Abstract
Monoclonal Ig deposition disease (MIDD) is a rare complication of monoclonal gammopathy characterized by deposition of monoclonal Ig light chains and/or heavy chains along the glomerular and tubular basement membranes. Here, we describe a unique case of IgD deposition disease. IgD deposition is difficult to diagnose, because routine immunofluorescence does not detect IgD. A 77-year-old man presented with proteinuria and renal failure, and kidney biopsy analysis showed a nodular sclerosing GN with extensive focal global glomerulosclerosis, tubular atrophy, and interstitial fibrosis. Immunofluorescence was negative for Ig deposits, although electron microscopy showed deposits in the glomeruli and along tubular basement membranes. Laser microdissection of glomeruli and mass spectrometry of extracted peptides showed a large spectra number for IgD, and immunohistochemistry showed intense glomerular and tubular staining for IgD. Together, these findings are consistent with IgD deposition disease. Bone marrow biopsy analysis showed 5% plasma cells, which stained for IgD. The patient was treated with bortezomib and dexamethasone, which resulted in improvement of hematologic parameters but no improvement of renal function. The diagnosis of IgD deposition disease underscores the value of laser microdissection and mass spectrometry in further evaluating renal biopsies when routine assessment fails to reach an accurate diagnosis.
Keywords: immunology and pathology, renal pathology, multiple myeloma
A 77-year-old man of Eastern European descent presented with proteinuria and severe renal insufficiency 6 months ago. The patient had numerous comorbidities, which included chronic obstructive pulmonary disease associated with pulmonary hypertension and bronchiectasis, coronary artery disease, mild aortic stenosis, arterial hypertension, and dyslipidemia.
At presentation, the patient complained of fatigue, weight loss, and lower extremity swelling. His BP was normal, and other than the edema, the physical examination was unremarkable. His serum creatinine level was 2.2 mg/dl (194 μmol/L), with an eGFR of 29 ml/min. Additional evaluation showed nephrotic range proteinuria of 5.3 g/d. Microscopic examination of the urine revealed >100 red blood cells per high-power field. Other significant laboratory findings included a mild normocytic anemia with a hemoglobin concentration of 11.4 g/dl and a decreased serum albumin at 2.5 g/dl. Serum calcium level was normal. Serological studies for antinuclear antibody and antineutrophil cytoplasmic antibodies were negative, and complement levels (C3 and C4) were in the normal range.
Serum protein electrophoresis showed no M spike on initial evaluation. However, the serum protein immunofixation studies revealed a monoclonal λ-band. Serum λ-free light-chain level was elevated at 2303 mg/L, with a κ:λ ratio of 0.02. A 24-hour urine collection revealed the presence of a monoclonal λ-light chain. Bone studies did not show any lytic bone lesion.
A bone marrow biopsy was performed, and it showed normal cellularity with a mild monoclonal plasmacytosis (5%) and a negative Congo red stain. Fluorescence in situ hybridization analysis of bone marrow aspirate did not show 17p13 deletion, and too few plasma cells were present to proceed to additional fluorescence in situ hybridization analysis.
The patient underwent a renal biopsy to determine the cause of proteinuria and renal insufficiency.
