Abstract
Background:
Gastric cancer (GC) is one of the most frequently occurring digestive tract cancers and fewer chemotherapeutic drugs for GC have shown promising results. In this study, we investigated the anti-tumor activity of shikonin, a natural compound isolated from the Chinese plant Lithospermum erythrorhizon, against the human GC cell line HGC-27.
Materials and Methods:
HGC-27 cells treated with shikonin at a concentration of 30μM or above showed significant growth inhibition compared to control cells. Shikonin-treated cells also underwent apoptosis as detected by flow cytometric analysis and microscopic examination of cellular morphology. Further investigation into the underlying mechanism of apoptosis by western blot showed that the shikonin promoted the activation of poly-(ADP-ribose)-polymerase, caspase-3 and caspase-9 following 24 h or 48 h of treatment time, as well as the activation of caspase-8, but only after 48 h of treatment time. Furthermore, the levels of mitochondrial membrane potential, B-cell lymphoma 2 (Bcl-2) and Bcl-extra large were reduced following shikonin treatment while the level of Bax was increased. In addition, shikonin also caused a significant reduction of the protein Survivin, while having little effect on the expression on X-linked inhibitor of apoptosis protein.
Conclusion:
Taken together, these results showed that the shikonin exhibited its anti-tumor activity against HGC-27 cells through inhibiting cell growth and promoting apoptosis by targeting mitochondrial-related signaling pathway. Our finding may represent a positive step in finding a natural and effective compound that could be important implication for future development of chemotherapeutic and/or chemopreventive agent against GC.
Keywords: Apoptosis, caspases, HGC-27 cells, mitochondrial pathway, shikonin
INTRODUCTION
Gastric cancer (GC) is one of the most frequently occurring digestive tract cancers. It has a poor prognosis and a high mortality rate worldwide.[1,2,3] In Asia and parts of South America in particular, GC is the most common epithelial malignancy and is a leading cause of cancer-related mortality.[4] Surgery plus chemotherapy remains the first-line of therapy. Despite rapid advances in surgical procedures and radiotherapy/chemotherapy, fewer chemotherapeutic drugs for GC have shown promising results.[5] So, there is an urgent need to search for and develop more effective anti-GC drugs. In recent years, compounds isolated from traditional Chinese herbs have been recognized as the most valuable resource in the development of novel and advanced cancer therapeutic drugs.[6,7,8,9]
Shikonin [Figure 1], one of the major naphthoquinone pigments isolated from the Chinese plant Lithospermum erythrorhizon,[10] exhibits many biological activities such as anti-microbial,[11] anti-inflammatory,[12] and anti-tumor[13] activities. The anti-tumor activity of shikonin was first demonstrated in murine sarcoma-180.[14] Recently, accumulated evidences have shown that the shikonin exerts its anti-tumor activity mainly through inhibiting cell growth and inducing apoptosis.[15] We have previously reported that the shikonin can inhibit the proliferation of human breast cancer cells and induce apoptosis in vitro.[16] Shikonin also induces apoptosis in hepatocellular carcinoma cells via reactive oxygen species.[17] In human papillary thyroid carcinoma cells, shikonin induces apoptosis through mitochondrial pathway,[18] whereas in human glioma cell lines, shikonin induces apoptosis through multiple mechanisms, including the generation of reactive oxygen species, depletion of glutathione and disruption of mitochondrial transmembrane potential.[19] However, the anti-tumor activity of shikonin on GC cells has not yet been reported. Thus, the aim of the present study was to elucidate the effect of shikonin on human GC cells and explore the potential underlying mechanism of its action. The results demonstrated that the shikonin could inhibit the proliferation of the human gastric cancer cell line HGC-27, and promote apoptosis by targeting mitochondrial-related signaling pathways.
Figure 1.
