Abstract
High-sensitivity troponin T (hsTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) strongly predict heart failure (HF) in the general population. However, the interpretation of levels of these biomarkers as predictors of HF is uncertain among patients with CKD. Here, we investigated whether hsTnT and NT-proBNP are associated with incident HF among patients with CKD. In a prospective cohort analysis, we studied 3483 people with CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study recruited from June of 2003 to August of 2008 who were free of HF at baseline. We used Cox regression to examine the association of baseline levels of hsTnT and NT-proBNP with incident HF after adjustment for demographic factors, traditional cardiovascular risk factors, markers of kidney disease, pertinent medication use, and mineral metabolism markers. At baseline, hsTnT levels ranged from ≤5.0 to 378.7 pg/ml, and NT-proBNP levels ranged from ≤5 to 35,000 pg/ml. Compared with those who had undetectable hsTnT, participants in the highest quartile (>26.5 ng/ml) had a significantly higher rate of HF (hazard ratio, 4.77; 95% confidence interval, 2.49 to 9.14). Similarly, compared with those in the lowest NT-proBNP quintile (<47.6 ng/ml), participants in the highest quintile (>433.0 ng/ml) experienced a substantially higher rate of HF (hazard ratio, 9.57; 95% confidence interval, 4.40 to 20.83). In conclusion, hsTnT and NT-proBNP were strongly associated with incident HF among a diverse cohort of individuals with mild to severe CKD. Elevations in these biomarkers may indicate subclinical changes in volume and myocardial stress that subsequently contribute to clinical HF.
Keywords: cardiovascular disease, heart failure, kidney disease
Heart failure (HF) is the most common cardiovascular complication among patients with CKD, and it imposes significant morbidity and mortality.1,2 Among people with CKD, subclinical cardiac dysfunction (e.g., early changes in left ventricular structure and function) confers poor prognosis, but its early diagnosis remains difficult.3,4 The cardiac biomarkers high-sensitivity troponin T (hsTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) have been shown to predict HF in the general population.5,6 NT-proBNP is secreted from cardiac myocytes in response to myocardial stretch from pressure or volume overload,7 and levels increase with increasing left ventricular mass.8–10 Through complementary physiologic pathways, concentrations of hsTnT may become elevated in response to myocardial injury or remodeling or left ventricular hypertrophy (LVH).11,1
The use of hsTnT and NT-proBNP to aid in the diagnosis of HF among patients with CKD has been limited because of concerns that elevated levels may be caused by reduced renal excretion.8,13 To better understand the importance of elevated concentrations of hsTnT and NT-proBNP among persons with CKD, we examined their associations with incident HF events among participants of an ongoing observational study: the Chronic Renal Insufficiency Cohort (CRIC) Study. We hypothesized that elevations in hsTnT and NT-proBNP would be independently associated with incident HF among persons with mild to severe CKD.
Results
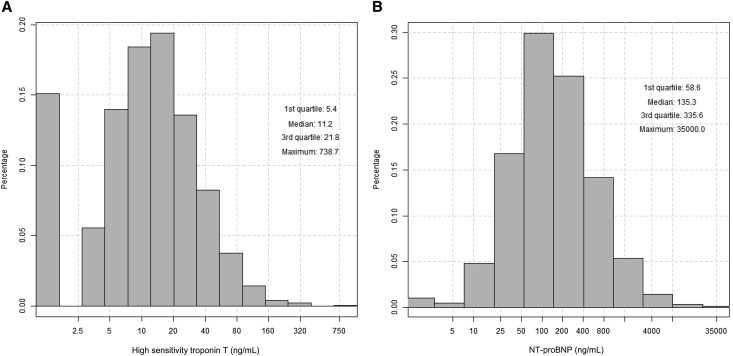
Among participants in our study, mean age was 57.9 years, eGFR was 45.7 ml/min per 1.73 m2, 45.6% were women, 40.0% identified as black, and 46.4% had diabetes. hsTnT ranged from ≤5 to 738.7 pg/ml, and NT-proBNP levels ranged from ≤5 to 35,000 pg/ml (Figure 1). Median (interquartile range) levels were 11.2 (5.4–21.8) pg/ml for hsTnT and 135.3 (58.6–335.6) pg/ml for NT-proBNP. Higher hsTnT levels were significantly associated with older age, lower eGFR, higher proteinuria, diabetes, higher systolic BP, greater diuretic use, higher fibroblast growth factor 23 (FGF23), lower ejection fraction (EF), and higher left ventricular mass index (LVMI) (Table 1). Similar associations were observed for NT-proBNP (Table 2).
Figure 1.
Distributions of cardiac biomarkers among participants without HF in the CRIC Study. (A) Distribution of hsTnT. (B) Distribution of NT-proBNP.
Table 1.
