Abstract
There are limited data regarding intermediate-term outcomes in patients with persistent BK viremia. Other viral infections have been implicated in the development of allosensitization through heterologous immunity, but the relationship between BK viremia and donor-specific antibodies (DSAs) is unexplored. In 2008, we initiated routine post-transplant BK viremia and DSA screening at our center; 785 kidney or kidney–pancreas transplant recipients were included in our study. Of these recipients, 132 (17%) recipients developed BK viremia during the study period. The median duration of BK viremia was 140 days (interquartile range=40–393 days), and persistent BK viremia was defined as lasting ≥140 days. Kaplan–Meier curves were generated to assess differences in patient and allograft survival on the basis of BK viremia status; survival was modeled using Cox proportional hazard regression. After a median follow-up of 3 years, there was no significant difference in terms of patient (hazard ratio [HR], 0.83; 95% confidence interval [95% CI], 0.28 to 2.49) or allograft survival (HR, 0.80; 95% CI, 0.37 to 1.73) between patients with and without BK viremia, which was confirmed in a time-varying analysis. In our logistic regression model, persistent BK viremia was strongly associated with the development of class II (HR, 2.55; 95% CI, 1.30 to 4.98) but not class I (HR, 1.13; 95% CI, 0.46 to 2.77) DSAs. These data suggest that persistent BK viremia does not negatively affect intermediate-term patient or allograft survival but is associated with increased risk for de novo DSA, although the exact mechanism is unclear.
Keywords: kidney transplantation, risk factors, transplant outcomes
BK virus is a double-stranded DNA polyomavirus first isolated from a kidney recipient in 1971.1 Primary infection is usually acquired in childhood, and latency is established in the uroepithelium.2 Although seldom a concern in immunocompetent hosts, BK viremia may be detected in 10%–30% of kidney recipients.3–5 The best elucidated risk factor for BK virus infection in kidney recipients is the overall degree of immunosuppression, with lymphodepleting antibody induction as well as tacrolimus- and mycophenolic acid (MPA)-based regimens considered by some to be especially permissive.6–8 If untreated, BK viremia can progress to BK nephropathy, impaired allograft function, and graft loss.9
There is currently no effective antiviral prophylaxis or therapy for BK virus. Guidelines10–12 recommend early post-transplant screening to detect viruria or viremia before the development of overt nephropathy. This strategy, coupled with reduction of maintenance immunosuppression on detection of viremia, often leads to rapid BK viral clearance13,14 and is the accepted standard. However, two recent studies15,16 suggest that approximately 50% of patients infected with BK develop persistent viremia, despite immunosuppression reduction. The effect of persistent BK viremia on patient outcomes is unclear. Although allograft survival was not affected in either series, patient survival was reduced in one study,15 and there was more graft dysfunction in the other study,16 albeit only in patients with persistently high viral loads.
BK infection frequently impairs graft function through direct viral cytopathic injury; however, it has also been associated with rejection.16 There are three theories put forth to explain the relationship between BK virus and rejection. (1) Overimmunosuppression from intensified therapy to treat rejection can predispose to subsequent BK viremia. (2) Rejection may complicate overaggressive immunosuppression reduction to treat BK viremia. (3) BK virus may mediate allosensitization and rejection through heterologous immunity. Both Epstein–Barr Virus and cytomegalovirus (CMV) are examples of viruses that have been strongly linked to acute and chronic antibody-mediated injury through this mechanism in organ transplant recipients.17 The development of de novo donor-specific antibodies (DSAs) after transplant is now a widely used harbinger of subclinical alloreactivity and often precedes overt antibody-mediated rejection by months to years.18,19 We hypothesized that persistent BK viremia is a risk factor for and precedes the development of DSAs.
Commencing in 2008, our center, which uses a standardized depleting antibody induction and tacrolimus–MPA–prednisone maintenance regimen, initiated a protocol of routine BK viremia and DSA screening starting at 3 months post-transplant. This study was performed to examine the effect of any BK viremia treated by immunosuppression reduction on patient and graft outcomes in our population, including testing our hypothesis relating persistent BK viremia to de novo DSA.
Results
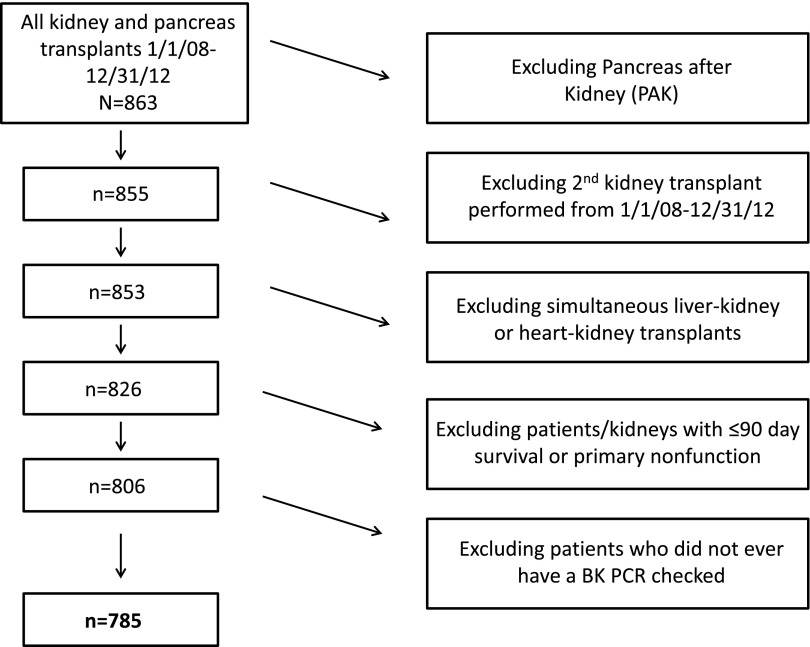
From January 1, 2008, to December 31, 2012, 863 kidney, pancreas, and kidney–pancreas transplants were performed at our institution (Figure 1). Eight patients received a pancreas after kidney transplant; 27 patients received a simultaneous liver–kidney or heart–kidney transplant. Two patients received a second kidney transplant during the study period. Twenty patients either experienced primary nonfunction or did not have at least 90 days patient or allograft survival. Twenty-one patients did not ever have a BK viral load checked in the post-transplant period. In total, 785 patients met study criteria and were included in the analysis.
Figure 1.
Most patients transplanted at our center were included in the study. Creation of the patient cohort. Pancreas transplants alone, multi-organ transplants (except kidney-pancreas) and those with less than 90 day survival were excluded from the analysis.
