Abstract
Some β-blockers are efficiently removed from the circulation by hemodialysis (“high dialyzability”) whereas others are not (“low dialyzability”). This characteristic may influence the effectiveness of the β-blockers among patients receiving long-term hemodialysis. To determine whether new use of a high-dialyzability β-blocker compared with a low-dialyzability β-blocker associates with a higher rate of mortality in patients older than age 66 years receiving long-term hemodialysis, we conducted a propensity-matched population-based retrospective cohort study using the linked healthcare databases of Ontario, Canada. The high-dialyzability group (n=3294) included patients initiating atenolol, acebutolol, or metoprolol. The low-dialyzability group (n=3294) included patients initiating bisoprolol or propranolol. Initiation of a high- versus low-dialyzability β-blocker was associated with a higher risk of death in the following 180 days (relative risk, 1.4; 95% confidence interval, 1.1 to 1.8; P<0.01). Supporting this finding, we repeated the primary analysis in a cohort of patients not receiving hemodialysis and found no significant association between dialyzability and the risk of death (relative risk, 1.0; 95% confidence interval, 0.9 to 1.3; P=0.71). β-Blocker exposure was not randomly allocated in this study, so a causal relationship between dialyzability and mortality cannot be determined. However, our findings should raise awareness of this potentially important drug characteristic and prompt further study.
Keywords: hemodialysis, cardiovascular disease, mortality, pharmacokinetics
Nearly half of all deaths among patients receiving hemodialysis are caused by cardiovascular disease.1 In the general population, β-adrenergic receptor antagonists (β-blockers) reduce cardiovascular mortality,1,2 so by extension, these drugs are recommended for the same indications among patients receiving hemodialysis.3 However, the extent to which individual β-blockers are removed from the circulation by hemodialysis, referred to as “dialyzability,” varies considerably within this class. Acebutolol, atenolol, and metoprolol have high dialyzablility,4–9 whereas