Abstract
Monocytes and kidney-resident macrophages are considered to be involved in the pathogenesis of renal ischemia-reperfusion injury (IRI). Several subsets of monocytes and macrophages are localized in the injured tissue, but the pathologic roles of these cells are not fully understood. Here, we show that CD169+ monocytes and macrophages have a critical role in preventing excessive inflammation in IRI by downregulating intercellular adhesion molecule-1 (ICAM-1) expression on vascular endothelial cells. Mice depleted of CD169+ cells showed enhanced endothelial ICAM-1 expression and developed irreversible renal damage associated with infiltration of a large number of neutrophils. The perivascular localization of CD169+ monocytes and macrophages indicated direct interaction with blood vessels, and coculture experiments showed that the direct interaction of CD169+ cell-depleted peripheral blood leukocytes augments the expression levels of ICAM-1 on endothelial cells. Notably, the transfer of Ly6Clo monocytes into CD169+ cell-depleted mice rescued the mice from lethal renal injury and normalized renal ICAM-1 expression levels, indicating that the Ly6Clo subset of CD169+ monocytes has a major role in the regulation of inflammation. Our findings highlight the previously unknown role of CD169+ monocytes and macrophages in the maintenance of vascular homeostasis and provide new approaches to the treatment of renal IRI.
Keywords: acute renal failure, immunology and pathology, ischemia-reperfusion, macrophages
Renal ischemia-reperfusion injury (IRI) is a mouse model of AKI, and it is manifested by enhanced fluid filtration and leukocyte plugging in capillaries.1 The severity of renal injury in this model is determined by not only the number of tubular epithelial cells undergoing cell death caused by hypoxia but also, the degree of endothelial activation and subsequent influx of inflammatory blood cells, including neutrophils and monocytes.2,3 The pathologic roles of monocytes and macrophages have been recently highlighted in renal IRI.4 During the early phase of IRI, Ly6Chi monocytes infiltrate injured tissue and are considered to differentiate into inflammatory macrophages. These macrophages produce a set of inflammatory cytokines, such as TNF-α and IL-1β. Depletion of macrophages by injection of clodronate liposome or deficiency of CCR2 chemokine prevented IRI, indicating the critical roles of inflammation induced by macrophages in IRI.5,6 However, kidney macrophages are also considered to promote the tissue repair and regeneration during the late phase of IRI.7,8 Because several subsets of monocytes and macrophages are located in the IRI tissue, it is very important to determine the pathologic roles of each subset to understand the pathogenesis of IRI.
Monocytes in peripheral blood are heterogeneous in terms of phenotype, function, and fate. It is widely accepted that they differentiate from myeloid progenitor cells in the bone marrow (BM). There are two distinct subsets of peripheral monocytes that differ in the expression levels of CX3CR1 and Ly6C.9,10 CX3CR1hiLy6Clo monocytes crawl along the surface of endothelial cells to maintain endothelial integrity; thus, they are referred to as patrolling monocytes.11,12 Although CX3CR1hi monocytes are thought to maintain vascular homeostasis, their roles in disease development in vivo remain unknown. A second population of peripheral blood monocytes, CX3CR1loLy6Chi monocytes, is the so-called inflammatory monocytes, because they are recruited selectively to the inflamed tissue, where they differentiate into macrophages and dendritic cells.13–15
A subset of macrophages that express CD169 resides in lymphoid organs, such as spleen, lymph node, and BM.16–18 Previously, we reported that CD169+ macrophages in the marginal zone of spleen and lymph node sinus capture apoptotic cells and induce dead cell antigen-specific immune responses.19–21 It is also reported that some other tissue-resident macrophages express CD169, but their physiologic or pathologic roles remain unclear.
In this study, we identified a subset of peripheral blood monocytes and kidney-resident F4/80+ macrophages using the mice in which CD169+ cells and their descendants were visualized. We found that the transient depletion of these cells results in the exacerbation of renal IRI. We categorized CX3CR1hiLy6CloCD169+ cells as vascular-resident monocytes/macrophages that suppress the excessive activation of endothelial cells. This finding may pave the way to the establishment of a novel therapeutic approach to the suppression of tissue injury in a broad range of human ischemic diseases.
Results
Identification of Novel Subsets of Peripheral Blood Monocytes and Kidney-Resident Macrophages
To explore the distribution of CD169+ cells outside secondary lymphoid organs, we generated mice that harbor the improved Cre recombinase gene22 in the CD169 loci (CD169-Cre mice) and crossed those mice with ROSA26-yellow fluorescent protein (YFP) reporter mice23 to analyze recombinase activity (Supplemental Figure 1). In those CD169-Cre×ROSA26-YFP mice (hereafter, CD169-Cre-YFP mice), YFP reporter visualizes CD169+ cells and their descendants independently of continuous or transient CD169 expression. Consistent with previous findings obtained by immunohistochemical staining with anti-CD169 antibody, YFP+ cells localized at the marginal zone of the spleen (Supplemental Figure 2). We further confirmed gene recombination in the cultured BM cells. As shown in Supplemental Figure 3, CD169-expressing BM-derived macrophages cultured with M-CSF became YFP-positive. The time course suggests that it takes a few days to complete gene recombination.
To our surprise, we detected also gene recombination in peripheral blood Gr-1int-lo, the CD11b+ fraction that contains Ly6Chi and Ly6Clo monocytes (Figure 1A). Flow cytometry analysis with Ly6C antibody revealed that both subpopulations of Ly6Chi and Ly6Clo monocytes include YFP-positive cells. These YFP-positive cells expressed CD115, another marker of blood monocytes (Supplemental Figure 4). We decided to focus on characterizing the novel subset of YFP+(CD169+)Gr-1int-loCD11b+ monocytes in additional experiments.
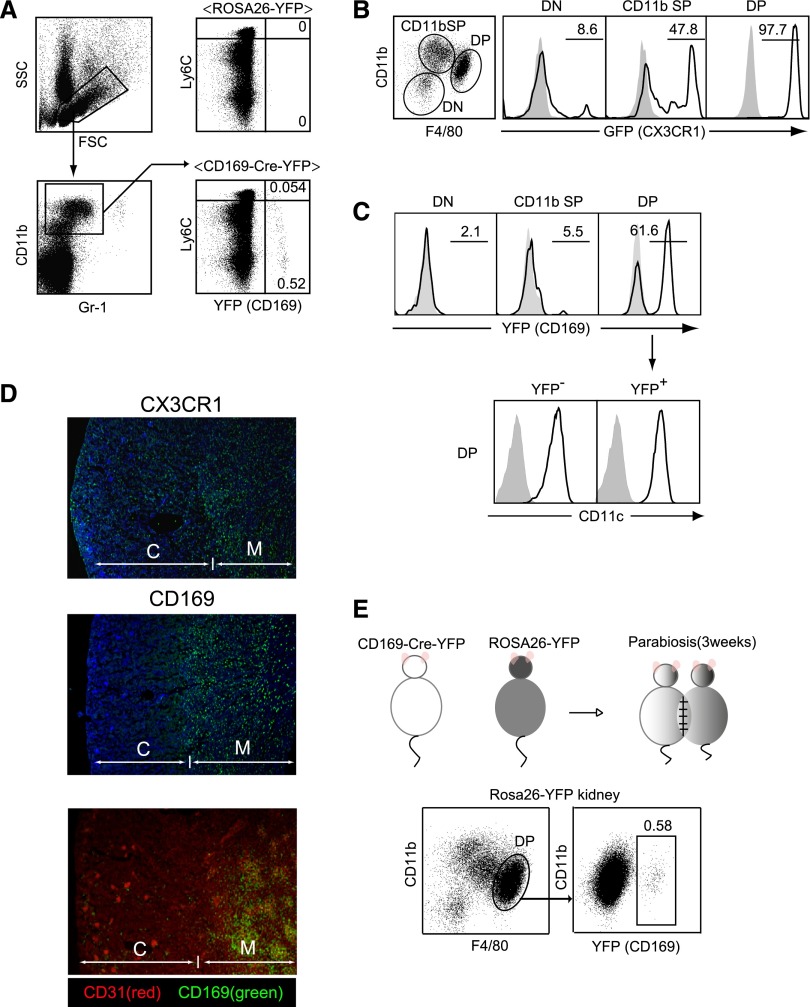
Figure 1.