Baseline Characteristics of the Cohort
The cohort was predominantly men (62%), with a mean age at transplant of 50.7 years (SD=13.5 years); 35% of patients were African American. Most patients (71%) received a deceased donor kidney and induction with rabbit antithymocyte globulin (rATG; 88%). The vast majority of patients were on a maintenance immunosuppression regimen consisting of tacrolimus (96%), mycophenolate mofetil (MMF)/MPA (99%), and prednisone (100%). Sensitized patients with a panel reactive antibody (PRA)≥30% comprised 18% of the cohort, and 95 (12%) patients had a history of a prior kidney transplant outside the study period. Allograft loss occurred in 43 patients, in whom just over one half (n=22) were because of patient death. The most common causes of ESRD included glomerular disease (25%) and diabetes (25%); 12 patients in the cohort were HIV-positive, 51 patients were infected with hepatitis C virus (HCV), and 9 patients were hepatitis B virus surface antigen-positive.
BK Virus Prevalence and Characteristics
During the study period, 132 (17%) patients had detectable BK viremia, with 17 (13%) patients having only a single positive BK PCR. Routine screening detected 117 infections, with another 15 infections diagnosed in the course of evaluation of allograft dysfunction. Median peak BK viral load was 4 log copies/ml (interquartile range [IQR]=3.5–4.7). Of 298 for-cause biopsies performed during the study, 12 patients were diagnosed with BK nephropathy at a median of 190 days (IQR=160–223) post-transplant. The median time to BK viremia detection was 137.9 days (IQR=94.3–216.0), and 48% of patients were diagnosed by the 3-month routine screening time point. The median duration of BK viremia was 140 days (IQR=40–393), and 68 (52%) of the infected patients met criteria for persistent BK viremia. Fourteen patients with BK viremia that did not clear the virus, despite the antimetabolite discontinuation and tacrolimus minimization strategy were converted to cyclosporin A (CsA). Six of these patients remained viremic, despite target CsA trough levels of 35–50 ng/ml.
Clinical Features of Patients with Detectable BK Viremia
An unadjusted analysis of the entire cohort is shown in Table 1, with patients without viremia compared with their counterparts with any detectable BK viremia as well as patients with persistent BK viremia. Compared with the nonviremic cohort, patients with any detectable BK viremia were older than patients without viremia (53.4±12.4 versus 50.2±13.7 years, P=0.01). There was no significant difference in terms of the sex or racial composition of the three groups. Similarly, there were no significant differences with respect to donor type, ureteral stent placement, induction immunosuppression, level of sensitization, prior transplant, or pretransplant diabetes prevalence between the viremic and nonviremic recipients. The vast majority of patients received tacrolimus as the initial calcineurin inhibitor (CNI); however, among 19 recipients treated with CsA, BK viremia was more prevalent. Significantly, more patients with BK viremia had acute rejection (T cell- or antibody-mediated) than patients without viremia. Because BK viremia detection had a slight bimodal distribution with a small second peak at 12 months post-transplant (Figure 2), we compared baseline clinical and demographic characteristics of these patients with later-onset viremia with those characteristics of patients whose viremia was detected at 3 months, although we found no significant differences (Supplemental Table 1).
Table 1.
Baseline characteristics of the patient cohort
| Variable | BK Never Positive (n=653) | BK Ever Positive (n=132) | P Valuea | Persistent BK (n=68) | P Valueb |
|---|---|---|---|---|---|
| Mean age (yr)±SD | 50.2±13.7 | 53.4±12.4 | 0.01 | 53±12.5 | 0.10 |
| Men | 399 (61.1%) | 86 (65.1%) | 0.38 | 46 (67.7%) | 0.29 |
| African-American race | 235 (36.0%) | 41 (31.1%) | 0.28 | 22 (32.4%) | 0.55 |
| Etiology of ESRD (%) | 0.09 | 0.01 | |||
| Diabetes | 159 (24.3%) | 34 (25.8%) | 17 (25%) | ||
| Polycystic kidney disease | 71 (10.9%) | 20 (15.1%) | 11 (16.2%) | ||
| GN | 173 (26.5%) | 27 (20.5%) | 9 (13.2%) | ||
| Obstruction | 29 (4.4%) | 3 (2.2%) | 0 (0%) | ||
| Hypertension | 97 (14.9%) | 12 (9.1%) | 8 (11.7%) | ||
| FSGS | 69 (10.6%) | 20 (15.2%) | 14 (20.6%) | ||
| Unknown/other | 55 (8.4%) | 16 (12.1%) | 9 (13.2%) | ||
| Pretransplant diabetes | 171 (26.2%) | 40 (30.3%) | 0.33 | 18 (26.5%) | 0.96 |
| Simultaneous kidney–pancreas transplant | 24 (3.7%) | 6 (4.5%) | 0.62 | 3 (4.4%) | 0.73 |
| Prior renal transplant | 81 (12.4%) | 13 (9.8%) | 0.41 | 5 (7.4%) | 0.32 |
| PRA≥30% at transplant | 114 (17.5%) | 27 (20.4%) | 0.42 | 13 (19.1%) | 0.74 |
| rATG induction | 573 (87.7%) | 116 (87.9%) | 0.97 | 62 (91.2%) | 0.56 |
| Initial CNI | <0.001 | <0.001 | |||
| Tacrolimus | 643 (98.5%) | 123 (93.2%) | 62 (91.2%) | ||
| CsA | 10 (1.5%) | 9 (6.8%) | 6 (8.8%) | ||
| Ureteral stent | 107 (16.4%) | 20 (15.1%) | 0.73 | 8 (11.8%) | 0.32 |
| HCV positive | 39 (6.0%) | 12 (9.1%) | 0.19 | 5 (7.4%) | 0.60 |
| Deceased donor | 456 (69.8%) | 101 (76.5%) | 0.12 | 51 (75%) | 0.38 |
| Expanded criteria donor | 67 (14.8%) | 17 (16.8%) | 0.61 | 8 (15.7%) | 0.71 |
| CMV viremia | 49 (7.5%) | 14 (10.6%) | 0.33 | 8 (18.6%) | 0.32 |
| Acute rejection | 70 (10.7%) | 24 (18.2%) | 0.02 | 13 (19.1%) | 0.04 |
| Class I DSA | 47 (7.2%) | 14 (10.6%) | 0.17 | 7 (11.1%) | 0.36 |
| Class II DSA | 67 (10.3%) | 22 (16.7%) | 0.03 | 16 (25%) | 0.001 |
| Median days to class I DSA | 187 (32–746) | 273 (106–732) | 0.53 | 344 (62–590) | 0.90 |
| Median days to class II DSA | 252 (36–691) | 383 (202–759) | 0.07 | 356 (175–485) | 0.59 |
| Serum creatinine 3 mo (mg/dl; median) | 1.4 (1.1–1.7) | 1.4 (1.1–1.6) | 0.54 | 1.41 (1.2–1.6) | 0.68 |
| Serum creatinine 6 mo (mg/dl; median) | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) | 0.98 | 1.4 (1.1–1.6) | 0.83 |
| Serum creatinine 12 mo (mg/dl; median) | 1.3 (1.0–1.6) | 1.3 (1.1–1.5) | 0.43 | 1.3 (1.1–1.5) | 0.88 |
| Serum creatinine 24 mo (mg/dl; median) | 1.3 (1.0–1.6) | 1.3 (1.1–1.5) | 0.64 | 1.3 (1.2–1.5) | 0.45 |
| Serum creatinine 36 mo (mg/dl; median) | 1.3 (1.0–1.6) | 1.3 (1.0–1.6) | 0.51 | 1.4 (1.1–1.6) | 0.23 |
P value for comparison of BK never positive versus BK positive.