Renal Biopsy
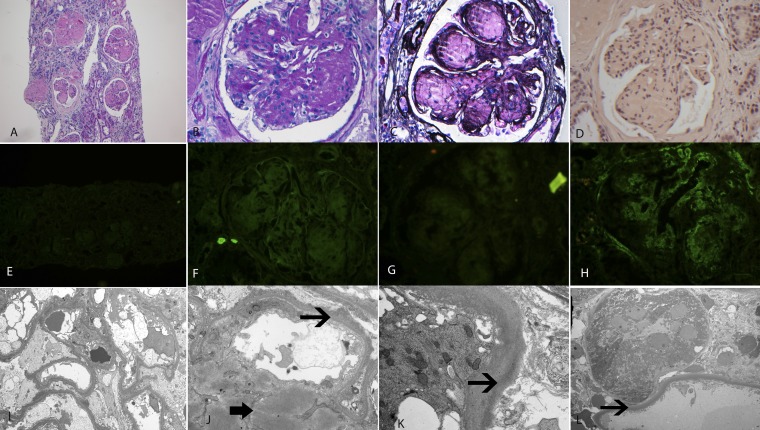
The kidney biopsy consisted of a single core of renal cortex. There were 27 glomeruli present, of which 18 (66.6%) glomeruli were globally sclerosed. The glomeruli showed marked mesangial expansion with granular, smudgy silver-negative, and periodic acid–Schiff-positive material, resulting in the formation of mesangial nodules. The glomerular basement membranes were thickened. Some of the loops showed subendothelial expansion with cellular elements and new basement membrane formation resulting in segmental double contours. The glomeruli did not show any evidence of crescent formation, fibrinoid necrosis, or thrombosis. Basement membrane spikes or pinholes were not present along the capillary walls. The interstitium showed moderate patchy interstitial inflammation with mostly mononuclear cells. The tubular basement membranes were thickened. There was extensive tubular atrophy and interstitial fibrosis present, and approximately 70%–80% of the sample showed tubular atrophy and interstitial fibrosis. Arteries and arterioles showed severe sclerosis of the intima with narrowing of the vascular lumen. Congo red stain was negative for amyloid in the glomeruli, interstitium, and vessels. Immunofluorescence studies were negative for IgA, IgG, IgM, C1q, and κ- and λ-light chains in the glomeruli, interstitium, and vessels. The glomeruli showed granular staining for C3 (2+) in the mesangium and along capillary walls. Electron microscopy showed mesangial expansion by smudgy powdery deposits. Vague fibrillary substructure was also noted. Powdery punctate electron dense deposits were also noted along the glomerular and tubular basement membranes. The light, immunofluorescence, and electron microscopy findings are shown in Figure 1. The preliminary kidney biopsy diagnosis on the basis of the above findings was an uncharacterized deposition disease, presumably related to the monoclonal gammopathy.
Figure 1.
Kidney biopsy findings. Light microscopy shows a nodular sclerosing GN. (A) Hematoxylin and eosin, ×10. (B) Periodic acid–Schiff, ×40. (C) Silver methenamine, ×40. (D) Congo red, ×40. Congo red stain is negative for amyloid. Immunofluorescence microscopy showing negative staining for IgG, λ- and κ-light chains ([E] IgG, ×40; [F] λ, ×40; and [G] κ-light chains, ×40), and moderate (2+) granular mesangial and capillary wall staining for C3 ([H] ×40). Electron microscopy showing powdery dense deposits in the mesangium (thick arrow) and along the glomerular and tubular basement membranes (thin arrows). Original magnification, ×2900 in I, ×12,600 in J, ×11,000 in K, ×1400 in L.
Laser Microdissection and Mass Spectrometry
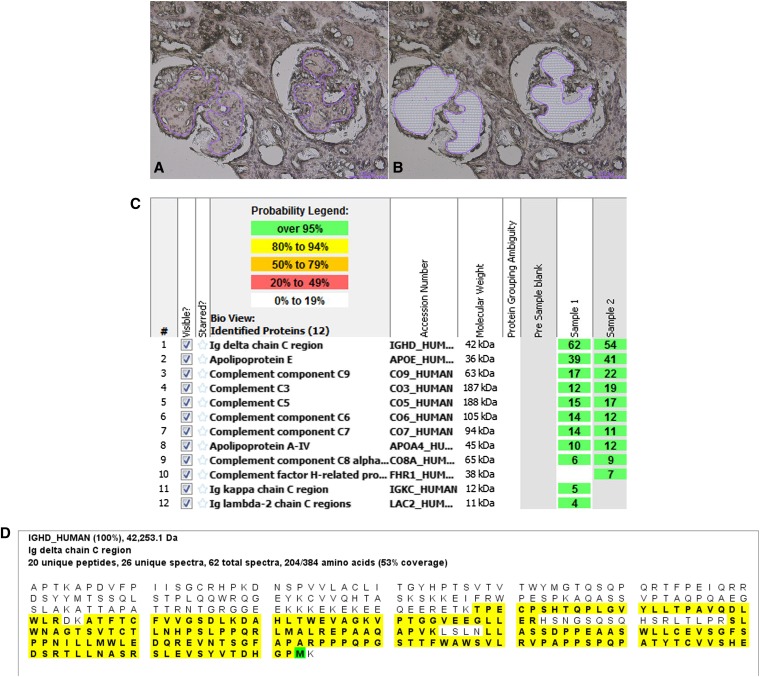
