Abstract
Background:
The extraction method of bioactive compounds is an important step in the manufacturing of herbal medicines, because secondary metabolites with therapeutic potential are usually found in small quantities in plant materials.
Objective:
Due the potential of Apeiba tibourbou Aubl, this study aimed to evaluate the impact of the extraction method on the quality of herbal extract and optimize the extraction of fatty acid, rosmarinic (Ra) and caffeic (Ca) acid from A. tibourbou.
Materials and Methods:
Determinations of residual moisture (Rm), proteins (Pt), lipids (Lp), total fiber (Tf), and carbohydrate (Cy) were performed in triplicate samples according assessment of antioxidant capacity. Extraction of fatty acids was carried out by two different methods: (i) By shoxlet and (ii) bligh and dyer. The optimized conditions were determined by surface response methodology (RSM), and the criterion of desirability was the maximum extraction of Ra and Ca.
Results:
The method of bligh and dyer was able to extraction more total Lp than the shoxlet. However, the extraction of fatty acid was different for the two methods. The optimized conditions to extract RA and Ca was calculated by RSM, 42°C, 30% (alcohol degree) and 24 min, this conditions maximize simultaneously the extraction of Ca (0, 04%) and Ry (1.89),
Conclusion:
It was observed that the extraction method alters the chemical composition of extract, and it is possible to extract Ca and Ra from A. tibourbou's leaves using ultrasound-assisted extraction.
Keywords: Herbal extract, methods of extraction, surface response methodology
INTRODUCTION
Medicinal plants are important as raw material to new medicines, however that this fact becomes reality is necessary studies to prove their efficacy, safety and source of raw material.[1,2] Often the chemical and medicinal potential of the plant is discarded because there is no standardization of raw material, generating results not accurate, with low reproducibility. Now there are advanced techniques to aid in the standardization of raw materials, e.g. High-performance liquid chromatography-mass spectrometry (HPLC-MS), Gas chromatography-MS (GC-MS), Raman and infrared spectroscopy, however extraction techniques have not advanced. This is a problem for accurate evaluation of the potential of a species.[1,3,4]
The extraction is an important step because the choices of extraction conditions determine the quality and the yield of the constituents. Plants are complex samples, so it is often necessary to employ various methods of extraction to evaluate the chemical profile of the species.[5]
Conventional extraction methods such as (i) heating maceration, (ii) refluxing, (iii) Soxhlet extraction, (iv) supercritical fluids,[6,7,8,9,10] generally require long extraction time, organic solvents toxics that may have potential negative on human health and environment.[11,12,13] Heating boiling or refluxing can be made use for extraction of natural products; the disadvantages are the loss of substances of interest due to hydrolysis, oxidation and ionization during the extractive process. In recent years, some new extraction methods have been employed for the extraction of natural products from herbals, including ultrasound-assisted extraction microwave-assisted extraction supercritical fluid extraction.[6,7,8,9,10]
Due the potential of Apeiba tibourbou Aubl, this study aimed to evaluate the impact of the extraction method on the quality of herbal extract and optimize the extraction of fatty acid, rosmarinic (Ra) and caffeic (Ca) acid from A. tibourbou. This species is popularly known as “pente de macaco” have great interest by the population, due many properties such as: Avoid hair fall, spasms and rheumatism, the leaves are rich in Ra and Ca, which are potent anti-inflammatory.
Plant material
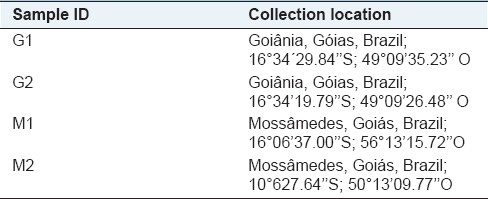
The fruits and leaves of A. tibourbou Aubl were collected in Goiânia, Goiás, Brazil and Serra Dourada, Mossâmedes, Goiás, Brazil. The species was identified by Ph.D Edemilson Cardoso da Conceição, and a voucher was deposited in the Herbarium of the Universidade Federal de Goiás (#40119) [Table 1].
Table 1.
Location collection of samples de Apeiba tibourbou
Chemical characterization of leaves
Determinations of residual moisture (Rm), polyphenols (Py) and flavonoids (Fn), were performed in triplicate samples according Assessment of Antioxidant Capacity (AOAC) (1998).[14] AOA was performed 2,2-diphenyl-1-picrylhydrazyl * free radical method described by (Roberta 2007).[15] Ra and Ca were quantified by HPLC. The separation method was adapted from Oliveira (2013)[16] was used Zorbax Eclipse eXtra-Dense Bonding-C18, de 25 cm × 4, 6 mm × 5 μm, mobile phase methanol (A): Water (1% acetic acid) (B), 0–7 min (40% of A), 7–11 min (40–80% of A), 11–23 min (80% of A), 23–24 min (80–90% of A), and flux was 1.0 mL/min. Separated Ra and Ca peaks were identified by comparing the individual standard with the retention time and the 330 nm spectra. The methodology was validated in accordance to United States Pharmacopeial[17] (data not shown).