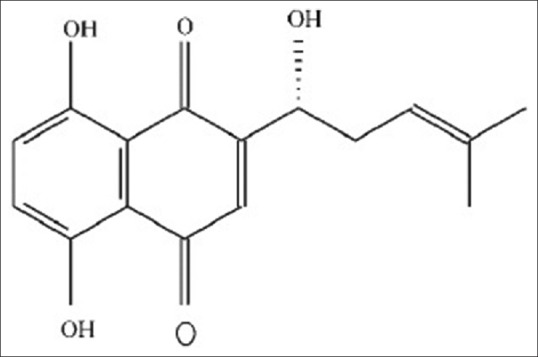
Structure of shikonin
MATERIALS AND METHODS
Reagents
Shikonin (purity > 98%) was obtained from the Beijing Institute of Biologic Products (Beijing, China). Fetal bovine serum (FBS) was purchased from TBD Biotechnology Development (Tianjin, China). RPMI 1640 was from Gibco (Grand Island, USA). Propidium iodide (PI), Hoechst-33258 and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical (St. Louis, MO, USA). Rhodamine-123 (Rh-123) was obtained from Molecular Probes (Eugene, OR, USA). The antibodies against poly-(ADP-ribose)-polymerase (PARP), caspase-3, caspase-9 and caspase-8 were purchased from Cell Signaling Technology (Beverly, MA). The antibodies against B-cell lymphoma 2 (Bcl-2), Bax, Bcl-extra large (Bcl-xL), Survivin, X-linked inhibitor of apoptosis protein (XIAP) and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
HGC-27 cells were obtained from the Institute of Biochemistry and Cell Research, China Life Science Academy (Shanghai, China). The cells were cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL) and maintained at 37°C in the presence of 5% CO2 in a humidified atmosphere.
Cell growth inhibition assay
The growth inhibitory effect of shikonin on HGC-27 cells was measured by MTT assay as previously described.[16] In brief, the cells were seeded in 96-well plates at a density of 4 × 103 cells/well. After 12 h of incubation, the cells were treated with different concentrations (1, 3, 10, 30 and 100 μM) of shikonin. As a control group, dimethyl sulfoxide (DMSO) was added instead (final concentration of 0.1%). After 24 or 48 h, the cells were incubated with MTT (0.25 mg/ml) for 3 h at 37°C. The purple formazan crystals were dissolved by addition of 100 μl DMSO, and the absorbance of the plate was read at 490 nm using a plate reader.
Flow cytometric analysis
HGC-27 cells (1 × 106 cells) were harvested and washed once in cold phosphate buffered saline (PBS). The cell pellets were fixed in 70% ethanol and washed in cold PBS, and then suspended in PI solution (1 mL) containing 50 μg/mL of PI, 0.1% (w/v) sodium citrate, 0.1% (v/v) triton X. The samples were incubated at 4°C in the dark and analyzed by a FACScan flow cytometer (Becton Dickinson).[16]
Morphological changes
After treatment with shikonin, HGC-27 cells were harvested by trypsinization and washed twice with PBS. The cells were fixed with 3.7% paraformaldehyde for 2 h at room temperature, washed once with PBS, and then stained with 167 μM Hoechst-33258 for 30 min at 37°C. At the end of the incubation, the cells were washed and resuspended in PBS. The nuclear morphology of the cells was observed under a fluorescence microscope.[20]
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential (MMP) was assessed by the retention of Rh-123, a membrane-permeable fluorescent cationic dye.[21] Briefly, cells treated with various concentrations of shikonin were collected and washed with PBS followed by incubation with 0.5 μg/mL Rh-123 in the dark for 30 min at 37°C. The cells were then washed with PBS and analyzed by FACScan with excitation and emission wavelength of 495 and 535 nm, respectively.[21,22]
Determination of protein changes
Western blot analysis for detecting the changes in the levels of certain proteins was performed using cells that had been treated with shikonin (0, 3, 10, and 30 μM) for both 24 and 48 h. Cells were lysed with radio immune precipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, 1 mM phenylmethyl sulfonyl fluoride, 100 μM leupeptin, and 2 μg/ml aprotinin, pH 8.0) and samples of the extract (50 μg total protein) were resolved in 8–12% SDS-polyacrylamide gels. After electrophoresis, the proteins in the gels were transferred to nitrocellulose membranes, and the membranes were stained with 0.2% Ponceau S red to assure equal protein loading and transferring. The membranes were blocked with 5% nonfat milk, followed by incubation with the appropriate primary antibody at 4°C for overnight. The membranes were incubated with horseradish peroxidase-conjugated secondary antibody. Positive bands were visualized using enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences, UK).[21,22]
Data analysis
Data were expressed as mean ± SEM of three experiments. Statistical significance (P < 0.05) was assessed by one-way ANOVA followed by Dunnett's t-test (SPSS 13.0 software, SPSS, Chicago, IL, USA).
