well as ADMA (intact FGF-23–based model: HR for 0.1 µmol/L, 1.73 [95% CI, 1.30 to 2.31; P<0.001]; c-terminal FGF-23–based model: HR for 0.1 µmol/L, 1.70 [95% CI, 1.27 to 2.27; P<0.001]) were significant predictors of CKD progression (doubling of baseline serum creatinine, dialysis therapy, or kidney transplantation) (Supplemental Appendix 3B). This has already been shown in former analyses in this cohort.10,21
Figure 2.
Kaplan–Meier survival curves for renal events according to quartiles of intact FGF-23 in the Southern Italy cohort.
Effect Modification by ADMA on the FGF-23–Renal Events Relationship in the Two Cohorts
In the Southern Italy cohort, both on univariate (P<0.01) and multivariate (P<0.01) (see Figure 3, upper panel) analyses a significant inverse interaction was found between intact FGF-23 and ADMA for explaining incident events in CKD progression. This analysis (Table 3) revealed that the risk of progression associated with a fixed increase in ADMA levels (0.1 μmol/L) was closely dependent on intact FGF-23 values. In fact, the risk excess for renal events associated with ADMA was highest in patients with low intact FGF-23 levels and progressively decreased as intact FGF-23 increased (Figure 3, upper panel). Thus, effects of FGF-23 and ADMA on CKD progression depend on their plasma levels. In other words, when FGF-23 levels are low, an increase in plasma ADMA levels translates into a higher risk of renal events, while such an effect is nullified at high FGF-23 levels. A survival analysis that considered a combined endpoint of renal events or death showed that the effect modification by intact FGF-23 of the relationship between ADMA and this composite outcome (P<0.01) was of similar magnitude and went in the same direction of that observed for renal events.
Figure 3.
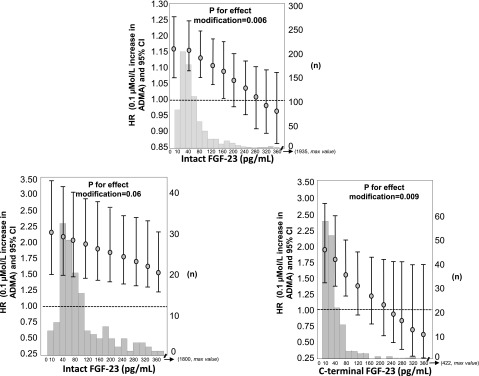
Effect modification by intact FGF-23 on the relationship between ADMA (0.1 µmol/L increase) and the relative risk of renal events in the Southern Italy cohort (top panel). Data were adjusted for all variables listed in Table 3, including ADMA and intact FGF-23 as single variables. In the bottom panels, the effect modification by intact FGF-23 and c-terminal FGF-23 on the ADMA-renal events link in the MMKD cohort is reported. Data were adjusted for ADMA and intact FGF-23 or c-terminal FGF-23 as single variables, as well as for age, sex, proteinuria and GFR. Data are HR, 95% CI, and P value. Histograms of intact and c-terminal FGF-23 in the two cohorts are also shown in the background.
Table 3.