Optimization of extraction of rosmarinic acid and caffeic acid
The ultrasound-assisted extraction (UEA) was performed in an ultrasonic bath (USC-4800, 50 kHz, 200W Instrument Company, Brazil) and was used flask volumetric (10 mL) with 25 mg of powdered leaves and the 10 mL of hydroethanolic mixture. The flask volumetric was partially immersed in an ultrasonic bath and submitted to ultrasound energy for a specific time. The extracts obtained were filtered and then analyzed by HPLC.
The influence of extraction method on yield of Ra and Ca were evaluation using a factorial drawing 33 (Box-Behnken) with 15 experimental runs, including three replicates at the center point. The factorial design matrix contained temperature 30, 40 and 50°C (X1), ethanol/water 20, 50 and 80% (v/v) (X2) and time extraction 10.20 and 30 min (X3). Experimental data were fitted to a polynomial model, and regression coefficients obtained [Equation. 1]. The M1 was used to optimization because had higher amounts of Ra and Ca.
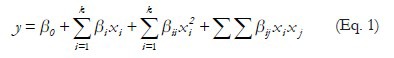
Where y is the dependent variable; β0 is the constant term; k number of variables; βi represents the coefficients of linear parameters; βii represents the coefficients of quadratic terms; βij represents the coefficients of interaction parameters. The Design expert 7.0 software was used to generate response surfaces. In order to verify the predictive capability of the model, optimum conditions were established by surface response methodology (RSM) and comparisons between the predicted results and the practical values were done by experimental rechecking using those presumed optimal conditions.
Optimization of extraction parameters
The optimized conditions were determined by RSM, and the criterion of desirability was the maximum extraction of Ra and Ca.
Evaluation of degradation of rosmarinic acid and caffeic acid by ultrasound
A previous study of stability was done with Ra and Ca (1 mg/mL), it was kept for 40 min in ultrasound bath (50°C) (USC-4800, 50 kHz, 200W Instrument Company, Brazil) A control solution in the same contraction was made and the areas of chemical marker were compared by HPLC.
Chemical characterization of seeds
Determinations of Rm, proteins (Pt), lipids (Lp), total fiber (Tf), and carbohydrate (Cy) were performed in triplicate samples according AOAC (1998). Extraction of fatty acids was carried out by two different methods: (i) By shoxlet (LpS), and (ii) bligh and dyer (LpBD) (1959).
Fatty acids were analyzed on GC model Varian 3900 A, fitted with a capillary column CP WA × 30 m and was used under the following conditions: Carrier gas, nitrogen with a flow rate of 2.5 μL/min; column temperature, 5 min hold for 90°C, 90–250°C at 5°C/min, 30 min hold at 280°C; injector temperature, 240°C; volume injected, 2 μL. The MS operating parameters were as follows: Ionization potential, 70 μeV; ion source temperature, 280°C; quadrupole 100°C, speed 2000 μ amu/s.[1]
RESULTS
The chemical profile was different to all samples leaves, M1 had the highest levels of polypenols (22.9% w/w), flavonoids (7.9% w/w), Ca 0.04 and rosmarinc (1.2% w/w) acid, therefore the antioxidant activity 45.3% was higher [Table 2].
Table 2.
Elemental analysis of leaves from Apeiba tibourbou
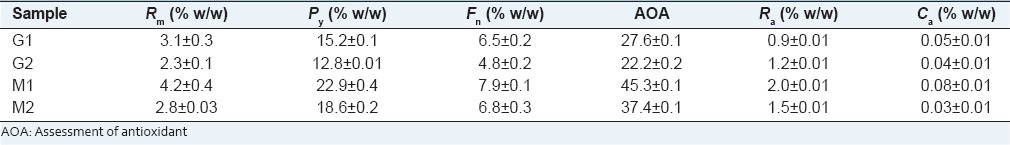
The AOA of these compounds was related another works,[4,18] this compounds are used as nutrients in the treatment and prevention of diseases, however, there are a great variety of substances that can act in synergy in protecting cells and tissues.[19,20] The importance concerning the performance of antioxidants in vitro or in vivo depends on the factors types free radicals formed, where and how these radicals are generated, analysis and methods for identifying damage and optimal dosages for protection.[20]
The protein content of 9–16% (w/w), Lp 22.8–28.2% (w/w), fiber 5.5–7.2 and CY 3.9–6.1% (w/w), are lower than the other species of the Brazilian Cerrado.[1] The method of extraction of Lp had influence on the yield and composition of fatty acids found in the oil [Tables 3 and 4].