bisoprolol and propranolol have low dialyzablility.10–14 This characteristic could theoretically affect patient outcomes by lowering the average plasma concentration achieved in patients receiving high-dialyzability agents. We conducted this study to test the hypothesis that among patients receiving long-term hemodialysis, initiation of high-dialyzability β-blockers was associated with higher risks of death and cardiovascular events compared with low-dialyzability β-blockers.
Results
Baseline Characteristics
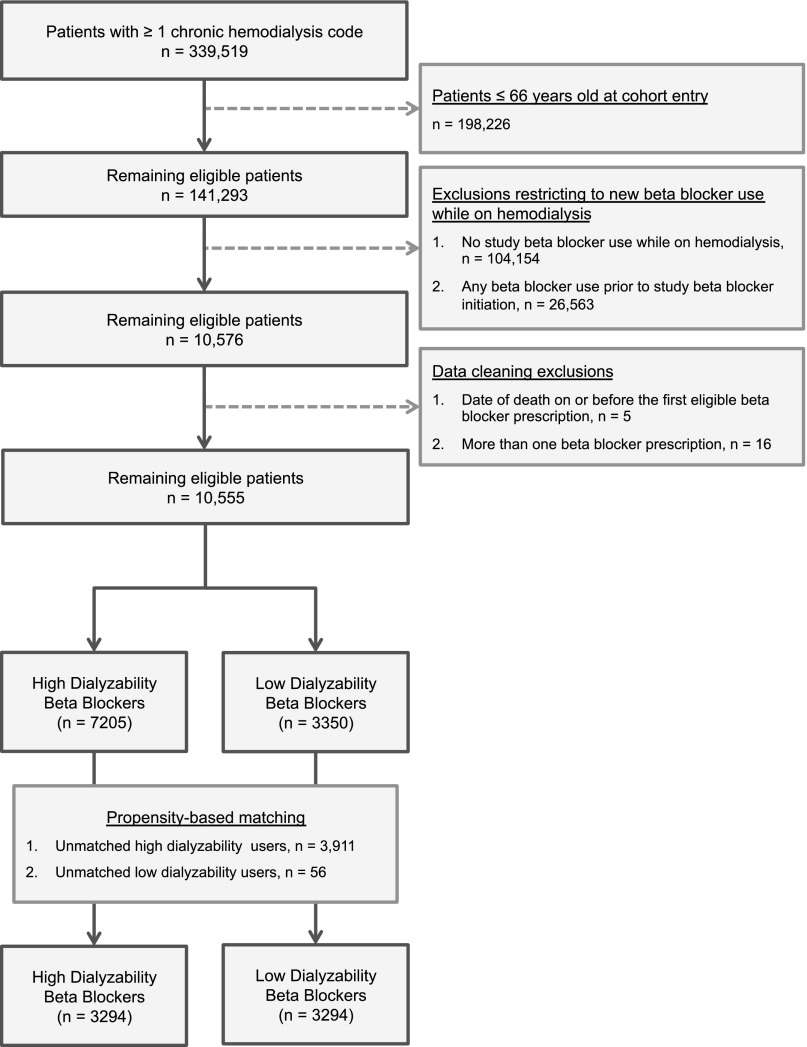
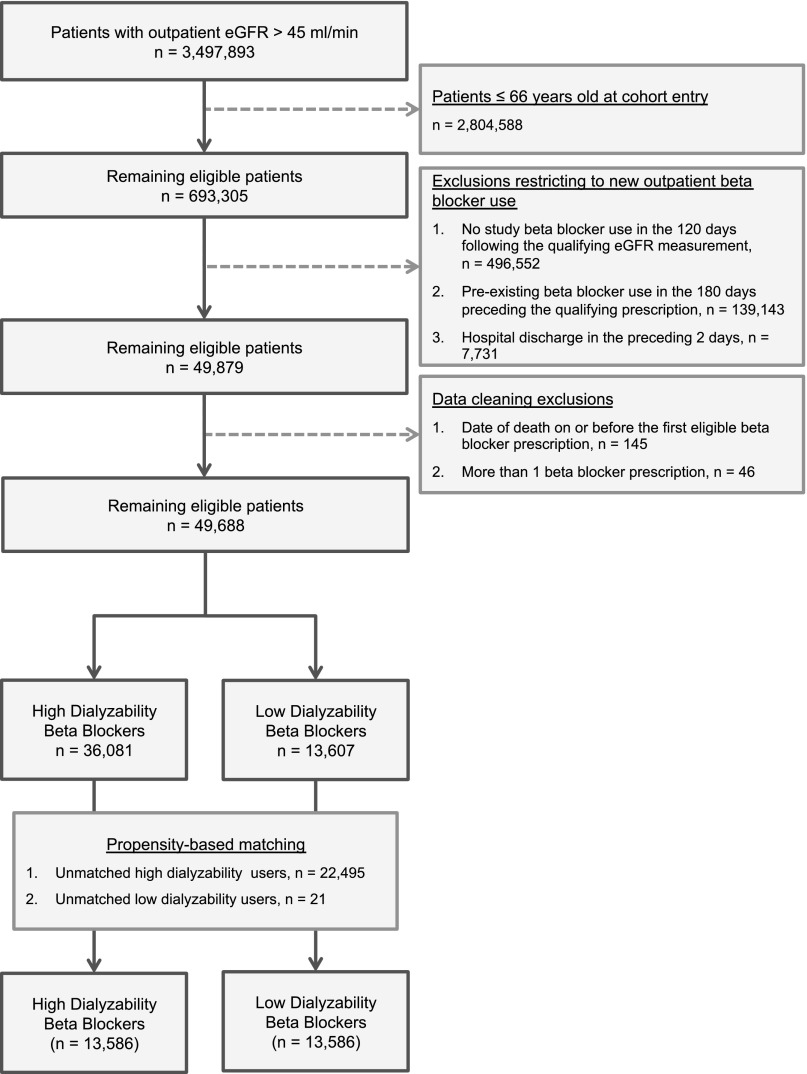
Figures 1 and 2 show how the hemodialysis and nondialysis cohorts were assembled. In both cohorts, high-dialyzability β-blockers (acebutolol, atenolol, metoprolol) were prescribed twice as frequently as low-dialyzability β-blockers (bisoprolol, propranolol). Table 1 shows the baseline characteristics of the hemodialysis cohort before and after matching (Supplemental Table 1 shows the baseline characteristics of the nondialysis cohort). Propensity matching removed many small but significant imbalances in the unmatched cohorts, particularly in the baseline prevalence of heart failure (standardized difference decreased from 20% to 1%), peripheral vascular disease (from 25% to 1%), and diabetes mellitus (from 18% to 2%). Matching resulted in loss of 3967 (37.6%) patients from the hemodialysis cohort (n=56 in the low-dialyzability group and n=3911 in the high-dialyzability group), and 22,516 (45.3%) patients from the nondialysis cohort (n=21 in the low-dialyzability group and n=22,495 in the high-dialyzability group). In both cohorts, atenolol or metoprolol accounted for >95% of high-dialyzability prescriptions and bisoprolol accounted for more than 80% of low-dialyzability prescriptions. A similar proportion of each group in the hemodialysis cohort received a kidney transplant during follow-up, 16 (0.5%) patients using low-dialyzability β-blockers and 22 (0.7%) patients using high-dialyzability β-blockers.
Figure 1.
Flow diagram showing the assembly of the long-term hemodialysis cohort.
Figure 2.
Flow diagram showing the assembly of the nondialysis cohort.
Table 1.