Baseline demographic, clinical, and biochemical characteristics of CRIC Study participants without HF at baseline by category of hsTnT
| Characterisitics | hsTnT categories (n), pg/mla | P Value | ||||
|---|---|---|---|---|---|---|
| ≤5 (n=814) | >5–9.3 (n=668) | >9.3–15.0 (n=667) | >15.0–26.5 (n=667) | >26.5 (n=667) | ||
| Age, yr | 52.6 (11.8) | 56.8 (11.3) | 60.8 (9.9) | 61.1 (9.6) | 59.3 (10.4) | <0.001 |
| Women | 556 (68.3%) | 348 (52.1%) | 278 (41.7%) | 234 (35.1%) | 171 (25.6%) | <0.001 |
| Race/ethnicity | <0.001 | |||||
| Non-Hispanic white | 417 (51.2%) | 326 (48.8%) | 310 (46.5%) | 262 (39.3%) | 177 (26.5%) | |
| Non-Hispanic black | 280 (34.4%) | 252 (37.7%) | 247 (37.0%) | 288 (43.2%) | 326 (48.9%) | |
| Hispanic | 76 (9.3%) | 58 (8.7%) | 84 (12.6%) | 93 (13.9%) | 144 (21.6%) | |
| Other | 41 (5.0%) | 32 (4.8%) | 26 (3.9%) | 24 (3.6%) | 20 (3.0%) | |
| eGFR, ml/min per 1.73 m2b | 56.9 (18.6) | 49.3 (16.0) | 44.8 (12.8) | 40.5 (13.8) | 34.8 (12.3) | <0.001 |
| 24-hour urine protein, g/d | 0.09 (0.06–0.3) | 0.1 (0.06–0.5) | 0.1 (0.07–0.6) | 0.3 (0.09–1.2) | 0.9 (0.2–3.3) | <0.001 |
| Diabetes | 166 (20.4%) | 223 (33.4%) | 322 (48.3%) | 373 (55.9%) | 531 (79.6%) | <0.001 |
| History of MI/stroke/PAD | 94 (11.5%) | 143 (21.4%) | 178 (26.7%) | 243 (36.4%) | 252 (37.8%) | <0.001 |
| Current smoker | 112 (13.8%) | 84 (12.6%) | 85 (12.7%) | 81 (12.1%) | 86 (12.9%) | 0.92 |
| Alcohol use | 591 (72.6%) | 444 (66.5%) | 432 (64.8%) | 401 (60.1%) | 362 (54.3%) | <0.001 |
| Body mass index, kg/m2 | 30.9 (7.9) | 31.3 (7.5) | 32.0 (7.8) | 32.9 (7.8) | 32.6 (7.4) | <0.001 |
| Systolic BP, mmHg | 119.0 (16.9) | 124.8 (19.7) | 129.4 (20.5) | 131.9 (23.1) | 139.1 (24.4) | <0.001 |
| Diastolic BP, mmHg | 71.8 (11.3) | 72.5 (12.0) | 71.4 (12.7) | 71.1 (13.3) | 72.8 (13.8) | 0.08 |
| Hemoglobin, g/dl | 13.0 (1.6) | 13.0 (1.8) | 12.8 (1.8) | 12.4 (1.7) | 11.8 (1.6) | <0.001 |
| LDL cholesterol, mg/dl | 110.6 (34.3) | 103.7 (32.8) | 101.1 (33.0) | 100.3 (36.5) | 102.1 (38.9) | <0.001 |
| HDL cholesterol, mg/dl | 52.4 (16.7) | 48.8 (15.0) | 47.4 (15.4) | 44.5 (14.0) | 45.7 (15.1) | <0.001 |
| ACE/ARB use | 423 (52.5%) | 457 (68.8%) | 469 (70.6%) | 509 (76.8%) | 484 (73.3%) | <0.001 |
| Diuretic use | 299 (37.1%) | 336 (50.6%) | 391 (58.9%) | 442 (66.7%) | 480 (72.7%) | <0.001 |
| β-Blocker use | 251 (31.1%) | 282 (42.5%) | 323 (48.6%) | 360 (54.3%) | 375 (56.8%) | <0.001 |
| FGF23, RU/ml | 107.7 (74.6–166.0) | 121.6 (85.8–194.1) | 138.6 (96.6–215.9) | 152.9 (103.4–239.4) | 198.0 (128.5–334.8) | <0.001 |
| Serum phosphorus, mg/dl | 3.6 (0.6) | 3.6 (0.6) | 3.6 (0.6) | 3.7 (0.6) | 4.1 (0.8) | <0.001 |
| Total PTH, pg/ml | 41.0 (30.0–61.2) | 45.9 (31.5–70.0) | 48.8 (33.0–78.0) | 61.0 (39.2–93.2) | 79.8 (46.0–136.0) | <0.001 |
| EF from echocardiogramc | 56.0 (6.4) | 55.9 (6.8) | 55.4 (7.4) | 54.2 (8.3) | 52.9 (8.3) | <0.001 |
| LVMI, g/m2.7c | 45.0 (10.4) | 47.1 (10.9) | 51.1 (12.5) | 54.7 (14.0) | 59.2 (14.4) | <0.001 |
MI, myocardial infarction; PAD, peripheral arterial disease; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; RU, relative units.
Detectable levels of hsTnT were divided into quartiles.
eGFR calculated using serum creatinine and cystatin C (47).
From the year 1 clinic visit.
Table 2.
Baseline demographic, clinical, and biochemical characteristics of CRIC Study participants without HF at baseline by quintile of NT-proBNP
| Characterisitics | NT-proBNP Quintile (n), pg/mla | P Value | ||||
|---|---|---|---|---|---|---|
| ≤47.6 (n=697) | >47.6–95.8 (n=697) | >95.8–188.8 (n=696) | >188.8–433.0 (n=697) | >433.0 (n=696) | ||
| Age, yr | 52.9 (11.7) | 57.2 (10.8) | 58.9 (11.2) | 59.9 (10.5) | 60.5 (10.0) | <0.001 |
| Women | 232 (33.3%) | 330 (47.3%) | 338 (48.6%) | 370 (53.1%) | 317 (45.5%) | <0.001 |
| Race/ethnicity | <0.001 | |||||
| Non-Hispanic white | 300 (43.0%) | 306 (43.9%) | 325 (46.7%) | 309 (44.3%) | 252 (36.2%) | |
| Non-Hispanic black | 317 (45.5%) | 295 (42.3%) | 264 (37.9%) | 254 (36.4%) | 263 (37.8%) | |
| Hispanic | 45 (6.5%) | 62 (8.9%) | 88 (12.6%) | 103 (14.8%) | 157 (22.6%) | |
| Other | 35 (5.0%) | 34 (4.9%) | 19 (2.7%) | 31 (4.4%) | 24 (3.4%) | |
