Abstract
Objective:
The objective was to investigate the endothelial nitric oxide synthase (eNOS/NO) pathway is involved or not in the protective effects of glycyrrhizic, ferulic, paeoniflorin, cinnamic (GFPC) in myocardial ischemia-reperfusion injury Sprague-Dawley rats.
Materials and Methods:
Ischemia-reperfusion (I/R) model was made by ligating the left anterior descending branch of the coronary artery for 30 min and releasing for 120 min, then the left ventricular apical was fixed and sliced, morphological changes of myocardial microvascular endothelial cell (MMVEC) was observed by electron microscopy, apoptosis index of MMVEC was observed by means of TUNEL, serum NO was tested by methods of nitrate reduction, lactate dehydrogenase (LDH), creatine kinase MB (CK-MB) was detected by automatic biochemical analyzer; Phosphorylated eNOS (PeNOS) and inducible NOS (iNOS) protein were measured by means of western blot.
Results:
In positive product control group, the serum levels of NO, LDH, CK-MB significantly increased (P < 0.05); MMVEC apoptosis was significantly decreased (P < 0.05); incidence of area at risk decreased significantly (P < 0.05); PeNOS protein increased (P < 0.05); iNOS protein decreased significantly (P < 0.05).
Conclusion:
Ischemic preconditioning of GFPC from GFPC plays a protective role in I/R heart through regulating the eNOS/NO signal pathway by increasing the PeNOS protein expression and decreasing the expression of iNOS protein.
Keywords: Cinnamic, endothelial nitric oxide synthasee, ferulic, glycyrrhizic, inducible nitric oxide synthase, myocardial ischemia- reperfusion injury, myocardial microvascular endothelial cell, paeoniflorin
INTRODUCTION
DangGuiSiNi Decoction (DGSND) is used to treat blood deficiency and cold syncope in shanghanlun, also a Chinese classical decoction. Investigations have shown that DGSND have effects on anticoagulation, dilation of blood vessels, analgesia, and anti-inflammation.[1] Paeoniflorin,[2,3,4,5] ferulic acid,[6] licorice acid,[7] cinnamic acid[8] are four components[9] were found have protective effects on ischemia-reperfusion (I/R) injury in rats. Ischemic preconditioning can significantly ease the I/R injury, previously we selected the best combination from DGSND against I/R injury by means of orthogonal design: Glycyrrhizic acid 50 mg/kg, ferulic acid 400 mg/kg, paeoniflorin 100 mg/kg, cinnamic acid 400 mg/kg[10] (GFPC), then to study whether the endothelial nitric oxide synthase (eNOS/NO) pathway is involved in the cardioprotective effects and the mechanisms of GFPC.
MATERIALS AND METHODS
Myocardial ischemia-reperfusion
The study protocol was approved by the Ethical Committee of Clinical Research in Jinan University. Sprague-Dawley (SD) rats (250–300 g, from Guangdong laboratory animal central) were maintained for at least 1 week before the experiments. The rats were anesthetized with sodium pentobarbital (40 mg/kg intraperitoneally), respiration with a fraction of inspired oxygen of 0.80, left anterior-descending (LAD) was ligated with a 4-0 silk suture. After 30 min of ischemia, the ligation was loosened. Rats were killed at 180 min of reperfusion.[11]
The reliability and stability of the model were observed by electrocardiogram changes and myocardial hematoxylin and eosin staining.
Experimental groups
Thirty-two SD rats were divided into sham group (animals were subjected to entire surgical procedure and the silk suture was passed beneath the coronary artery, but the LAD was not ligated), I/R group (heart subject to ischemia-reperfusion), positive product control (PPC) group (heart subjected to I/R preconditioning with GFPC), PPC + L group (heart subjected to I/R preconditioning with GFPC and pretreated with L-NG-Nitroarginine methyl ester (L-NAME), the eNOS inhibitor, Nω-Nitro-L-arginine methylester, 15 min before reperfusion, 30 mg/kg), 8/group.
Nitrate reduction for nitric oxide, creatine kinase MB, lactate dehydrogenase
Serum NO level was tested by methods of nitrate reduction according to the instruction of the kit. Serum lactate dehydrogenase (LDH), creatine kinase MB levels were detected by automatic biochemical analyzer.
Apoptosis
Left ventricular tissue (4 mm × 4 mm × 4 mm, close to the apex cordis) was fixed in 4% paraformaldehyde (1:10), embedded in paraffin and cut into sections 4 μm in thickness for observation of apoptosis (TUNEL), and calculating the apoptotic index (each slice count positive apoptotic nuclei of five high-power field/total nuclei, the mean value for the apoptosis index).