To determine the composition of the glomerular deposits, we performed liquid chromatography and tandem mass spectrometry (MS) on peptides extracted from the glomeruli after laser microdissection (LMD) of the paraffin-embedded material. LMD/MS analysis of the amyloid has been previously described.1,2 Briefly, the glomeruli were microdissected by using laser capture techniques. Two samples (sample 1 and 2) were dissected, and each sample contained at least two glomeruli. Peptides extracted from the microdissected tissue were then subjected to liquid chromatography MS. MS raw data files were queried using three different algorithms (Sequest, Mascot, and X!Tandem). The results were combined and assigned peptide and protein probability scores in Scaffold (Proteome Software Inc., Portland, OR). For each sample, a list of proteins on the basis of peptides identified by MS was generated. The MS data show spectra that match to a particular protein on the basis of the amino acid sequence available in the database. Some of the peptides from different proteins can be common and shared depending on the homology of their amino acid sequence. However, unique peptides and spectra are distinctive to the particular protein. The Spectra value indicates the total number of mass spectra collected by MS and matched to the protein using the proteomics software. A higher number of mass spectra is indicative of greater abundance and will typically yield greater amino acid sequence coverage. A higher mass spectra value also indicates a higher confidence in the protein identification. Peptide identifications were accepted if they could be established at >90.0% probability as specified by the Peptide Prophet algorithm.3,4 By LMD/MS, we identified large spectra numbers for the Ig-δ (IgD) C region (average spectra number of 58) and moderate spectra numbers of factors of complement factors, indicating activation and accumulation of components of the classic and terminal pathways of complement. Taken together with the renal biopsy findings, the data supported the diagnosis of IgD heavy-chain deposition disease. The LMD/MS findings are shown in Figure 2.
Figure 2.
Laser microdissection and mass spectrometry results. LMD/MS. Light microscopy showing (A) glomeruli marked for dissection and (B) empty space after microdissection (hematoxylin and eosin, ×20). (C) LMD/MS results. Scaffold 2 display of proteomic data. The figure shows proteomics data from two microdissected samples (samples 1 and 2) from the biopsy showing the presence of large spectra numbers for IgD. Apolipoproteins E and A-IV are also present. In addition, spectra for C9, C3, C5, C6, and C7 are also detected. The probability number (>95% is highlighted by green, and 80%–94% is highlighted by yellow) indicates essentially the percentage of homology between peptides detected in the specimens and the published amino acid sequences of their corresponding proteins. (D) Representative sequence (peptide) coverage of the IgD detected in the microdissected sample showing 62 total spectra, 26 unique spectra, and 20 unique peptides for 53% coverage of IgD protein with 100% probability. The figure shows the entire IgD peptide sequence; the peptides highlighted in yellow represent the unique peptides found in the microdissected sample that map to IgD.
Immunohistochemistry
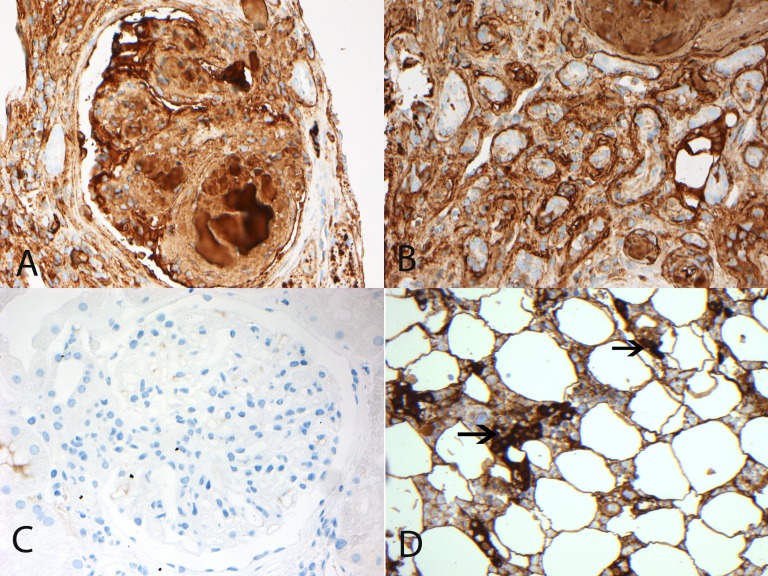
We performed immunohistochemistry staining for IgD on the paraffin-embedded material to validate the LMD/MS results. There was intense glomerular and tubular basement membrane staining for IgD (Figure 3).