Baseline characteristics of the Hemodialysis Cohort
| Baseline Characteristics | Unmatched Cohort | Propensity-Matched Cohort | ||||
|---|---|---|---|---|---|---|
| High Dialyzability (Acebutolol, Atenolol, Metoprolol)a (n=7205) | Low Dialyzability (Propranolol, Bisoprolol)a (n=3350) | Standardized Differences (%) | High Dialyzability (Acebutolol, Atenolol, Metoprolol)a (n=3294) | Low Dialyzability (Propranolol, Bisoprolol)a (n = 3294) | Standardized Differences (%) | |
| Mean age±SD (yr) | 75.7±6.5 | 75.5±6.5 | 3 | 75.6±6.4 | 75.7±6.5 | 1 |
| Women, n (%) | 3429 (47.6) | 1636 (48.4) | 2 | 1617 (49.1) | 1617 (49.1) | 0 |
| Rural residence, n (%) | 812 (11.3) | 315 (9.4) | 6 | 391 (11.9) | 311 (9.4) | 8 |
| General measures of comorbidity (measured in the year before the index dateb) | ||||||
| Median no. of distinct prescription drugs (IQR) | 15 (10–21) | 13 (8–18) | 24c | 13 (8–19) | 13 (8–19) | 2 |
| Median duration of hemodialysis (first hemodialysis to index dateb) (IQR) (d) | 91 (16–684) | 24 (12–372) | 2 | 26 (12–438) | 24 (12–380) | 1 |
| Comorbidities (measured in the 5 yr before the index dateb), n (%) | ||||||
| Coronary artery diseased | 4907 (68.1) | 2070 (61.8) | 13c | 2069 (62.8) | 2055 (62.4) | 1 |
| Coronary revascularization | 1740 (24.2) | 617 (18.4) | 14c | 632 (19.2) | 615 (18.7) | 1 |
| Heart failure | 2921 (40.5) | 1031 (30.8) | 20c | 1037 (31.5) | 1022 (31.0) | 1 |
| Arrhythmiae | 2114 (29.3) | 860 (25.7) | 8 | 878 (26.7) | 853 (25.9) | 2 |
| Aortic aneurysm repair or bypass | 134 (1.9) | 32 (1.0) | 8 | 26 (0.8) | 32 (1.0) | 2 |
| Peripheral vascular disease | 1007 (14.0) | 216 (6.5) | 25c | 222 (6.7) | 216 (6.6) | 1 |
| Stroke or TIA | 426 (5.9) | 149 (4.5) | 7 | 152 (4.6) | 149 (4.5) | 0 |
| Diabetes mellitus | 2331 (32.4) | 815 (24.3) | 18c | 841 (25.5) | 811 (24.6) | 2 |
| β-blocker dosef (measured at the index dateb) | ||||||
| Low dose, n (%) | 6204 (86.1) | 3046 (90.9) | 15c | 2996 (91.0) | 2996 (91.0) | 0 |
| Medications (measured in the 180 d before the index dateb), n (%) | ||||||
| αBlockers | 678 (9.4) | 202 (6.0) | 13c | 222 (6.7) | 202 (6.1) | 2 |
| ACE inhibitors | 3217 (44.7) | 1446 (43.2) | 3 | 1436 (43.6) | 1419 (43.1) | 1 |
| ARB | 1648 (22.9) | 847 (25.3) | 6 | 784 (23.8) | 837 (25.4) | 4 |
| CCB | 3466 (48.1) | 1341 (40.0) | 16c | 1321 (40.1) | 1330 (40.4) | 1 |
| Digoxin | 555 (7.7) | 274 (8.2) | 2 | 237 (7.2) | 270 (8.2) | 4 |
| Statins | 4110 (57.0) | 1920 (57.3) | 1 | 1916 (58.2) | 1894 (57.5) | 1 |
| Warfarin | 1860 (25.8) | 913 (27.3) | 3 | 854 (25.9) | 905 (27.5) | 4 |
| β-Blockers | ||||||
| Acebutolol | 229 (3.2) | – | – | 88 (2.7) | – | – |
| Atenolol | 1948 (27.0) | – | – | 900 (27.3) | – | – |
| Metoprolol | 5028 (69.8) | – | – | 2306 (70.0) | – | – |
| Bisoprolol | – | 3223 (96.2) | – | – | 3169 (96.2) | – |
| Propranolol | – | 127 (3.8) | – | – | 125 (3.8) | – |
| Propensity score probability (propensity of receiving a high-dialyzability β-blocker) | ||||||
| Mean±SD | 0.71±0.15 | 0.61±0.13 | 72c | 0.62±0.12 | 0.62±0.13 | 2 |
| Median (IQR) | 0.73 (0.59–0.84) | 0.59 (0.52–0.70) | 72c | 0.59 (0.52–0.70) | 0.59 (0.52–0.70) | 2 |
“Acebutolol” refers to acebutolol HCl; “metoprolol” refers to metoprolol tartrate; “bisoprolol” refers to bisoprolol fumarate
“Index date” is defined as the day of the first new study β-blocker prescription filled during hemodialysis.
Significant standardized differences (≥10%).
“Coronary artery disease” includes myocardial infarction, angina, and percutaneous coronary interventions
“Arrhythmia” includes brady and tachyarrhythmias.
Definitions of “high” or “low” dose were based on the recommendations found in each product’s monograph. High doses: acebutolol, ≥400 mg/d; atenolol, >50 mg/d; metoprolol, >100 mg/d; propranolol, ≥160 mg/d; bisoprolol, >5 mg/d.
TIA, transient ischemic attack; IQR, interquartile range, ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CCB, calcium-channel blocker.
Primary Outcome: Mortality
In the hemodialysis cohort, the relative risk (RR) of death was 40% higher in patients prescribed a high-dialyzability β-blocker than in those prescribed a low-dialyzability β-blocker (RR, 1.4; 95% confidence interval [95% CI], 1.1 to 1.8; P<0.01) (Table 2). The absolute risk increase was 1.4%, giving a number needed to harm of 71. In the nondialysis cohort the dialyzability of the prescribed β-blocker was not associated with a significant difference in mortality (RR, 1.0; 95% CI, 0.9 to 1.3; P=0.71).