P value for comparison of BK never positive versus persistent BK viremia.
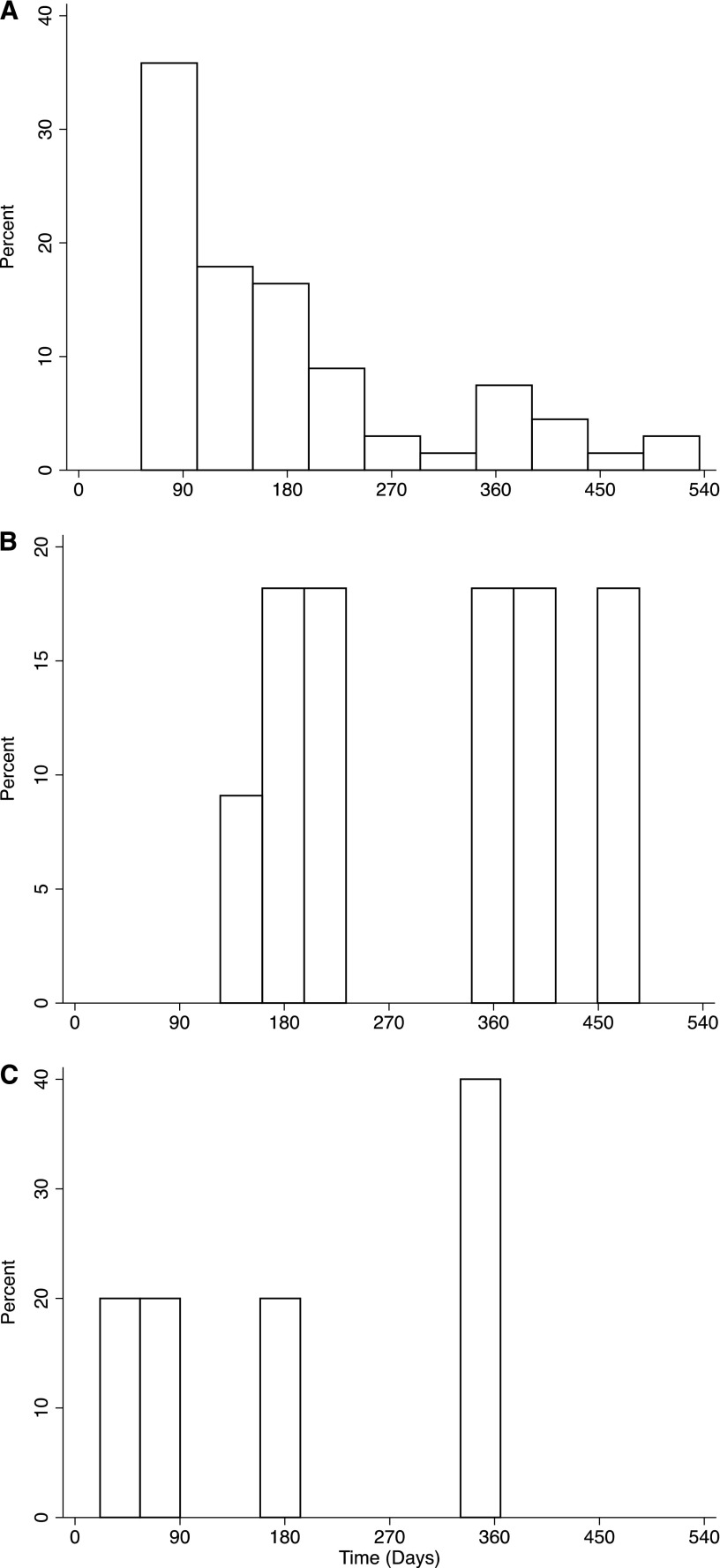
Figure 2.
BK viremia precedes DSA class II. Histogram of time to detection of (A) BK viremia (median=137.7 days; IQR=95–238), (B) de novo class II DSA (median=356 days; IQR=175–485), and (C) de novo class I DSA (median=344 days; IQR=62–590) in patients with persistent BK viremia. Among patients with persistent viremia who developed de novo class II DSA, BK virus was detected before the DSA in 17.6% of them.
Development of DSA
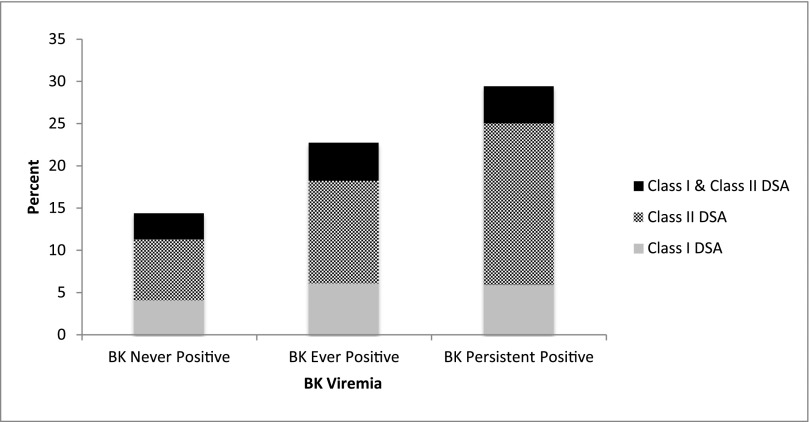
In 710 patients in whom DSA was measured, de novo DSA developed in 124 (90 patients during routine screening and 34 patients during investigation of allograft dysfunction) patients, including 35 (4%) patients with class I DSA alone, 63 (8%) patients with class II DSA alone, and 26 (3%) with both classes I and II DSA. Median class I mean fluorescence intensity (MFI) was 1900 (IQR=1000–3600), and median class II MFI was 3000 (IQR=1200–14,000). Overall, DSA and class II DSA positivity was higher in patients with BK viremia than patients without viremia (P=0.03), whereas the percentage of patients with class I DSA did not differ significantly between these groups (P=0.17) (Figure 3). In patients with BK viremia, detection of BK virus (median time=137.9 days) preceded development of de novo class I DSA (median time=273 days) and class II DSA (median time=383 days). Median trough tacrolimus levels measured on 15 separate occasions in viremic patients both before and after BK virus detection during the first 2 post-transplant years were not significantly different at any of these time points when stratified by DSA status (Supplemental Table 2).
Figure 3.
Class II DSA was most common in patients with persistent BK viremia. Proportion of patients who developed a positive DSA (overall, class I, or class II) stratified by BK viral status. The proportion of patients with a positive class II DSA in the BK ever-positive group compared with the BK never-positive group was significantly greater (P=0.03) along with the proportion of patients in the persistent BK group compared with the BK never-positive group (P=0.001).