Figure 3.
Immunohistochemistry staining for IgD. (A and B) Glomerular and tubular deposits of IgD. Immunohistochemistry studies showing (A) glomerular and (B) tubular staining for IgD. (C) Control case of diabetic nodular glomerulosclerosis showing no glomerular staining for IgD. (D) Immunohistochemistry showing plasma cells staining for IgD on bone marrow biopsy. Arrows point to IgD staining plasma cells. Original magnification, ×40.
Kidney Biopsy Diagnosis
The kidney biopsy diagnosis was IgD heavy-chain deposition disease.
Clinical Follow-Up
The patient was treated with subcutaneous bortezomib (1.3 mg/m2) on days 1, 8, 15, and 22 and oral dexamethasone (20 mg on the same days and days after) for 5-week cycles. Monoclonal IgD-λ was not measurable on serum protein electrophoresis, and total IgD level was not measured at time of diagnosis. However, serum λ-free light chain was significantly increased (2303 mg/L at diagnosis), the levels of which allowed for evaluation of response to treatment. Subsequent staining for IgD of bone marrow biopsy showed IgD-positive plasma cells (Figure 3D). After two cycles, there was at least a very good partial response to treatment, and serum λ-light chain level was significantly decreased to 62.2 mg/L, with a normalization of the κ:λ ratio (0.93). Although serum IgD levels were not checked at the time of diagnosis, the serum IgD was within normal limits (76 kIU/L, normal<100) after two cycles of treatment. Unfortunately, the patient’s renal function continued to decline (serum creatinine=7.45 mg/dl), and dialysis was initiated.
Discussion
Monoclonal gammopathy consists of a heterogeneous group of disorders characterized by clonal proliferation of Ig producing B lymphocytes or plasma cells.5,6 The proliferating cells secrete Ig, which can be detected in the blood or urine as monoclonal Ig (M protein). The M protein usually consists of a heavy chain and a light chain (κ- or λ-light chains), although in some instances, the neoplastic cells may synthesize only the heavy or light chain alone. The clinical hematologic spectrum is wide and includes malignancies, such as multiple myeloma and Waldenström macroglobulinemia, clonal- and paraprotein-related disorders, such as light-chain (AL) amyloidosis, and the incidentally detected premalignant plasma cell dyscrasia termed monoclonal gammopathy of undetermined significance.7,8
Renal involvement may occur as a complication of the monoclonal gammopathy. Renal accumulation of the monoclonal Ig can result in cast nephropathy, amyloidosis, monoclonal Ig deposition disease (MIDD), proximal light-chain tubulopathy, including Fanconi syndrome, and the recently described monoclonal gammopathy-associated proliferative GN.9,10 MIDD is a relatively rare complication of monoclonal gammopathy and is characterized by deposition of monoclonal Ig along the glomerular and tubular basement membranes. MIDD is classified into light-chain deposition disease, where the deposits are composed of light chains only, heavy-chain deposition disease, where the deposits are composed of heavy chains only, and light- and heavy-chain deposition disease, where the deposits are composed of both light and heavy chains. Light-chain deposition disease is the most common; the deposits are most commonly composed of κ-light chains. However, the deposits of heavy-chain deposition disease are typically composed of γ-chain and rarely, α- and μ-chains, and they often lack the C (H)1 domain.11–16
The diagnosis of MIDD is on the basis of the presence of a nodular sclerosing GN on light microscopy, diffuse staining of monoclonal light/heavy chains along the glomerular and tubular basement membranes on immunofluorescence microscopy, and powdery/punctate dense deposits along the glomerular and tubular basement membranes on electron microscopy.14 The nodular sclerosing glomerulopathy is typically present in advanced stages of the disease and not diagnostic in itself; it is the detection monoclonal Ig along the tubular and glomerular basement membranes that is pathognomonic for the diagnosis of MIDD.