Table 2.
All-cause mortality (conditional logistic regression model)
| Variable | Patients (n) | No. of Events (%) | RR (95% CI) | P Value |
|---|---|---|---|---|
| Hemodialysis cohort | ||||
| High-dialyzability β-blockers | 3294 | 182 (5.5) | 1.4 (1.1 to 1.8) | <0.01 |
| Low-dialyzability β-blockers | 3294 | 135 (4.1) | 1 (referent) | |
| Nondialysis cohort | ||||
| High-dialyzability β-blockers | 13,586 | 186 (1.4) | 1.0 (0.9 to 1.3) | 0.71 |
| Low-dialyzability β-blockers | 13,586 | 179 (1.3) | 1 (referent) |
Secondary Outcomes: Cardiovascular Disease
Supplemental Table 2 shows the results of the analyses of secondary outcomes assessed in the hemodialysis cohort. The composite of death, myocardial infarction, or heart failure occurred more frequently in those prescribed a high-dialyzability β-blocker (RR, 1.2; 95% CI, 1.0 to 1.5; P=0.03), but this was driven by the increased risk of death because no significant differences in the risk of myocardial infarction or heart failure were observed when each outcome was assessed separately.
Additional Analyses
Dose Stability of β-Blockers
Our hypothesis depended on the combination of high-dialyzability β-blockers and hemodialysis resulting in lower average degrees of β blockade. If prescribing physicians titrated the dose to a clinical effect such as heart rate, the effect of dialyzability would be diluted. To assess this, we examined the change in β-blocker dose during the 180-day observation period and expressed the change standardized to the initial dose ([initial dose−final dose]/initial dose×100). We observed very little change in dose over the 180 days. The mean change in the standardized dose difference was −0.17% for low-dialyzability β-blockers and −0.15% for high-dialyzability β-blockers. We also recorded the frequency and direction of dose changes within the cohort. Doses were stable in 72.5% of the low-dialyzability group and in 72.2% of the high-dialyzability group. Doses increased in only 8.5% of the low-dialyzability group and 10.1% of the high-dialyzability group.
β-Blocker Indications
Although most β-blockers in our study have similar indications, propranolol and acebutolol have unique properties. Propranolol is a nonselective β-blocker with many noncardiac indications, and acebutolol has intrinsic sympathomimetic activity. To eliminate the influence of these special indications and properties, we repeated the primary analysis with propranolol users, acebutolol users, and their matches removed from the cohort. This resulted in the loss of 211 patients from each group and no substantial change in the estimated risk of death associated with high-dialyzability agents (RR, 1.4; 95% CI, 1.1 to 1.7; P<0.01).
Tracer Outcome
We tested the specificity of our findings by determining each group’s risk of admission with bowel obstruction, an outcome we did not expect to be influenced by the dialyzability of the prescribed β-blocker. We identified admissions with bowel obstruction using corresponding diagnostic codes in any of the Canadian Institute for Health Information–Discharge Access Database (CIHI-DAD)’s diagnostic fields (International Classification of Diseases, Ninth Revision, [ICD-9] code, 560; ICD, 10th revision [ICD-10] code, K56). Compared with low-dialyzability β-blockers, we found no significant increase in the risk of bowel obstruction with high-dialyzability agents (RR, 1.8; 95% CI, 0.9 to 3.7; P=0.09).
Cox Proportional Hazards Model
When we repeated the primary outcome analyses with Cox regression stratifying on matched sets, the results were materially similar (low-dialyzability, 8.5 deaths per 100 person-years; high-dialyzability, 11.5 deaths per 100 person-years; hazard ratio, 1.3 [95% CI, 1.1 to 1.7], P=0.02) (Supplemental Table 3).
Post hoc Analysis: Ventricular Arrhythmia
We initially believed that ventricular arrhythmia would have very low sensitivity as an outcome measure; however, we conducted this analysis as a proxy for the risk of sudden cardiac death. We defined ventricular arrhythmia using the ICD-10 code I490. The difference in the risk of ventricular arrhythmia did not reach statistical significance in the hemodialysis cohort (RR, 1.3; 95% CI, 0.9 to 2.0; P=0.20) or the nondialysis cohort (RR, 1.1; 95% CI, 0.7 to 1.5; P=0.79) (Table 3).
Table 3.
Ventricular arrhythmia (conditional logistic regression model)
| Variable | Patients (n) | No. of Events (%) | RR (95% CI) | P Value |
|---|---|---|---|---|
| Hemodialysis cohort | ||||
| Hemodialysis cohort | ||||
| High-dialyzability β-blockers | 3294 | 52 (1.6) | 1.3 (0.9 to 2.0) | 0.20 |
| Low-dialyzability β-blockers | 3294 | 40 (1.2) | 1 (referent) | |
| Nondialysis cohort | ||||
| High-dialyzability β-blockers | 13,586 | 65 (0.5) | 1.1 (0.7 to 1.5) | 0.79 |
| Low-dialyzability β-blockers | 13,586 | 62 (0.5) | 1 (referent) | |
Discussion
Among patients receiving chronic hemodialysis, we observed a significantly higher risk of death in those prescribed a high-dialyzability β-blocker compared with those prescribed a low-dialyzability β-blocker. We did not see this association among patients not receiving hemodialysis. The mechanism of death did not appear to be related to an excess risk of myocardial infarction or heart failure among the high-dialyzability β-blocker group; however, the confidence intervals around the risk of ventricular arrhythmia suggest that a more sensitive outcome measure could have yielded a statistically significant result.