Identification of novel subsets of monocytes and kidney macrophages using CD169-Cre-YFP mice. (A) YFP-positive cells in peripheral blood. White blood cells obtained from CD169-Cre-YFP mice or ROSA26-YFP mice were stained for CD11b, Gr-1, and Ly6C. Numbers indicate the frequency (percentage) of YFP+ cells among the Gr-1int-loCD11b+ fraction. Data are representative of three independent experiments. FSC, forward scatter; SSC, side scatter. (B and C) Kidney myeloid cells in (B) CX3CR1-GFP mice and (C) CD169-Cre-YFP mice. CD11b+ and/or CD11c+ cells in kidneys were stained with Gr-1, CD11b, F4/80, and CD11c antibodies. Numbers indicate the frequency (percentage) of GFP+ or YFP+ cells in CD11b and F4/80 double-negative (DN), CD11b single-positive (SP), or CD11b and F4/80 double-positive (DP) cells. (C, lower panel) CD11c expression in DP cells in CD169-Cre-YFP mice. Shaded area represents renal cells from (B) WT or (C, upper panel) ROSA26-YFP mice or (C, lower panel) staining without CD11c. Experiments were independently repeated at least three times. (D) Localization of renal CX3CR1+ macrophages and CD169+ macrophages. (Top and middle panels) Cryosections of kidneys from (top panel) CX3CR1-GFP and (middle panel) CD169-Cre-YFP mice were stained with anti-GFP antibody (green) and 4′,6-diamidino-2-phenylindole (blue). (Bottom panel) Cryosections of kidneys from CD169-Cre-YFP mice were stained with anti-GFP antibody (green) and CD31 (clone: MEC13.3; red). Note that the glomeruli in the cortex are densely stained with the anti-CD31 Ab, a marker of vascular endothelial cells. C, cortex; M, medulla. (E) CD11b+, F4/80+ kidney macrophages are derived from blood monocytes. Parabiotic mice were generated from CD169-Cre-YFP and ROSA26-YFP mice. Three weeks later, the frequency of YFP+ cells in the DP macrophages in ROSA26-YFP mouse kidneys was analyzed. The numbers indicates the frequency (percentage) of YFP+ cells among DP macrophages.
Macrophages are classified into cells originating from yolk sac or fetal liver and hematopoietic stem cells.24–27 The major population of tissue macrophages, which includes Kupffer cells,28 Langerhans cells of the skin,29,30 and lung alveolar macrophages,31 proliferates locally independent of the BM. In contrast, the maintenance of tissue macrophages in the kidney and the intestine is dependent on constant CX3CR1+ monocyte supply from blood stream.9,11,32 Consistent with those reports, all of the CD11b+F4/80+ macrophages and nearly 50% of CD11b+F4/80lo cells in the kidney express CX3CR1, supporting the CX3CR1+ monocyte origin of kidney myeloid cells (Figure 1B). This finding led us to ask if any of the kidney macrophage subsets are YFP-positive in CD169-Cre-YFP mice. Among three subpopulations, 60% of CD11b+F4/80+ macrophages, all of which were CX3CR1+, were YFP-positive, whereas two other subpopulations, CD11b+ F4/80lo and CD11b− fractions, did not contain YFP+ cells (Figure 1C). Both YFP-positive and YFP-negative macrophages in the DP subpopulation were CD11c-positive. Immunohistochemical observation revealed the localization of YFP+ cells exclusively in the medullary interstitium (Figure 1D, middle and bottom panels), whereas CX3CR1+ cells distributed throughout the cortex and the medulla (Figure 1D, top panel). We next tried to reveal the origin of the YFP-positive kidney macrophages. For this purpose, we parabiotically joined CD169-Cre-YFP mice and ROSA26-YFP mice. Three weeks after parabiosis, a part of CD11b+F4/80+ kidney macrophages became YFP-positive (Figure 1E) in ROSA26-YFP mice, indicating that this subpopulation of kidney macrophages, at least in part, is derived from blood monocytes.
Depletion and Recovery Kinetics of Peripheral Blood and Kidney-Resident CD169+ Cells in CD169-DTR Mice
To understand the turnover kinetics of CD169+ cells in peripheral blood and the kidney, we injected diphtheria toxin (DT) into CD169-DTR mice and analyzed the frequency of CD169+ cells up to 7 days after the injection. The percentages of Gr-1int-loCD11b+ cells dropped from 14.5% to 4.3% 1 day after the DT injection (Figure 2, A and B) followed by a decrease of CD11b+F4/80+ kidney macrophages at day 2 (Figure 2, C and D). The frequency of CD11b+F4/80lo cells was unchanged in the kidney during this period, indicating selective depletion of resident macrophages. To further confirm the depletion of CD169+ cells among the CX3CR1+ monocytes and kidney macrophages, we injected DT into CD169-DTR mice that were crossed with CX3CR1-green fluorescent protein (GFP) mice.33 In peripheral blood, the DT injection induced approximately 57% (from 1.94 to 0.82) and 36% (from 6.6 to 4.2) reduction of CX3CR1hi and CX3CR1lo monocytes, respectively (Figure 2E) and 50% (from 68 to 34) reduction of kidney CD11b+CX3CR1+ macrophages (Figure 2F, upper panel). Notably, the depletion was restricted to the CD11b+F4/80+ macrophage fraction in the kidney (Figure 2F, lower panel). The degree of peripheral blood monocyte reduction was more profound than the frequency of YFP-positive cells among peripheral blood CD11b+ cells. This difference could be explained by a delayed gene recombination (Supplemental Material). Consistent with the results from CD169-Cre-YFP mice, these data show that CX3CR1+ monocytes and CX3CR1+CD11b+F4/80+ kidney macrophages are composed of CD169+ and CD169− fractions.
