Abstract
Objective:
The aim was to study and identify free radicals scavenging and antihyperglycemic principles in fruit of Piper attenuatum.
Materials and Methods:
Bioassay guided identification of extracts possessing potent free radical scavenging activity, and isolation of compounds was done. Chloroform extract of P. attenuatum possessing potent radical scavenging activity was also evaluated for antihyperglycemic activity following oral glucose tolerance test in rats.
Results:
Nine neolignans namely, denudatin B (1), iso-4’, 5’-dimethoxy-3, 4-methylenedioxy-2’-oxo-Δ3’,5’,8’-8.1’-lignan (2), lancifolin D (3), denudatin A (4), wallichinin (5), piperenone (6), lancifolin C (7), 2-oxo-piperol B (8), piperkadsin A (9) and a crotepoxide (10) was identified in Chloroform extract of P. attenuatum. Neolignans (1-9) displayed potent 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical and piperkadsin A (9) also displayed 1, 1-diphenyl-2-picrylhydrazyl radical scavenging activity. Analysis of structure-activity relationship revealed that presence of furan ring and methoxy groups is an important criterion to influence 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging potentials. Chloroform extract of P. attenuatum fruit could not display antihyperglycemic activity following oral glucose tolerance test in rats.
Conclusion:
Neolignans present in P. attenuatum fruits are potent free radical scavengers and this is the first report identifying these compounds and activities in this fruit.
Keywords: 2, 2’-Azinobis (3-ethyl benzthiazoline-6-sulphonic acid) radical; chloroform extract; 2, 2-Diphenyl-1-picrylhydrazyl radical; glucose tolerance test; Piper attenuatum
INTRODUCTION
Piper species have been used in traditional medicinal systems for thousands of years, including Chinese and Indian systems, as well as in folklore medicines of Latin America and West Indies.[1] For nearly five decades, investigations into natural products chemistry of genus Piper have led to identification of structurally and pharmacologically important scaffolds that have spurred novel synthetic methodology and inspired many chemists and biochemists to speculate on plausible biogenetic relationships among these diverse skeletal.[2] Efforts on phytochemical investigations of medicinal plants from genus Piper in Piperaceae family from our laboratory has resulted in identification and isolation of the series of structurally intriguing compounds with potential biological activities.[3] Piper attenuatum, also known as oval leaved pepper[4] is advocated in traditional medicines as rubefacient, effective for headache and muscular pains. Roots macerated in water are used as diuretic.[5] However, chemical compositions and relevant biological activities in this medicinal plant have not yet been explored.
In this communication, we reported isolation of compounds in P. attenuatum fruit and evaluated their free radical scavenging and the antihyperglycemic activity. Based on free radicals scavenging potentials and structural characteristics, structure-activity relationship of compounds is discussed.
MATERIALS AND METHODS
General procedures
The solvents used were all of AR grade and were distilled under positive pressure of dry nitrogen atmosphere where necessary. Thin-layer chromatography (TLC) performed on Merck silica gel 60 F254 plates. Visualization was performed using with 5% H2SO4 solution followed by heating. Column chromatography was performed on silica gel (60–120 mesh). Melting points were recorded on Fisher Johns apparatus and were uncorrected. FABMS was recorded on VG Auto Spec-M instrument, USA. IR spectra were recorded on Nicolet spectrometer. 1H NMR and 13C NMR spectra obtained on Varian 200, 400 MHz and Bruker 300 MHz spectrometers using tetramethylsilane as an internal standard. HMBC, HSQC and NOSEY experiments were done on Oxford 500 MHz spectrometer.
Plant material
The fruits of P. attenuatum were collected from Tirumala, Chitoor Dist. (Andhra Pradesh, India) in the month of November 2011 and identification was made by Prof. K. Madhava Chetty, Department of Botany, Sri Venkateswara University, Tirupati. A voucher specimen of the plant is deposited in the herbarium, Department of Botany with an accession number 526.
Extraction and isolation
The dried plant material (5 kg) was powdered and extracted with chloroform by cold maceration for 2 days. The resulting chloroform extract was evaporated to dryness under reduced pressure, affording syrupy residue (12 g). Then this chloroform extract was subjected to column chromatography on a silica gel column (60–120 mesh, 150 cm × 15 cm) and eluted with a stepwise gradient of hexane-EtOAc (99:1, 98:2, 92:8, 90:10, 88:12 by volume) to afford a total of 102 fractions of 50 ml each. Column fractions were analyzed by TLC (silica gel 60 F254, hexane/EtOAc, 85:15), and fractions with similar TLC patterns were combined to give eight major fractions (F1–F8). Fraction F2 was further purified using ether/hexane (15:85) to give 1 (32 mg). Fraction F3 (32 g) was chromatographed on silica gel (100-200 mesh) using EtOAc/hexane (2:98) to give 2 (22 mg) and 3 (18 mg). Fraction F4 (36 g) was purified by CC on silica gel (100–200 mesh) using acetone/hexane (5:95%) to give 4 (21 mg). Fraction F5 was purified by CC two give subfractions A and B. Sub fraction A was further purified on silica gel (230–400 mesh) using CHCl3/ether (95:5) to give 5 (33 mg). Sub fraction B was purified by HPLC using Atlantis DC-18 column (150 mm × 4.6 mm [5 μ]) and H2O/CH3OH (30:70) as eluents to give 6 (10 mg) and 7 (12 mg). Fraction F6 was purified by CC on silica gel G (230–400 mesh) using ether/CHCl3 to give pure 8 (8 mg) and 9 (16 mg). Fraction F7 was dissolved in EtOAc/hexane (1:1), which resulted in the formation of crystals that were then filtered and washed with hexane to give 10 (4.2 g).