RESULTS
Shikonin exhibited growth inhibitory activity against HGC-27 cells
The effect of shikonin on the growth of HGC-27 cells was investigated by MTT assay. Shikonin inhibited the growth of HGC-27 cells in a concentration-dependent manner [Figure 2]. Compared to control cells, significant inhibition occurred only at 10 μM and above. At 100 μM shikonin (the highest concentration tested), about 85% of growth was inhibited in the case of 24 h and exposure and 90% when the exposure time was increased to 48 h. Much more intense growth inhibition was also obtained at 48 h compared to 24 h when 30 μM shikonin was used (20% vs. 60%). Thus, the anti-growth effect of shikonin against HGC-27 cells also exhibited a time-dependent effect. The IC50 of a 24 h and 48 h time course for HGC-27 cells was 47.70 μM and 25.78 μM, respectively.
Figure 2.
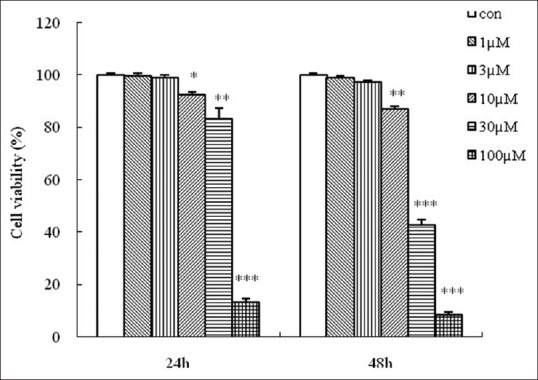
Growth inhibition of HGC-27 cells by shikonin. HGC-27 cells were treated with different concentrations (1, 3, 10, 30 or 100 μM) of shikonin for 24 h and 48 h. Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and cell viability was expressed as a percentage of surviving cells relative to control cells (no treatment). Data are the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared to control cells
Shikonin induced apoptosis in HGC-27 cells
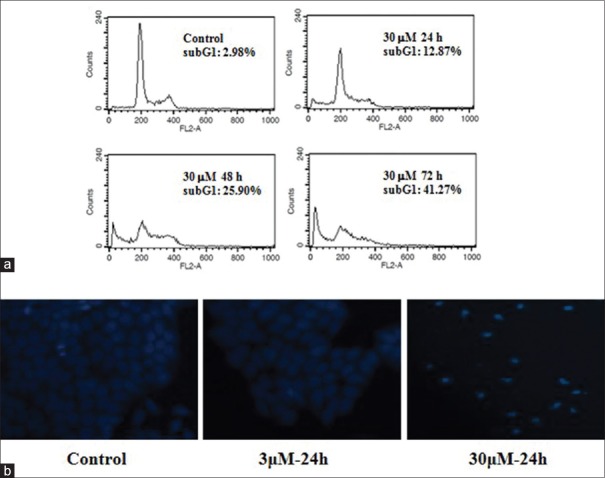
To determine whether the growth-inhibition effect exerted on HGC-27 cells was also accompanied with apoptosis, shikonin-treated cells were subjected to flow cytometry analysis complemented with PI staining. For cells that were treated with 30 μM shikinon for 24, 48 and 72 h, the characteristic hypodiploid DNA content peak (subG1) could be seen. These cells also showed an increase in the percentage of subG1 cells from 2.98% in the case of control to 12.87%, 25.90%, and 41.27% in the case of 24-, 48- and 72-h exposure to shikinon, respectively [Figure 3a].
Figure 3.
Shikonin-induced apoptosis in HGC-27 cells. (a) Sub-G1 DNA content in HGC-27 cells as analyzed by flow cytometry after treatment without or with shikonin. (b) Morphology of HGC-27 cells following treatment without or with shikonin. Cells were first stained with Hochest-33258 and then observed under a fluorescence microscope
Hoechst-33258 fluorescence-staining confirmed that the shikonin-treated cells underwent apoptosis, and morphological changes that are associated with apoptosis, such as chromatin condensation and formation of apoptotic bodies were all clearly visible [Figure 3b].