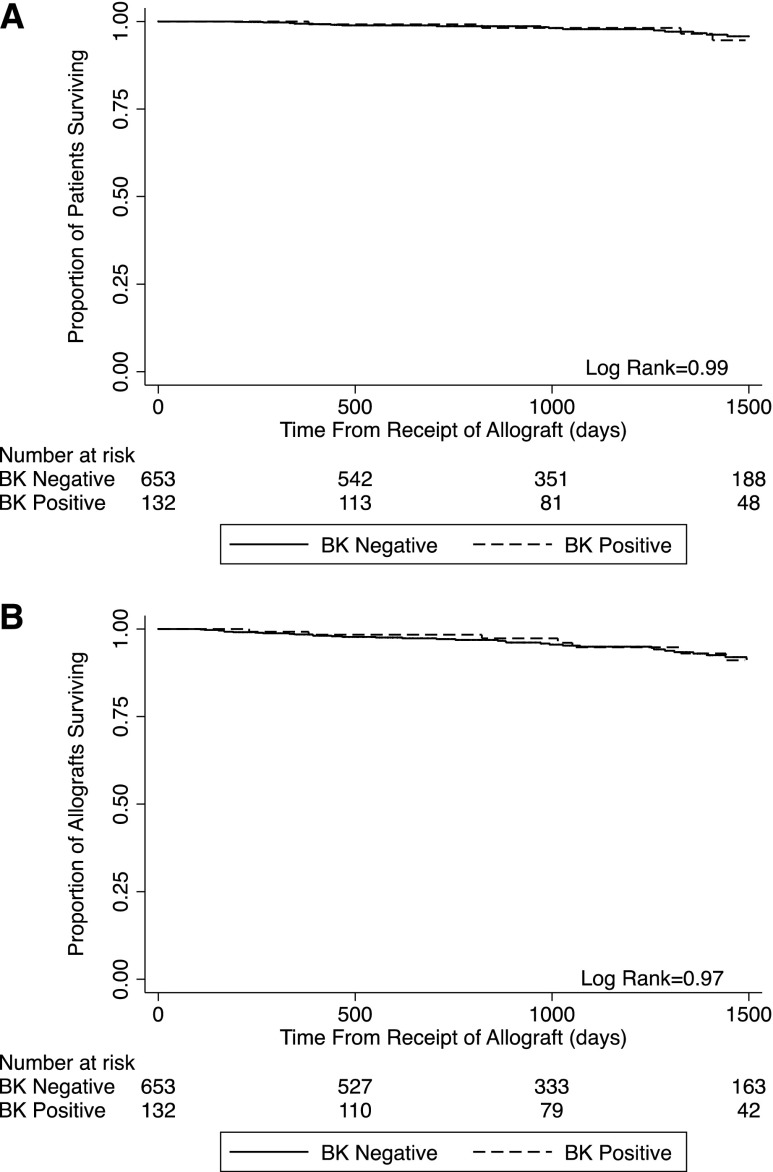
After a median follow-up of the cohort of 1104 days (IQR=653–1562), there were no significant differences in serum creatinine levels at 3, 6, 12, 24, or 36 months between the patient groups. Patient survival (97.0% in the BK+ group versus 97.2% in the uninfected group) and allograft survival (93.9% in the BK+ group versus 94.6% in the uninfected group) were, likewise, not different between the BK-infected and uninfected cohorts (Figure 4).
Figure 4.
BK viremia does not adversely affect patient or allograft survival. (A) Kaplan–Meier estimates of time to patient death stratified by BK viral status (BK positive versus BK never positive). (B) Kaplan–Meier estimates of time to all-cause allograft loss stratified by BK viral status (BK positive versus BK never positive).
Patient and Graft Loss: Multivariable Regression Models
Univariable and multivariable Cox regression models were fit for graft loss and patient mortality (Tables 2–4). In the univariable analysis for graft loss, age>65 years, presence of HCV infection, prior kidney transplant, prior nonrenal transplant, class I DSA, and any acute rejection were all significantly associated. Although BK viremia was not associated with graft loss (hazard ratio [HR], 1.02; 95% confidence interval [95% CI], 0.47 to 2.19), it was retained in the multivariable model, because it was the exposure of interest. Highest BK viral load was also tested in the univariable model and not significantly associated with allograft loss (HR, 0.57; 95% CI, 0.24 to 1.37). In the multivariable model, only age>65 years, HCV infection, and acute rejection remained significantly associated with graft loss, whereas BK viremia was not (HR, 0.80; 95% CI, 0.37 to 1.73).
Table 2.
Univariable and multivariable Cox regressions
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| All-cause allograft loss | ||||||
| BK ever positive | 1.02 | 0.97 | 0.47 to 2.19 | 0.80 | 0.58 | 0.37 to 1.73 |
| Age≥65 yr | 2.48 | 0.008 | 1.27 to 4.83 | 3.05 | 0.001 | 1.55 to 5.99 |
| HCV positive | 3.81 | <0.001 | 1.83 to 7.94 | 3.42 | 0.001 | 1.61 to 7.30 |
| Acute rejection | 5.99 | <0.001 | 3.24 to 11.09 | 6.23 | <0.001 | 3.41 to 11.39 |
| Prior renal transplant | 2.13 | 0.04 | 1.02 to 4.43 | |||
| Prior nonrenal transplant | 3.70 | 0.03 | 1.14 to 11.98 | |||
| Class I DSA | 2.47 | 0.05 | 1.02 to 6.01 | |||
| Patient death | ||||||
| BK ever positive | 0.99 | 0.99 | 0.33 to 2.93 | 0.83 | 0.74 | 0.28 to 2.49 |
| Age≥65 yr | 4.48 | 0.001 | 1.91 to 10.49 | 7.14 | <0.001 | 2.86 to 17.82 |
| HCV positive | 5.29 | 0.001 | 2.07 to 13.53 | 8.99 | <0.001 | 3.28 to 24.67 |
| Prior renal transplant | 2.31 | 0.10 | 0.85 to 6.27 | 3.45 | 0.02 | 1.24 to 9.59 |
| Prior nonrenal transplant | 8.13 | 0.001 | 2.40 to 27.53 | |||
Table 4.
Time-varying analysis for death
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| BK_TV | 0.94 | 0.96 | 0.13 to 7.10 | 0.83 | 0.86 | 0.11 to 6.23 |
| HCV positive | 5.29 | 0.001 | 2.07 to 13.53 | 8.76 | <0.001 | 3.22 to 23.84 |
| Age≥65 yr | 4.48 | 0.001 | 1.92 to 10.49 | 7.20 | <0.001 | 2.88 to 17.99 |
| Prior renal transplant | 2.31 | 0.10 | 0.85 to 6.27 | 3.41 | 0.02 | 1.23 to 9.45 |
In a similar analysis performed for death-censored allograft loss, African-American race (HR, 2.86; 95% CI, 1.28 to 6.36), HCV infection (HR, 3.57; 95% CI, 1.34 to 9.50), class I DSA (HR, 3.30; 95% CI, 1.08 to 10.13), and acute rejection (HR, 13.74; 95% CI, 6.06 to 31.15) were all significant in the univariable analysis. However, in the multivariable analysis, only acute rejection (HR, 13.1; 95% CI, 5.73 to 30.08) was significantly associated, whereas BK viremia was not (HR, 0.78; 95% CI, 0.29 to 2.08).