Determination of free radicals scavenging antioxidant potentials
2, 2’-Azinobis (3-ethyl benzthiazoline-6-sulphonic acid) radical scavenging assay
Scavenging of 2, 2’-Azinobisn (3-ethyl benzthiazoline-6-sulphonic acid) cation was performed as described by Walker and Everett[6] with suitable modifications. Briefly, 100 mL stock solution of ABTS+ (0.5 mM) was prepared by addition of 1 mL potassium persulfate [6.89 mM in PBS (pH 8.0)]. Mixture was stored in the dark for 16 h. 10 μL of test sample (5 mg/mL in dimethyl sulfoxide [DMSO]) was added to 190 μL of ABTS+ in 96-well microplate. Absorbance was recorded at 734 nm on BioTeksynergy4 multi-mode microplate reader. Trolox was taken as a standard. For the estimation of ABTS+ scavenging concentration 50% (SC50) various dilutions of the test samples were prepared and end-point absorbance (734 nm) was measured after 15 min incubation in the dark. Percentage scavenging of ABTS+ by test samples was calculated as follows ([Absorbance control − absorbance test]/absorbance control) × 100. Suitable regression analysis was applied for calculation of SC50.
2, 2-Diphenyl-1-picrylhydrazyl radical scavenging
Assay for scavenging of free radical 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) was done as reported earlier.[7] Briefly, in a 96-well microplate, 20 μL of test sample (5 mg/mL in DMSO), 100 μL of 0.1M tris-HCl buffer (pH 7.4) and 100 μL DPPH solution (0.5 mM in methanol) were added. The reaction mixture was shaken well. DPPH decolorization was recorded (517 nm) on BioTeksynergy4 multi-mode microplate reader. Percentage scavenging of DPPH by test samples was calculated as described above.
Animal experiment
Male Wistar rats weighing between 180-210 g were procured from the National Institute of Nutrition ((CPCSEA Reg.No. 154, Government of India), Hyderabad and housed in IICT animal house (temperature 22°C ± 1°C and relative humidity 50% ±15% with an alternating 12 h light and dark cycle). Food and water were provided ad libitum. Experimental protocols were approved by Institutional Animal Ethical Committee.
Oral glucose tolerance test
Influence of chloroform extract on time-dependent plasma glucose levels following single dose oral glucose feeding was evaluated adopting the method reported earlier.[7] In brief, rats were kept overnight fasting. Next day morning blood was collected from retro-orbital plexus in ethylenediaminetetraacetic acid containing tubes and plasma glucose levels for basal ‘0’ h values were measured by glucose-oxidase method using Auto blood analyzer instrument (Bayer Express Plus, USA). Rats were divided into three groups viz. Control, Test and Standard group (six rats in each group). Test group received crude chloroform extract of P. attenuatum fruits suspended in acacia and distilled water orally (250 mg/kg body weight). The control group received only distilled water. The standard group received standard drug glibenclamide orally (5 mg/kg body weight). After 30 min, all the animals were administered glucose dissolved in distilled water (2 g/kg body weight). Blood was collected at the intervals of 30, 60, 90 and 120th min postglucose feeding. Plasma glucose levels were measured as described above.
Statistical analysis
Statistical analysis was carried out using Graph Pad Prism 5 software (San Diego, California, USA). Data were compared applying one-way ANOVA followed by Dunnett's multiple comparison tests. Values were considered as statistically significant at P < 0.05.
RESULTS AND DISCUSSION
Nine neolignans (1-9) and crotepoxide (10) were isolated from crude chloroform extract of P. attenuatum. After comparing their physicochemical and spectrometric data with those reported in the literature, they were identified as known compounds and confirmed as denudatin B(1) iso-4’, 5’-dimethoxy-3, 4-methylenedioxy-2’-oxo-Δ3’,5’,8’-8.1’-lignan (2),[8,9] lancifolin D (3),[10] denudatin A (4),[11] wallichinine (5),[12] piperenone (6),[13] lancifolin C (7),[12] 2-oxo-piperol B (8),[14] piper kadsin A (9),[15] and crotepoxide (10).[16] Structure of these compounds is shown in [Figure 1].
Figure 1.