Shikonin activates caspases and decreases mitochondrial membrane potential level in HGC-27 cells
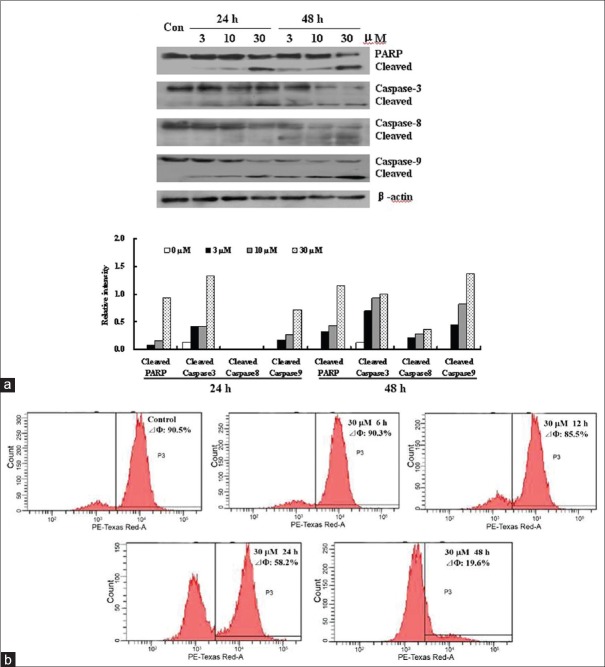
Since caspases are known to play critical roles in the initiation and maintenance of apoptosis,[23] activation of caspases was used to explore the mechanism of shikonin-induced apoptosis in HGC-27 cells. As shown in Figures 4a, shikonin (3, 10, 30 μM) treatment resulted in the activation of PARP and caspase-3 after 24 h and 48 h, as indicated by the presences of cleaved fragments of both enzymes. Moreover, caspase-9, acting on the mitochondrial pathway, was also activated in HGC-27 cells after treatment with shikonin for 24 h and 48 h, whereas caspase-8 was activated only after 48 h of treatment with the same concentrations of shikonin. Since the activation of caspase-9 is linked to the loss of MMP,[21,24] we also examined the effect of shikonin on the level of MMP using flow cytometry after staining the cells with the cationic dye Rh-123 in HGC-27 cells. Obvious decrease in MMP level was evident in shikonin-treated HGC-27 cells, and the decrease appeared to exhibit a time-dependent manner [Figure 4b].
Figure 4.
Effects of shikonin on the levels of poly-(ADP-ribose)-polymerase (PARP), caspases and mitochondrial membrane potential (MMP) in HGC-27 cells. (a) Changes in the levels of activated PARP, caspase-3, caspase-8 and caspase-9. HGC-27 cells were treated with shikonin (3, 10 or 30 μM) for 24 h or 48 h, and the total proteins were extracted and analyzed by Western blot analysis. (b) Changes in the level of MMP. HGC-27 cells were treated with shikonin (30 μM) for 6, 12, 24 and 48 h. Disruption of MMP was determined according to changes in fluorescence density using rhodamine-123
Effect of shikonin on the levels of B-cell lymphoma 2 family, X-linked inhibitor of apoptosis protein and Survivin
To examine the relationship between shikonin treatment and mitochondrial disruption, changes in the levels of members of the Bcl-2 family proteins were investigated.[25] Proteins of the Bcl-2 family play a critical role in the mitochondrial-dependent activation of caspases. As shown in Figure 5, the levels of Bcl-2 and Bcl-xL in HGC-27 cells were reduced following shikonin treatment while the level of Bax was increased. This suggested that the activation of the mitochondrial pathway might be caused by the upregulation of Bax and downregulation of Bcl-2 and Bcl-xL following exposure to shikonin.
Figure 5.