Unadjusted and fully adjusted HRs of the FGF-23-ADMA interaction for the risk of renal events in the Southern Italy cohort
| Variable | Units of Increase | Unadjusted HR (95% CI), P Value | Fully Adjusted ( HR [95% CI], P Value |
|---|---|---|---|
| Intact FGF-23 | 20 pg/ml | 1.15 (1.06 to 1.24), P<0.001 | 1.17 (1.06 to 1.29), P=0.001 |
| ADMA | 0.1 µmol/L | 1.20 (1.11 to 1.29), P<0.001 | 1.17 (1.07 to 1.28), P=0.001 |
| Intact FGF-23×ADMA interaction | 20 pg/ml × 0.1 µmol/L | 0.988 (0.980 to 0.997), P=0.008 | 0.984 (0.973 to 0.995), P=0.006 |
| eGFR | 0.1 µmol/L | 0.97 (0.96 to 0.98), P<0.001 | |
| Age | 1 yr | 0.99 (0.98 to 1.00), P=0.25 | |
| Antihypertensive treatment | 0=no; 1=yes | 1.41 (0.52 to 3.86), P=0.50 | |
| Triglycerides | 20 mg/dl | 1.00 (0.99 to 1.01), P=0.50 | |
| Hemoglobin | 1 g/dl | 0.95 (0.87 to 1.04), P=0.30 | |
| Albumin | 1 g/dl | 0.69 (0.50 to 0.96), P=0.03 | |
| Phosphate | 1 mg/dl | 1.00 (0.84 to 1.20), P=0.97 | |
| PTH | 20 pg/ml | 1.04 (1.01 to 1.07), P<0.01 | |
| 1,25(OH)2D | 1 pg/ml | 1.00 (0.98 to 1.01), P=0.86 | |
| 25(OH)D | 1 pg/ml | 0.98 (0.95 to 1.00), P=0.07 | |
| Urinary protein | 1 g/24 hr | 1.21 (1.12 to 1.30), P<0.001 | |
| Sex | 0=F; 1=M | 1.12 (0.84 to 1.49), P=0.45 | |
| Oral hypoglycemic therapy | 0=no; 1=yes | 0.95 (0.63 to 1.42), P=0.80 | |
| Insulin therapy | 0=no; 1=yes | 1.04 (0.73 to 1.48),P=0.84 | |
| Calcium | 1 mg/dl | 0.77 (0.64 to 0.93), P<0.01 | |
| Body mass index | 1 kg/m2 | 0.99 (0.96 to 1.02), P=0.38 | |
| Systolic pressure | 1 mmHg | 1.01 (0.99 to 1.02), P=0.08 | |
| LDL cholesterol | 1 mg/dl | 1.00 (0.99 to 1.01), P=0.76 | |
| C-reactive protein | 1 mg/L | 0.99 (0.97 to 1.00), P=0.10 |
The validity of this interaction was externally assessed in the MMKD study, in which ADMA and both intact and c-terminal FGF-23 were available. Univariate (P=0.11) and multivariate (P=0.06) (Figure 3, bottom panel on the left) Cox regression analyses showed a trend for intact FGF-23 modifying the relationship between plasma ADMA and renal events, but it did not reach statistical significance. When c-terminal FGF-23 instead of intact FGF-23 was used for the analysis, we observed very clear evidence for an effect modification (P for interaction for crude and adjusted analysis=0.001 and 0.009, respectively) (Figure 3, bottom right panel). Thus, also in the MMKD cohort, the risk excess for renal events associated with ADMA was highest in patients with low FGF-23 levels and progressively decreased as FGF-23 increased.
The analysis testing the interaction of SDMA and l-arginine/ADMA ratio on the risk of renal events in the MMKD cohort showed that c-terminal FGF-23 significantly modified the relationship between SDMA and renal events (P for the effect modification=0.001) (Figure 4), and this was also true for the link between l-arginine/ADMA ratio and renal events (P for the effect modification=0.001) (Figure 4). To assess whether the relationship between renal events with SDMA×c-terminal FGF-23 interaction and l-arginine/ADMA ratio×c-terminal FGF-23 interaction was independent of ADMA×c-terminal FGF-23 interaction, we constructed a saturated Cox model including all these interaction terms. Of note, both SDMA×c-terminal FGF-23 interaction and l-arginine/ADMA ratio×c-terminal FGF-23 interaction remained significant (P<0.01) also in Cox models including the ADMA×c-terminal FGF-23 interaction term; this finding indicates that such effect modifications were fully independent of each other. No effect modification was found between SDMA (P=0.10) and l-arginine/ADMA ratio (P=0.18) with intact FGF-23 in the MMKD cohort.
Figure 4.
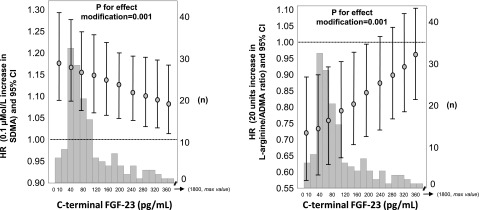
Effect modification by c-terminal FGF-23 on the relationship between SDMA (left panel) and l-arginine/ADMA ratio and renal events in the MMKD cohort. For left panel, data were adjusted for SDMA and c-terminal FGF-23 as single variables as well as for age, sex, proteinuria, and GFR. For right panel, data were adjusted for l-arginine/ADMA ratio and c-terminal FGF-23 as single variables as well as for age, sex, proteinuria, and GFR. Data are HR, 95% CI, and P value. Histograms of c-terminal FGF-23 are also shown in the background.