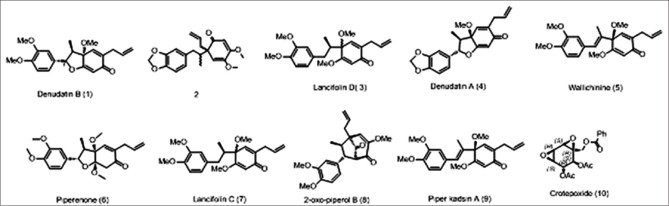
Compounds isolated from fruits of Piper attenuatum
Spectral data
Denudatin B (1)
Oil (32 mg); C21H24O5 ESI-MS m/z 357.2 (M+ + H) [α]D24 + 8.0 (c 0.40, CHCl3); IR (KBr) νmax cm− 1: 1671, 1643, 1624, 1517, 1459, 1370, 1269, 1239, 1186, 1139, 1067, 1029; 1H NMR (300 MHz, CDCl3): δ 1.12 (3H, d, J 7.5 Hz, H-9), 2.69 (1H, q, J 7.5, 7.3 Hz, H-8), 3.04 (3H, s, OMe-3'), 3.15 (2H, d, J 6.6 Hz, H-7'), 3.89 and 3.90 (3H each, s, OMe-3 and OMe-4), 5.09 (1H, s, Ha-9'), 5.13 (1H, d, J 4.3 Hz, Hb-9'), 5.24 (1H, s, H-7), 5.84–5.87 (1H, m, H-8'), 5.88 (1H, s, H- 5'), 6.21 (1H, s, H-2'), 6.82–6.91 (2H, m, H-2 and H-5) and 7.01 (1H, d, J 2.0 Hz, H-6). 13C NMR (75 MHz, CDCl3): δ 16.2 (C-9), 33.3 (C-7'), 46.8 (C-8), 50.4 (OMe-3'), 55.74 (OMe-3 and OMe-4), 81.1 (C-3'), 94.6 (C-7), 104.6 (C-5'), 108.9 (C-2), 110.6 (C-5), 117.2 (C-6), 118.1 (C-9'), 131.8 (C-1), 132.6 (C-2'), 135.1 (C-8'), 142.7 (C-1'), 148.6 and 148.8 (C-4 and C-3) 173.0 (C-4') and 187.0 (C-6').
Iso-4', 5'-dimethoxy-3, 4-methylenedioxy-2'-oxo-Δ3' 5', 8'-8.1'-lignan (iso-6- [2-(1, 3 benzodioxol-5-yl)-1-methylethyl]-3,4-dimethoxy-6-(2-propenyl)-2,4-cyclohexadien-1-one) (2)
Pale yellow oil (22 mg); C21H24O5 ESI-MS m/z 357.2 (M+ + H); [α]D24 + 0.8 (c 0.12, CHCl3); IR (KBr) νmax cm− 1: 1736, 1662, 1632, 1608, 1580, 1490, 1449, 1405, 1243, 1230, 1171 and 1037; 1H NMR (300 MHz, CDCl3): δ 0.88 (3H, d, J 6.9 Hz, H-9), 2.00 (1H, dd, J 6.0, 5.3 Hz, Ha-7), 2.25–2.41 (2H, m, H-8 and Ha-7'), 2.49–2.62 (2H, m, Hb-7 and Hb-7'), 3.76 (3H, s, OMe-4'), 3.82 (3H, s, OMe-3'), 4.90–5.05 (2H, m, H-9'), 5.24 (1H, s, H-5'), 5.57 (1H, s, H-2'), 5.45–5.55 (1H, m, H-8'), 5.90 (2H, s, OCH2O), 6.53 (1H, dd, J 6.0, 1.5 Hz, H-6), 6.60 (1H, d, J 1.5 Hz, H-2) and 6.70 (1H, d, J 8.3 Hz, H-5); 13C NMR (75 MHz, CDCl3): δ 12.7 (C-9), 38.3 (C-7), 43.6 (C-7'), 44.7 (C-8), 55.4 (OMe-4'), 56.5 (OMe-5'), 56.6 (C-1'), 100.7 (OCH2O), 103.1 (C-5'), 107.8 (C-2'), 109.0(C-2), 109.5 (C-5), 117.8 (C-9'), 121.9 (C-6), 132.9 (C-8'), 134.5 (C-1), 145.5 (C-3'), 147.4 (C-3), 147.8 (C-4), 166.2 (C-4') and 203.5 (C-6').