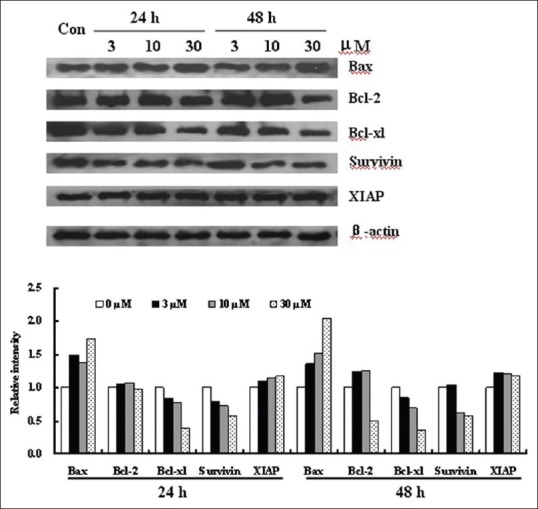
Effect of shikonin on apoptosis related proteins. HGC-27 cells were treated with shikonin (3, 10 and 30 μM) for 24 h and 48 h. Total cell lysates or cytosolic fractions were prepared and the levels of B-cell lymphoma 2 family proteins as well as of Surivin and XIAP were determined by western blot
In addition, the protein levels of XIAP and Survivin, both of which are inhibitors of caspase-9 and caspase-3,[26] were also investigated. Shikonin significantly reduced the protein level of Survivin while having little effect on the level of XIAP [Figure 5] consistent with the data obtained for caspase-9 and caspase-3.
DISCUSSION
Tumors are diseases resulting primarily from cell proliferation disorder and obstruction of cell apoptosis. Thus, finding compounds that can induce cell apoptosis would be important in the search for potential chemotherapeutic agents.[21,27,28] Of particular interest are potential anti-cancer compounds from natural sources, because these compounds are considered safe.[29,30] The present study demonstrated for the first time that the natural product shikonin was able to inhibit the proliferation of human gastric cancer HGC-27 cells and induce these cells to undergo apoptosis.
Apoptosis, a biological process that removes cancerous or virally infected cells from the body of an animal can be triggered through two classical apoptosis signaling pathways: The extrinsic pathway (death receptor pathway) and the intrinsic pathway (the mitochondrial pathway).[31] It is executed by caspases, a family of intracellular aspartate-specific cysteine proteases, which amplify the apoptotic signal and proteolytically process numerous cellular target molecules with different functions.[32,33] The multiple apoptotic stimuli can activate upstream caspase-9 and/or caspase-8, and the activated caspase-9 and/or 8 in turn cleave and activate the executioner caspase-3.[34] Following the activation of caspase-3, some specific substrates of this enzyme, such as PARP are cleaved, eventually leading to apoptosis.[21,35] Shikonin treatment resulted in the formation of active cleaved fragments of PARP, caspase-3, caspase-8 and caspase-9 in a concentration-and time-dependent manner. The fact that caspase-9 was cleaved earlier than caspase-8 after shikonin treatment indicated that caspase-9, rather than caspase-8, might play an important role in shikonin-induced apoptosis. Cleavage of caspase-9 plays a key role in the mitochondria-associated apoptotic pathway while caspase-8 is usually activated through the death receptor-mediated pathway.[31] The result of the current study suggested that the shikonin probably induced apoptosis in GC cells mainly through the mitochondrial-related pathway.
The activation of caspase-9, which is closely linked to the loss of MMP,[36] was also confirmed by the ability of shikonin to decrease the level of MMP in HGC-27 cells treated with this compound [Figure 4b].
The intrinsic apoptotic pathway hinges on the balance of activity between pro- and anti-apoptotic members of the Bcl-2 superfamily proteins, which act to regulate the permeability of the mitochondrial membrane as well as the activation of caspases-9.[31,37,38,39,40] Among these Bcl-2 family proteins, Bcl-2 can stabilize the mitochondria permeability to prevent the release of cytochrome c and activation of caspase-9, whereas Bax interacts with pore proteins on the mitochondrial membrane to increase the membrane permeability, which leads to the release of cytochrome c from mitochondria, activation of caspase-9 and initiation of the caspase-activation pathway to initiate apoptosis.[21,34] Here, we observed that the shikonin induced the down-regulation of Bcl-2 and up-regulation of Bax in HGC-27 cells. In addition, the level of anti-apoptosis proteins Bcl-xL was also down-regulated after treatment with shikonin. These results suggested that the shikonin-induced down-regulation of MMP level and activation of caspase-9 and caspase-3 were due, at least in part, to the disruption of a balance between Bax and Bcl-2, as well as the down-regulation of Bcl-xL.