In the Southern Italy cohort there was no interaction between FGF-23 and ADMA for death or cardiovascular events (i.e., stroke, heart failure, angina episodes, myocardial infarction, transient ischemic attacks, atrial fibrillation/flutter, arrhythmia, pulmonary embolism, peripheral vascular diseases, and sudden death) (data not shown). The same analysis could not be done in the MMKD cohort because of an insufficient number of cardiovascular events and deaths, which can be explained by a lower age distribution of the patients in the MMKD cohort.
Discussion
In this study FGF-23 and ADMA interacted in predicting CKD progression in a large cohort of patients with CKD. This interaction was robust and largely independent of classic and CKD-specific risk factors, such as GFR, anemia, C-reactive protein, and major mineral metabolism biomarkers, including serum phosphate and fractional phosphate excretion, PTH, 1,25(OH)2D, and 25(OH)D. Furthermore, and most important, these findings were highly consistent with those in the MMKD study cohort (i.e., the cohort in which the association between FGF-23 and ADMA with CKD progression were described for the first time10,21). It must be emphasized that replication of an effect modification in an independent cohort is rare and underscores the validity of the finding. Furthermore, these data go along with biologic knowledge that both FGF-23 and ADMA interfere with the NO system and that inhibition of NO is a powerful mechanism prompting renal function loss in experimental models.
Dietary phosphate load may itself induce endothelial dysfunction in experimental clinical studies in healthy humans,31 whereas no such direct evidence exists for FGF-23. In the present study adjustment for serum phosphate or for a better indicator of phosphate burden (such as fractional phosphate excretion) did not modify the ADMA-FGF-23 interaction. Yet both serum phosphate and fractional phosphate excretion are fairly imprecise biomarkers of global phosphate balance. Therefore, on the basis of our observational data we cannot exclude the possibility that the true second actor of the interaction we described is phosphate rather than FGF-23. This issue clearly deserves to be specifically examined in experimental studies.
FGF-23 has been intensively investigated over the past few years, and it is suspected as a risk factor for a variety of clinical outcomes, spanning from bone mineral disorders to cardiovascular and renal disease.2,5 Several cohort studies have examined the relationship between FGF-23 and renal outcomes.10–15
In line with findings in the Chronic Renal Insufficiency Cohort cohort11 and in the MMKD study10 from analyses not considering the FGF-23–ADMA interaction, we confirmed that the FGF-23–CKD progression relationship was independent of (1) classic risk factors and (2) CKD-specific risk factors, such as proteinuria and the GFR, that are known to belong to the strongest predictors of CKD progression, as well as (3) major biomarkers of CKD mineral and bone disorder, including serum phosphate, 1,25(OH)2 D, and PTH. These results further highlight the potential relevance of FGF-23 as a novel risk factor for evolution toward kidney failure in CKD.