| eGFR, ml/min per 1.73 m2b | 58.1 (16.8) | 49.7 (16.4) | 45.2 (15.3) | 40.6 (13.8) | 35.0 (12.4) | <0.001 |
| 24-hour urine protein, g/d | 0.1 (0.06–0.3) | 0.1 (0.07–0.6) | 0.2 (0.07–0.7) | 0.2 (0.08–1.1) | 0.7 (0.1–2.8) | <0.001 |
| Diabetes | 218 (31.3%) | 291 (41.8%) | 315 (45.3%) | 362 (51.9%) | 429 (61.6%) | <0.001 |
| History of MI/stroke/PAD | 79 (11.3%) | 121 (17.4%) | 172 (24.7%) | 215 (30.8%) | 323 (46.4%) | <0.001 |
| Current smoker | 66 (9.5%) | 76 (10.9%) | 83 (11.9%) | 99 (14.2%) | 124 (17.8%) | <0.001 |
| Alcohol use | 511 (73.3%) | 496 (71.2%) | 433 (62.2%) | 419 (60.1%) | 371 (53.3%) | <0.001 |
| Body mass index, kg/m2 | 31.8 (6.6) | 32.0 (7.5) | 32.1 (8.4) | 32.0 (8.3) | 31.6 (7.7) | 0.76 |
| Systolic BP, mmHg | 118.9 (15.2) | 124.0 (18.5) | 125.0 (18.7) | 131.8 (22.0) | 142.5 (26.1) | <0.001 |
| Diastolic BP, mmHg | 73.0 (11.1) | 72.4 (11.4) | 70.1 (11.8) | 71.3 (13.2) | 72.7 (14.9) | <0.001 |
| Hemoglobin, g/dl | 13.6 (1.6) | 12.9 (1.5) | 12.6 (1.6) | 12.3 (1.7) | 11.8 (1.9) | <0.001 |
| LDL cholesterol, mg/dl | 107.7 (34.0) | 104.5 (35.0) | 104.0 (35.5) | 101.8 (34.1) | 101.2 (37.8) | 0.005 |
| HDL cholesterol, mg/dl | 47.1 (14.2) | 48.2 (15.3) | 49.0 (15.8) | 48.8 (16.8) | 46.7 (15.7) | 0.02 |
| ACE/ARB use | 457 (66.2%) | 467 (67.6%) | 479 (69.1%) | 481 (69.5%) | 458 (66.3%) | 0.56 |
| Diuretic use | 292 (42.3%) | 369 (53.4%) | 404 (58.3%) | 401 (57.9%) | 482 (69.8%) | <0.001 |
| β-Blocker use | 157 (22.8%) | 242 (35.0%) | 305 (44.0%) | 405 (58.5%) | 482 (69.8%) | <0.001 |
| FGF23, RU/ml | 99.6 (73.4–144.8) | 122.7 (86.2–183.7) | 133.2 (94.6–206.0) | 159.3 (107.4–244.5) | 209.1 (136.2–344.0) | <0.001 |
| Serum phosphorus, mg/dl | 3.5 (0.6) | 3.6 (0.6) | 3.7 (0.7) | 3.8 (0.7) | 3.9 (0.7) | <0.001 |
| Total PTH, pg/ml | 41.0 (29.4–56.0) | 47.8 (32.6–77.3) | 51.5 (32.0–78.6) | 57.0 (37.0–94.0) | 74.0 (46.0–123.4) | <0.001 |
| EF from echocardiogramc | 55.6 (6.4) | 56.1 (6.1) | 55.6 (7.0) | 54.8 (7.7) | 52.2 (9.5) | <0.001 |
| LVMI, g/m2.7c | 45.3 (10.4) | 48.6 (11.4) | 49.9 (12.3) | 52.4 (13.2) | 59.7 (15.4) | <0.001 |
MI, myocardial infarction; PAD, peripheral arterial disease; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; RU, relative units.
NT-proBNP categories were defined by quintiles.
eGFR calculated using serum creatinine and cystatin C (47).
From the year 1 clinic visit.
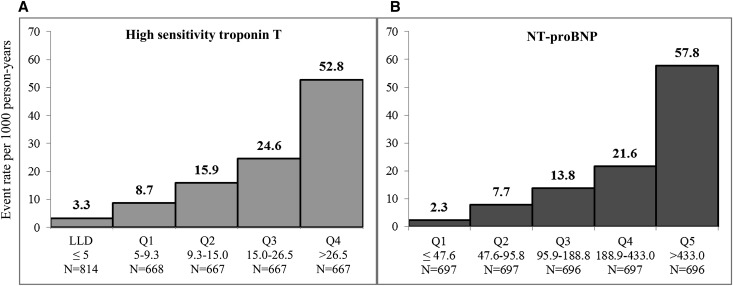
Over a median follow-up of nearly 6 years, 320 incident HF events were identified (n=237 definite and n=83 probable events). In total, 582 participants were censored because of ESRD, and 300 participants were censored because of death. Crude incident HF rates increased steadily across categories of both cardiac biomarkers (Figure 2). hsTnT and NT-proBNP were both strong predictors of incident HF, which remained significant, even after adjustment for an extensive set of covariates (Table 3). After adjustment for medication usage and novel cardiovascular risk factors (model 2 in Table 3), compared with those with undetectable hsTnT levels, rates of incident HF increased incrementally, with 2- to 5-fold higher rates across categories of detectable levels. Similarly, compared with the lowest quintile of NT-proBNP, rates of incident HF increased incrementally and were 3- to 10-fold higher across remaining quintiles. After adjustment for NT-proBNP, the risk of incident HF associated with hsTnT remained unchanged. Similarly, after adjustment for hsTnT, the association between incident HF and NT-proBNP was attenuated only slightly (Table 3). Spline models did not detect any nonlinear relationships or meaningful thresholds for either of the cardiac biomarkers overall or in models stratified by baseline eGFR, sex, or race (data not shown).
Figure 2.
Crude rates of incident HF per 1000 person-years of follow-up in the CRIC Study across categories of hsTnT and NT-proBNP. Events rates for hsTnT are shown for those with undetectable levels (≤5) and then by quartile among detectable levels; event rates for NT-proBNP are shown by quintile. LLD, lower limit of detection; Q, quartile.
Table 3.