The multivariable Cox regression models for patient death are shown in Table 2. Age>65 years, HCV infection, prior kidney transplant, and prior nonrenal transplant were all associated with patient death in the univariable analysis. In the multivariable analysis, only age>65 years, HCV infection, and prior kidney transplant remained significant. Neither BK viremia (HR, 0.83; 95% CI, 0.28 to 2.49) nor highest BK viral load (HR, 0.32; 95% CI, 0.07 to 1.45) was associated with patient death.
We next explored whether the duration of BK viremia was associated with patient and graft survival by examining the change in BK viral status at each of the screening time points and analyzing it as a time-varying covariate (Tables 3 and 4). In the univariable analysis for allograft loss, using BK as a time-varying variable (noted as BK_TV in the tables), we found that BK was not associated with graft loss (HR, 0.42; 95% CI, 0.06 to 3.07) but was associated with age>65 years, HCV infection, prior kidney transplant, acute rejection, and class I DSA. In the multivariable analysis, only HCV infection, acute rejection, and age>65 years remained significantly associated. BK modeled in a time-varying fashion was not associated with allograft loss (HR, 0.35; 95% CI, 0.05 to 2.55). The time-varying analysis for patient death revealed similar results, with HCV, age>65 years, and prior kidney transplant being significantly associated with patient death, whereas BK viremia was not (HR, 0.83; 95% CI, 0.11 to 6.23).
Table 3.
Time-varying analysis for all-cause allograft loss
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| BK_TV | 0.42 | 0.39 | 0.06 to 3.07 | 0.35 | 0.30 | 0.05 to 2.55 |
| Age≥65 yr | 2.48 | 0.008 | 1.27 to 4.83 | 3.23 | 0.001 | 1.64 to 6.36 |
| HCV positive | 3.80 | <0.001 | 1.82 to 7.93 | 3.43 | 0.002 | 1.60 to 7.35 |
| Prior renal transplant | 2.13 | 0.04 | 1.02 to 4.43 | 2.04 | 0.06 | 0.97 to 4.29 |
| Acute rejection | 6.23 | <0.001 | 3.40 to 11.39 | 5.66 | <0.001 | 3.04 to 10.52 |
| Class I DSA | 2.47 | 0.05 | 1.02 to 6.00 | |||
BK Viremia and Development of De Novo DSA Multivariable Models
We fit logistic regression models to investigate whether BK virus was associated with allosensitization in terms of any de novo DSA formation as well as development of de novo classes I and II DSA individually (Tables 5 and 6). With BK virus ever positive included as a covariate in the model, it was significantly associated with de novo DSA formation (odds ratio [OR], 1.82; 95% CI, 1.23 to 2.92; P=0.01). When the model was fit with persistent BK viremia as a covariate, persistent BK viremia was also significantly associated with the development of any DSA (OR, 2.56; 95% CI, 1.45 to 4.52) in the univariable analysis as well as male recipient, African-American race, man donor, PRA≥30, and acute rejection. In the multivariable model, persistent BK viremia remained significantly associated with any de novo DSA (OR, 2.53; 95% CI, 1.40 to 4.59) as well as African-American race, PRA≥30, and acute rejection.
Table 5.
Univariable and multivariable logistic regressions for development of class II DSA
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | P Value | 95% CI | OR | P Value | 95% CI | |
| Persistent BK viremia | 2.81 | <0.001 | 1.51 to 5.24 | 2.55 | 0.01 | 1.30 to 4.98 |
| HCV | 1.98 | 0.10 | 0.88 to 4.48 | |||
| Donor age (yr) | 0.99 | 0.05 | 0.97 to 1.00 | |||
| PRA≥30 | 2.17 | <0.001 | 1.28 to 3.67 | 1.78 | 0.05 | 1.01 to 3.13 |
| Acute rejection | 3.07 | <0.001 | 1.73 to 5.45 | 2.62 | 0.01 | 1.42 to 4.83 |
| Class I DSA | 6.84 | <0.001 | 3.75 to 12.48 | 5.96 | <0.001 | 3.19 to 11.16 |
Table 6.
Univariable and multivariable time-varying analyses for class II DSA
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | P Value | 95% CI | HR | P Value | 95% CI | |
| Persistent BK_TV | 2.22 | 0.02 | 1.12 to 4.39 | 2.20 | 0.02 | 1.11 to 4.36 |
| African American | 1.46 | 0.09 | 0.95 to 2.26 | |||
| Donor age, yr | 0.98 | 0.07 | 0.97 to 1.00 | |||
| Donor, men | 1.77 | 0.01 | 1.12 to 2.80 | |||
| Expanded criteria donor | 0.41 | 0.08 | 0.15 to 1.11 | |||
| Prior kidney transplant | 1.65 | 0.09 | 0.93 to 2.94 | |||
| PRA≥30 | 2.13 | 0.002 | 1.32 to 3.44 | 2.32 | 0.003 | 1.32 to 4.06 |
| Class I DSA | 5.02 | <0.001 | 3.07 to 8.19 | |||
| Acute rejection | 2.54 | <0.001 | 1.53 to 4.22 | 1.82 | 0.07 | 0.96 to 3.46 |
In the analysis of risk factors for development of class II DSA (Table 5), BK ever positive was not significantly associated with class II DSA (OR, 1.54; 95% CI, 0.88 to 2.73; P=0.13), whereas persistent BK viremia (OR, 2.81; 95% CI, 1.51 to 5.24) as well as HCV, donor age, PRA≥30, class I DSA, and acute rejection were all significantly associated with class II DSA; in the multivariable analysis, only persistent BK viremia (OR, 2.55; 95% CI, 1.30 to 4.98), acute rejection, PRA≥30, and class I DSA remained significant. To further examine the relationship between persistent BK and class II DSA, we performed a time-varying analysis for the development of class II DSA (Table 6). In the univariable analysis, persistent BK viremia, African-American race, donor age, donor sex, expanded criteria donor kidney, prior kidney transplant, PRA≥30, class I DSA, and acute rejection were all associated with class II DSA; in the multivariable analysis, only persistent BK viremia (HR, 2.20; 95% CI, 1.11 to 4.36; P=0.02), PRA≥30, and acute rejection remained significantly associated with development of class II DSA.
Neither BK ever positive nor persistent BK viremia was significantly associated with the development of class I DSA in the univariable (OR, 1.51; 95% CI, 0.65 to 3.51) or multivariable (OR, 1.13; 95% CI, 0.46 to 2.77) logistic regression or time-varying models. Similarly, no association was found between the MFI of class I or II DSA and either presence of BK viremia or BK viral load.