Lancifolin D (3)
Oil (18 mg); C22H28O5 ESI-MS m/z: 373.2 (M+ + H), 395.2 (M+ + Na); [α]D24 − 12.5 (c 0.08, CHCl3); IR (KBr) νmax cm− 1: 1725, 1662, 1632, 1608, 1514, 1459, 1261, 1225, 1157, 1079, 1029. 1H NMR (300 MHz, CDCl3): δ 0.99 (3H, d, J 6.7 Hz, H-9), 1.91 (1H, d, J 13 Hz, Ha-7), 2.07 (1H, dd, J 13, 5 Hz, Hb-7), 2.37–2.48 (1H, m, H-8), 3.12 (3H, s, 3'-OMe), 3.16 (2H, d, J 6.6 H-7'), 3.72 (3H, s, OMe-4'), 3.84 (6H, s, OMe-3 and OMe-4), 5.12–5.19 (2H, m, H-9'), 5.70 (1H, s, H-5'), 5.8–6.0 (1H, m, H-8'), 6.34 (1H, s, H-2'), 6.54-6.61 (2H, m, H-2 and H-5) and 6.74 (1H, d, J 7.7 Hz, H-6). 13C NMR (75 MHz, CDCl3): δ 13.9 (C-9), 33.0 (C-7'), 37.2 (C-7), 42.5 (C-8), 52.4 (OMe-3'), 55.7(OMe-4'), 55.9 (OMe-3 and OMe-4), 79.7 (C-3'), 105.8 (C-5'), 110.9 (C-2), 111.9 (C-5), 117.1 (C-9'), 120.9 (C-6), 132.7 (C-1), 135.1 (C-8'), 139.1 (C-2'), 141.1 (C-1'), 147.2 (C-4), 148.6 (C-3), 173.0 (C-4') and 186.3 (C-6').
Denudatin A (4)
Oil (21 mg); C20H20O5, ESI-MS m/z: 341 (M+ + H), 363 (M+ + Na); [α]D24 + 11.6 (c 0.12, CHCl3); IR (KBr) νmax cm− 1: 1668, 1622, 1492, 1448, 1250, 1216, 1176, 1041; 1H NMR (300 MHz, CDCl3): δ 1.12 (3H, d, J 7.0 Hz, H-9), 2.11–2.16 (1H, m, H-8), 3.12 (3H, s, OMe-3'), 3.14–3.24 (2H, m, H-7'), 5.07–5.18 (2H, m, H-9'), 5.30 (1H, d, J 9.4 Hz, H-7), 5.81 (1H, s, H-5'), 5.84–5.93 (1H, m, H-8'), 5.98 (2H, s, OCH2O), 6.25 (1H, br s, H-2') and 6.74–6.83 (3H, m, H-2, H-5, H-6). 13C NMR (75 MHz, CDCl3): δ 6.7 (C-9), 33.5 (C-7'), 50.7 (C-8), 51.1 (OMe-3'), 77.7 (C-3'), 91.3 (C-7), 101.3 (OCH2O),102.8 (C-5'), 106.7 (C-2), 108.2 (C-5), 117.2 (C-9'), 120.9 (C-6), 131.1 (C-2'), 131.2 (C-1), 135.1 (C-8'), 143.0 (C-1'), 148.2 (C-4), 148.3 (C-3), 174.5 (C-4') and 187.0 (C-6').
Wallichinine (5)
Oil (33 mg); C22H28O5, ESI-MS (m/z): 371 (M+ + H), 393 (M+ + Na); [α]D24 − 4.7 (c 0.40, CHCl3); IR (KBr) νmax cm− 1: 1744, 1659, 1637, 1608, 1513, 1457, 1353, 1256, 1227, 1156, 1081, 1026; 1H NMR (300 MHz, CDCl3): δ 1.68 (3H, s, H-9), 3.10–3.18 (2H, m, H-7'), 3.25 (3H, s, OMe-3'), 3.80 (3H, s, OMe-4'), 3.86 (3H, s, OMe-3), 3.88 (3H, s, OMe-4), 5.04–5.20 (2H, m, H-9'), 5.82 (1H, s, H-5'), 5.84–5.96 (1H, m, H-8'), 6.16 (1H, s, H-2'), 6.77–6.90 (3H, m, H-2, H-5 and H-6) and 6.96 (1H, s, H-7). 13C NMR (75 MHz, CDCl3): δ 14.1 (C-9), 32.7 (C-7'), 52.0 (OMe-3'), 55.36 (OMe-3), 55.38 (OMe-4), 55.7 (OMe-4'), 105.2 (C-5'), 110.2 (C-2), 112.0 (C-5), 116.6 (C-9'), 121.1 (C-6), 126.6 (C-7), 129.8 (C-1), 132.0 (C-8), 134.5 (C-8'), 139.1 (C-1'), 140.7 (C-2'), 147.3 (C-3), 147.9 (C-4), 171.8 (C-4') and 186.6 (C-6').