Associations of cardiac biomarkers and incident HF in the CRIC Study
| Cardiac Biomarker | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value | |
| Continuous predictors | ||||||
| Log(hsTnT) per 1 SD (1.12) increase | 1.60 (1.36 to 1.88) | <0.001 | 1.50 (1.27 to 1.78) | <0.001 | 1.27 (1.07 to 1.52) | 0.008 |
| Log(NT-proBNP) per 1 SD (1.39) increase | 2.30 (1.99 to 2.66) | <0.001 | 2.11 (1.80 to 2.47) | <0.001 | 1.99 (1.69 to 2.35) | <0.001 |
| Categorical predictorsd | ||||||
| hsTnT, pg/ml (reference: undetectable) | ||||||
| >5–9.3 | 1.75 (0.96 to 3.22) | <0.001 | 1.96 (1.02 to 3.79) | <0.001 | 1.98 (1.03 to 3.83) | 0.001 |
| >9.3–15.0 | 2.47 (1.38 to 4.39) | 2.70 (1.44 to 5.05) | 2.51 (1.34 to 4.71) | |||
| >15.0–26.5 | 2.72 (1.52 to 4.88) | 2.96 (1.57 to 5.59) | 2.47 (1.31 to 4.66) | |||
| >26.5 | 4.85 (2.68 to 8.80) | 4.77 (2.49 to 9.14) | 3.71 (1.93 to 7.13) | |||
| NT-proBNP, pg/ml (reference: ≤47.6) | ||||||
| >47.6–95.8 | 2.71 (1.28 to 5.75) | <0.001 | 2.71 (1.23 to 5.98) | <0.001 | 2.57 (1.17 to 5.68) | <0.001 |
| >95.8–188.8 | 4.11 (1.99 to 8.50) | 3.72 (1.72 to 8.04) | 3.41 (1.58 to 7.39) | |||
| >188.8–433.0 | 5.73 (2.80 to 11.74) | 5.47 (2.55 to 11.74) | 4.76 (2.21 to 10.23) | |||
| >433.0 | 11.83 (5.75 to 24.34) | 9.57 (4.40 to 20.83) | 8.07 (3.70 to 17.64) | |||
Model 1 adjusted for age, sex, race/ethnicity, clinical center, diabetes status, self-reported cardiovascular disease at baseline, current smoking, alcohol use, log(24-hour urine total protein excretion), eGFR, systolic BP, body mass index, LDL, and HDL.
Model 2 adjusted for model 1 variables plus hemoglobin, angiotensin-converting enzyme/angiotensin receptor blocker use, diuretic use, β-blocker use, serum phosphorus, log(PTH), and log(FGF23).
Model 3 adjusted for model 2 variables plus the alternative cardiac biomarker (e.g., adjusted for NT-proBNP and hsTnT in the models and vice versa).
Detectable levels of hsTnT were divided into quartiles; NT-proBNP categories were defined by quintiles.
Using covariates, including demographic and traditional cardiovascular risk factors (model 1 in Table 3), a predictive model without inclusion of hsTnT and NT-proBNP generated a C statistic of 0.779. Models additionally adjusting for hsTnT and NT-proBNP separately produced C statistics of 0.789 and 0.810 (P=0.001), respectively. Finally, inclusion of both cardiac biomarkers in this model with clinical data generated a C statistic of 0.812.
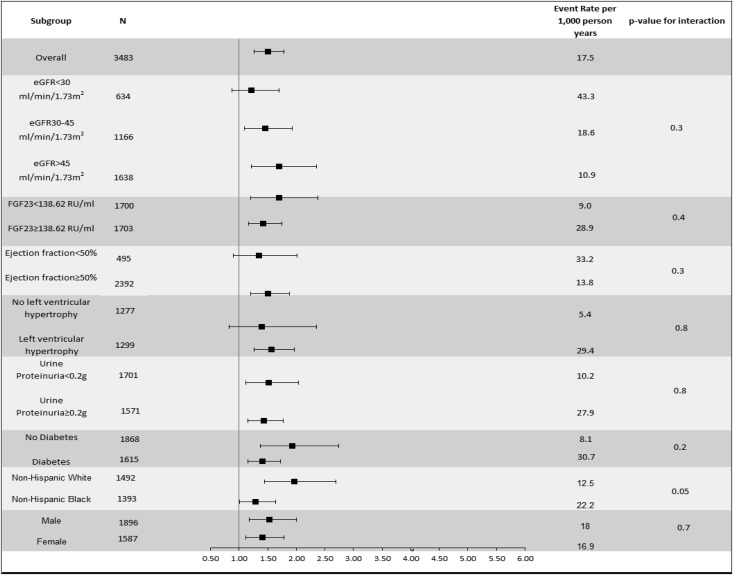
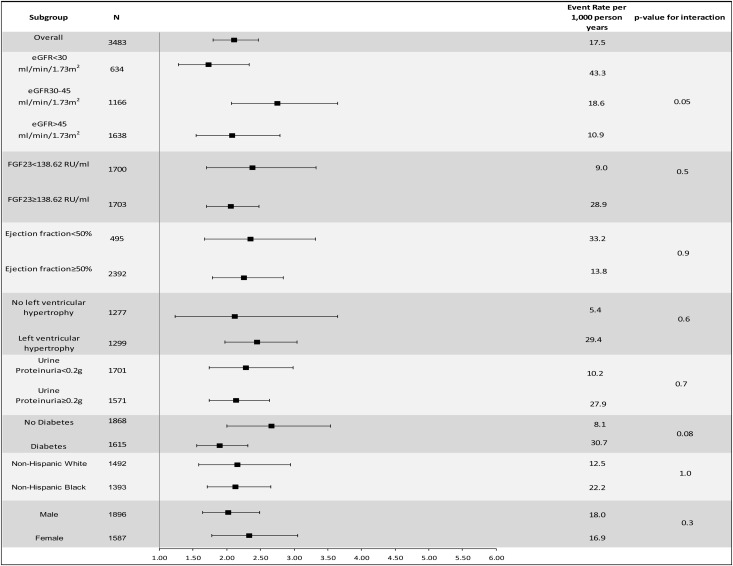
Levels of kidney function, FGF23, EF, presence of LVH, proteinuria, diabetes status, race/ethnicity, and sex did not modify the association of hsTnT or NT-proBNP with incident HF (each P value for interaction≥0.05) (Figures 3 and 4).