Table 3.
Elemental analysis of seeds from Apeiba tibourbou
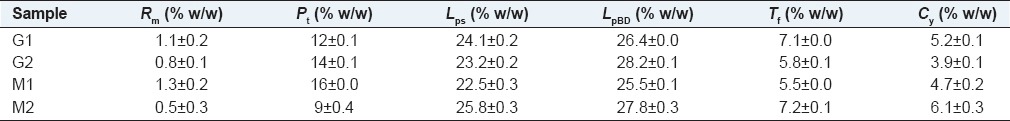
Table 4.
Profile of fatty acids found in seed's Apeiba tibourbou
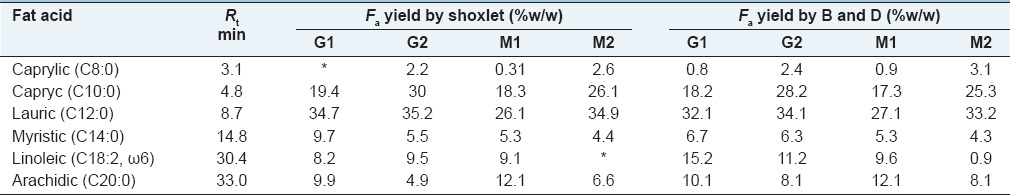
The method of bligh and dyer was able to extraction more total Lp (increase 5%) than the shoxlet. However, the extraction of fatty acid was different for the two methods [Table 3]. Six fatty acids (Fa) were identified [Table 4], five Fa were unsaturated (caprylic, capryc, lauric, myristic, arachidic) and one sutured (linoleic acid). The majority Fa in all samples was lauric acid 27.1–35.2% (w/w), and minority Fa was caprylic acid 0–2.6% (w/w). Table 4 shows that the shoxlet does not extract caprylic from G1 and linoleic acid from M1.
The efficiency of extraction depends on solvent and sample characteristics, temperature, time of extraction, and particle size.[1] The shoxlet method uses hexane, and this solvent is more efficient to extraction of apolar acids, whereas methanol and chloroform used in blingh and dyer method extraction fatty acids neutral, polar and not polar, high temperatures increase the solubility of fatty acid on solvent, but can degrade same compounds by peroxidation or hydrolysis reactions, thus cold extraction methods with bligh and dyer are more suited to preserve the lipid profile.[21]
Due a wide hydrophobicity range of Fa, the extraction with a single solvent is inefficient, neutral Lp form covalently bond, and it can be extracted by apolar solvents. The polar Lp form forces electrostatic and hydrogen bonds with Pt, and to breaking this kind of bonds is required polar solvents as methanol.[21] Single extraction method to characterize the lipid profile is not inefficient, the adequate use more methods.
The stability study shows that Ca and Ra were not altered by the action of ultrasound, there was a range of <0.5% between the sample content and the control.
The UEA values are summarized in Table 5 caffeic acid yield (CaY) ranged 0.016–0.048% (w/w) and rosmarinic acid yield (RaY) 1.3–2.0% (w/w), the analysis of variance in Table 6. This analysis showed that the factors X2, X3 and X22 were significant to extract Ca, and to Ra, X2, X3, interaction between X1. X3 and X2. X3, no linear interaction X12, X22, and X32 were significant.
Table 5.
Box–Behnken factorial design matrices and result of UAE
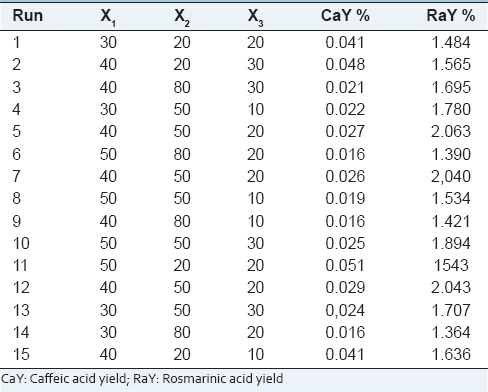
Table 6.