Interpretation
With only one randomized controlled trial of a β-blocker in hemodialysis,15 treatment recommendations must be based largely on nondialysis trial data.2 However, important differences exist between patients who do and do not require long-term hemodialysis, one of which may be the opportunity for drug removal during dialysis. Our findings should prompt further investigation into the pharmacokinetics and pharmacodynamics of drugs commonly used in this population.
Strengths
To our knowledge, this is the first study to examine the potential effect of dialyzability on patient outcomes. No randomized trials have compared high- and low-dialyzability β-blockers in patients receiving hemodialysis. We are confident in our study’s findings for several reasons. First, the findings in the nondialysis cohort support the physiologic basis for the effect we observed in the hemodialysis cohort. Second, indication bias is unlikely to have substantially influenced our findings because we studied drugs with similar indications and the imbalances between groups were minimized through propensity-based matching. When we excluded β-blockers with alternate indications (propranolol, acebutolol), there was no material effect on our findings. Third, because β-blocker doses remained constant during the observation period we are reassured that the effects of high-dialyzability were not being lost to physicians titrating to clinical effect.
Limitations
Our results must be interpreted in the context of the study’s limitations. We relied on health administrative data to ascertain exposure and outcome data. Although the databases are reliable for the data they contain, they do not provide information on some potentially important parameters such as heart rate, BP, the timing of drug ingestion, or the hemodialysis prescription. Although indication bias was limited because all patients were prescribed β-blockers, we could not eliminate its influence. Atenolol, metoprolol, and bisoprolol made up the bulk of prescriptions in our study, and their evidence-based indications differ. We can infer from the baseline characteristics that hypertension and coronary artery disease were the most common indications for β-blockade in our cohort (Table 1). For this indication, practice guidelines do not differentiate among these agents.16–18 However, 30% of the cohort had heart failure, and in this setting, bisoprolol is the only study drug proven to lower mortality.19 Although extended-release metoprolol carries similar evidence,20 this cannot be extrapolated to the short-acting formulation we studied.21
Our study lacked a control group to whom β-blockers were not prescribed. This group would have allowed us to determine whether high-dialyzability β-blockers were more protective than no β-blocker at all. However, the findings from such an analysis would have been strongly influenced by indication bias and the overall interpretation of our results (to choose low- instead of high-dialyzability β-blockers) would not have been altered. Furthermore, observational data have shown associations between β-blocker use and decreased mortality in patients receiving dialysis,22–24 although this is not the case in patients with CKD not requiring hemodialysis.25
Among patients receiving hemodialysis, sudden cardiac death is a common cause of death that occurs less frequently with β-blocker use.1,23 Therefore, sudden cardiac death may have accounted for the excess mortality we observed with the use of high-dialyzability β-blockers. However, because sudden cardiac death is more likely to happen at night,26 it occurs outside the health care system and patients do not receive diagnostic administrative codes. This makes sudden cardiac death difficult to quantify using administrative data. We attempted to circumvent this issue by assessing the risk of ventricular arrhythmia in a post hoc analysis but were limited by a low event rate related to the code’s low sensitivity.27
Bisoprolol’s high degree of β-1 selectivity may offer an alternative biologic explanation for our findings. Bisoprolol’s ratio of β-1 to β-2 selectivity is 13.5, compared with only 2.3 for metoprolol and 4.7 for atenolol.28,29 The hypotensive effects of β-2 antagonism, which are more pronounced with metoprolol and atenolol, may explain the excess risk of perioperative stroke seen with these agents,30,31 but not with bisoprolol.32 The same mechanism may have contributed to the excess mortality we observed.
Carvedilol is an important β-blocker for patients receiving hemodialysis. It has low dialyzability and has been proven effective in a randomized trial of hemodialysis-dependent patients with dilated cardiomyopathy and symptomatic heart failure.15 However, we did not include carvedilol in our analysis because in our jurisdiction, access to this medication is restricted to those with documented severe symptomatic heart failure with a recent exacerbation and a left ventricular ejection fraction less than 35%. These restrictions would have produced a strong indication bias.
Finally, we recognize that the dialyzability of a drug is a complex interaction among many aspects of its pharmacokinetics and the dialysis prescription.33,34 Although a drug’s volume of distribution, molecular weight, and protein binding are readily available, the literature lacks data on factors such as the degree of red blood cell binding and changes in hepatic metabolism. Furthermore, studies describing changes in the elimination half-life of drugs during hemodialysis (Supplemental Table 4) have two important limitations. First, none were conducted using modern high-flux, high-efficiency dialysis membranes, and second, the amount of drug removed during hemodialysis is only a proxy for the true variable of interest, which is the degree of β blockade achieved. Overall, we determined dialyzability based on the balance of all available data (Table 4), and although we are confident with the conclusions we reached, the evidence leaves room for debate.
Table 4.