Electron microscopy
The sample of tissue (1 mm × 1 mm × 1 mm) was collected from the apex, placed on ice, and then fixed in 2.5% glutaraldehyde at 4°C for 24 h. The myocardium was then washed in phosphate-buffered saline, embedded in epoxy resin, and immersed in Epon812. Longitudinal ultrathin sections collected with an LKB-V microtome (Bromma, Sweden) were stained with uranium acetate, folic acid lead, and captured with a transmission electron microscope (H-600, Hitachi, Japan).
Western blotting
Phosphorylation of eNOS and inducible NOS (iNOS) protein were measured by the means of western blotting. Extracting about 200 mg tissues from the left ventricular, protein were fractionated on 10% sodium dodecyl sulfate-polyacrylamide gels in running buffer at 90 V and then electroblotted to nitrocellulose membranes. Membranes were blocked then incubated overnight at 4°C with the following primary antibodies (Santa Cruz Biotech, CA). Then membranes were washed three times in Tween-20 and incubated with the corresponding secondary antibody (Santa Cruz Biochemicals). Immunoreactive bands were visualized with the chemiluminescence kit (Santa Cruz Biochemicals) according to the instructions.
Statistics analysis
All values are presented as the mean ± standard error of the mean Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 15.0, Analysis of Variance (ANOVA) of orthogonal design, one-way and two-way ANOVA for multiple group comparisons, Chi-square test for data count. P < 0.05 was considered significant.
RESULTS
Effect of glycyrrhizic, ferulic, paeoniflorin, cinnamic on serum nitric oxide, lactate dehydrogenase, creatine kinase MB levels
As shown in Figure 1a-c, Compared with sham group, serum NO level of I/R group significantly decreased (P < 0.05); compared with the I/R group, serum NO level of PPC group significantly increased (P < 0.05); compared with PPC group, serum NO level of PPC + L group significantly decreased (P < 0.05). Rats in I/R group, the serum level of LDH, CK were significantly higher than that in the sham group (P < 0.05), serum LDH, CK levels in PPC group were significantly lower than I/R group (P < 0.05), serum LDH, CK levels in PPC + L group were significantly higher than PPC group (P < 0.05).
Figure 1.

(a) Serum nitric oxide level statistical analysis. (b) Serum creatine kinase MB level statistical analysis. (c) Serum lactate dehydrogenase level statistical analysis
Effect of glycyrrhizic, ferulic, paeoniflorin, cinnamic on myocardial apoptosis
Compared with sham group seen in Figures 2 and 3, I/R group myocardial microvascular endothelial cell (MMVEC) apoptosis rate was significantly higher (P < 0.05); compared with the I/R group, PPC group MMVEC apoptosis was significantly decreased (P < 0.05); compared with the PPC group, PPC + L group MMVEC apoptosis rate was increased (P < 0.05).
Figure 2.
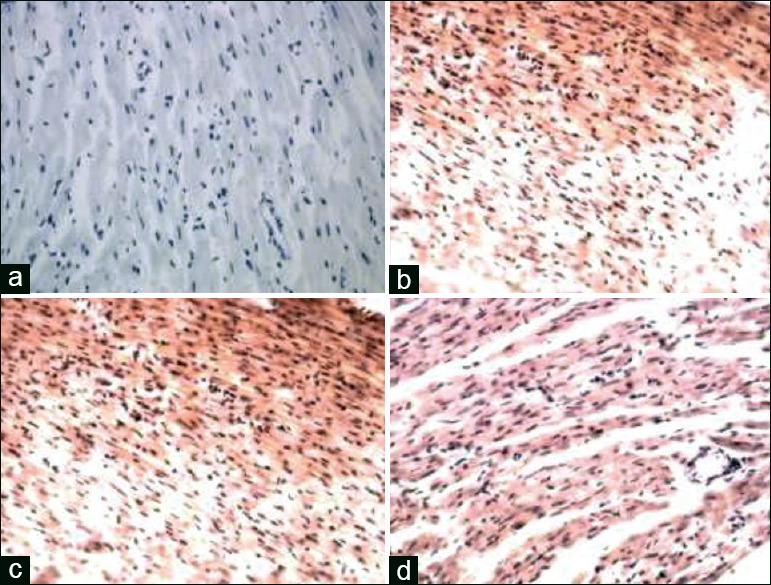
(a) Sham group myocardial microvascular endothelial cell (×200). (b) Ischemia-reperfusion group myocardial microvascular endothelial cell (×200). (c) Positive product control group myocardial microvascular endothelial cell (×200). (d) Positive product control group myocardial microvascular endothelial cell (×200)
Figure 3.