We describe a patient with monoclonal gammopathy presenting with proteinuria and renal failure whose kidney biopsy was difficult to characterize. The kidney biopsy showed a nodular sclerosing GN, immunofluorescence studies showed no Ig deposits, and electron microscopy revealed powdery deposits along the glomerular and tubular basement membranes. Taken together with monoclonal Ig noted in serum and urine, the findings were suggestive of an Ig deposition disease, although immunofluorescence studies were negative for Ig deposits. To characterize the deposits, we microdissected the glomeruli and analyzed the glomerular proteins by MS, which showed large spectra for IgD. Furthermore, IgD deposition was confirmed by immunohistochemistry. Taken together with kidney biopsy findings, this represents a unique case of IgD heavy-chain deposition disease. To the best of our knowledge, there has been one previously reported autopsy case of nodular sclerosing glomerulopathy in the setting of an IgD myeloma.17 Our case is unique and highlights several important points.
IgD and Monoclonal Gammopathy
IgD monoclonal gammopathy is not detected on routine immunofixation studies, because antisera to IgD is not used in the test. However, IgD can be detected by immunofixation or immunodiffusion using antisera to IgD.18 IgD monoclonal gammopathy is extremely rare.8,18 Although IgD heavy-chain deposition associated with an IgD monoclonal gammopathy has not been described, both IgD myeloma and IgD amyloidosis have been described in the literature. IgD myeloma is rare and accounts for <2%–3% of myelomas.19–21 Patients with IgD myeloma tend to present more often with features of advanced disease, significant renal dysfunction, and large amounts of Bence Jones proteinuria. IgD amyloidosis is also rare, and in two recent large studies, serum IgD monoclonal protein was identified in <1% of patients with AL amyloidosis.18,22 Gertz et al.18 and Roussel et al.22 suggested a lower frequency of renal and cardiac involvement in IgD amyloidosis, although the overall survival of patients was similar to AL amyloidosis.
Nodular Sclerosing GN
IgD heavy-chain disease poses a diagnostic dilemma, because routine immunofluorescence studies do not include antibodies to detect IgD. IgD heavy-chain deposition appears as nodular sclerosing GN, and the presence of powdery deposits along the glomerular and tubular basement membranes should raise the possibility of an unusual light-/heavy-chain deposition disease in the absence of positive immunofluorescence studies. Differential diagnosis of nodular glomerulosclerosis includes diabetic nodular glomerulosclerosis, amyloidosis, light- and heavy-deposition diseases, chronic membranoproliferative GN, chronic thrombotic microangiopathy, and idiopathic nodular glomerulosclerosis related to chronic hypertension and smoking.23 Amyloidosis, light- and heavy-deposition diseases, and membranoproliferative GN in the setting of monoclonal gammopathy may be related to monoclonal Ig deposition. The kidney biopsy findings are distinctive in each entity, but all of which show the monoclonal Ig on immunofluorescence studies. However, nodular glomerulosclerosis noted in diabetes, chronic hypertension, and smoking and chronic thrombotic microangiopathy are negative on immunofluorescence studies. The presence of a monoclonal Ig on serum electrophoresis/immunofixation studies and negative kidney biopsy immunofluorescence studies should raise suspicion for an unusual Ig, such as a truncated Ig or Ig not evaluated by immunofluorescence studies. The correct clinical context and laboratory evaluation are extremely important in determining the underlying etiology in these cases.