ADMA is an endogenous inhibitor of the NO synthase. The predictive role of this methylarginine for adverse renal outcomes was noted in the MMKD study21 as well as in a cohort of elderly patients with CKD of varying severity.20 NO inhibition has profound effects on kidney function, including impaired ability to dispose of a sodium load, decrease in renal blood flow, and increase in filtration fraction32 and TGF-β–mediated renal fibrosis.24
The nitric oxide system is fundamental for vasoregulation. In previous studies in patients with CKD we found that high ADMA and high FGF-23 were associated with altered endothelium-dependent response to forearm ischemia.16,26 These studies showed that high ADMA is associated with an important attenuation of the link between FGF-23 and endothelium-dependent vasodilatation, suggesting that these factors may share a common pathway disturbing endothelial function in CKD.26 These observations, along with previous studies linking these two biomarkers with CKD progression, formed a strong a priori hypothesis for modeling the combined effect of FGF-23 and ADMA on the risk for progression to kidney failure in the first cohort of the present study. In line with the hypothesis that interference with the NO system is critical to explain the predictive power of FGF-23 for renal outcomes, we documented that FGF-23 was a powerful effect modifier for the risk of CKD progression associated with ADMA. ADMA is the most abundant endogenous inhibitor of NO system,33 and it dose-dependently inhibits NO synthase at concentration in the range of values actually attained in patients with CKD.34 FGF-23 and the associated co-receptor Klotho impinge on the same system. Indeed, Klotho knockout mice35,36 (i.e., animal models characterized by high plasma levels of FGF-2337) do not express NO synthase in the vascular system and exhibit almost abolished response to acetylcholine, the strongest stimulant of NO activity.36 Our findings show that ADMA has a variable influence on renal outcomes across the spectrum of FGF-23 values because it is associated with a higher risk for CKD progression only when FGF-23 levels are in the low-normal range (i.e., when the expression of NO synthase is not suppressed by FGF-23–Klotho). This possibility has biologic plausibility because the gene expression of NO synthase is almost entirely abolished when FGF-23 levels are elevated, such as in the Klotho knockout mice.35,36 Therefore, in this situation minimal or no effect of ADMA on NO synthesis can be envisaged and vice versa. Thus, FGF-23 and ADMA act jointly rather than independently on NO-dependent mechanisms, impinging upon renal integrity and renal function. Their competitive interaction can be seen as an experiment where we injected a fixed dose of ADMA at progressively increasing levels of FGF-23. Because high FGF-23–Klotho almost completely suppresses NO synthase,35,36 ADMA at high FGF-23 levels has no effect on the risk of CKD progression, an outcome strongly influenced by the NO system.24
The observation that in the MMKD cohort not only ADMA but also SDMA (i.e., a methylarginine that inhibits NO synthesis by reducing cellular l-arginine uptake) showed a competitive interaction with c-terminal FGF-23 adds circumstantial support to our interpretation that these interactions depend on the interference of FGF-23–Klotho with the NO system. Of note, the interaction between FGF-23 and ADMA was specific for the risk of renal events because no such interaction emerged for cardiovascular events and death in the Southern Italy cohort, emphasizing the peculiar sensitivity of the kidney to disturbances impinging upon the NO system.38,39
Our findings observed in two independent cohorts of different age range, eGFRs, and geographic provenances indicate that future studies focusing on the risk for CKD progression attributable to FGF-23 and ADMA should consider both biomarkers rather than just one of them at a time. Our data bridging a classic endothelial toxin such as ADMA with a biomarker of mineral bone disorders such as FGF-23 provide a novel framework for the study of the bone-vascular connection. Experimental studies (in vitro and in animal models) and observational studies in other conditions characterized by altered circulating levels of these compounds are the necessary steps to elucidate the FGF-23–ADMA interaction highlighted in the present study.
We consider it a strength of our study that we could show the interaction between FGF-23 and ADMA in two independent cohorts, considering the pre-established, different renal endpoint definitions. As repeatedly emphasized, the observational design of our study makes it impossible to prove causality of the associations between FGF-23, ADMA, and kidney disease progression. As discussed, phosphate and fractional phosphate excretion are imperfect biomarkers of underlying phosphate burden. ADMA was measured by different methods in the two cohorts, and the MMKD study is rather small study and did not include diabetic patients. Because only white patients were included in our cohorts, it remains to be elucidated whether these findings can be confirmed in other ethnic groups.
Concise Methods
The study protocol was in conformity with the ethical guidelines of our institutions, and written informed consent was obtained from each participant. The Ethical Committees of our institutions formally approved the study.