Each group of left ventricular apex myocardial microvascular endothelial cell apoptosis rate (%) comparison. *versus sham group P < 0.05, *versus I/R group P < 0.05, #versus positive product control group P < 0.05
Morphological changes of myocardial microvascular endothelial cell by electron microscope
Electron microscope: In sham group, mitochondrial membrane integrity of MMVEC, crest particle exists, within the folds close, no bubble, regular nuclear membrane, chromatin uniform, no concentration phenomenon, nucleolus exists [Figure 4a]; mitochondrial of I/R group swelling, membrane Irregular [Figure 4b], loose vacuoles within the wrinkle, ridge particles disappeared, and irregular nuclear membrane [Figure 4c], chromatin condensation and margination, nucleolar disappearance, or even apoptotic bodies [Figure 4d]; compared with I/R group, PPC group significantly improved in symptoms; PPC + L group worse than PPC group.
Figure 4.

(a) Sham group myocardial microvascular endothelial cell figure. (b) Ischemia-reperfusion group myocardial microvascular endothelial cell. (c) Positive product control group myocardial microvascular endothelial cell. (d) Positive product control group myocardial microvascular endothelial cell
The incidence of reperfusion arrhythmias
Compared with the sham group, incidence of area at risk (RA) of I/R group significantly increased (P < 0.05), Compared with the I/R group, incidence of RA of PPC group decreased significantly (P < 0.05); but compared with the PPC group, the incidence of RA of the PPC + L group changes were not statistically significant (P > 0.05). GFPC can significantly reduce the incidence of RA in rat myocardial ischemia-reperfusion injury (MIRI) [Figure 5].
Figure 5.
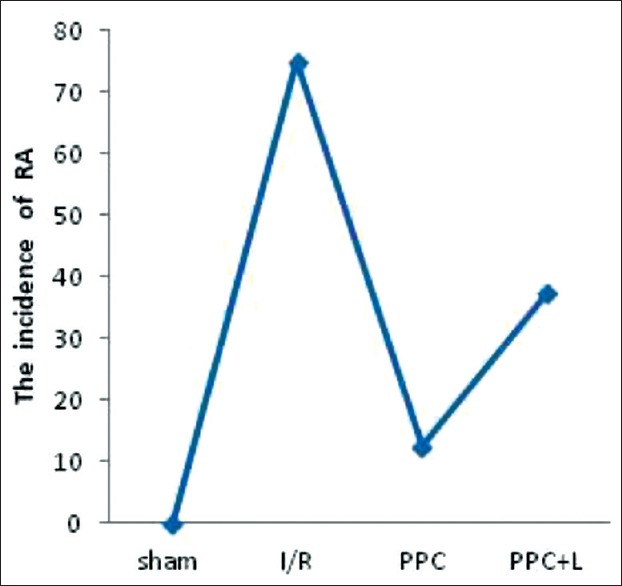
Multiple comparisons among groups of sample rate. *versus sham group P < 0.05, Δversus I/R group P < 0.05
Effect of glycyrrhizic, ferulic, paeoniflorin, cinnamic on endothelial nitric oxide synthase and inducible nitric oxide synthase
In Figures 6 and 7, PeNOS and iNOS protein of different groups in rat hearts. A. significant reduction in amount of PeNOS protein was observed in the I/R group (P < 0.05 vs. the sham group); After administration of GFPC, the amount of PeNOS protein was increased in PPC (P < 0.05 vs. the I/R group); and in the PPC + L group, the amount of PeNOS protein was reduced significantly (P < 0.05 vs. the PPC group); the amount of iNOS protein increased significantly in the I/R group (P < 0.05 vs. the sham group); After administration of GFPC, the amount of iNOS protein was decreased in PPC (P < 0.05 vs. the I/R group); and between the PPC and PPC + L, the amount of iNOS protein changes was not significant (P > 0.05).
Figure 6.
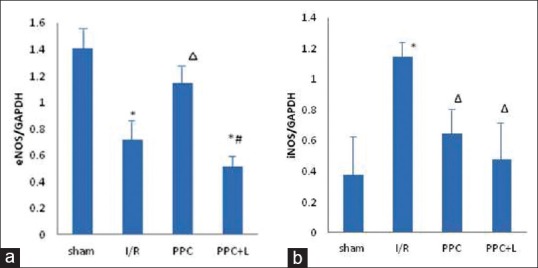
(a) Protein endothelial nitric oxide synthase protein expression (b) Inducible nitric oxide synthase protein expression. *versus sham group P < 0.05, versus I/R group P < 0.05, #versus positive product control group P < 0.05
Figure 7.