Dialyzability of study β-blockers
| β-Blocker | Industry Sources | Review Articles | Dialyzability Categorization for This Study | ||||
|---|---|---|---|---|---|---|---|
| Product Monograph Statements (Lexicomp and Compendium of Pharmaceuticals and Specialties)a | Dialysis of Drugs 2013b | Levin et al.38 | Chazot and Jean12 | Chen et al.10 | Redon et al.43 | ||
| Bisoprolol | “Bisoprolol is not dialyzable. Dose replacement or adjustment is not necessary in patients undergoing dialysis” | Not significantly dialyzable | Not dialyzable | Not dialyzable | Not dialyzable | Not dialyzable | Low dialyzability |
| Propranolol | “Propranolol is not substantially removed by hemodialysis” | Not significantly dialyzable | Not dialyzable | – | Not dialyzable | – | Low dialyzability |
| Acebutolol | “Acebutolol and its major metabolite are dialyzable” | Dialyzable | Dialyzable | Not dialyzable | Not dialyzable | Not dialyzable | High dialyzability |
| Atenolol | “Moderately dialyzable (20% to 50%) via hemodialysis; administer dose postdialysis or administer 25 – 50 mg supplemental dose” | Dialyzable | Dialyzable | Dialyzable | Dialyzable | Dialyzable | High dialyzability |
| Metoprolol | No statement | Dialyzable | Dialyzable | Dialyzable | Dialyzable | Not dialyzable | High dialyzability |
These sources summarize data from drug manufacturers’ product monographs.
This source is sponsored by Sanofi S.A. (Paris, France) and compiles available scientific and industry data on drug dialyzability.
In conclusion, we found that among patients receiving long-term hemodialysis, the risk of death was significantly higher among those prescribed high-dialyzability β-blockers than those prescribed low-dialyzability β-blockers. The importance of dialyzability of β-blockers and other medications used to treat patients receiving long-term dialysis should be investigated further.
Concise Methods
Study Design and Setting
We conducted a one-to-one matched population-based retrospective cohort study using health administrative data from Ontario, Canada. The Ontario Health Insurance Plan is the single payer for 13 million residents who receive universal access to hospital and physician services. Those older than 65 years of age also receive prescription drug coverage. The Research Ethics Board at Sunnybrook Health Sciences Centre approved the protocol, and we have reported it according to established guidelines for observational studies.35
Data Sources
We used linked databases housed at the Institute for Clinical Evaluative Sciences. We ascertained vital statistics, including mortality, for people issued a provincial health card from the Registered Persons Database. We used the Ontario Drug Benefits database to ascertain prescription drug exposure and drug-related baseline characteristics. This database records prescription drug dispensing for patients older than age 65 years and has a basic error rate of <1%.36 We identified admissions to hospital and baseline characteristics using the CIHI-DAD. We used the Ontario Health Insurance Plan database to ascertain information on physician services.
β-Blocker Dialyzability
The dialyzability of β-blockers is determined by several parameters, including molecular weight, the degree of protein binding, water solubility, and the volume of distribution.33,34,37 To categorize β-blockers according to dialyzability, we first consulted each drug’s product monograph and looked for statements relating to dialyzability or dosing in the setting of hemodialysis. This yielded clear statements regarding dialyzability for all study drugs except metoprolol (Table 4). To supplement these data, we consulted the 2013 Dialysis of Drugs handbook.37 This resource agreed with the product monographs but also listed metoprolol as dialyzable. We then searched MEDLINE and EMBASE for review articles that addressed drug dosing in hemodialysis. This search yielded four peer-reviewed articles that discussed the dialyzability of β-blockers (Table 4). The categorization of atenolol, bisoprolol, and propranolol was consistent across these four publications; however, the categorization of acebutolol and metoprolol varied. Using the review article reference lists and primary search terms, we identified articles that described the pharmacokinetics of β-blockers in patients receiving hemodialysis. The key findings of these studies are presented in Supplemental Table 4, and their important limitations are summarized in Supplemental Table 5. On the balance of the industry data, we concurred with Levin et al. and categorized propranolol and bisoprolol as “low dialyzability” and acebutolol, atenolol, and metoprolol as “high dialyzability.”38 Note that carvedilol is a low-dialyzability β-blocker, but we did not include it our analysis because its coverage in Ontario is limited to patients with echocardiographic and symptomatic evidence of advanced heart failure. We considered the addition of an intermediate dialyzability category with labetalol, nadolol, pindolol, and timolol, but these agents were too infrequently used in Ontario to yield meaningful results. At the time the study was conducted, nebivolol was not available in Canada.
Patients
Hemodialysis Cohort
We assembled a cohort of patients who received their first study β-blocker while receiving hemodialysis. To accomplish this, we used physician billing records from April 1, 2002, to March 31, 2011, to identify all patients who received long-term hemodialysis. Because Ontario residents older than age 65 years receive prescription drug coverage, we restricted enrollment to patients older than 66 years to ensure at least 1 year of drug use data before inception. We then identified patients who filled a prescription for one of the five study β-blockers. To identify β-blocker use during hemodialysis, we excluded prescriptions that were not preceded within 30 days by a long-term hemodialysis code. To ensure β-blocker use was new, we excluded patients who filled any β-blocker prescription within 120 days before the first prescription filled during hemodialysis.