Piperenone (6)
Oil (10 mg) C22H28O6, ESI-MS (m/z): 411 (M+ + Na); [α]D24−-30.0(c 0.04, CHCl3); IR (KBr) νmax cm− 1: 1738, 1686, 1514, 1462, 1374, 1310, 1264, 1236, 1140, 1108, 1029; 1H NMR (300 MHz, CDCl3): δ 0.98 (3H, d, J 6.8 Hz H-9), 2.64 (1H, d, J 16.6 Hz, Ha-7'), 2.76–3.00 (1H, m, H-8), 3.12 (2H, d, J 6.8 Hz, H-5'), 3.28 (1H, d, J 16.6 Hz, Hb-7'), 3.40 (3H, s, OMe-3'), 3.55 (3H, s, OMe-4'), 3.85 (3H, s, OMe-3), 3.87 (3H, s, OMe-4), 4.17 (1H, d, J 10.5 Hz, H-7), 5.00–5.30 (2H, m, H-9'), 5.68–6.04 (1H, m, H-8'), 6.44 (1H, s, H-6), 6.81 (1H, s, H-2) and 6.90 (1H, s, H-5). 13C NMR (75 MHz, CDCl3): δ 8.7 (C-9), 29.2 (C-7'), 33.0 (C-8), 42.8 (C-5'), 48.5 (OMe-3'), 52.0 (OMe-4'), 55.2 (OMe-3), 55.4 (OMe-4), 81.4 (C-3'), 85.0 (C-7), 101.5 (C-4'), 109.5 (C-2), 110.3 (C-5), 117.0 (C-9'), 119.6 (C-6), 131.8 (C-1), 134.2 (C-8'), 138.1(C-2'), 142.8 (C-1'), 148.8 (C-4), 148.6 (C-3) and 193.7 (C-6').
Lancifolin C (7)
Oil (12 mg). C22H28O5 ESI-MS m/z: 373.2 (M+ + H), 395.2 (M+ +Na); [α]D24 + 95.0 (c 0.04, CHCl3); IR (KBr) νmax cm− 1: 1664, 1636, 1608, 1514, 1459, 1262, 1224, 1151; 1H NMR (300 MHz, CDCl3): δ 0.59 (3H, d, J 5.9 Hz, H-9), 2.05 (1H, d, J 12.9 Hz, Ha-7), 2.25–2.36 (1H, m, H-8), 3.12–3.16 (2H, m, H-7'), 3.17 (3H, s, OMe-3'), 3.38 (1H, d, J 12.9 Hz, Hb-7), 3.76 (3H, s, OMe-4'), 3.84 (3H, s, OMe-3), 3.87 (3H, s, OMe-4), 5.09–5.19 (2H, m, H-9'), 5.73 (1H, s, H-5'), 5.84–5.95 (1H, m, H-8'), 6.34 (1H, s, H-2') and 6.66–6.84 (3H, m, H-2, H-5 and H-6). 13C NMR (75 MHz, CDCl3): δ 13.5 (C-9), 33.1 (C-7'), 36.7 (C-7), 43.4 (C-8), 52.7 (OMe-3'), 55.9 (OMe-4'), 55.9 (OMe-3), 56.0 (OMe-4), 80.3 (C-3'), 105.3 (C-5'), 111.0 (C-2), 112.2 (C-5), 117.1 (C-9'), 121.3 (C-6), 133.4 (C-1), 135.2 (C-8'), 138.7 (C-2'), 141.5 (C-1'), 147.3 (C-4), 148.8 (C-3), 173.2 (C-4') and 186.4 (C-6').
2-oxo-piperol B (8)
Oil (8 mg); C21H24O5, ESI-MS (m/z): 357 (M+ + H); [α]D24−-4.0 (c 0.32, CHCl3);; IR (KBr) νmax cm− 1: 3305, 1761, 1695, 1603, 1516, 1459, 1261, 1163, 1145, 1026; 1H NMR (300 MHz, CDCl3): δ 0.99 (3H, d, J 6.8, H-9), 2.08 (1H, q, J 6.79, 13.5 Hz, H-8), 2.20–2.44 (1H, m, H-7), 2.46–2.64 (2H, m, H-7'), 3.55 (1H, d, J 1 Hz, H-3'), 3.73 (3H, s, OMe-3), 3.84 (3H, s, OMe-4), 3.86 (3H, s, OMe-5'), 5.04–5.28 (1H, m, H-9'), 5.78 (1H, s, H-6'), 5.84–6.08 (1H, m, H-8'), 6.52 (1H, d, J 1.5 Hz, H-2), 6.64 (1H, dd, J 2.2, 6.0 Hz, H-6) and 6.80 (1H, d, J 7.5 Hz, H-5). 13C NMR (75 MHz, CDCl3): δ 13.7 (C-9), 35.0 (C-7'), 46.4 (C-8), 49.5 (C-7), 55.3 (OMe-3), 55.4 (OMe-4), 56.9 (OMe-5'), 55.4 (C-1'), 69.8 (C-3'), 109.8 (C-2), 111.0 (C-5), 117.5 (C-6'), 118.6 (C-9'), 118.8 (C-6), 132.6 (C-8'), 133.5 (C-1), 147.8 (C-3), 148.8 (C-4), 152.0 (C-5'), 189.8 (C-4') and 202.3 (C-2').