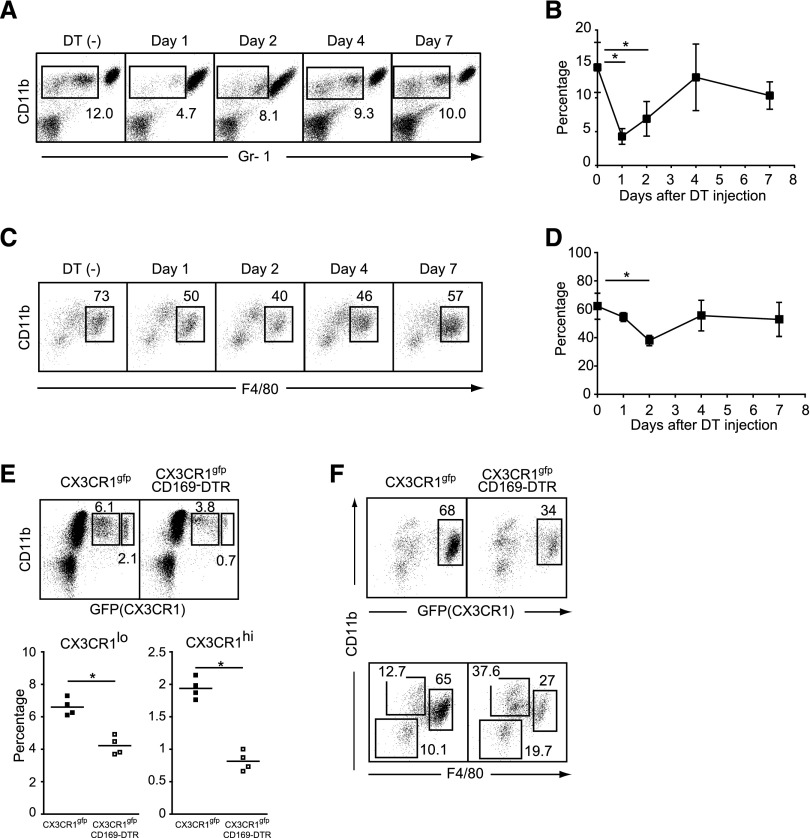
Figure 2.
Selective depletion of monocytes and kidney macrophages in CD169-DTR mice. (A) White blood cells of DT-treated CD169-DTR mice at the indicated time points were stained with CD11b and Gr-1 antibodies. Numbers indicate the frequency (percentage) of CD11b+Gr-1int-lo monocytes among white blood cells. Average percentages of CD11b+Gr-1int-lo monocytes (three to five mice per group) are plotted with SD in B. Mean percentage of CD11b+Gr-1int-lo cells was compared by one-way ANOVA. *P<0.05. (C) Kidney macrophages of DT-treated CD169-DTR mice at the indicated time points were stained for Gr-1, CD11b, and F4/80 antibodies. Numbers indicate the frequency (percentage) of CD11b+F4/80+ kidney macrophages among CD11b+ and/or CD11c+ cells in kidneys. Average percentages of CD11b+, F4/80+ kidney macrophages (four mice per group) are plotted with SD in D. Mean percentage of CD11b+F4/80+ cells was compared by one-way ANOVA. *P<0.05. (E) Depletion of monocytes in CX3CR1-GFP×CD169-DTR mice. DT was injected into CX3CR1-GFP mice (upper left panel) or CX3CR1-GFP×CD169-DTR mice (upper right panel). Twenty-four hours later, white blood cells were stained for CD11b. Numbers indicate the frequency (percentage) of CX3CR1lo or CX3CR1hi cells among white blood cells. (Lower panel) Average percentages of CX3CR1lo and CX3CR1hi cells are plotted. ▪, CX3CR1-GFP; □, CX3CR1-GFP × CD169-DTR. *P<0.05. (F) Depletion of kidney macrophages in CX3CR1-GFP × CD169-DTR mice. DT was injected into (left panel) CX3CR1-GFP or (right panel) CX3CR1-GFP×CD169-DTR mice. Two days after the DT injection, kidney macrophages were stained for CD11b and F4/80. Numbers indicate (upper panel) the frequency (percentage) of CX3CR1-positive cells among CD11b+ and/or CD11c+ cells in kidneys or (lower panel) the frequency (percentage) of CD11b and F4/80 double-negative, CD11b single-positive, or CD11b and F4/80 double-positive cells among CD11b+ and/or CD11c+ cells in kidneys. Data are representative of three independent experiments.
Role of CD169+ Cells in the Development of Renal IRI
To further investigate the roles of CD169+ monocytes in peripheral blood and kidney macrophages under an inflammatory condition, we performed renal IRI. In this model, clamping of unilateral renal pedicles combined with contralateral nephrectomy induces tubular necrosis and massive infiltration of polymorphonuclear cells in the interstitium that manifest as a clinical symptom of AKI in wild-type (WT) mice. The improved surgical approach to kidney pedicles from retroperitoneum enabled us to perform 1-hour clamping for AKI followed by complete recovery in WT mice. Surprisingly, the depletion of CD169+ cells resulted in progressive renal injury by IR (Figure 3, A–D). WT mice showed transient increases of serum creatinine and BUN levels, both of which returned to their basal levels within 2 days from IR (Figure 3B). Tissue injury in WT mice was characterized by transient loss of brush border and casts in the lumen that will recover within 7 days. Moderate interstitial infiltration was also observed (Figure 3C, center panel). However, kidneys of CD169-DTR mice presented with progressive renal dysfunction (Figure 3B) and larger necrotic lesion than WT mice (Figure 3D, left panel) caused by sustained tissue injury that is characterized by tubular necrosis and inflammatory cell infiltration (Figure 3D, center panel), and they could not survive the IR-induced AKI (Figure 3A). CD169-DTR mice survived unilateral IRI of the kidney, but the kidneys from those mice were totally destructed at day 7 (Figure 3D, right panel). Damage was most prominent in the corticomedullary junction. In parallel with the development of AKI, we observed substantial increases of proinflammatory cytokine in the kidneys of CD169-DTR mice compared with WT mice after IR (Figure 3E). Collectively, these results suggest that CD169+ cells in peripheral blood and the kidney contribute to the suppression and/or resolution of IRI. To deny the possibility that the depletion of CD169+ cells results in inflammation, even in the preinjury state of DT-treated CD169-DTR mice, we assessed vascular hyperpermeability of the kidneys. As shown in Supplemental Figure 5A, there were no significant differences in the leakage of Evans blue in the kidneys between DT-treated WT and DT-treated CD169-DTR mice. In addition, there was no increase in the number of neutrophils between these mice in the preinjury state (Supplemental Figure 5B).