Discussion
In this study, we describe the outcomes of 785 kidney or kidney–pancreas recipients who were managed early post-transplant with routine serum BK virus screening and immunosuppression reduction by protocol for any detectable viremia. With a median of 3 years post-transplant follow-up, there were four notable findings of this study. (1) Consistent with prior studies, most BK viremia occurs within the first 3 post-transplant months. (2) Although immunosuppression reduction is an effective way to clear the virus from the blood, it is usually a gradual process. (3) Compared with uninfected patients, neither the presence nor the duration of BK viremia had a deleterious effect on patient or graft survival. (4) Persistent BK viremia was associated with an increased risk of developing de novo DSA.
During the study period, 132 (17%) patients developed BK viremia, and 12 of them had biopsy-proven BK nephropathy. Almost one half of the patients with BK viremia were identified at the 3-month screening time point, consistent with other reports.3,4,14 In contrast to some prior studies,7,8,14 our multivariate analysis did not find an association between development of BK and choice of induction or maintenance immunosuppression; however, this result may be because of the overwhelmingly predominant use of rATG induction and tacrolimus-mycophenolate–based immunosuppression in our cohort. Other previously identified risk factors,9,10,20,21 such as sex, race, and ureteral stent use, were not shown to be significantly associated with BK viremia in our cohort. We believe that this finding reflects the fact that the BK viremia risk imparted by burden of immunosuppression dwarfs these other factors. This notion is underscored by the fact that both older recipient age and treatment for rejection, exposures known to diminish immunity, were significantly associated with BK viremia. We also noted no association between other potentially immunomodulatory infections, such as HIV, HCV, and CMV, and BK viremia.
As an opportunistic infection, BK virus is strongly related to overall immune status. In our cohort, where the immunosuppression regimen was uniform in almost all patients, reduction of the antirejection burden was effective in bringing about viral clearance, consistent with established guidelines. However, our study highlights that this process is a gradual process, with more than one half of infected patients still viremic 4 months after commencing immunosuppression reduction. This finding is in contrast to CMV infection, for example, where immunosuppression reduction coupled with therapies that disrupt viral replication typically render patients aviremic within a few weeks. Persistent BK viremia, therefore, has the potential to limit practitioners’ decision-making capacities regarding upward or downward titration of immunosuppression during this entire period, especially given the lack of efficacious antiviral therapy. It is possible that, during this phase of persistent viremia in the setting of gradual viral clearance, there is a tipping point, where the net state of immunosuppression changes from one of over- to underimmunosuppression, predisposing to allosensitization and acute rejection. For this reason, the development of effective preventive antiviral therapies is urgently needed to help mitigate this risk.
In our cohort, 78% of infected patients were still viremic 4 weeks after diagnosis and initiation of immunosuppression reduction, with 61.5% of patients remaining viremic at 3 months. In contrast to a prior study by Hardinger et al.,15 in which persistent viremia occurred in 48% of the cohort and was associated with inferior patient survival, we did not find that persistent BK viremia negatively affected either patient or allograft survival. There are two possible explanations for the differences in rates of persistent viremia between the study by Hardinger et al.15 and our study. (1) The study by Hardinger et al.15 screened weekly for the first 16 weeks and therefore, detected and intervened earlier. (2) Although rATG was widely used in both studies, one third of the patients in the study by Hardinger et al.15 were on CsA as the calcineurin inhibitor, and a high proportion were receiving azathioprine rather than MMF; hence, the overall immunosuppression burden was likely lower in their population.
One of the most novel aspects of our study is that we have examined the relationship between BK viremia and the development of de novo DSA. There was a statistically significant increase in the risk of any de novo DSA in patients with persistent BK viremia (HR, 2.53; 95% CI, 1.40 to 4.59; P<0.01) and an increase in the risk of class II DSA in particular (OR, 2.55; 95% CI, 1.30 to 4.98; P=0.01). The exact mechanism behind this association, although intriguing, is unclear. It may represent an unanticipated consequence of overaggressive reduction of immunosuppression that is not adequately replenished after BK virus is cleared. Alternatively, analogous to what has been observed with other viruses, such as CMV, de novo DSA formation could occur as a manifestation of heterologous immunity by which BK-specific T cells provide help for alloreactive B cells. The observed temporal relationship between the early development of BK viremia followed by later appearance of class II DSA is suggestive of a causal link between them. All patients with BK viremia in our study received a standard immunosuppression regimen before virus detection and had their immunosuppression reduced after detection according to a standard minimization protocol, regardless of DSA status. Moreover, trough tacrolimus levels during the first 24 post-transplant months, measured before and after BK virus detection in all patients with viremia, were not significantly different when stratified by DSA status (Supplemental Tables 1 and 2). Despite seemingly standardized immunosuppression management in all patients, we cannot further quantify the overall immunosuppression burden of patients to more clearly understand the relationship between BK viremia and DSA in the absence of a universally accepted immunostat. Additional prospective studies using translational research methodology will be required to elucidate the exact mechanism of DSA development in these patients. Regardless of the mechanism of DSA development in the setting of BK viremia, it is widely accepted that de novo DSA represents a threat to long-term allograft survival.
Our study has several strengths. It describes the prevalence of BK viremia in the modern immunosuppressive era in a cohort almost exclusively induced with rATG and maintained on tacrolimus–MPA–prednisone and the concomitant effect of a standardized immunosuppression reduction approach on BK viremia. Furthermore, to better assess the effect of duration of BK infection on outcomes and more completely replicate what occurs in clinical practice, we have fit regression models that included BK viremia as a time-varying covariate to account for changes in BK viral status over time.
There are also several limitations to our study. First, although the BK virus screening protocol was instituted prospectively, this study is a single-center retrospective study. However, unlike most prior studies in this area, we have examined BK virus-related outcomes in a transplant population treated with a homogeneous immunosuppressive regimen that reflects the most widely used combination of therapies in the United States. Second, 21 (2.7%) patients did not ever have a serum BK viral load measured as part of routine clinical care, and 32 (4.1%) patients had only one BK viral load measurement during the study period. We believe that these patients not tested or tested one time would have been less likely to have their immunosuppression reduced in the absence of a clear clinical indication, such as BK viremia. Even if undiagnosed BK viremia had been present in these patients not tested or tested one time, a standard, unmodified immunosuppression regimen would be expected to be permissive of BK viral replication, leading to BK nephropathy, attendant allograft dysfunction, and a for-cause biopsy indication, which would have ultimately unmasked BK viremia. Therefore, we believe that the likelihood of missed BK viremia in these nontested patients was low. Third, because our BK virus screening protocol only commenced at 3 months post-transplant, it is plausible that, by having missed an opportunity for earlier BK virus diagnosis and immunosuppression intervention, we selected out for patients with persistent viremia. Evidence against this selection, however, comes from a recent study,16 where a protocol BK virus screening and management strategy starting 1 month post-transplant showed that the time to initial detection of BK virus was not different between patients with transient or persistent viremia. Fourth, because of the retrospective collection of data, we had no information regarding BK genotype and donor and recipient BK serostatus; moreover, because we did not biopsy all patients with viremia, we cannot determine the true prevalence of BK nephropathy in our study. However, because for-cause biopsies are routinely performed in all patients with allograft dysfunction, it is very unlikely that clinically significant BK nephropathy culminating in graft dysfunction or graft loss was missed during the period of the study. Fifth, we cannot exclude the contribution of residual confounding to our findings from unmeasured factors.