Piper kadsin A (9)
Oil (16 mg), C21H24O5, ESI-MS m/z: 357.2 (M+ +H), 379.2 (M+ +Na) [α]D24 − 12.5 (c 0.04, CHCl3); IR (KBr) νmax cm− 1: 3395, 1659, 1632, 1607, 1513, 1457, 1356, 1269, 1223, 1159, 1081; 1H NMR (300 MHz, CDCl3): δ 1.65 (3H, s, H-9), 3.09–3.17 (2H, m, H-7'), 3.25 (3H, s, OMe-3'), 3.80 (3H, s, OMe-4'), 3.88 (3H, s, OMe-3), 5.06–5.15 (2H, m, H-9'), 5.61 (1H, br s, OH-4), 5.82 (1H, s, H-5'), 5.85–6.00 (1H, m, H-8'), 6.15 (1H, s, H-2'), 6.77–6.91 (3H, m, H-2, H-5 and H-6) and 6.94 (1H, s, H-7). 13C NMR (75 MHz, CDCl3): δ 14.0 (C-9), 32.7 (C-7'), 52.5 (OMe-3'), 55.8 (OMe-3), 56.1 (OMe-4'), 79.9 (C-3'), 105.7 (C-5'), 111.8 (C-2), 114.0 (C-5), 117.0 (C-9'), 122.2 (C-6), 127.1 (C-7), 129.6 (C-1), 132.2 (C-8), 134.9 (C-8'), 139.5 (C-1'), 141.2 (C-2'), 144.4 (C-4), 146.0 (C-3), 172.3 (C-4') and 187.0 (C-6').
Crotepoxide (10)
White crystal (4.2 g), mp 146°C-148°C, [α]D26 + 190.4 (c 1.00, CHCl3); IR (KBr) νmax cm− 1: 1754, 1727, 1451, 1373, 1275, 1236, 1120, 1068, 1045, 903, 719; 1H NMR (300 MHz, CDCl3): δ 1.99 (3H, s, OAc-3), 2.12 (3H, s, OAc-4), 3.05 (1H, dd, J 1.5, 2.2 Hz, H-2), 3.38 (1H, dd, J 2.2, 3.7 Hz, H-1), 3.60 (1H, d, J 2.2 Hz, H-7), 4.16 (1H, d, J 12.1 Hz, H-6a), 4.54 (1H, d, J 12.1 Hz, H-6b), 4.94 (1H, dd, J 1.1, 7.5 Hz, H-3), 5.64 (1H, d, J 9.0 Hz, H-4), 7.47 (2H, t, J 7.5 Hz), 7.62 (1H, t, J 7.5 Hz) and 8.02 (2H, d, J 7.5 Hz). 13C NMR (75 MHz, CDCl3): δ 20.6 (C-Ac-CH3), 48.0 (C-1), 52.5 (C-2), 53.8 (C-7), 59.4 (C-5), 62.4 (C-6), 69.5 (C-4), 70.4 (C-3), 128.5, 129.2, 129.8, 133.4, 165.6, 169.5 and 169.9 (CO-Ac).
Free radicals scavenging and antioxidant activities
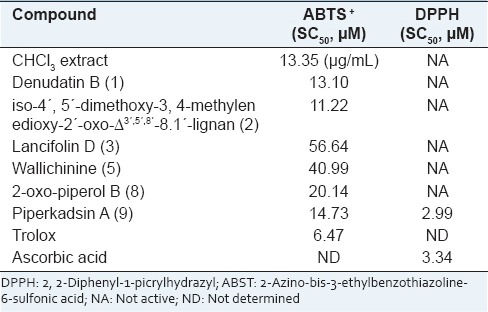
2, 2’-Azinobis (3-ethyl benzthiazoline-6-sulphonic acid) is oxidized to its radical cation ABTS+ by reaction with potassium persulphate[17] and represent peroxyl radicals. The ABTS+ cation is intensely colored, and the ability of test material to reduce its color represents radical scavenging capacity. ABTS+ cation is soluble in both aqueous and organic solvents and is not affected by ionic strength, so can be used in multiple media to determine both hydrophilic and lipophilic antioxidant capacities of extracts or body fluids.[18] The DPPH· radical is one of the few stable organic nitrogen radicals, which bears a deep purple color. It is commercially available and does not have to be generated before assay like ABTS+. This assay is also based on measurement of reducing ability of antioxidants toward DPPH· radical. Both the ABTS+ and DPPH· tests are simple to adapt and rapid in performing analyses. Hence, they are widely used in antioxidant screening. Ohlyan et al.[19] have identified the presence of ABTS+ scavenging activity and anticancer properties in various extracts of fruits of P. attenuatum. Our study finds that chloroform extract of its fruit bears potent antioxidant activity and identifies that Neolignans present in this extract are potent molecules for ABTS+ cation scavenging. However, only Piperkadsin A (9) could scaveng DPPH· radical. Figure 2a and b represents concentration dependent ABTS and DPPH radical scavenging potential of the compounds. Evaluation of free radical scavenging concentration SC50 [Table 1] revealed that all the neo-lignans displayed the ABTS+ radical scavenging activity, but only Piperkadsin A (9) could display DPPH· scavenging activity [Table 1]. The results indicated that the presence of methoxyl groups in either of the rings significantly affected compounds ABTS+ radical scavenging potential as absence of methoxyl groups in crotepoxide (10) drastically reduced its DPPH· and ABTS+ radical scavenging capacity.
Figure 2.