Nondialysis Cohort
We assembled a cohort of patients not requiring dialysis who filled a new study β-blocker prescription during the same time period as the hemodialysis cohort. To accomplish this, we first restricted enrollment to patients who had an outpatient eGFR≥45 ml/min per 1.73 m2. We then applied a set of inclusion criteria analogous to those used for the hemodialysis cohort.
Outcomes
Outcomes were ascertained identically for the hemodialysis and nondialysis cohorts, and the primary, secondary, and additional outcomes were specified before the analysis. All outcomes were assessed in the 180 days after the index β-blocker prescription. We chose this duration of follow-up on the basis of our findings that the median (interquartile range) duration of continuous use was 471 (85–646) days for high-dialyzability β-blockers and 508 (78–752) days for low-dialyzability β-blockers. We chose a short observation period of 180 days to minimize the likelihood of dropout or crossover between exposure groups.
Primary Outcome
The Registered Persons Database has a sensitivity of 97.8% and specificity of 100% for the finding of death.39
Secondary Outcomes
We defined hospital admissions as being due to a myocardial infarction when the appropriate diagnostic codes appeared in the “Most Responsible Diagnosis” field of the CIHI-DAD (ICD-10 I21 or I22). This field records the single diagnosis that contributed most to the patient’s length of stay in the hospital. We defined admission due to heart failure the same way (ICD-10 I50.0). We also assessed each component of the composite outcome individually. The coding of cardiovascular disease in the CIHI-DAD is highly specific (92.8%–96.8%) and moderately sensitive (58.5%–88.8%).40
Additional Analyses
We conducted additional analyses to support the findings of our primary analysis. We examined the stability of β-blocker dosing during the 180-day observation period. We tested the specificity of our findings using a tracer outcome of bowel obstruction. We repeated the primary outcome analyses with Cox regression stratifying on matched sets. Because of the unique properties and indications associated with propranolol and acebutolol, we repeated the primary analysis without these drugs. As a proxy for sudden cardiac death, we conducted a post hoc analysis of hospital admission with ventricular arrhythmia. The rationale, methods, and results of the additional analyses are presented together in the Results section.
Statistical Analyses
For both the hemodialysis and nondialysis cohorts, we compared the prevalence of baseline characteristic between the high- and low-dialyzability groups using standardized differences, which describe a difference between group means as percentages of the pooled standard deviation. Standardized differences >10% represent meaningful imbalances.41,42 After this comparison, we pair-matched low-dialyzability patients to high-dialyzability patients in a one-to-one ratio based on age (±3 years), sex and propensity score (±0.2 SD). We estimated propensity scores using a logistic regression model in which high-dialyzability β-blocker use was the dependent variable. Independent variables included age, year of index, sex, comorbid conditions (coronary artery disease, peripheral vascular disease, abdominal aortic aneurysm, diabetes mellitus, heart failure, stroke, or transient ischemic attack), general measures of comorbidity (duration of dialysis, number of unique prescriptions in the last year), and concomitant medications (angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, calcium-channel blockers, statins). Comparisons between dialyzability groups were subsequently made within the matched cohorts. We used conditional logistic regression analyses to estimate odds ratios and 95% CIs. Odds ratios were interpreted as RRs (which was appropriate given the incidences observed). We conducted all analyses with SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Disclosures
M.A.W. received salary support from the Academic Medical Organization of Southwestern Ontario. R.S.S. received salary support from the Fonds de la Recherche en Santé du Québec. D.G.H. received salary support from the Canadian Institute of Health Research New Investigator Award. The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by these organizations is intended or should be inferred.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Salimah Shariff for her assistance with programming and data acquisition.
The Canadian Institute of Health Research funded this project. It was conducted at the Institute for Clinical Evaluative Sciences (ICES) Western University Site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-term Care. Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “β-Blockers in Dialysis Patients: A Nephrocardiology Perspective,” on pages 774–776.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014040324/-/DCSupplemental.