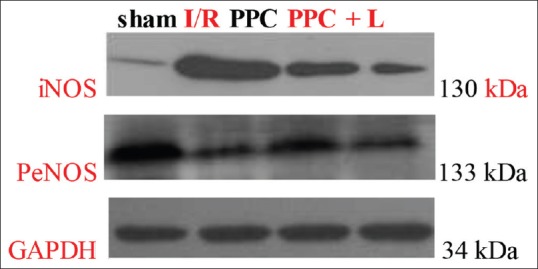
Protein endothelial nitric oxide synthase and inducible nitric oxide synthase of different groups
DISCUSSION
Nitric oxide was found as a vascular relaxing factor.[12] L-arginine-NO pathway contributed to free radical generation, which lead to I/R injury. NO synthase inhibitors decreased coronary sinus free radical concentration and tissue peroxynitrite formation in an ischemic-reperfusion canine model.[13] Various competitive inhibitors of the NOS enzyme have been shown to reduce I/R injury in various settings by reducing myocardial infarct size and improving myocardial contractile function.[14,15] L-arginine aggravated myocardial staining through production of peroxynitrite.[16] Some studies have shown that an important protective role of NO in the ischemic preconditioning.[17,18] NO precursor arginine ameliorated the endothelial dysfunction resulting from global I/R sequences in an isolated working rat heart model.[19] In the basic state, NO released from vascular endothelial cells plays an important role in maintaining the cardiovascular system at a relaxation state, regulating blood pressure, ameliorating coronary artery vascular tone and I/R injury. The generation of nitric oxide contributes to the marked antiarrhythmic effects of preconditioning in the canine myocardium, probably through elevation of cyclic guanosine monophosphate.[20]
Apoptosis is considered to be an important part of MIRI, and in vitro studies have shown that I/R can lead to apoptosis in VEC,[21,22] slow down the process of apoptosis may improve the prognosis of MIRI, so find the way to block apoptosis signal transduction pathway is very important.[23] The combination use of GFPC playing a multi-target inhibition role of anti-apoptosis of MMVEC.[24] The results of our study provided the experimental evidence that iNOS expression elevate, while eNOS expression reduce in I/R myocardium. During reperfusion, the resulting formation of NO decreases. GFPC could increase the expression level of eNOS protein, and then promote the L-arginine, NO precursor synthesize nitric oxide. At the same time, GFPC decreases the expression level of iNOS and protein. A large number of iNOS will produce the toxicity NO and inflammatory factors, which will aggravate ischemia-reperfusion injury. Preconditioning with GFPC plays a protective role on ischemic myocardium by increasing eNOS and inhibiting iNOS. But after administration of L-NAME, eNOS inhibitor, phosphorylation of eNOS protein was inhibited. Hence, the generation of NO reduced. It is concluded from these results that the generation of NO is based on the changes of NOS isozyme. Preconditioning of GFPC plays a major protective role on myocardial ischemia in MIRI through the eNOS/NO pathway.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China Grant (8117-3189) and science and Scientific and technological project of Henan Province. (122102310-473).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Qian GQ, Peng X, Cai C, Zhao GP. Effect on eNOS/NO Pathway in MIRI rats with preconditioning of GFPC from Dang Gui Si Ni decoction. Pharmacognosy Res. 2014;6:133–7. doi: 10.4103/0974-8490.129032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai HY, Lin YT, Chen CF, Tsai CH, Chen YF. Effects of veratrine and paeoniflorin on the isolated rat aorta. J Ethnopharmacol. 1999;66:249–55. doi: 10.1016/s0378-8741(98)00236-0. [DOI] [PubMed] [Google Scholar]
- 3.He X, Xing D, Ding Y, Li Y, Xu L, Du L. Effects of cerebral ischemia-reperfusion on pharmacokinetic fate of paeoniflorin after intravenous administration of Paeoniae Radix extract in rats. J Ethnopharmacol. 2004;94:339–44. doi: 10.1016/j.jep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Chen DM, Xiao L, Cai X, Zeng R, Zhu XZ. Involvement of multitargets in paeoniflorin-induced preconditioning. J Pharmacol Exp Ther. 2006;319:165–80. doi: 10.1124/jpet.106.104380. [DOI] [PubMed] [Google Scholar]