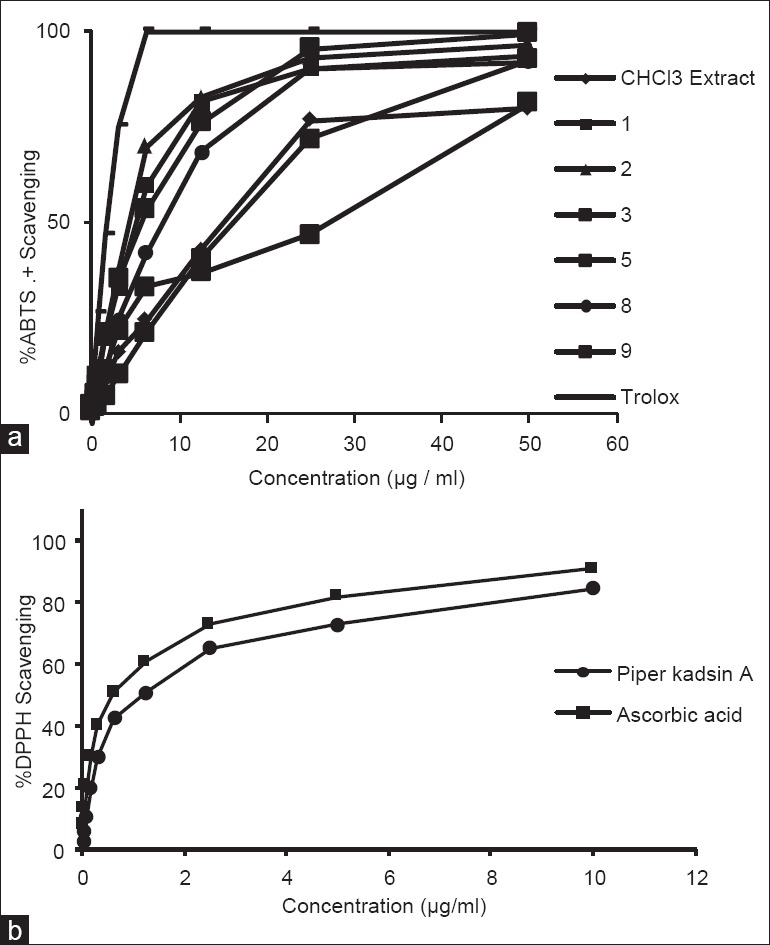
(a) Concentration dependent 2, 2’-Azinobis (3-ethyl benzthiazoline-6-sulphonic acid)+ scavenging pattern of chloroform extract of Piper attenuatum fruits and compounds isolated from the extract (b) Concentration dependent 2, 2-Diphenyl-1-picrylhydrazyl free radical scavenging pattern of piper kadsin A (9)
Table 1.
Concentrations required for scavenging ABTS+ and DPPH radicals by 50% (SC50) by the extract and compounds isolated from Piper attenuatum
Hyperglycemia is known to induce oxidative stress and increased generation of free radicals in the body.[20] For compound present in chloroform extract displayed potent free radical scavenging activity, we also studied its effect on glycemic activity by oral administration following oral glucose tolerance test in rats.[21] The results indicated that before glucose feeding could not influence glycemic values over time [Figure 3a] and overall 2 h glycemic load [Figure 3b] in rats.
Figure 3.
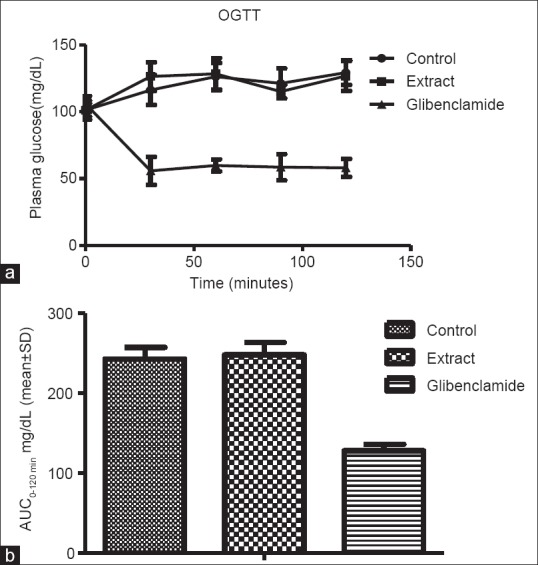
(a) Influence of chloroform extract of Piper attenuatum on postprandial plasma glucose level of rats following oral glucose tolerance test. 250 mg/kg body weight dose of extract and 5 mg/kg-body weight dose of standard antihyperglycemic drug glibenclamide was used in the study (b) Two hours glycemic loads under Influence of chloroform extract of Piper attenuatum following oral glucose tolerance test in normal rats. Area under the curve (mg/dL/hr) represent per hour postprandial glycemic load. ANOVA followed by Dunnett's multiple comparison tests was applied to compare difference between the groups
In summary, the nine neolignans (1-9) were isolated and identified for the first time from P. attenuatum fruits and displayed potent ABTS+ scavenging activity. Involvement of furan ring and R-configuration of methoxy groups present in neolignans were found major players in influencing ABTS+ scavenging potentials. Piperkadsin A (9) displayed potent DPPH· scavenging activity due to the presence of OH-group on benzene ring. The antioxidant rich chloroform extract of fruits of P. attenuatum did not display antihyperglycemic activity in rats after glucose feeding.