Patients
The first cohort included 759 patients with stage 2–5 CKD consecutively recruited from 22 nephrology units in Southern Italy. Patients were enrolled between October 2005 and October 2008. The selection criteria and the detailed clinical characteristics of this cohort were described in detail elsewhere.40,41 All patients were in stable clinical condition, and none had intercurrent infections or acute inflammatory processes. Inclusion criteria were nonacute or rapidly evolving renal diseases, age ranging from 18 to 75 years, nontransplanted, nonpregnant, and not affected by cancer or diseases in the terminal phase. The MMKD study (validation cohort) included 227 nondiabetic white patients (age ranging from 18 to 65 years) with primary CKD and mild to moderate impairment of kidney function. These patients were recruited from eight nephrology departments in Germany, Austria, and South Tyrol (Italy), as previously described.10 Before inclusion in the study, the patients had to have stable kidney function for at least 3 months. Patients were excluded from the study in case of serum creatinine >6 mg/dl (531 µmol/L); diabetes mellitus of any type; malignancy, liver, thyroid, or infectious diseases; nephrotic syndrome (defined as daily proteinuria >3.5 g/1.73 m2); organ transplantation; immunosuppressive treatment, allergy to ionic contrast media; treatment with fish oil or erythropoietin; and pregnancy. From the baseline cohort, 173 patients had follow-up for as long as 7 years. FGF-23 values were not available for 4 patients. Therefore, 173 patients are part of the present study. The demographic and clinical characteristics of the MMKD Study are summarized in Supplemental Appendix 1.
Laboratory Measurements
In the Southern Italy cohort, serum lipids, albumin, calcium, phosphate, glucose, PTH, and hemoglobin were measured by standard methods in routine clinical laboratories. Serum C-reactive protein was measured by a high-sensitivity commercially available RIA kit and serum and urine creatinine by the Jaffé method implemented in multichannel analyzers. ADMA42 was measured by ELISA (DLD, Diagnostika Gmbh, Hamburg, Germany) that we specifically validated in patients with CKD43; intact FGF-23 was measured an ELISA kit (Kainos Laboratories, Tokyo, Japan). Both 1,25(OH)2D and 25(OH)D were measured in serum by an RIA kit (Immunodiagnostic Systems Ltd., Bordon, UK). All patients underwent a 24-hour urinary collection for the measurement of proteinuria. In the MMKD study, FGF-23 was quantified by the human c-terminal as well as the intact ELISA (Immutopics, San Clemente, CA), and plasma concentrations of ADMA were measured by a liquid chromatography–mass spectrometry method.44 In this cohort the GFR was measured in all patients by iohexol clearance.45 In the MMKD cohort, SDMA and l-arginine were also available and were measured as reported elsewhere.21
Study Outcomes
The predefined study endpoint in the Southern Italy cohort was a composite renal endpoint: a decrease in GFR of >30% (estimated by Modification of Diet in Renal Disease 186 formula), dialysis, or kidney transplantation.39 In the validation cohort (MMKD study), the predefined renal endpoint was doubling of baseline serum creatinine and/or terminal renal failure necessitating RRT (dialysis therapy or kidney transplantation).10
Statistical Analyses
Normally distributed data were summarized as mean±SD, non-normally distributed data as median and IQR, and binary data as percentages. Comparisons between groups were made by unpaired t test, Mann–Whitney test, or chi-squared test, as appropriate. The relationship between two continuous variables was investigated by Pearson product moment correlation coefficient (r). The association between binary and continuous variables was assessed by point-biserial correlation coefficient. Continuous variables having a positively skewed distribution were log transformed (lg10) before the correlation study. The Grubbs test46 detected a significant outlier (P<0.01) with intact FGF-23 levels of 50,275 pg/ml, a figure more than 800 times higher than the median value. To avoid any analytical distortion due to this extreme value, this observation was excluded from data analysis.