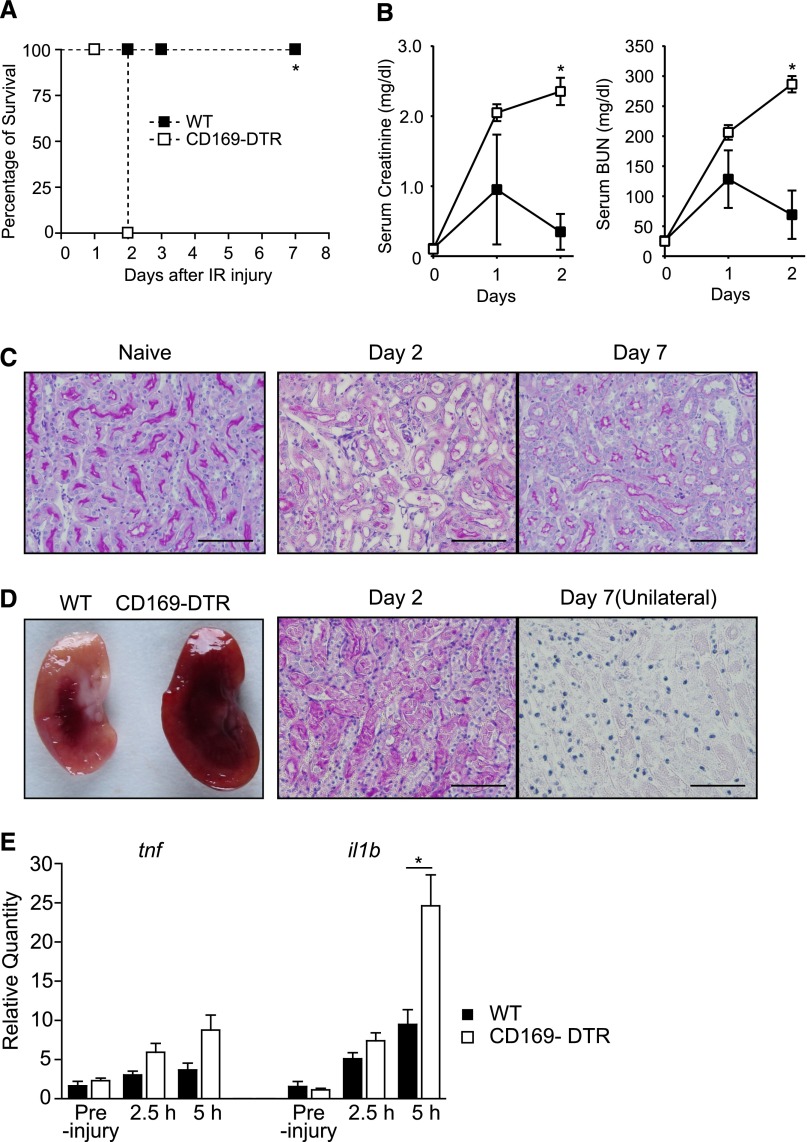
Figure 3.
Severe IRI in CD169-DTR mice. (A and B) DT was intraperitoneally injected into WT or CD169-DTR mice. Thirty-six hours later, clamping of the unilateral renal pedicle for 1 hour followed by contralateral nephrectomy (bilateral IR) was performed in the mice. (A) Survival rate of IR mice. n=6 WT mice or n=7 CD169-DTR mice. *P< 0.001. Data are representative of three independent experiments. (B) Serum creatinine and BUN concentrations in IR mice. The average values of five WT or three CD169-DTR mice are plotted with SD. ▪, WT; □, CD169-DTR. Mean serum creatinine and BUN concentrations of WT and CD169-DTR mice at different time points were compared by two-way ANOVA. *P<0.05. (C) Microscopic observation of naïve and IR kidneys in WT mice. Bilateral IR was performed in WT mice. Paraffin sections were stained with periodic acid–Schiff (PAS). Scale bars, 50 μm. (D) Macroscopic and microscopic observations of IR kidneys. Bilateral IR was performed in WT or CD169-DTR mice as described in A and B. (Left panel) Macroscopic observation of kidneys on day 1 after IR. (Center panel) Bilateral or (Right panel) unilateral IR was performed in the DT-treated CD169-DTR mice. Paraffin sections of kidneys on (center panel) day 2 or (right panel) day 7 (unilateral) were stained with PAS. Unilateral IR was performed in CD169-DTR mice to avoid death after bilateral IR in those mice. Scale bars, 50 μm. (E) Inflammatory cytokine mRNA levels in kidneys. Bilateral IR was performed in WT or CD169-DTR mice as described in A and B. Quantity of tnfa and il1b mRNA levels in the kidneys relative to WT preinjury mice at the indicated time points after IR were determined by quantitative PCR. Mean±SD, n=5 mice/group. *P<0.05.
Aggravation of Renal Injury in the Absence of CD169+ Cells Is Induced by Accumulated Neutrophils
Immunohistochemistry revealed larger number of neutrophils in the kidney of CD169-DTR mice than WT mice after IR (Figure 4A). Flow cytometry also confirmed increase in the absolute number of neutrophils (Figure 4B). These findings suggest that the infiltrated neutrophils are responsible for the development of lethal AKI in CD169-DTR mice. To confirm this hypothesis, we depleted neutrophils in peripheral blood and the kidney by using anti–Gr-1 antibody before IR. CD169-DTR mice were rescued by the neutrophil depletion (Figure 4, C and D, Supplemental Figure 6). These results indicate that neutrophil accumulation promoted by the absence of CD169+ cells is the primary cause of AKI.
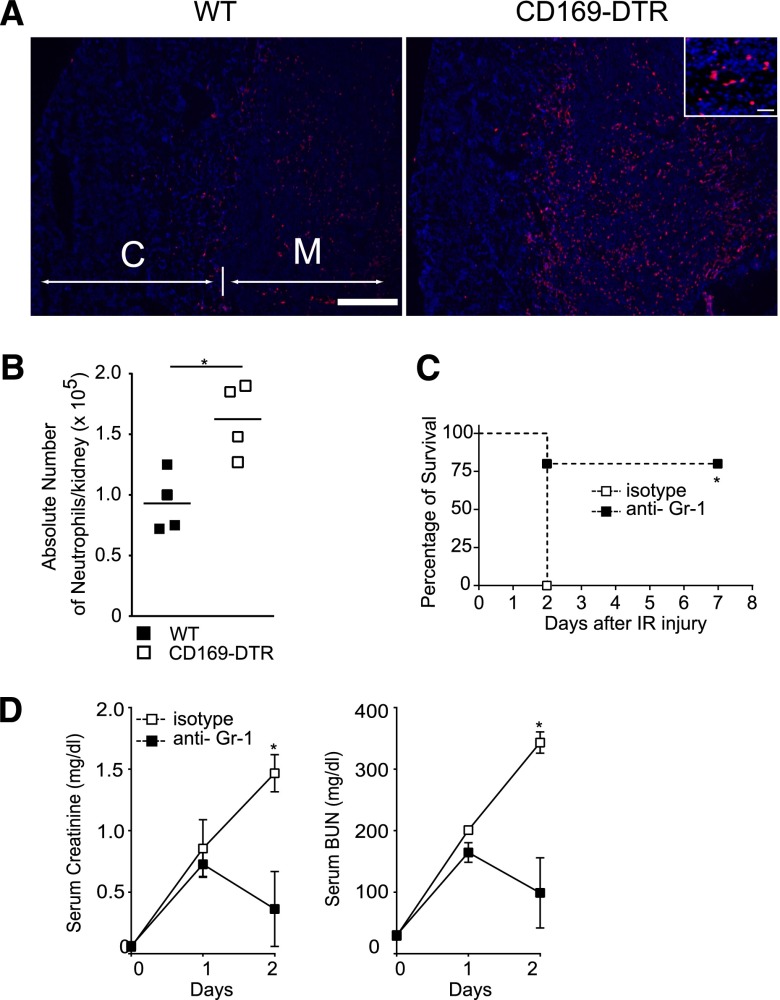
Figure 4.