Figure 3.
Multivariable-adjusted association of hsTnT (per 1 SD increase) and incident HF in specific subgroups. Adjusted for age, sex, race/ethnicity, clinical center, diabetes status, self-reported cardiovascular disease at baseline, current smoking, alcohol use, log(urine total protein excretion), eGFR, systolic BP, body mass index, LDL, HDL, hemoglobin, angiotensin-converting enzyme/angiotensin receptor blocker use, diuretic use, β-blocker use, serum phosphorus, log(PTH), and log(FGF23).
Figure 4.
Multivariable-adjusted association of NT-proBNP (per 1 SD increase) and incident HF in specific subgroups. Adjusted for age, sex, race/ethnicity, clinical center, diabetes status, self-reported cardiovascular disease at baseline, current smoking, alcohol use, log(urine total protein excretion), eGFR, systolic BP, body mass index, LDL, HDL, hemoglobin, angiotensin-converting enzyme/angiotensin receptor blocker use, diuretic use, β-blocker use, serum phosphorus, log(PTH), and log(FGF23).
In sensitivity analyses, incremental adjustment for EF and LVMI in addition to model 2 factors resulted in similar estimates of the association of both cardiac biomarkers and incident HF (Supplemental Table 1). Finally, when the study population included only participants free of any cardiovascular disease at baseline, the association of hsTnT with incident HF in this subgroup was similar to that observed in the primary analysis (Supplemental Table 2). The association between NT-proBNP was even stronger, resulting in a nearly 20-fold higher rate of HF among those in the highest quintile of NT-proBNP compared with the lowest quintile (Supplemental Table 2).
Discussion
We examined the associations between baseline levels of hsTnT and NT-proBNP and subsequent incident HF in a large, well characterized, multicenter cohort of individuals with mild to moderate CKD. Participants with detectable hsTnT levels had up to a 5-fold higher rate of incident HF, even after adjustment for a number of potentially confounding factors. Study participants with the highest NT-proBNP levels had a nearly 10-fold higher rate of incident HF. These associations remained robust, even after adjustment for the alternative biomarker, suggesting that, although hsTnT and NT-proBNP are complementary, they may be indicative of distinct biologic pathways for HF.
Participants in the highest quartile of hsTnT had a 5-fold risk of incident HF in our study population. Patients with kidney disease often have increased hsTnT levels, even in the absence of clinically suspected acute myocardial ischemia, which has led to speculation on whether troponin may cleared by the kidney.14,15 In particular, hsTnT (versus troponin I) is more frequently elevated in patients with kidney disease.16 However, we found that, even after adjustment for eGFR, there was a strong association between higher levels of hsTnT and incident HF. Our findings are consistent with a previous analysis of 8000 participants with albuminuria in the Prevention of Renal and Vascular End-Stage Disease (PREVEND) Study, which showed that hsTnT was associated with incident cardiovascular events, even after adjustment for eGFR and severity of albuminuria.17 Prior studies have also shown that elevated hsTnT is associated with abnormal left ventricular structure and function in patients with12,18 and without CKD.11 Among participants in the CRIC Study, those with the highest quartile of detectable hsTnT had a 2-fold higher odds of LVH compared with those in the lowest quartile.12 Our findings were similar after excluding participants with any cardiovascular disease (including coronary heart disease) at baseline. There are several possible mechanisms that may explain elevated hsTnT levels in patients with CKD, including (but not limited to) previous myocardial infarction/unrecognized coronary ischemia, cardiac stress from increased filling pressures (e.g., volume), ventricular fibrosis, LVH, left ventricular dilation, inflammation, endothelial dysfunction, and other cardiac injury.19–21 Prior studies of hsTnT among patients with CKD are limited, and outcomes have been restricted to coronary heart disease and mortality or have included patients on chronic dialysis.22,23 We showed that small, early increases in hsTnT may represent subclinical injury that progresses to HF in patients with mild to severe CKD.
We observed that even modest elevations in NT-proBNP were associated with significantly increased rates of incident HF. Our results remained robust, even after adjustment for eGFR and proteinuria and in subgroups stratified by eGFR, proteinuria, and diabetic status. NT-proBNP regulates BP and body fluid volume by its natriuretic and diuretic actions, arterial dilation, and inhibition of the renin-aldosterone-angiotensin system,24 and increased levels of this marker likely reflect myocardial stress induced by subclinical changes in volume or pressure, even in persons without clinical heart disease. Our findings are consistent with prior studies of the general population that have shown NT-proBNP to be strongly associated with HF.25,26 In sensitivity analyses, we excluded participants who reported cardiovascular disease at baseline to reduce the role of other biologic processes, such as clinical atherosclerosis, that may confound our findings. Interestingly, the association between NT-proBNP and incident HF was even stronger in this subgroup.
There has been reluctance to adopt the use of NT-proBNP as a biomarker for HF in the CKD population because of the concern that elevated levels might simply be a reflection of reduced clearance from diminished GFR.8,27 However, similar to our findings, several studies have shown that NT-proBNP levels are strongly associated with cardiovascular events, independent of eGFR levels, in patients with CKD.28–30 In the African-American Study of Kidney Disease and Hypertension, a study of hypertensive black patients with CKD, for each doubling of NT-proBNP, there was a 70% increased risk of HF.31 In a study of 104 patients with CKD but without HF, elevated BNP was associated with a 6-fold higher rate of subsequent HF.29 In the PREVEND Study, although there was a cross-sectional association between eGFR and albuminuria with NT-proBNP, the association of NT-proBNP and cardiovascular events was independent of eGFR level.17 Our study expands on these previous studies by showing a very strong association of NT-proBNP with incident HF among a large, diverse, and more generalizable cohort of patients with CKD. In contrast to previous studies, we were able to evaluate the association between NT-proBNP and incident HF across important subgroups and adjust for numerous traditional and novel cardiovascular risk factors.