ACKNOWLEDGEMENTS
This work was financially supported by NaPAHA project grant CSC-0130 from the Council of Scientific and Industrial Research, New Delhi, India under CSIR-Network program.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kirtikar KR, Basu BD. III. New Delhi, India: Periodical Experts Book Agency; 1933. In: Indian Medicinal Plants; p. 2128. [Google Scholar]
- 2.Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, et al. Phytochemistry of the genus Piper. Phytochemistry. 2007;46:591–73. [Google Scholar]
- 3.Rao VR, Suresh G, Suresh Babu K, Raju SS, Vishnuvardhan MV, Ramakrishna S, et al. Novel dimeric amide alkaloids from Piper chaba Hunter: Isolation, cytotoxic activity, and their biomimetic synthesis. Tetrahedron. 2011;67:1885–92. [Google Scholar]
- 4.Pullaiah T. III. New Delhi: Regency Publications; 2006. Encyclopedia of World Medicinal Plants; p. 1539. [Google Scholar]
- 5.Khare CP. New York: Springer Science+Business Media, LLC; 2007. Indian Medicinal Plants; p. 489. [Google Scholar]
- 6.Walker RB, Everette JD. Comparative reaction rates of various antioxidants with ABTS radical cation. J Agric Food Chem. 2009;25(57):1156–61. doi: 10.1021/jf8026765. [DOI] [PubMed] [Google Scholar]
- 7.Ranga Rao R, Tiwari AK, Prabhakar Reddy P, Suresh Babu K, Ali AZ, Madhusudana K, et al. New furanoflavanoids, intestinal alpha-glucosidase inhibitory and free-radical (DPPH) scavenging, activity from antihyperglycemic root extract of Derris indica (Lam.) Bioorg Med Chem. 2009;17:5170–5. doi: 10.1016/j.bmc.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 8.Green TP, Wiemer DF. Four neolignan ketones from Piper capense. Phytochemistry. 1991;30:3759–62. [Google Scholar]
- 9.Prasad AK, Tyagi OD, Wengel J, Boll PM, Olsen CE, Gupta S, et al. Lignans and neolignans from stems of Piper wightii. Tetrahedron Lett. 1994;50:10579–86. [Google Scholar]
- 10.Tyagi OD, Prasad AK, Wengel J, Boll PM, Olsen CE, Parmar VS, et al. Neolignans from Piper schmidtii and reassignment of the structure of schmiditin. Acta Chem Scand. 1995;49:142–8. [Google Scholar]
- 11.Iida T, Ichino K, Ito K. Neolignans from Magnolia denudata. Phytochemistry. 1982;21:2939–41. [Google Scholar]
- 12.Han GQ, Wei LH, Li CL, Qiao L, Jia YZ, Zheng QT. The isolation and identification of PAF inhibitors from Piper wallichii (Miq.) Hand-Mazz and P. hancei Maxim. Yao Xue Xue Bao. 1989;24:438–43. [PubMed] [Google Scholar]
- 13.Matsui K, Munakata K. Structure of piperenone. Agric Biol Chem. 1976;40:1113–8. [Google Scholar]
- 14.Ribeiro AB, Bolzani VS, Yoshida M, Santos LS, Eberlin MN, Silva DH. A new neolignan and antioxidant phenols from Nectandra grandiflora. J Braz Chem Soc. 2005;16:526–30. [Google Scholar]
- 15.Lin LC, Shen CC, Shen YC, Tsai TH. Anti-inflammatory neolignans from Piper kadsura. J Nat Prod. 2006;69:842–4. doi: 10.1021/np0505521. [DOI] [PubMed] [Google Scholar]
- 16.Shing TK, Tam EK. Enantiospecific synthesis of (+)-crotepoxide,(+)-boesenoxide,(+)-β-senepoxide,(+)-pipoxide acetate, (-)-iso-crotepoxide, (-)-senepoxide and (-)-tingtanoxide from (-)-quinic acid. J Org Chem. 1988;63:1547–54. [Google Scholar]
- 17.Suresh G, Tiwari AK, Radha Krishna Murthy M, Anand Kumar D, Rajendra Prasad K, Ranga Rao R, et al. New advanced glycation end-products inhibitors from Dichrostachys cinerea Wight and Arn. J Nat Med. 2012;66:213–6. doi: 10.1007/s11418-011-0557-3. [DOI] [PubMed] [Google Scholar]
- 18.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 19.Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51:6657–62. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- 20.Ohlyan R, Kandale A, Deora GS, Rathore V, Chahal J. In vitro screening of dry fruit extracts of Piper attenuatum for antioxidant and anticancer activity. Med Chem Res. 2013;22:1365–70. [Google Scholar]
- 21.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–72. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]