BK viremia is a common complication of kidney transplantation. With intermediate-term follow-up, persistent BK viremia does not have a negative effect on patient or allograft survival. Although immunosuppression reduction is an effective strategy for treating BK viremia, viral clearance is typically gradual and may predispose to de novo DSA, although the exact mechanism is unclear. Future treatment strategies should focus on simultaneously clearing BK viremia while mitigating allosensitization risk.
Concise Methods
Subjects
A retrospective cohort study of all consecutive kidney or simultaneous kidney–pancreas recipients transplanted at the University of Pennsylvania from January 1, 2008, to December 31, 2012, was conducted (Figure 1). Patients were included in the study if they had at least 6 months of post-transplant follow-up and at least one serum BK virus DNA measurement performed post-transplant. Patients lacking any serum BK virus DNA measurements ever or whose kidney allografts survived for <90 days (the first time point in our protocol for routine BK virus screening) as well as recipients of pancreas alone, pancreas after kidney, simultaneous liver–kidney, or simultaneous heart–kidney transplantation were excluded from the analysis. This study was approved by the institutional review board (Institutional Review Board Protocol 818550).
Kidney Transplant Protocol
Immunosuppression Protocol
Triple therapy-based maintenance immunosuppression comprised a CNI in conjunction with an antimetabolic agent and prednisone. The CNI was almost always tacrolimus; modified CsA was used if patients had been on it at the time of transplant (e.g., from a prior transplant) or were deemed by the clinical team to be at particular risk for tacrolimus-related toxicity. Target tacrolimus trough levels were (1) weeks 0–12: 8–12 ng/ml, (2) weeks 12–24: 6–8 ng/ml, (3) weeks 24–48: 5–7 ng/ml, and (4) beyond 48 weeks: 4–6 ng/ml. Target CsA trough levels were (1) weeks 0–12: 150–250 ng/ml, (2) weeks 12–24: 150–200 ng/ml, (3) weeks 24–48: 100–200 ng/ml, and (4) beyond 48 weeks: 50–100 ng/ml. MMF was the antimetabolite used in the vast majority of patients, and it was administered at 1 g/d in divided doses, as tolerated, and adjusted to maintain white blood cell count>3.5×106/L; the dose was titrated downward for adverse drug reactions. Steroids were initially given a bolus methylprednisolone (500 mg) intraoperatively. Prednisone was started postoperative day 1 and tapered according to center protocol to 5 mg daily by 30 days post-transplant. Induction therapy generally consisted of rabbit antithymocyte globulin (Thymoglobulin; Genzyme, Cambridge, MA) dosed at 1.5 mg/kg intravenously over 4–6 hours, commencing intraoperatively before reperfusion and infused daily for 2–3 days with an additional two to three doses over the subsequent 10 days in the outpatient setting to a total of three to five doses according to immunologic risk. At the discretion of the transplant team, basiliximab (20 mg; Simulect; Novartis Pharmaceuticals Corp., East Hanover, NJ) administered intraoperatively and again on postoperative day 3 was occasionally used, especially in patients with low immunologic risk. Patients were considered higher immunologic risk if they were highly sensitized (complement-dependent cytoxicity or calculated PRA>30%), had a prior transplant, or were of African-American race; these patients were generally administered five doses of rATG.
Immunoprophylaxis
All patients received prophylaxis against Pneumocystis jiroveci pneumonia with Sulfamethoxazole-Trimethoprim (400–80 mg) for 24 weeks unless allergic, in which case patients were given atovaquone (1500 mg) daily for 24 weeks. Patients who were both donor and recipient CMV-naïve received valacyclovir (500 mg) once daily for 12 weeks for viral prophylaxis. Patients who were CMV-seropositive were treated with prophylactic valganciclovir (450 mg) daily (dose adjusted for Cockcroft–Gault creatinine clearance≤40 ml/m) for 12 weeks; seronegative recipients of kidneys from seropositive donors received prophylaxis with valganciclovir (900 mg) daily (dose adjusted for Cockcroft Gault creatine clearance≤60 ml/m).
Routine Laboratory Testing
Patients underwent routine laboratory monitoring two times per week for weeks 1–4 post-transplant, weekly for weeks 5–12, biweekly for weeks 12–24, and monthly thereafter until week 48. Beyond week 48, patients have laboratory testing done every 1–2 months unless otherwise indicated.
Indications for Kidney Transplant Biopsy
Kidney transplant biopsies were only performed for cause. Any patient with an increase in serum creatinine≥0.3mg/dl above baseline without an alternate explanation (elevated CNI trough level, urinary tract infection, or obstruction) was considered for renal biopsy. Patients with persistent proteinuria>1 g/d were also referred for biopsy.
Diagnosis and Treatment of Rejection
All rejection episodes were diagnosed by renal transplant biopsy. All biopsies were read by a renal pathologist and interpreted according to the Banff 2005 classification. Borderline acute T cell rejection was treated with methylprednisolone (500 mg; three doses) at the discretion of the nephrologist. Banff 1a acute T cell rejection was treated with methylprednisolone (500 mg; three doses). Banff 1b acute T cell rejection was treated with solumedrol or rATG (1–1.5 mg/kg; one to three doses) at the discretion of the nephrologist. Banff grade 2 or 3 acute T cell rejection was treated with rATG (1–1.5 mg/kg; one to three doses). Acute antibody-mediated rejection was treated with plasmapheresis (three to five sessions every other day), 100 mg/kg intravenous Ig (Gammagard; Baxter Healthcare Corp., Westlake Village, CA) after each plasmapheresis session and 500 mg/kg intravenous Ig after the last plasmapheresis session, and one to two doses of 375 mg/m2 rituximab (Rituxan; Genentech Inc., South San Francisco, CA) at the discretion of the treating nephrologist.