Southern Italy Cohort
The independent relationship between eGFR, FGF-23, and ADMA in this cohort was investigated by introducing into the same linear regression model all variables that met criteria to be confounders,47 as well as all variables that correlated with intact FGF-23, ADMA, and eGFR with P≤0.10 at univariate analysis. Tested covariates included traditional risk factors (age, sex, smoking, glucose and diabetes, lipids, BP, and cardiovascular comorbidities); factors specific for CKD (24-hour urinary protein, calcium, phosphate and phosphate clearance normalized by eGFR, hemoglobin, and albumin); 1,25(OH)2D and 25(OH)D;PTH; body mass index; C-reactive protein; and treatment with antihypertensive drugs, insulin, and oral hypoglycemic agents. The presence of outliers was assessed by the Grubbs test.46
The relationship between intact FGF-23 and the incidence rate of renal events was investigated by Kaplan–Meier survival analysis and by univariate and multiple Cox regression models. In the survival analysis, patients who died (n=42) were censored at the date of death. In the multiple Cox regression model we included intact FGF-23, ADMA, and eGFR and all variables that met criteria to be confounders.47 We also tested the full list of variables considered above in a saturated model. The Cox regression model did not violate the proportional hazards assumption. The effect modification of intact FGF-23 on the relationship between ADMA (0.1 µmol/L increase) and renal events was investigated by simultaneously including the following into the same multiple Cox model: intact FGF-23 (in pg/ml), ADMA (in µmol/L), and their interaction term (intact FGF 23×ADMA). The HR for renal events of a fixed increase in ADMA (0.1 µmol/L) across values for intact FGF-23 was calculated by the linear combination method. In the survival analysis, data were expressed as HR (95% CIs) with P values. To assess whether the competitive risk of death could affect the FGF-23×ADMA interaction, a sensitivity analysis was performed by considering a combined end point: renal events or death.48
MMKD Cohort (Validation Cohort)
In the MMKD cohort, the analysis of the effect modification by ADMA on the relationship between c-terminal FGF-23 and renal events was investigated by using the same analytical approach adopted for the Southern Italy cohort. Because of the relatively low number of renal events in this cohort (n=63), we adjusted the intact and c-terminal FGF-23–ADMA interaction only for four major confounders (age, sex, proteinuria, and GFR measured by iohexol clearance). In the MMKD cohort, an interaction analysis was also carried out for SDMA and l-Arginine/ADMA ratio. Additional information and analyses in this cohort are provided in the Supplemental Appendices.
Data analysis was performed by using a standard statistical package (SPSS for Windows, versions 9.01 and 21; SPSS Inc., Chicago, IL) as well as R software, version 3.0.1, and Stata software, version 11.0 (StataCorp., College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
Members of the Southern Italy CKD cohort Working Group are Maimone I, Cicchetti T, Plutino D, Santoro O, Grandinetti F, Natale G, Tramontana D, Pugliese A, Chiarella S, Pinciaroli A, Audino A, Mannino M, Catalano F, Mafrica A, Pisano A, Caridi G, Marino F, Parlongo G, Garozzo M, Fatuzzo P, Rapisarda F, D'Anello E, Fabiano F, Bruzzese V, Caglioti A, Papalia T, Gullo M, Fersini S, Pinna M, Campo S, Palma L, Mancuso F. Contributors to the Mild to Moderate Kidney Disease Study: Kuen E, Ritz E, König P, Kraatz G, Mann JFE, Müller GA, Neyer U, Köhler H, Riegler P.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013121355/-/DCSupplemental.
References
- 1.Quarles LD: Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutiérrez OM, Wolf M, Taylor EN: Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol 6: 2871–2878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirza MA, Alsiö J, Hammarstedt A, Erben RG, Michaëlsson K, Tivesten A, Marsell R, Orwoll E, Karlsson MK, Ljunggren O, Mellström D, Lind L, Ohlsson C, Larsson TE: Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol 31: 219–227, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez OM: Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: Updating the “trade-off” hypothesis. Clin J Am Soc Nephrol 5: 1710–1716, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD: A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS ONE 7: e44161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanchi C, Locatelli M, Benigni A, Corna D, Tomasoni S, Rottoli D, Gaspari F, Remuzzi G, Zoja C: Renal expression of FGF23 in progressive renal disease of diabetes and the effect of ACE inhibitor. PLoS ONE 8: e70775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P, MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semba RD, Fink JC, Sun K, Cappola AR, Dalal M, Crasto C, Ferrucci L, Fried LP: Serum fibroblast growth factor-23 and risk of incident chronic kidney disease in older community-dwelling women. Clin J Am Soc Nephrol 7: 85–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, Hoeper MM, Haller H, Fliser D: Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation 109: 172–177, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kielstein JT, Simmel S, Bode-Böger SM, Roth HJ, Schmidt-Gayk H, Haller H, Fliser D: Subpressor dose asymmetric dimethylarginine modulates renal function in humans through nitric oxide synthase inhibition. Kidney Blood Press