Furthermore, we did not observe any differences in the associations or thresholds of each cardiac biomarker with risk of incident HF in important subgroups. Prior investigations have suggested that thresholds for troponin, for example, may be higher in men (versus women)32,33 and blacks (versus whites).33 Interestingly, it has been suggested that NT-proBNP levels may be higher in women (versus men)34 and blacks (versus whites).35 Similar to our findings, NT-proBNP had similar associations with incident HF across sex and race/ethnicity groups in the Multi-ethnic Study of Atherosclerosis Study.36
We adjusted for various physiologic parameters as well as novel risk factors that are particularly relevant to patients with CKD without significant effect on our findings. Although LVH and reduced EF are strongly associated with adverse outcomes, including HF, among patients with CKD,37,38 the risk of HF in persons with elevated hsTnT and NT-proBNP with incident HF was similar among participants without reduced EF or LVH and unchanged after adjustment for LVMI and EF. These data suggest that hsTnT and NT-proBNP may detect subclinical disease even before abnormalities of cardiac structure or function. Adjustment for a number of novel risk factors, including indicators of mineral metabolism (e.g., FGF23 and serum phosphorus) previously shown to be strong predictors of HF,39 also did not significantly alter our findings.
Our study had numerous strengths. We prospectively followed a racially/ethnically diverse large population of well characterized individuals with CKD but without known clinical HF. We adjusted for a number of possible confounding factors, including mineral metabolism markers, LVH, and EF quantified from research echocardiograms. Study participants experienced a relatively large number of incident HF events. These outcomes were carefully adjudicated using a centralized process with accepted guidelines. Our study had limitations as well. We determined HF and cardiovascular disease at baseline on the basis of self-report. Incident HF was only on the basis of HF hospitalizations; we were not able to adjudicate outpatient HF. Although we relied on local treating physicians to make a diagnosis of HF and central physician adjudication to confirm the diagnoses of HF, we recognized that it is often clinically challenging to differentiate between HF and volume overload from kidney disease. Enrollment into the CRIC Study was not consecutive at each clinical site but on the basis of a combination of chart and laboratory database review, physician referral, and clinical screenings, which may introduce some bias into the study population. Each cardiac biomarker was measured only one time; thus, we did not examine changes in each biomarker over time. We were not able to draw conclusions on the association of other isoforms of troponin or BNP in this analysis, which may differ in their associations with outcomes in patients with CKD. Finally, we studied research volunteers, and therefore, our results may not be generalizable to all patients with CKD.
In conclusion, among patients with mild to moderate CKD, elevated levels of hsTnT and NT-proBNP were strongly associated with incident HF, even after adjustment for a broad range of traditional and novel cardiovascular risk factors, and they may indicate early subclinical changes in volume and myocardial stress that subsequently contribute to clinical HF. These associations remained robust after adjustment for the alternative biomarker, suggesting that, although hsTnT and NT-proBNP are complementary, they may represent distinct biologic pathways to HF. Additional studies are needed to develop and validate risk prediction tools for clinical HF in the setting of CKD and determine the potential role of these biomarkers in a comprehensive HF risk prediction and prevention strategy.
Concise Methods
Study Population
We studied a subgroup of men and women with mild to severe CKD enrolled in the CRIC Study. In total, 3939 participants were enrolled into the CRIC Study between June of 2003 and August of 2008 at seven clinical centers across the United States (Ann Arbor/Detroit, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland, CA). Eligible participants were identified through a combination of chart and laboratory database review, physician referral, and clinic screenings. Details on study design and baseline characteristics of the participants were previously published.40,41 All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites.
Inclusion/Exclusion Criteria
Men and women were eligible for the study if they were between 21 and 74 years of age and met the following age-specific eGFR criteria: 20–70 ml/min per 1.73 m2 for individuals ages 21–44 years, 20–60 ml/min per 1.73 m2 for individuals ages 45–64 years, and 20–50 ml/min per 1.73 m2 for individuals ages 65–74 years. Exclusion criteria included New York Heart Association class III/IV HF and polycystic kidney disease among others previously described.40 In total, 3483 participants were included in this study after excluding persons with missing data for hsTnT or NT-proBNP (n=84) and persons who self-reported HF at baseline (n=372).
Data Collection
Main Predictors
hsTnT and NT-proBNP were measured at baseline from EDTA plasma stored at −70°C using a chemilluminescent microparticle immunoassay (www.roche-diagnostics.us) on the ElecSys 2010. hsTnT was measured using the highly sensitive assay with a range of detectable values from 5 to 10,000 pg/ml.42 The coefficient of variation (CV) was 6.0% at a level of 26 pg/ml and 5.4% at a level of 2140 pg/ml. The value at the 99th percentile cutoff from a healthy reference population was 13 pg/ml for hsTnT with a 10% CV.42 The range of values for NT-proBNP was from 5 to 35,000 pg/ml, and the CV was 9.3% at a level of 126 pg/ml and 5.5% at a level of 4319 pg/ml.
Outcomes
The primary study outcome was incident HF over the time from study entry to March 31, 2012. HF was identified by asking study participants biannually if they were hospitalized, and selected hospitals or health care systems were queried for qualifying encounters. The first 30 discharge codes were identified for all hospitalizations, and codes relevant to HF resulted in retrieval of medical records by study personnel for centralized adjudicated review. At least two study physicians reviewed all possible HF events and deaths using medical records and guidelines on clinical symptoms, radiographic evidence of pulmonary congestion, physical examination of the heart and lungs, and when available, central venous hemodynamic monitoring data and echocardiographic imaging. HF was confirmed when both reviewers agreed on a probable or definite occurrence of HF on the basis of modified clinical Framingham criteria.43 Patients were censored at ESRD, death, or loss to follow-up. ESRD was identified through participant self-report and ascertainment by the US Renal Data System. Deaths were identified from report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and information from the Social Security Death Master File.