Depletion of neutrophils rescues CD169-DTR mice from lethal AKI. (A and B) Large numbers of neutrophils accumulated in kidneys of CD169-DTR mice subsequent to IR. (A) Bilateral IR was performed in DT-treated WT or CD169-DTR mice. Neutrophils in the kidneys on day 1 were detected immunohistochemically by anti-Ly6G (red) and 4′,6-diamidino-2-phenylindole (blue) staining. C, cortex; M, medulla. Scale bars, 1 mm in low power fields; 50 μm in Inset. (B) Absolute numbers of CD11b+, Gr-1+ neutrophils were quantitated by a flow cytometer. Each symbol represents data from an individual animal, and the bars indicate average values. *P<0.05. (C and D) Injection of anti–Gr-1 antibody suppresses IR-induced AKI in CD169-DTR mice. DT was injected intraperitoneally into CD169-DTR mice. Twenty-four hours later, 100 μg anti–Gr-1 antibody or rat IgG2B isotype control was injected intravenously into these mice. Bilateral IR was performed 36 hours after DT injection. (C) Survival rate of IR mice. n=5 mice/each group. *P<0.05. (D) Serum creatinine and BUN concentrations. The average values of six anti–Gr-1–injected mice or three isotype antibody-injected mice are plotted with SD. Mean serum creatinine and BUN concentrations of WT and CD169-DTR mice at different time points were compared by two-way ANOVA. *P<0.05.
CD169+ Cells Suppress Intercellular Adhesion Molecule-1 Expression on Endothelial Cell Surface to Prevent Accumulation of Neutrophils
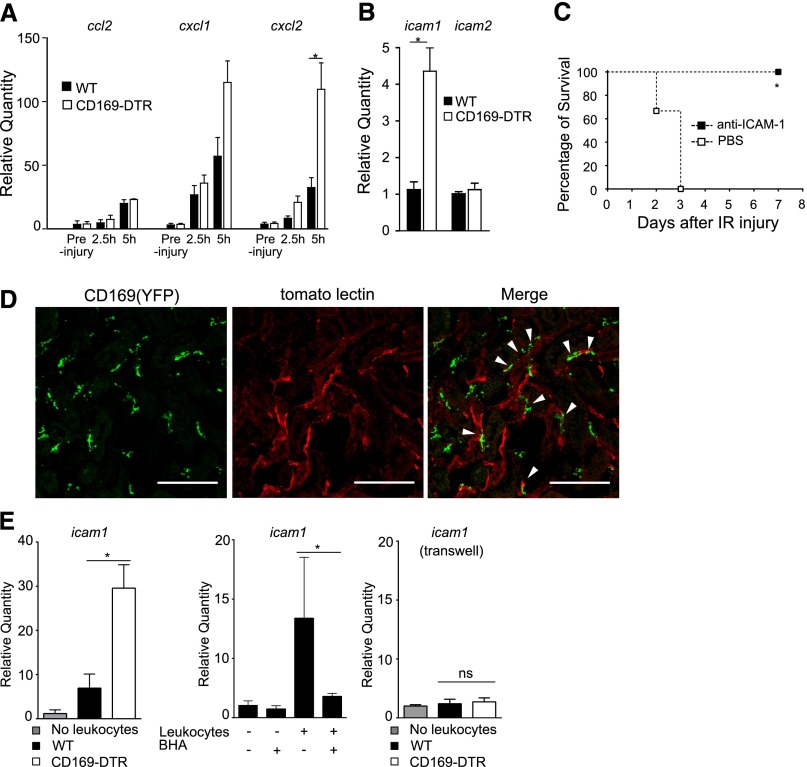
We next sought signals that drive neutrophil accumulation in the kidney. Such chemokines as CXCL1 and CXCL2 are responsible for neutrophil recruitment to an inflammatory site. In parallel with previous results (Figure 4, A and B), the production of CXCL2 was higher in the kidneys of CD169-DTR mice than in WT kidneys after IR (Figure 5A). However, we could not answer whether the upregulation of those chemokines was the direct outcome of CD169+ cell depletion or whether the upregulation represented one of the secondary results of inflammation, because the chemokine expression was similar in preinjury kidneys of WT and CD169-DTR mice. To further explore the primary cause of neutrophil accumulation, we quantitated the expression levels of adhesion molecules. We found that the depletion of CD169+ cells resulted in the substantial increase of intercellular adhesion molecule-1 (ICAM-1) mRNA expression even before IRI (Figure 5B). As shown in Figure 5C, the injection of blocking anti–ICAM-1 antibody before IR into CD169-DTR mice rescued them from lethal AKI. These results suggest that the suppression of ICAM-1 expression on endothelial cell surface by vascular CD169+ cells under physiologic conditions is critical for the maintenance of tissue homeostasis.
Figure 5.
Upregulated ICAM-1 expression in the absence of CD169+ cells exacerbates AKI. (A) Chemokine expression in IR kidneys. DT was injected intraperitoneally into WT or CD169-DTR mice. Thirty-six hours later, bilateral IR was performed. Quantities of ccl2, cxcl1, and cxcl2 mRNA levels in the kidneys relative to WT preinjury mice were determined by quantitative PCR. n=5 mice/group. Mean±SD. *P<0.05. (B) Enhanced expression of ICAM-1 in kidneys of CD169-DTR mice. Quantity of icam1 and icam2 mRNA levels in the kidneys of DT-treated WT or CD169-DTR mice relative to WT mice without DT were determined by quantitative RT-PCR. n=8 mice/each group. The average values are plotted with SD. *P<0.05. (C) Blockade of ICAM-1 suppresses the development of AKI in CD169-DTR mice. DT was injected intraperitoneally into CD169-DTR mice. Twenty-four hours later, 100 μg anti–ICAM-1 (clone KAT1) antibody or PBS was injected intravenously into these mice. Thirty-six hours after the DT injection, bilateral IR was performed. Survival rate is shown. n=3 mice/group. *P<0.05. (D) CD169+ cells localize in the perivascular area of the renal interstitium. DyLight 488-labeled tomato lectin was injected intravenously into CD169-Cre-YFP mice 5 minutes before removing the kidney. Cryosections of the kidneys were stained for YFP (green) and observed under a confocal microscope. White arrows indicate the CD169-positive macrophages, which are located along the capillary vessels. z-Stack images were processed by ImageJ software. Red signal indicates tomato lectin on endothelium. Scale bar, 100 μm. (E) Peripheral blood leukocytes from CD169-DTR mice augment ICAM-1 expression levels in cultured endothelial cells. (Left panel) Peripheral blood leukocytes from DT-treated WT or CD169-DTR mice were cocultured with UV♀2 cells for 18 hours. icam1 mRNA levels of these cells were determined by quantitative RT-PCR. (Center panel) Peripheral blood leukocytes from DT-treated CD169-DTR mice were cocultured with UV♀2 cells for 18 hours in the presence or absence of butylated hydroxyanisole (BHA). (Right panel) Peripheral blood leukocytes from DT-treated WT or DT-treated CD169-DTR mice were cocultured with UV♀2 cells for 18 hours using the transwell plate. The average values are plotted with SD. Data are representative of two or three independent experiments. *P<0.05.