Res 27: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, Ozgurtas T, Aydin A, Eyileten T, Ozcan O, Acikel C, Tasar M, Genctoy G, Erbil K, Vural A, Zoccali C: The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis 47: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Bai Y, Sun L, Du L, Zhang T, Xin W, Lan X, Du G: Association of circulating levels of asymmetric dimethylarginine (ADMA) with carotid intima-media thickness: Evidence from 6168 participants. Ageing Res Rev 12: 699–707, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C: Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, Ritz E: Asymmetric dimethylarginine and progression of chronic kidney disease: The mild to moderate kidney disease study. J Am Soc Nephrol 16: 2456–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hanai K, Babazono T, Nyumura I, Toya K, Tanaka N, Tanaka M, Ishii A, Iwamoto Y: Asymmetric dimethylarginine is closely associated with the development and progression of nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant 24: 1884–1888, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Lajer M, Tarnow L, Jorsal A, Teerlink T, Parving HH, Rossing P: Plasma concentration of asymmetric dimethylarginine (ADMA) predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 31: 747–752, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Mihout F, Shweke N, Bigé N, Jouanneau C, Dussaule JC, Ronco P, Chatziantoniou C, Boffa JJ: Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF-β1 synthesis. J Pathol 223: 37–45, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Mirza MA, Larsson A, Lind L, Larsson TE: Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 205: 385–390, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 78: 679–685, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucsi I, Ujszaszi A, Wolf MS, Kielstein JT, Molnar MZ: Serum FGF23 levels are indepently associated with serum asymmetric dimethylarginine (ADMA) levels in kidney transplant recipients. [Abstract] J Am Soc Nephrol 23: 803A, 2012 [Google Scholar]
- 29.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ: Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol 13: 806–816, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Çakar M, Altun B, Yenicesu M, Carrero JJ: Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant 26: 3537–3543, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E: Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoccali C: Asymmetric dimethylarginine (ADMA): A cardiovascular and renal risk factor on the move. J Hypertens 24: 611–619, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Leiper JM: The DDAH-ADMA-NOS pathway. Ther Drug Monit 27: 744–746, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL: Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R: Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 248: 324–329, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Saito Y, Ohyama Y, Masuda H, Sumino H, Kuro-o M, Nabeshima Y, Nagai R, Kurabayashi M: Production of nitric oxide, but not prostacyclin, is reduced in klotho mice. Jpn J Pharmacol 89: 149–156, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE: Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 23: 1641–1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baylis C, Qiu C: Importance of nitric oxide in the control of renal hemodynamics. Kidney Int 49: 1727–1731, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA: Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoccali C, Leonardis D, Enia G, Postorino M, Mallamaci F, MAURO study working group : The MAURO study: Multiple intervention and audit in renal diseases to optimize care. J Nephrol 21: 20–22, 2008 [PubMed] [Google Scholar]
- 41.Leonardis D, Mallamaci F, Enia G, Postorino M, Tripepi G, Zoccali C, MAURO Study Investigators : The MAURO study: Baseline characteristics and compliance with guidelines targets. J Nephrol 25: 1081–1090, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, Cooke JP, Böger RH: Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin Chem Lab Med 42: 1377–1383, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Pecchini P, Malberti F, Mieth M, Quinn R, Tripepi G, Mallamaci F, Maas R, Zoccali C, Ravani P: Measuring asymmetric dimethylarginine (ADMA) in CKD: A comparison between enzyme-linked immunosorbent assay and liquid chromatography-electrospray tandem mass spectrometry. J Nephrol 25: 1016–1022, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Martens-Lobenhoffer J, Bode-Böger SM: Simultaneous detection of arginine, asymmetric dimethylarginine, symmetric dimethylarginine and citrulline in human plasma and urine applying liquid chromatography-mass spectrometry with very straightforward sample preparation. J Chromatogr B Analyt Technol Biomed Life Sci 798: 231–239, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Bostom AG, Kronenberg F, Ritz E: Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol 13: 2140–2144, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Grubbs FE: Procedures for detecting outlying observations in samples. Technometrics 11: 1:21, 1969 [Google Scholar]
- 47.Jager KJ, Zoccali C, Macleod A, Dekker FW: Confounding: What it is and how to deal with it. Kidney Int 73: 256–260, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Allignol A, Schumacher M, Wanner C, Drechsler C, Beyersmann J: Understanding competing risks: A simulation point of view. BMC Med Res Methodol 11: 86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.