Covariates
At the baseline study visit, participants provided information on their sociodemographic characteristics, medical history, medication usage, and lifestyle behaviors. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other. Alcohol use was dichotomized as none versus any in the past 12 months. Anthropometric measurements and BP were assessed using standard protocols.44 Body mass index was derived as weight in kilograms divided by height in meters squared. Serum creatinine was measured using an enzymatic method on an Ortho Vitros 950 at the CRIC Central Laboratory and standardized to isotope dilution mass spectrometry-traceable values.45,46 Additional assays measured serum cystatin C, serum phosphorus, 24-hour urine total protein, glucose, LDL cholesterol (mathematically derived), HDL, cholesterol, FGF23, and total parathyroid hormone (PTH). The aforementioned assays were performed at the CRIC Central Laboratory, with the exception of PTH (measured at Scantibodies Laboratory, Inc.) and hemoglobin (locally measured). Diabetes mellitus was defined as a fasting glucose>126 mg/dl, a nonfasting glucose>200 mg/dl, or use of insulin or other antidiabetic medication. eGFR was calculated from serum creatinine and cystatin C using a CRIC Study equation.47
Echocardiograms were performed 1 year after enrollment in 2327 participants in our study population and provided data on left ventricular EF, LVMI, and LVH.48 Cardiac structure and function were assessed as previously described.49,50 In brief, assessments were performed using two-dimensional images and a standard imaging protocol according to American Society of Echocardiography guidelines51 and quantified centrally by a highly trained Registered Diagnostic Cardiac Sonographer.
Statistical Analyses
Summary statistics and distributions of hsTnT and NT-proBNP were generated. Study variables were described overall and across categories of hsTnT (category 1: undetectable values [≤5 pg/ml]; categories 2–5: quartiles of detectable values) and NT-proBNP (quintiles) using standard measures. Differences in characteristics across hsTnT and NT-proBNP categories were compared using ANOVA, chi-squared test, and Wilcoxon rank sum test as appropriate. Because of their skewed distributions, 24-hour urine total protein, PTH, and FGF23 were log transformed.
Crude incident HF event rates were calculated across hsTnT and NT-proBNP categories. Cox proportional hazards models were fit for HF, and follow-up was censored at ESRD, end of administrative follow-up, loss to follow-up, or death, whichever occurred first. Participants who reached ESRD were censored from analyses to address potential differences in the relationship of these biomarkers to incident HF among patients undergoing dialysis. We performed a series of nested Cox proportional hazard models with sequential adjustment for potential confounders as follows. Model 1 adjusted for demographic factors, including age, sex, and race/ethnicity, and traditional cardiovascular risk factors, including diabetes status, self-reported cardiovascular disease at baseline, current smoking, alcohol use, log(24-hour urine total protein excretion), eGFR, systolic BP, body mass index, and LDL and HDL levels. Model 2 included the factors in model 1 as well as pertinent medication use and novel cardiovascular risk factors: hemoglobin, use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and β-blockers, and serum phosphorus, log(PTH), and log(FGF23) levels. Model 3 included the factors in model 2 plus hsTnT and NT-proBNP together. All models were adjusted for CRIC Clinical Center. The cardiac biomarkers were modeled in terms of hazards ratios per 1 SD increase of the log-transformed marker level and also across discrete categories. Spline models were fit to explore for any nonlinear relationships in the study population overall and models stratified by baseline eGFR, sex, and race. C statistics, which quantify our ability to discriminate cases from noncases, were reported from the multivariable-adjusted model 1 before and after the addition of the two cardiac biomarkers.
We explored effect modification by an a priori-selected set of baseline characteristics, including baseline level of kidney function (eGFR<30, 30–44, and ≥45 ml/min per 1.73 m2), FGF23 (above and below the median value of 138.62 relative units/ml), EF (<50% and ≥50%), presence of LVH, 24-hour urine total protein excretion (a median value of <0.2 or ≥0.2 g/d), diabetes mellitus, race (non-Hispanic black and non-Hispanic white), and sex (men and women).
Two sensitivity analyses were performed. The first analysis recapitulated model 2 with incremental adjustment for EF and LVMI among 2327 participants who had an echocardiogram performed. Because the echocardiograms were performed 1 year after study entry, the follow-up time was reset from the time of echocardiogram rather than study entry (which was used in the primary analysis). A second analysis included participants in the CRIC Study who did not report cardiovascular disease (e.g., myocardial infarction, revascularization, stroke, and peripheral arterial disease) at baseline and adjustment for all model 2 predictors (n=2329).
All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K23-DK088865 to N.B., K01-DK092353 to A.H.A., 1DK066488 to M.G.S., and K24-DK02651 to H.I.F.). Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under a cooperative agreement from the NIDDK (Grants U01-DK060990, U01-DK060984, U01-DK061022, U01-DK061021, U01-DK061028, U01-DK060980, U01-DK060963, and U01-DK060902). In addition, this work was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) Grant UL1-TR000003, Johns Hopkins University Grant UL1-TR-000424, University of Maryland Grant GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland Grant UL1-TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research Grant UL1-TR000433, University of Illinois at Chicago Grant CTSA UL1-RR029879, Tulane University Translational Research in Hypertension and Renal Biology Grant P30-GM103337, and Kaiser Permanente NIH/National Center for Research Resources Grant UCSF-CTSI UL1 RR-024131. This work was not supported by any industry funds.
The study was conducted on behalf of the CRIC Study Investigators: Lawrence J. Appel (John Hopkins), A.S.G., J.H., J.W.K., J.P.L., H.I.F., Akinlolu Ojo (University of Michigan), Mahboob Rahman (Case Western Reserve University), and Raymond R. Townsend (University of Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010108/-/DCSupplemental.