The inhibitors of apoptosis (IAP) are a family of functionally-and structurally-related proteins, which serve as endogenous IAP.[41] The best-characterized IAPs are XIAP and Survivin, which inhibit caspase activation, thereby leading to negative regulation of apoptosis.[42,43] Shikonin could decrease the expression levels of Survivin in vitro [Figure 5]. Taken together, the data demonstrated that, besides directly activating caspase-3 and caspase-9, the down-regulation of Survivin might be an additional mechanism by which shikonin might induce apoptosis in HGC-27 cells.
SUMMARY
The present study demonstrated for the first time that the shikonin could inhibit the proliferation of the human gastric cancer cells, HGC-27, probably through inducing apoptosis by targeting mitochondrial-related signaling pathway, which included the down-regulation of Bcl-2, Bcl-xL and Surivin, as well as the up-regulation of Bax. These findings suggested that the shikonin could potentially be considered as a chemotherapeutic and/or chemopreventive agent for GC. Further study that addresses the anti-tumor activity of shikonin in an animal model will provide greater support for the potential usefulness of this compound as an anti-GC drug.
ACKNOWLEDGMENTS
This research is supported by the National Natural Science Foundation of China (81102432), the Fundamental Research Funds for the Central Universities (N130420004, N130120001), Training Program Foundation for the Distinguished Young Scholars of University in Liaoning Province (LJQ2012088) and Fund for long-term training of young teachers in Shenyang Pharmaceutical University (ZCJJ2013409).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Yuan X, Zhou Y, Wang W, Li J, Xie G, Zhao Y, et al. Activation of TLR4 signaling promotes gastric cancer progression by inducing mitochondrial ROS production. Cell Death Dis. 2013;4:e794. doi: 10.1038/cddis.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li ZM, Pu YW, Zhu BS. Blockade of NF-kB nuclear translocation results in the inhibition of the invasiveness of human gastric cancer cells. Oncol Lett. 2013;6:432–436. doi: 10.3892/ol.2013.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM, Chen LG, et al. Oridonin induces apoptosis in gastric cancer through Apaf-1, cytochrome c and caspase-3 signaling pathway. World J Gastroenterol. 2012;18:7166–74. doi: 10.3748/wjg.v18.i48.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Flower A, Ritchie A, Liu J, Molassiotis A, Yu H, et al. Oral Chinese herbal medicine (CHM) as an adjuvant treatment during chemotherapy for non-small cell lung cancer: A systematic review. Lung Cancer. 2010;68:137–45. doi: 10.1016/j.lungcan.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Yang X, Zeng X, Eslick GD. Traditional Chinese medicinal herbs in the treatment of patients with esophageal cancer: A systematic review. Gastroenterol Clin North Am. 2009;38:153–67. doi: 10.1016/j.gtc.2009.01.006. x. [DOI] [PubMed] [Google Scholar]
- 8.Molassiotis A, Potrata B, Cheng KK. A systematic review of the effectiveness of Chinese herbal medication in symptom management and improvement of quality of life in adult cancer patients. Complement Ther Med. 2009;17:92–120. doi: 10.1016/j.ctim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhu AK, Zhou H, Xia JZ, Jin HC, Wang K, Yan J, et al. Ziyuglycoside II-induced apoptosis in human gastric carcinoma BGC-823 cells by regulating Bax/Bcl-2 expression and activating caspase-3 pathway. Braz J Med Biol Res. 2013;46:670–5. doi: 10.1590/1414-431X20133050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang IC, Huang YJ, Chiang TI, Yeh CW, Hsu LS. Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biol Pharm Bull. 2010;33:816–24. doi: 10.1248/bpb.33.816. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki K, Abe H, Yoshizaki F. In vitro antifungal activity of naphthoquinone derivatives. Biol Pharm Bull. 2002;25:669–70. doi: 10.1248/bpb.25.669. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka S, Tajima M, Tsukada M, Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod. 1986;49:466–9. doi: 10.1021/np50045a014. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 14.Sankawa U, Ebizuka Y, Miyazaki T, Isomura Y, Otsuka H. Antitumor activity of shikonin and its derivatives. Chem Pharm Bull (Tokyo) 1977;25:2392–5. doi: 10.1248/cpb.25.2392. [DOI] [PubMed] [Google Scholar]