LMD/MS
LMD/MS is new technique used as an ancillary study in the diagnosis of kidney diseases.24 It has found great use in the diagnosis and typing of amyloidosis, including detection of novel types of amyloidosis.25–29 Recently, the technique has also been used to determine the composition of deposits in proliferative GN, including immune complex-mediated GN and complement-mediated GN.2,30,31 Because conventional immunofluorescence microscopy was not helpful, we used LMD/MS to determine the composition of the deposits in our patient, and to our surprise, we found very large spectra numbers for IgD. Apolipoprotein E was also present and likely represents a component of the deposits. It is often present in deposits with a fibrillary substructure.26 In this case as well, a vague fibrillary substructure was noted on electron microscopy. To confirm the LMD/MS findings, we then stained the paraffin-embedded kidney biopsy material for IgD, which showed intense glomerular and tubular basement membrane staining for IgD. Surprisingly, there were only very small spectra numbers for light chains, indicating that it was primarily IgD heavy-chain disease and not mixed heavy- and light-chain deposition disease. This was substantiated by the negative immunofluorescence microscopy study for either κ- or λ-light chains. LMD/MS did not detect serum amyloid protein, thus ruling out amyloidosis and confirming the negative Congo red findings.
An interesting finding on LMD/MS was the presence of complement factors of the classic/lectin and terminal pathways of complement, suggesting activation of the classic or lectin pathway of complement by the IgD heavy chain. This was supported by the granular deposits of C3 noted on immunofluorescence studies. In one study, it was shown that IgD had complement fixing capabilities in a patient with IgD myeloma.32 Interestingly, IgD has also been shown to bind to T cell surface lectins.33 Thus, it is possible that the complement factors noted in the biopsy may be derived as a result of IgD activation of the classic or lectin pathway.
Treatment and Follow-Up
Treatment of IgD deposition disease with chemotherapy resulted in improvement of hematologic parameters, but the kidney function did not improve. This is likely because of the extensive chronic changes that had already set in by the time of diagnosis. Thus, early recognition, diagnosis, and treatment of IgD deposition disease are key factors that may help preserve renal function.
In summary, we present a patient with a unique case of IgD deposition disease who presented with nodular sclerosing GN with negative routine immunofluorescence microscopy. Evaluation of the kidney biopsy by LMD/MS revealed the accurate diagnosis of IgD deposition disease. IgD deposition disease seems to be a progressive disease that results in extensive focal global glomerulosclerosis, tubular atrophy, and interstitial fibrosis.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A: Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, 3rd, Zipfel PF, Dogan A, Smith RJ: Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int 75: 952–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi NH, Nakano Y, Tobe T, Mazda T, Tomita M: Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol 2: 413–417, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Nesvizhskii AI, Keller A, Kolker E, Aebersold R: A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ, 3rd: A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 346: 564–569, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Rajkumar SV: Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23: 3–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Dispenzieri A, Kyle RA: Monoclonal gammopathy of undetermined significance, Waldenström macroglobulinemia, AL amyloidosis, and related plasma cell disorders: Diagnosis and treatment. Mayo Clin Proc 81: 693–703, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ, 3rd: Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354: 1362–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bida JP, Kyle RA, Therneau TM, Melton LJ, 3rd, Plevak MF, Larson DR, Dispenzieri A, Katzmann JA, Rajkumar SV: Disease associations with monoclonal gammopathy of undetermined significance: A population-based study of 17,398 patients. Mayo Clin Proc 84: 685–693, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi S, Rajkumar SV: Monoclonal gammopathy-associated proliferative glomerulonephritis. Mayo Clin Proc 88: 1284–1293, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Kambham N, Markowitz GS, Appel GB, Kleiner MJ, Aucouturier P, D’agati VD: Heavy chain deposition disease: The disease spectrum. Am J Kidney Dis 33: 954–962, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Moulin B, Deret