References
- 1.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Curtis BM, Randell EW, Parfrey PS: Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5: 805–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada S, Ishii H, Takahashi H, Aoyama T, Morita Y, Kasuga H, Kimura K, Ito Y, Takahashi R, Toriyama T, Yasuda Y, Hayashi M, Kamiya H, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T: Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol 5: 1793–1798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM: Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 123: 1367–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL: Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 304: 2494–2502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K: Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 90: 195–203, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, Lamb EJ: B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 46: 610–620, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Mishra RK, Li Y, Ricardo AC, Yang W, Keane M, Cuevas M, Christenson R, deFilippi C, Chen J, He J, Kallem RR, Raj DS, Schelling JR, Wright J, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort Investigators : Association of N-terminal pro-B-type natriuretic peptide with left ventricular structure and function in chronic kidney disease (from the Chronic Renal Insufficiency Cohort [CRIC]). Am J Cardiol 111: 432–438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFilippi CR, Fink JC, Nass CM, Chen H, Christenson R: N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis 46: 35–44, 2005 [DOI] [PubMed] [Google Scholar]
- 11.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK: Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 304: 2503–2512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra RK, Li Y, DeFilippi C, Fischer MJ, Yang W, Keane M, Chen J, He J, Kallem R, Horwitz EJ, Rafey M, Raj DS, Go AS, Shlipak MG, CRIC Study Investigators : Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis 61: 701–709, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutamoto T, Kawahara C, Yamaji M, Nishiyama K, Fujii M, Yamamoto T, Horie M: Relationship between renal function and serum cardiac troponin T in patients with chronic heart failure. Eur J Heart Fail 11: 653–658, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA: Prevalence and determinants of troponin T elevation in the general population. Circulation 113: 1958–1965, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Wiessner R, Hannemann-Pohl K, Ziebig R, Grubitzsch H, Hocher B, Vargas-Hein O, Lun A, Schimke I, Liefeldt L: Impact of kidney function on plasma troponin concentrations after coronary artery bypass grafting. Nephrol Dial Transplant 23: 231–238, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Freda BJ, Tang WH, Van Lente F, Peacock WF, Francis GS: Cardiac troponins in renal insufficiency: Review and clinical implications. J Am Coll Cardiol 40: 2065–2071, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, Bakker SJ, Gansevoort RT, PREVEND study group : High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J 33: 2272–2281, 2012 [DOI] [PubMed] [Google Scholar]
- 18.deFilippi C, Seliger SL, Kelley W, Duh SH, Hise M, Christenson RH, Wolf M, Gaggin H, Januzzi J: Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem 58: 1342–1351, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Fichtlscherer S, Breuer S, Zeiher AM: Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: Further evidence for the existence of the “vulnerable” patient. Circulation 110: 1926–1932, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ooi DS, Isotalo PA, Veinot JP: Correlation of antemortem serum creatine kinase, creatine kinase-MB, troponin I, and troponin T with cardiac pathology. Clin Chem 46: 338–344, 2000 [PubMed] [Google Scholar]
- 21.Korff S, Katus HA, Giannitsis E: Differential diagnosis of elevated troponins. Heart 92: 987–993, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa M, Ishii J, Kitagawa F, Kanayama K, Takahashi H, Ozaki Y, Yuzawa Y: Prognostic value of highly sensitive troponin T on cardiac events in patients with chronic kidney disease not on dialysis. Heart Vessels 28: 473–479, 2013 [DOI] [PubMed] [Google Scholar]
- 23.deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, Christenson R, Uretsky B, Smiley M, Gold J, Muniz H, Badalamenti J, Herzog C, Henrich W: Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 290: 353–359, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D: N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int 71: 548–554, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Cleland JG, Taylor J, Freemantle N, Goode KM, Rigby AS, Tendera M: Relationship between plasma concentrations of N-terminal pro brain natriuretic peptide and the characteristics and outcome of patients with a clinical diagnosis of diastolic heart failure: A report from the PEP-CHF study. Eur J Heart Fail 14: 487–494, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P: N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 293: 1609–1616, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Yi S, Contreras G, Miller ER, Appel LJ, Astor BC: Correlates of N-terminal prohormone brain natriuretic peptides in African Americans with hypertensive chronic kidney disease: The African American Study of Kidney Disease and Hypertension. Am J Nephrol 29: 292–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagore R, Ling LH, Yang H, Daw HY, Chan YH, Sethi SK: Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol 3: 1644–1651, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takami Y, Horio T, Iwashima Y, Takiuchi S, Kamide K, Yoshihara F, Nakamura S, Nakahama H, Inenaga T, Kangawa K, Kawano Y: Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis 44: 420–428, 2004 [PubMed] [Google Scholar]
- 30.deFilippi CR, Seliger SL, Maynard S, Christenson RH: Impact of renal disease on natriuretic peptide testing for diagnosing decompensated heart failure and predicting mortality. Clin Chem 53: 1511–1519, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Astor BC, Yi S, Hiremath L, Corbin T, Pogue V, Wilkening B, Peterson G, Lewis J, Lash JP, Van Lente F, Gassman J, Wang X, Bakris G, Appel LJ, Contreras G: N-terminal prohormone brain natriuretic peptide as a predictor of cardiovascular disease and mortality in blacks with hypertensive kidney disease: The African American Study of Kidney Disease and Hypertension (AASK). Circulation 117: 1685–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, Ballantyne CM, de Lemos JA: Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 63: 1441–1448, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM: Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 49: 1331–1336, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, Levy D, Vasan RS, Wang TJ: Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol 108: 1341–1345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruger R, Schutte R, Huisman HW, Hindersson P, Olsen MH, Eugen-Olsen J, Schutte AE: NT-proBNP, C-reactive protein and soluble uPAR in a bi-ethnic male population: The SAfrEIC study. PLoS ONE 8: e58506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida AL, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA, Lima JA: N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail 5: 727–734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G: Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Wu IW, Hung MJ, Chen YC, Hsu HJ, Cherng WJ, Chang CJ, Wu MS: Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol 23: 181–188, 2010 [PubMed] [Google Scholar]
- 39.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA: Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 56: 254–261, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D: Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88: 107–115, 1993 [DOI] [PubMed] [Google Scholar]
- 44.National Center for Health Statistics (NCHS) : National Health and Nutrition Examination Survey Anthropometry Procedures Manual, Centers for Disease Control and Prevention, 2007. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf. Accessed August 1, 2014 [Google Scholar]
- 45.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Variability of creatinine measurements in clinical laboratories: Results from the CRIC study. Am J Nephrol 31: 426–434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI, CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, Hamm LL, Kusek J, Ojo A, Rahman M, Tao K, Wright JT, Xie D, Hsu CY, CRIC Study Investigators : A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I: Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367, 1989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.