We next tried to reveal the mechanisms of regulation of ICAM-1 expression by CD169+ cells. It was recently reported that the direct interaction between leukocytes and endothelial cells regulates ICAM-1 expression on endothelial cell surface.34 Therefore, we first analyzed the spatial relationship between CD169+ cells and blood vessels in the kidney. As shown in Figure 5D, the majority of YFP+ cells localized in close contact with blood vessel, suggesting their direct interaction with blood vessels. We also examined localization of CD169+ monocytes in blood vessels. When we injected anti-CD11b antibody into CD169-Cre-YFP mice, the frequency of YFP+ cells in Gr-1int-loCD11b+CD115+ fraction of peripheral blood leukocytes increased (Supplemental Figure 7). These results also suggest that CD169+ monocytes on vascular surface interact with the vascular endothelial cells in a CD11b-dependent manner.
To examine the possibility that the direct interaction of peripheral blood leukocytes regulates the expression of ICAM-1 on endothelial cells, we cocultured endothelial cells and peripheral blood leukocytes from the mice. We found that upregulation of ICAM-1 expression was higher in the endothelial cells cocultured with leukocytes obtained from DT-treated CD169-DTR mice than in those cocultured with leukocytes from WT mice (Figure 5E, left panel); 10 μM butylated hydroxyanisole suppressed ICAM-1 mRNA expression in endothelial cells cocultured with CD169-DTR leukocytes (Figure 5E, center panel), indicating the oxidant-dependent upregulation of ICAM-1. Intriguingly, the transwell experiments clearly showed that the direct interaction between these cells was definitely required for the upregulation of ICAM-1 expression on the endothelial cells (Figure 5E, right panel).
Transfer of Ly6Clo Monocytes Rescues CD169-DTR Mice from IR-Induced AKI
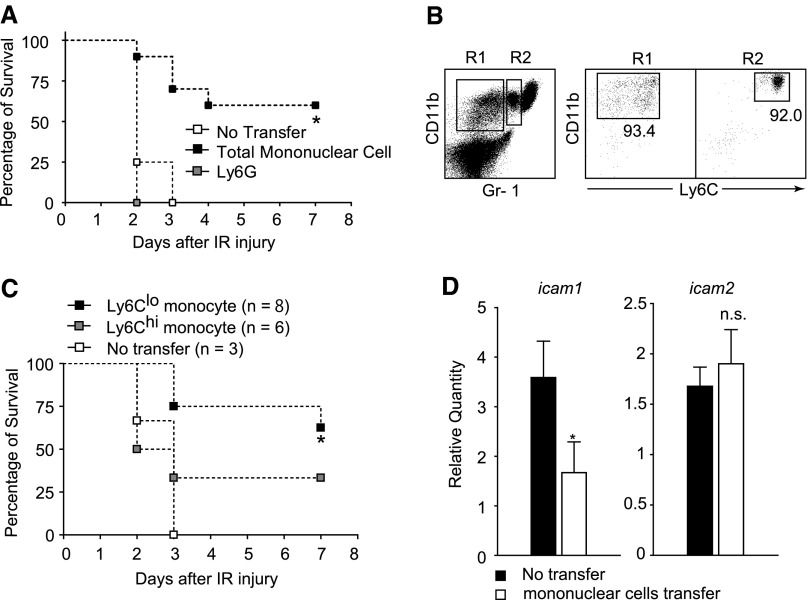
To prove anti-inflammatory activity of CD169-positive cells in tissue injury, we transferred total peripheral blood mononuclear cells from neutrophil-depleted WT mice into CD169-DTR mice before IR. As shown in Figure 6A, the injection of WT blood mononuclear cells improved the survival rate of CD169-DTR mice from lethal AKI by 60%, whereas the injection of WT Ly6Ghi neutrophils did not improve the survival rate. It is reported that Ly6Clo monocytes contribute to vascular homeostasis.12 To specify the monocyte subpopulation responsible for the amelioration of the IR-induced AKI, we injected fractionated Ly6Chi or Ly6Clo monocytes into CD169-DTR mice (Figure 6B). As shown in Figure 6C, the survival rate of CD169-DTR mice injected with Ly6Clo monocytes improved by 62.5% 7 days after IR. Contrary to our speculation, CD169-DTR mice injected with Ly6Chi monocytes were also partially rescued (33% at day 7) from the IR-induced AKI (Figure 6C). The amelioration of AKI in mice injected with Ly6Chi monocytes may be explained by the conversion of Ly6Chi monocytes into Ly6Clo monocytes in the host mouse.32 We further examined the ICAM-1 expression levels in the kidneys of mononuclear cell-transferred mice and found that the levels were normalized in these mice (Figure 6D). The result further supports the notion that Ly6Clo monocytes suppress ICAM-1 expression on endothelial cell surface.
Figure 6.
Transfer of Ly6Clo monocytes rescues CD169-DTR mice from lethal AKI. (A) Transfer of mononuclear blood cells rescues CD169-DTR mice from lethal IRI. Gr-1 antibody (100 μg) was injected intraperitoneally into WT mice to deplete neutrophils. Residual leukocytes from these mice were injected into CD169-DTR mice (▪). Ly6Ghi neutrophils were enriched from WT mice by magnetic sorting and injected into CD169-DTR mice (gray square). Twenty-four hours later, DT was injected intraperitoneally into the mice. Bilateral IR was performed 36 hours after DT injection. Survival rate is shown. n=4 mice without transfer, n=10 mice with monocyte transfer, and n=3 mice with Ly6G+ neutrophils transfer. *P<0.05. (B and C) Ly6Clo monocytes are primarily responsible for the suppression of IR-induced AKI. (B) Ly6Clo (n=8) or Ly6Chi (n=6) monocytes prepared from WT mice by using a cell sorter were injected intravenously into CD169-DTR mice. Twenty-four hours later, DT was injected intraperitoneally into the mice. Bilateral IR was performed 36 hours after DT injection. (C) Survival rate of IR mice. *P<0.05 compared with no transfer group. (D) Transfer of mononuclear cells suppresses the augmented expression of ICAM-1 in the kidneys of CD169-DTR mice. Transfer of mononuclear cells into CD169-DTR mice was performed as described in A. Twenty-four hours later, DT was injected intraperitoneally into the mice. Thirty-six hours later, ICAM-1 mRNA levels relative to those of WT kidneys were determined by quantitative RT-PCR. n=6 mice/group. The average values are plotted with SD. *P<0.05.
Discussion
In this study, we identified and characterized a novel population of peripheral blood monocytes and kidney-resident macrophages that express CD169. We showed that they reside along the length of blood vessel and suppress the activation state of endothelial cells under physiologic conditions. The analysis using CX3CR1-GFP mice crossed with CD169-DTR mice reveals that the CD169+ cell lineage constitutes a major compartment of CX3CR1+ monocytes and kidney-resident macrophages.