References
- 1.Wanner C, Krane V, März W, Olschewski M, Mann JFE, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Freemantle N, Cleland J, Young P, Mason J, Harrison J: Beta Blockade after myocardial infarction: Systematic review and meta regression analysis. BMJ 318: 1730–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45: S1–153, 2005 [PubMed] [Google Scholar]
- 4.Roux A, Aubert P, Guedon J, Flouvat B: Pharmacokinetics of acebutolol in patients with all grades of renal failure. Eur J Clin Pharmacol 17: 339–348, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Campese VM, Feinstein EI, Gura V, Mason WD, Massry SG: Pharmacokinetics of atenolol in patients treated with chronic hemodialysis or peritoneal dialysis. J Clin Pharmacol 25: 393–395, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Flouvat B, Decourt S, Aubert P, Potaux L, Domart M, Goupil A, Baglin A: Pharmacokinetics of atenolol in patients with terminal renal failure and influence of haemodialysis. Br J Clin Pharmacol 9: 379–385, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirch W, Köhler H, Mutschler E, Schäfer M: Pharmacokinetics of atenolol in relation to renal function. Eur J Clin Pharmacol 19: 65–71, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Seiler KU, Schuster KJ, Meyer GJ, Niedermayer W, Wassermann O: The pharmacokinetics of metoprolol and its metabolites in dialysis patients. Clin Pharmacokinet 5: 192–198, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Niedermayer W, Seiler KU, Wassermann O: Pharmacokinetics of antihypertensive drugs (atenolol, metoprolol, propranolol and clonidine) and their metabolites during intermittent haemodialysis in humans. Proc Eur Dial Transplant Assoc 15: 607–609, 1978 [PubMed] [Google Scholar]
- 10.Chen J, Gul A, Sarnak MJ: Management of intradialytic hypertension: The ongoing challenge. Semin Dial 19: 141–145, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Payton CD, Fox JG, Pauleau NF, Boulton-Jones JM, Ioannides C, Johnston A, Thomas P: The single dose pharmacokinetics of bisoprolol (10 mg) in renal insufficiency: The clinical significance of balanced clearance. Eur Heart J 8[Suppl M]: 15–22, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Chazot C, Jean G: Intradialytic hypertension: It is time to act. Nephron Clin Pract 115: c182–c188, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Stone WJ, Walle T: Massive propranolol metabolite retention during maintenance hemodialysis. Clin Pharmacol Ther 28: 449–455, 1980 [DOI] [PubMed] [Google Scholar]
- 14.Wood AJ, Vestal RE, Spannuth CL, Stone WJ, Wilkinson GR, Shand DG: Propranolol disposition in renal failure. Br J Clin Pharmacol 10: 561–566, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R: Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Campbell NRC, Poirier L, Tremblay G, Lindsay P, Reid D, Tobe SW, Canadian Hypertension Education Program : Canadian Hypertension Education Program: The science supporting New 2011 CHEP recommendations with an emphasis on health advocacy and knowledge translation. Can J Cardiol 27: 407–414, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS: ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction): Developed in Collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 116: 803–877, 2007 [DOI] [PubMed] [Google Scholar]
- 18.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E: 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Investigators C-I : The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 353: 9–13, 1999 [PubMed] [Google Scholar]
- 20.Hjalmarson A, Goldstein S: Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007, 1999 [PubMed] [Google Scholar]
- 21.Poole-Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A, Carvedilol Or Metoprolol European Trial Investigators : Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 362: 7–13, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL: Beta-Blocker use in long-term dialysis patients: Association with hospitalized heart failure and mortality. Arch Intern Med 164: 2465–2471, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Matsue Y, Suzuki M, Nagahori W, Ohno M, Matsumura A, Hashimoto Y: β-blocker prevents sudden cardiac death in patients with hemodialysis. Int J Cardiol 165: 519–522, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Nakao K, Makino H, Morita S, Takahashi Y, Akizawa T, Saito A, Asano Y, Kurokawa K, Fukuhara S, Akiba T, J-DOPPS Investigators Group : Beta-blocker prescription and outcomes in hemodialysis patients from the Japan Dialysis Outcomes and Practice Patterns Study. Nephron Clin Pract 113: c132–c139, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V: Effects of beta-adrenergic antagonists in patients with chronic kidney disease: A systematic review and meta-analysis. J Am Coll Cardiol 58: 1152–1161, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Ritz E, Bommer J: Cardiovascular problems on hemodialysis: Current deficits and potential improvement. Clin J Am Soc Nephrol 4[Suppl 1]: S71–S78, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S: Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf 20: 700–708, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Baker JG: The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 144: 317–322, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker JG: The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol 160: 1048–1061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Málaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P, POISE Study Group : Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet 371: 1839–1847, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH: Perioperative beta blockers in patients having non-cardiac surgery: A meta-analysis. Lancet 372: 1962–1976, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Ashes C, Judelman S, Wijeysundera DN, Tait G, Mazer CD, Hare GMT, Beattie WS: Selective β1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: A single-center cohort study of 44,092 consecutive patients. Anesthesiology 119: 777–787, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Sefer S, Degoricija V: About drug dialyzability. Acta Clin Croat 42: 257–267, 2003 [Google Scholar]
- 34.Atkinson A, Umans J: KDIGO Controversies Conference. Drug Prescribing in Kidney Disease: Initiative for Improved Dosing. 2010 Available at: http://kdigo.org/home/conferences/drug-prescribing-in-kidney-disease-initiative-for-improved-dosing-2010. Accessed September 13, 2012
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 61: 344–349, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D: Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 10: 67–71, 2003 [PubMed] [Google Scholar]
- 37.Bailie GR, Mason NA: Dialysis of Drugs. Saline, Michigan: Renal Pharmacy Consultants, LLC, 2013
- 38.Levin NW, Kotanko P, Eckardt K-U, Kasiske BL, Chazot C, Cheung AK, Redon J, Wheeler DC, Zoccali C, London GM: Blood pressure in chronic kidney disease stage5D—report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int 77: 273–284, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Jha P, Deboer D, Sykora K, Naylor CD: Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: a population-based comparison. J Am Coll Cardiol 27: 1335–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Austin PC, Daly PA, Tu JV: A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J 144: 290–296, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Comm Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 42.Mamdani M, Sykora K, Li P, Normand S-LT, Streiner DL, Austin PC, Rochon PA, Anderson GM: Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330: 960–962, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redon J, Martinez F, Cheung AK: Special considerations for antihypertensive agents in dialysis patients. Blood Purif 29: 93–98, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.