- 5.Kilgore KS, Tanhehco EJ, Park JL, Naylor KB, Anderson MB, Lucchesi BR. Reduction of myocardial infarct size in vivo by carbohydrate-based glycomimetics. J Pharmacol Exp Ther. 1998;284:427–35. [PubMed] [Google Scholar]
- 6.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–50. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian S, Bowyer MW, Egan JC, Knolmayer TJ. Attenuation of renal ischemia-reperfusion injury with selectin inhibition in a rabbit model. Am J Surg. 1999;178:573–6. doi: 10.1016/s0002-9610(99)00238-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Fu Y, Zhou L, Zhu L. Protection of cinnamic acid against cardiac ischemia-reperfusion injury [J] J Chin Med. 1999;14:68–9. [Google Scholar]
- 9.Zhao X, Gu Y, Song X, ZUO J, LI D, ZHAO C. HPLC method for simultaneous determination of four active ingredients of Dangguisini soup [J] J Shenyang Pharm Univ. 2008;25:200–3. [Google Scholar]
- 10.Qian G, Zhao G. Best combination of four active imgredients of anti-MIRI of DGSND. J Chin Med Mater. 2011;34:81–5. [Google Scholar]
- 11.Zhang J, Bian HJ, Li XX, Liu XB, Sun JP, Li N, et al. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16:307–15. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Bissing JW, Xu L, Ryan AJ, Martin SM, Miller FJ, Jr, et al. Nitric oxide synthase inhibitors decrease coronary sinus-free radical concentration and ameliorate myocardial stunning in an ischemia-reperfusion model. J Am Coll Cardiol. 2001;38:546–54. doi: 10.1016/s0735-1097(01)01400-0. [DOI] [PubMed] [Google Scholar]
- 14.Patel VC, Yellon DM, Singh KJ, Neild GH, Woolfson RG. Inhibition of nitric oxide limits infarct size in the in situ rabbit heart. Biochem Biophys Res Commun. 1993;194:234–8. doi: 10.1006/bbrc.1993.1809. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996;271:29223–30. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 16.Mori E, Haramaki N, Ikeda H, Imaizumi T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res. 1998;40:113–23. doi: 10.1016/s0008-6363(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 17.Hotta Y, Otsuka-Murakami H, Fujita M, Nakagawa J, Yajima M, Liu W, et al. Protective role of nitric oxide synthase against ischemia-reperfusion injury in guinea pig myocardial mitochondria. Eur J Pharmacol. 1999;380:37–48. doi: 10.1016/s0014-2999(99)00531-2. [DOI] [PubMed] [Google Scholar]
- 18.Jones KW, Flaherty MP, Tang XL, Qiu Y, Banerjee S, Bolli R. Ischemic preconditioning regulates expression of NOS in RNA in conscious rabbits via an NO-dependent mechanism (abstr) Circulation. 1998;98:I–620. [Google Scholar]
- 19.Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE, 3rd, Deaton DW, et al. Constitutive nitric oxide release is impaired after ischemia and reperfusion. J Thorac Cardiovasc Surg. 1995;110:1047–53. doi: 10.1016/s0022-5223(05)80173-4. [DOI] [PubMed] [Google Scholar]
- 20.Vegh A, Szekeres L, Parratt J. Preconditioning of the ischaemic myocardium; involvement of the L-arginine nitric oxide pathway. Br J Pharmacol. 1992;107:648–52. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita H, Morishita R, Nata T, Aoki M, Nakagami H, Taniyama Y, et al. Hypoxia-induced endothelial apoptosis through nuclear factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: In vivo evidence of the importance of NF-kappaB in endothelial cell regulation. Circ Res. 2000;86:974–81. doi: 10.1161/01.res.86.9.974. [DOI] [PubMed] [Google Scholar]
- 23.Chung MT, Cheng PY, Lam KK, Chen SY, Ting YF, Yen MH, et al. Cardioprotective effects of long-term treatment with raloxifene, a selective estrogen receptor modulator, on myocardial ischemia/reperfusion injury in ovariectomized rats. Menopause. 2010;17:127–34. doi: 10.1097/gme.0b013e3181b4c4ac. [DOI] [PubMed] [Google Scholar]
- 24.Pabla R, Curtis MJ. Effect of endogenous nitric oxide on cardiac systolic and diastolic function during ischemia and reperfusion in the rat isolated perfused heart. J Mol Cell Cardiol. 1996;28:2111–21. doi: 10.1006/jmcc.1996.0203. [DOI] [PubMed] [Google Scholar]