In sterile inflammation, activated vascular endothelial cells release an array of inflammatory mediators that upregulate the expression of adhesion molecules, such as ICAM-1, which promotes leukocyte recruitment to the site of inflammation.35 In addition, damaged cells release endogenous adjuvants that activate pattern recognition receptors on innate immune cells, including Toll-like receptors and intracellular NOD-like receptors, resulting in sterile inflammation. A recent study revealed that Ly6Clo monocytes survey the endothelial cell surface and sense a nucleic acid-mediated danger signal that is presented on the epithelial surface by Toll-like receptor 7.12 However, several reports showed the anti-inflammatory roles of Ly6Clo monocytes in various pathologic conditions.36,37 In this study, we revealed that the depletion of CD169+ monocytes enhanced ICAM-1 expression in the kidney in the steady state. Because depletion of CD169+ cells did not increase inflammatory cytokine production, vascular permeability, or the number of other macrophages and leukocytes in preinjured kidneys (Figures 2, 3E, and 5A, Supplemental Figure 5), the ICAM-1 upregulation was not due to systemic inflammation caused by depletion of these cells. These results strongly suggest the anti-inflammatory roles of the CD169+ monocytes by regulating ICAM-1 expression on the endothelial cells. Under inflammatory conditions, inflammatory cytokines cause ICAM-1 upregulation, resulting in an enhanced recruitment of neutrophils to the inflammatory site. Recently, it was reported that not only inflammatory cytokines but also, the direct interaction between neutrophils and endothelial cells regulate ICAM-1 expression on endothelial cell surface.34 Consistent with this report, expression of ICAM-1 was significantly higher on the surface of endothelial cells that were cocultured in vitro with CD169+ cell-depleted peripheral blood leukocytes than on those cocultured with WT leukocytes. Although the precise mechanism of ICAM-1 suppression remains elusive, this result highlights the suppressive role of CD169+ monocytes on endothelial cells.
In this study, because not only a subpopulation of monocytes but also, kidney-resident macrophages were depleted by DT injection into CD169-DTR mice, it is most likely that the kidney-resident CD169+ macrophages also play some role in the regulation of kidney inflammation. From this point of view, CD169 kidney macrophages may be categorized as M2 macrophages. Because CD169+ macrophages are located along blood vessels in kidneys, they may maintain the homeostasis of capillary endothelial cells in coordination with CD169+ monocytes. There are several works on the characterization of the mononuclear phagocyte system in the kidney. Mononuclear phagocyte system in the kidney is composed of mesangial and interstitial cells. Mesangial cells in the glomeruli are considered macrophages, because they show some phagocytic function, although they do not express panmacrophage marker F4/80. However, medullary interstitial macrophages that localize in direct contact with tubules express both macrophage marker F4/80 and DC marker CD11c.38,39 In addition, there are solid lines of evidence that infiltrating monocyte-derived macrophages initiate and propagate the renal IRI and that the inhibition of macrophage infiltration ameliorates renal damage.5,40 In contrast, we propose that kidney-resident CD169+ macrophages play protective roles in the IR-induced AKI. Intriguingly, YFP+ macrophages preferentially localize in the renal medulla. Proximal tubular cells in the corticomedullary border are considered susceptible to IRI.2,41 The correlation between the damaged site and the localization of CD169+ macrophages prompted us to speculate the protective role of those macrophages in the renal IRI. It was recently shown that macrophages are also involved in tissue repair after IRI.8,42,43 It would be very intriguing to examine the roles of those cells in tissue repair subsequent to IRI.
In conclusion, our study identified CD169+ monocytes/macrophages as a novel anti-inflammatory cell type in the initiation of IRI, which may be relevant to clinical treatment of human ischemic diseases.
Concise Methods
Mice
C57BL/6J (8–12 weeks old) male mice were obtained from CLEA Japan, Inc. CD169-DTR knockin mice (C57BL/6J background) were established in our laboratory. CX3CR1-GFP mice and ROSA26-YFP mice were kindly provided from D. Littman (New York University School of Medicine) and F. Costantini (Columbia University), respectively. All mice were housed under specific pathogen-free conditions in the RIKEN RCAI and Tokyo University of Pharmacy and Life Sciences animal facility. All experiments using the mice described herein were approved by the RCAI and Tokyo University of Pharmacy and Life Sciences animal use committee and performed in accordance with the applicable guideline and regulations.
For systemic CD169+ cell depletion, CD169-DTR mice were injected intraperitoneally with 20–40 μg DT/kg body wt (CALBIOCHEM) 24–36 hours before IR and experiments. The generation of CD169-Cre knockin mice was performed essentially as described previously.19 Briefly, the improved Cre recombinase gene with a polyA tail and the neomycin resistance gene were introduced by replacing the first 28-bp fragment including the endogenous ATG start site in exon 1 of the CD169 gene. HSV-thymidine kinase gene was inserted downstream of the 3′ arm to select against random integrants. Then, B6JN/1 ES cells were transfected with the linearized targeting vector by electroporation. G-418 and ganciclovir-resistant clones were screened for homologous recombination by Southern blot analysis. Germ-line chimeric mice were generated by aggregation methods. Chimeric mice with a high ES cell contribution were crossed with C57BL/6J mice to produce +/Cre mice. +/Cre mice were crossed to ROSA26-YFP mice.
Flow Cytometry Analyses
We evaluated kidney cells with a flow cytometer. To obtain single-cell suspensions, whole kidneys were minced and digested with a digestion mixture (500 μg/ml Collagenase IIs [Sigma-Aldrich] and 10 μg/ml DNase I [Roche] in 1× HBSS buffer containing Ca2+ and Mg2+) for 20 minutes at 37°C under vigorous agitation (200 rpm/min). The cells were suspended in 5 ml 2 mM EDTA in 1× HBSS buffer not containing Ca2+ and Mg2+ and further incubated for 20 minutes at 37°C under gentle agitation (50 rpm/min). Single-cell suspensions thus prepared were incubated with Fc-blocker (anti-CD16/32; 2.4G2). Then, CD11b- and/or CD11c-positive cells were enriched by magnetic sorting (Miltenyi).
For the analysis of peripheral blood mononuclear cells, peripheral blood was suspended in 2 mM EDTA in PBS. Then, red blood cells were lysed with deionized water. For flow cytometry analysis, cells were stained with the following antibodies: anti-CD11b (M1/70), anti-F4/80 (CI:A3–1), anti-CD11c (N418), anti-Gr-1 (RB6–8C5), and anti-Ly6C (AL-21). In some experiments, 7-AAD (BD Biosciences) was used to exclude dead cells. Then, the cells were analyzed by a flow cytometer (FACS Verse; BD Biosciences). The data were analyzed with FlowJo software (Tree Star, Inc.).
Parabiosis
Parabiotic mice were generated as reported44 from age- and weight-matched CD169-Cre-YFP and ROSA26-YFP mice. The separation was done 3 weeks after parabiotic operation. The frequency of YFP+ cells in the kidneys of each mouse was quantitated by a flow cytometer.
Model of Kidney IR
Renal IRI was performed essentially as described previously.45,46 Briefly, under anesthesia, a retroperitoneal incision was made. The left renal pedicle was clamped for 60 minutes using microaneurysm clamps (Fine Science Tools). After the clamp was removed, reperfusion of the left kidney was visually confirmed. A sham operation was performed in a similar manner, except for clamping of the left renal pedicle. Right nephrectomy was performed in some mice to induce bilateral IR. All experiments were performed under a warm lamp to maintain body temperature. To maintain fluid balance and volume status, mice were supplemented intraperitoneally with sterile 0.9% NaCl.
Assessment of Renal Damage
Renal injury after IR was assessed by measuring serum creatinine and BUN levels (measured by SRL). For histologic examination, kidneys were fixed in 10% buffered formalin and embedded in paraffin, and 5-μm-thick sections were stained with periodic acid–Schiff and observed under a light microscope (Olympus).