BK Virus Screening Detection and Management
Since January of 2008, our kidney transplant program has had a policy in place to prospectively screen sera of all recipients for BK virus DNA by PCR assay (Rotor-Gene Q assay; Qiagen, Valencia, CA) at 3, 6, and 12 months post-transplant and then yearly thereafter. BK viremia is defined by >2.6 log copies/ml in the serum, the lower limit of detection for the assay in our hospital laboratory. All patients with detectable viremia are managed in a standardized manner, which includes discontinuation of antimetabolite therapy at the time of initial detection followed by serial serum BK viral load measurements every 3–4 weeks. In patients whose BK viral load does not decline, despite discontinuation of the antimetabolite, the CNI dose is then downwardly titrated until the level of viremia starts to decrease. In patients whose BK viremia does not improve, despite the above measures, tacrolimus is switched to CsA at the discretion of the treating nephrologist. Patients with >4 log copies/ml BK viremia and evidence of allograft dysfunction are biopsied. BK nephropathy was diagnosed by light microscopy with tubular atrophy and fibrosis accompanied by an inflammatory interstitial infiltrate in addition to positive VP-1 capsid (PAB597) and SV40 immunohistochemical staining. Clearance of BK viremia is defined by three consecutive negative monthly tests. After BK viremia is cleared, the antimetabolite is reintroduced at the discretion of the treating nephrologist, and monitoring resumes at routine intervals. In our study, we defined persistent BK viremia as viremia lasting >140 days, the 50th percentile for viremia duration in our cohort, and 68 patients met this criteria. In our study, a median of three BK viral loads per patient (IQR=2–4) was checked at the predefined surveillance time points outside of testing on the basis of either indication or monitoring for viral clearance after discovery of detectable viremia as described above.
Screening for HLA Antibodies after Transplantation
Since January of 2008, our kidney transplant program has prospectively screened sera of all recipients for anti-HLA antibodies using single-antigen Luminex assays (One Lambda, Canoga Park, CA) at 1, 3, 6, and 12 months post-transplant and then yearly. Any DSA>800 MFI was reported as positive. Patients who develop a positive DSA will have either their antimetabolite or CNI dose increased at the discretion of the treating nephrologist; DSA monitoring continues as outlined above.
Statistical Analyses
The following clinical and demographic data were collected on patients in the study: sex, race, age at transplant, donor type (living, deceased, expanded criteria, donor after cardiac death, and Centers for Disease Control (CDC) high risk), prior transplant, PRA (complement dependent cytoxicity or calculated PRA), induction immunosuppression, maintenance immunosuppression, cause of ESRD, pretransplant diabetes mellitus, new-onset diabetes after transplantation, donor age, donor race, donor sex, HIV serostatus, HCV load, hepatitis B status, ureteral stent placement, CMV viremia, renal biopsy date and result, classes I and II DSA, serum creatinine, BK viremia as determined by PCR at 3, 6, 12, 24, 36, and 48 months, patient survival, and allograft survival. Data were analyzed using STATA, version 12.1 (Statacorp., College Station, TX). Continuous variables were analyzed using t test or Wilcox rank sum test as appropriate. Categorical variables were analyzed using chi-squared or Fisher exact test. Kaplan–Meier curves were generated to assess differences in patient and allograft survival on the basis of BK viremia status. Patient and allograft survival were then modeled using Cox proportional hazards regression. Any variable found to have a P value<0.10 in univariable analysis or known a priori to be a risk factor for BK viremia and patient or allograft survival was included in the multivariable analysis. BK viremia was retained in the multivariable models, regardless of statistical significance, because it is the exposure of interest. Given that the state of BK viremia changed over time, we modeled BK viremia as a time-varying covariate in a sensitivity analysis. For patients who were missing a BK value at a collection time point, the value was imputed by carrying the last measured observation forward. There were only a very small number of instances in which the flanking measurements were discordant, and models were tested imputing values as both positive and negative. The direction of the imputation in these cases did not significantly influence model outcomes.
Risk factors for the development of DSA were modeled using logistic regression. As outlined above, any variable found to have a P value<0.10 in the univariable model or known to be a risk factor for the development of DSA was included in the multivariable model. Standard model checking procedures were used to assess model fit.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Warren Bilker for his assistance with the statistical analysis.
K.A.F. is supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK090209.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014010119/-/DCSupplemental.
References
- 1.Gardner SD, Field AM, Coleman DV, Hulme B: New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1: 1253–1257, 1971 [DOI] [PubMed] [Google Scholar]
- 2.Dörries K: Molecular biology and pathogenesis of human polyomavirus infections. Dev Biol Stand 94: 71–79, 1998 [PubMed] [Google Scholar]
- 3.Alméras C, Vetromile F, Garrigue V, Szwarc I, Foulongne V, Mourad G: Monthly screening for BK viremia is an effective strategy to prevent BK virus nephropathy in renal transplant recipients. Transpl Infect Dis 13: 101–108, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Koukoulaki M, Grispou E, Pistolas D, Balaska K, Apostolou T, Anagnostopoulou M, Tseleni-Kotsovili A, Hadjiconstantinou V, Paniara O, Saroglou G, Legakis N, Drakopoulos S: Prospective monitoring of BK virus replication in renal transplant recipients. Transpl Infect Dis 11: 1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Thakur R, Arora S, Nada R, Minz M, Joshi K: Prospective monitoring of BK virus reactivation in renal transplant recipients in North India. Transpl Infect Dis 13: 575–583, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Suwelack B, Malyar V, Koch M, Sester M, Sommerer C: The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transplant Rev (Orlando) 26: 201–211, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Dharnidharka VR, Cherikh WS, Abbott KC: An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 87: 1019–1026, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hirsch HH, Vincenti F, Friman S, Tuncer M, Citterio F, Wiecek A, Scheuermann EH, Klinger M, Russ G, Pescovitz MD, Prestele H: Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: A prospective, randomized, multicenter study. Am J Transplant 13: 136–145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramos E, Drachenberg CB, Papadimitriou JC, Hamze O, Fink JC, Klassen DK, Drachenberg RC, Wiland A, Wali R, Cangro CB, Schweitzer E, Bartlett ST, Weir MR: Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol 13: 2145–2151, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, Limaye AP, Mihatsch MJ, Nickeleit V, Ramos E, Randhawa P, Shapiro R, Steiger J, Suthanthiran M, Trofe J: Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 79: 1277–1286, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM, Kidney Disease: Improving Global Outcomes : KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int 77: 299–311, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Hirsch HH, Randhawa P, AST Infectious Diseases Community of Practice : BK polyomavirus in solid organ transplantation. Am J Transplant 13[Suppl 4]: 179–188, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M: Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 10: 2615–2623, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, Torrence S, Schuessler R, Roby T, Gaudreault-Keener M, Storch GA: Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 5: 582–594, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hardinger KL, Koch MJ, Bohl DJ, Storch GA, Brennan DC: BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant 10: 407–415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elfadawy N, Flechner SM, Schold JD, Srinivas TR, Poggio E, Fatica R, Avery R, Mossad SB: Transient versus persistent BK viremia and long-term outcomes after kidney and kidney-pancreas transplantation. Clin J Am Soc Nephrol 9: 553–561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiello FB, Calabrese F, Rigotti P, Furian L, Marino S, Cusinato R, Valente M: Acute rejection and graft survival in renal transplanted patients with viral diseases. Mod Pathol 17: 189–196, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, Catrou PG, Bolin P, Kendrick WT, Kendrick SA, Harland RC, Terasaki PI: Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 95: 410–417, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J: Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347: 488–496, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Thomas A, Dropulic LK, Rahman MH, Geetha D: Ureteral stents: A novel risk factor for polyomavirus nephropathy. Transplantation 84: 433–436, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.