S, Mariette X, Kourilsky O, Imai H, Dupouet L, Marcellin L, Kolb I, Aucouturier P, Brouet JC, Ronco PM, Mougenot B: Nodular glomerulosclerosis with deposition of monoclonal immunoglobulin heavy chains lacking C(H)1. J Am Soc Nephrol 10: 519–528, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, D’Agati VD, Leung N: Renal monoclonal immunoglobulin deposition disease: A report of 64 patients from a single institution. Clin J Am Soc Nephrol 7: 231–239, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D’Agati VD: Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol 12: 1482–1492, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Alexander MP, Nasr SH, Watson DC, Méndez GP, Rennke HG: Renal crescentic alpha heavy chain deposition disease: A report of 3 cases and review of the literature. Am J Kidney Dis 58: 621–625, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Herrera GA: Renal lesions associated with plasma cell dyscrasias: Practical approach to diagnosis, new concepts, and challenges. Arch Pathol Lab Med 133: 249–267, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kubo Y, Morita T, Koda Y, Hirasawa Y: Two types of glomerular lesion in non-amyloid immunoglobulin deposition disease: A case report of IgD-lambda myeloma. Clin Nephrol 33: 259–263, 1990 [PubMed] [Google Scholar]
- 18.Gertz MA, Buadi FK, Hayman SR, Dingli D, Dispenzieri A, Greipp PR, Kumar SK, Lacy MQ, Lust JA, Leung N, Rajkumar SV, Russell SJ, Zeldenrust SR, Mikhael JR, Roy V, Kyle RA: Immunoglobulin D amyloidosis: A distinct entity. Blood 119: 44–48, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Zagouri F, Kastritis E, Symeonidis AS, Giannakoulas N, Katodritou E, Delimpasi S, Repousis P, Terpos E, Dimopoulos MA, Greek Myeloma Study Group : Immunoglobulin D myeloma: Clinical features and outcome in the era of novel agents. Eur J Haematol 92: 308–312, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Bladé J, Kyle RA: Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 13: 1259–1272, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Jancelewicz Z, Takatsuki K, Sugai S, Pruzanski W: IgD multiple myeloma. Review of 133 cases. Arch Intern Med 135: 87–93, 1975 [PubMed] [Google Scholar]
- 22.Roussel M, Sachchithanantham S, Gibbs SDJ, Venner CP, Pinney JH, Gillmore JD, Lachmann HJ, Hawkins PN, Wechalekar AD: Clinical profile and treatment outcomes of immunoglobulin D associated AL amyloidosis. Br J Haematol 162: 856–858, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Nasr SH, D’Agati VD: Nodular glomerulosclerosis in the nondiabetic smoker. J Am Soc Nephrol 18: 2032–2036, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Sethi S, Vrana JA, Theis JD, Dogan A: Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens 22: 273–280, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Sethi S, Vrana JA, Theis JD, Leung N, Sethi A, Nasr SH, Fervenza FC, Cornell LD, Fidler ME, Dogan A: Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int 82: 226–234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi S, Theis JD, Vrana JA, Fervenza FC, Sethi A, Qian Q, Quint P, Leung N, Dogan A, Nasr SH: Laser microdissection and proteomic analysis of amyloidosis, cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy. Clin J Am Soc Nephrol 8: 915–921, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sethi S, Theis JD, Shiller SM, Nast CC, Harrison D, Rennke HG, Vrana JA, Dogan A: Medullary amyloidosis associated with apolipoprotein A-IV deposition. Kidney Int 81: 201–206, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Sethi S, Theis JD, Quint P, Maierhofer W, Kurtin PJ, Dogan A, Highsmith EW, Jr.: Renal amyloidosis associated with a novel sequence variant of gelsolin. Am J Kidney Dis 61: 161–166, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Sethi S, Theis JD, Leung N, Dispenzieri A, Nasr SH, Fidler ME, Cornell LD, Gamez JD, Vrana JA, Dogan A: Mass spectrometry-based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin J Am Soc Nephrol 5: 2180–2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ: C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain D, Green JA, Bastacky S, Theis JD, Sethi S: Membranoproliferative glomerulonephritis: The role for laser microdissection and mass spectrometry. Am J Kidney Dis 63: 324–328, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Konno T, Hirai H, Inai S: Studies in IgD—I. Complement fixing activities of IgD myeloma proteins. Immunochemistry 12: 773–777, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Rudd P, Fortune F, Lehner T, Parekh R, Patel T, Wormald M, Malhotra R, Sim R, Dwek R: Lectin-carbohydrate interactions in disease. T-cell recognition of IgA and IgD; mannose binding protein recognition of IgG0. Adv Exp Med Biol 376: 147–152, 1995 [PubMed] [Google Scholar]