Quantitative PCR
Total RNA of kidneys was extracted using an RNAspin Mini RNA Isolation Kit (GE Healthcare) and then reverse-transcribed using a ReverTra Ace qPCR RT Master Mix Kit (TOYOBO), and cDNA was used for quantitative PCR by SYBR Green (TOYOBO). Primer sequences are listed in Supplemental Table 1. Relative amounts of mRNA were calculated by the ΔΔCt method using 18s rRNA as the internal control.
Immunohistochemistry
To detect neutrophils, kidneys were snap-frozen in OCT compound. Six-micrometer-thick cryosections were fixed in ice-cold acetone. Endogenous peroxidase activity and nonspecific binding in the sections were blocked by 3% H2O2. The sections were then incubated with a Biotin Blocking System (DAKO). The sections were stained with biotinylated anti-Ly6G antibody (clone: 1A8) using a TSA BIOTIN SYSTEM Kit (PerkinElmer Life and Analytical Sciences, Inc.).
For the detection of GFP or YFP, tissues were fixed in 4% paraformaldehyde/4% sucrose in 0.1 M phosphate buffer (pH 7.2), snap-frozen in OCT compound, and subsequently submerged in 10% and 20% sucrose for 1–16 hours each. Fourteen-micrometer-thick cryosections were stained with biotinylated anti-GFP (Abcam, Inc.) using the TSA BIOTIN SYSTEM Kit. Sections were observed under a fluorescent microscope (Keyence) or a confocal microscope (Olympus). In some experiments, DyLight 488-labeled Lycopersicon Esculentum (Tomato) lectin (Vector Laboratories) was injected intravenously into mice 5 minutes before euthanasia to visualize blood vessel. The z stack of confocal images was processed using ImageJ software (National Institutes of Health).
In Vitro Endothelium Activation Assay
The UV♀2 cells, a mouse vascular endothelial cell line, were obtained from RIKEN Cell Bank. The cells were seeded (5×105 cells/well) on six-well plates in DMEM/10% FCS and cultured overnight. Then, the cells were cocultured with total leukocytes obtained from either WT or DT-injected CD169-DTR mice for 18 hours.
In some experiments, to confirm the role of oxidants derived from leukocyte in the mechanism of ICAM-1 expression on the endothelial cells, butylated hydroxyanisole (100 μM; WAKO) was added into the coculture system. To exclude the influence of some humoral factors, endothelial cells and total leukocytes were cocultured using transwell plates (Corning). Endothelial cells were seeded (2.5×105 cells/well) on the bottom chamber in DMEM/10% FCS and cultured overnight. Then, the total leukocytes obtained from either WT or DT-injected CD169-DTR mice were added to the upper chamber and cocultured for 18 hours. Total RNA was isolated with an RNAspin Mini RNA Isolation Kit (GE Healthcare). ICAM-1 mRNA levels were determined by quantitative PCR.
Monocyte Isolation Using Cell Sorter
For monocyte transfer experiments, peripheral blood was obtained from WT mice. Red blood cells were then lysed with 1× Pharmlyse (Pharmingen) or distilled water. For transfer of monocyte subsets, white blood cells were stained with FITC–anti-mouse Ly6C (AL-21), PE–anti-mouse Gr-1 (RB6–8C5), and APC–anti-mouse CD11b (M1/70). Then, Ly6Clo or Ly6Chi monocytes were sorted by a cell sorter (SH-800; SONY). Sorted monocytes (3.5–6.0×104) were injected intravenously into a CD169-DTR mouse.
Statistical Analyses
For analyses, t test or ANOVA was used. Some datasets had a statistical difference in the variation between groups. The survival rate was estimated by the Kaplan–Meier method, and rates were compared using the log-rank test. Differences in values were considered significant at P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. F. Costantini for ROSA26-YFP mice, Dr. Rolf Sprengel for pBlue.iCre vector, Dr. D. Littman and Dr. S. Jung for CX3CR1-GFP mice, Dr. S. Nagata for MGM-5 cells, Dr. A. Kudo for CMG 14-12 cells, Dr. A. Maeshima for instruction of ischemia-reperfusion surgery, and Dr. K. Honda for interpretation of renal pathology. We also thank T. Suito for secretarial assistance.
This work was supported, in part, by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan, the Uehara Memorial Foundation, the Takeda Science Foundation, and the Naito Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “CD169+ Macrophages: Regulators of Neutrophil Trafficking to Injured Kidneys,” on pages 769–771.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014020195/-/DCSupplemental.
References
- 1.Carden DL, Granger DN: Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 255–266, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Eckle T: Ischemia and reperfusion—from mechanism to translation. Nat Med 17: 1391–1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day YJ, Huang L, Ye H, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: Role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H: CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 14: 2503–2515, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC: The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 12: 778–785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F: Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F: Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153: 362–375, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robben PM, LaRegina M, Kuziel WA, Sibley LD: Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med 201: 1761–1769, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serbina NV, Pamer EG: Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S: Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37: 1076–1090, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Crocker PR, Gordon S: Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med 169: 1333–1346, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS: Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208: 261–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N, Tanaka M, Zhao ZJ, Bergman A, Merad M, Frenette PS: CD169⁺ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med 19: 429–436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M: Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest 117: 2268–2278, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu CH, Miyake Y, Kaise H, Kitamura H, Ohara O, Tanaka M: Novel subset of CD8alpha+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol 182: 4127–4136, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, Kanagawa O, Fujii S, Tanaka M: CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity 34: 85–95, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Shimshek DR, Kim J, Hübner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R: Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 32: 19–26, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F: Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F: A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Cumano A, Godin I: Ontogeny of the hematopoietic system. Annu Rev Immunol 25: 745–785, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Orkin SH, Zon LI: Hematopoiesis: An evolving paradigm for stem cell biology. Cell 132: 631–644, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M: Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN: Kupffer cell heterogeneity: Functional properties of bone marrow derived and sessile hepatic macrophages. Blood 110: 4077–4085, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F: Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med 206: 3089–3100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG: Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 3: 1135–1141, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M: Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38: 792–804, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S: Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR: Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20: 4106–4114, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, Frey RS, Rahman A, Malik AB: Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem 277: 3404–3411, 2002 [DOI] [PubMed] [Google Scholar]
- 35.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P: Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330: 362–366, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Hamers AA, Vos M, Rassam F, Marinković G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ: Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res 110: 428–438, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ: The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hume DA, Gordon S: Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J Exp Med 157: 1704–1709, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J: The renal mononuclear phagocytic system. J Am Soc Nephrol 23: 194–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL: Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 294: F264–F271, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Swirski FK, Nahrendorf M: Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339: 161–166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takada Y, Hisamatsu T, Kamada N, Kitazume MT, Honda H, Oshima Y, Saito R, Takayama T, Kobayashi T, Chinen H, Mikami Y, Kanai T, Okamoto S, Hibi T: Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol 184: 2671–2676, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Furuichi K, Gao JL, Horuk R, Wada T, Kaneko S, Murphy PM: Chemokine receptor CCR1 regulates inflammatory cell infiltration after renal ischemia-reperfusion injury. J Immunol 181: 8670–8676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M: Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.