- 15.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, et al. Anti-cancer natural products isolated from Chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Y, Guo T, Wu C, He X, Zhao M. Effect of shikonin on human breast cancer cells proliferation and apoptosis in vitro. Yakugaku Zasshi. 2006;126:1383–6. doi: 10.1248/yakushi.126.1383. [DOI] [PubMed] [Google Scholar]
- 17.Gong K, Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: A potential new treatment for hepatocellular carcinoma. Free Radic Biol Med. 2011;51:2259–71. doi: 10.1016/j.freeradbiomed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Yin L, Chen J, Chen J. The apoptotic effect of shikonin on human papillary thyroid carcinoma cells through mitochondrial pathway. Tumour Biol. 2014;35:1791–8. doi: 10.1007/s13277-013-1238-5. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Lin ML, Ong PL, Yang JT. Novel multiple apoptotic mechanism of shikonin in human glioma cells. Ann Surg Oncol. 2012;19:3097–106. doi: 10.1245/s10434-012-2324-4. [DOI] [PubMed] [Google Scholar]
- 20.Ma EL, Zhao DM, Li YC, Cao H, Zhao QY, Li JC, et al. Activation of ATM-Chk2 by 16-dehydropregnenolone induces G1 phase arrest and apoptosis in HeLa cells. J Asian Nat Prod Res. 2012;14:817–25. doi: 10.1080/10286020.2012.694874. [DOI] [PubMed] [Google Scholar]
- 21.Ma E, Li Y, Zhang W, Wang X, Tai W, Li T, et al. 2(+/-)-7,8,3’,4’,5’- Pentamethoxyflavan induces G2/M phase arrest and apoptosis in HL60 cells. Toxicol In Vitro. 2009;23:874–9. doi: 10.1016/j.tiv.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Yang JY, Zhou F, Wang LH, Zhang W, Sha S, et al. Seed Oil of Brucea javanica induces apoptotic death of acute myeloid leukemia cells via both the death receptors and the mitochondrial-related pathways. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1155/2011/965016. 965016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson DW, Thornberry NA. Caspases: Killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 24.Brenner C, Kroemer G. Apoptosis. Mitochondria – The death signal integrators. Science. 2000;289:1150–1. doi: 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- 25.Borner C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–47. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 26.Mohapatra S, Chu B, Zhao X, Pledger WJ. Accumulation of p53 and reductions in XIAP abundance promote the apoptosis of prostate cancer cells. Cancer Res. 2005;65:7717–23. doi: 10.1158/0008-5472.CAN-05-0347. [DOI] [PubMed] [Google Scholar]
- 27.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 28.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–46. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 30.Bi X, Zhao Y, Fang W, Yang W. Anticancer activity of Panax notoginseng extract 20(S)-25-OCH3-PPD: Targetting beta-catenin signalling. Clin Exp Pharmacol Physiol. 2009;36:1074–8. doi: 10.1111/j.1440-1681.2009.05203.x. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Tai W, Li Y, Zhang L, Zhang W, Ma E, et al. 1-(3’,4’,5’- Trimethoxyphenyl)-3-(3”,4”- dimethoxy-2”- hydroxyphenyl)- propane with microtubule-depolymerizing ability induces G2/M phase arrest and apoptosis in HepG2 cells. Chem Biol Interact. 2010;188:161–70. doi: 10.1016/j.cbi.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, Wu L, Li L, Tashiro S, Onodera S, Ikejima T. p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375-S2 cells. J Pharmacol Sci. 2004;94:166–76. doi: 10.1254/jphs.94.166. [DOI] [PubMed] [Google Scholar]
- 34.Zou H, Li Y, Liu X, Wang X. An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–56. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–17. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 37.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 38.Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115–23. doi: 10.1016/s1044-579x(02)00129-3. [DOI] [PubMed] [Google Scholar]
- 39.Lavrik IN, Golks A, Krammer PH. Caspases: Pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–72. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 41.Gyrd-Hansen M, Meier P. IAPs: From caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–74. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 42.Kashkar H. X-linked inhibitor of apoptosis: A chemoresistance factor or a hollow promise. Clin Cancer Res. 2010;16:4496–502. doi: 10.1158/1078-0432.CCR-10-1664. [DOI] [PubMed] [Google Scholar]
- 43.Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]