Summary of factor effects and significances (P) ANOVA
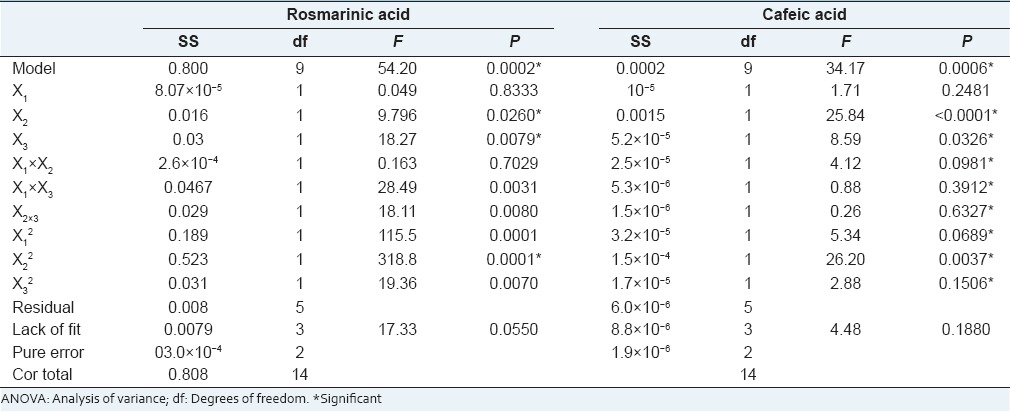
The Model F-value of 54.20 to Ra and 34.17 to Ca implies the model is significant. There is only a 0.02% (Ra) and 0.06% (Ca) chance that a “Model F-Value” this large could occur due to noise. To Ra Lack of Fit F-value was 17.33 implies there is a 5.5%, to Ca the “Lack of Fit F-value” was 4.48 implies there is a 18.8% chance this model to be confused with the noise.
Figures 1 and 2 shows the surface response plot for the CaY and RaY. When there are an increase in temperature (X1) and extraction time (X3) increases extraction of CaY, on the other hand increasing the proportion of ethanol decreases the efficiency of extraction. RaY extraction performance was a parable, when used medium levels the extraction increase [Figure 2].
Figure 1.
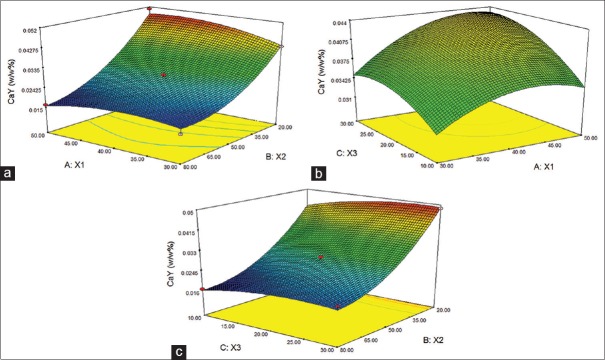
Response surface for the ultrasound assisted extraction (a,b and c) of caffeic acid (Ca) from A. tibourbou (CaY % = 0.027−0.014. X2+0.003. X3+0.007. X22 [Eq. 1])
Figure 2.
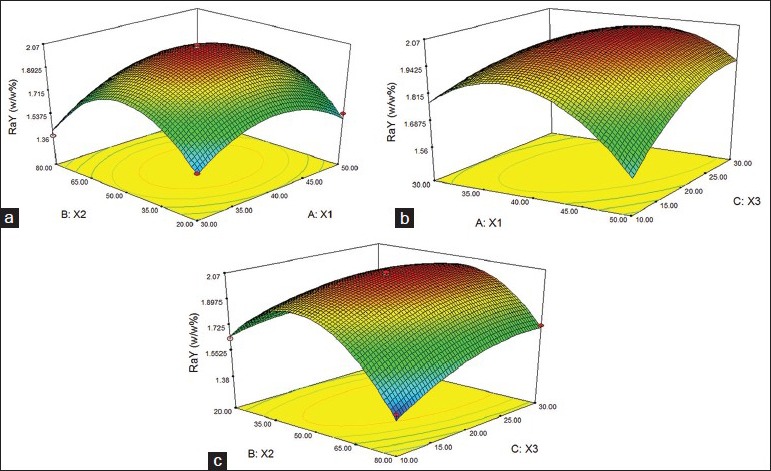
Response surface for the ultrasound assisted extraction (a,b and c) of Rosmarinic acid (Ra) from A. tibourbou (RaY%=2.05–0.04. X2+0.06. X3−0.01.X1.X2+0.11.X1.X3+ 0.09.X2.X3−0.23. X12−0.38. X22−0,09 X32 [Eq 2])
The theoretical optimized conditions was calculated by RSM, 42°C, 30% (alcohol degree) and 24 min, this conditions maximize simultaneously the extraction of Ca (0, 04%) and Ry (1.89), but the max extraction of RaY could be obtained using 50% of ethanol. Ra is more polar than Ca, than the extraction was favored with higher concentrations of alcohol.
The verification test showed that the Ra and Ca contents obtained from extraction under optimal conditions were 0.042 ± 0.01% w/w (n = 5) to Ca and 1.85 ± 0.08% w/w (n = 5). A good correlation between the theoretical results and the rechecked values confirmed that the response model [Equations 1 and 2] represented the expected optimization well.
CONCLUSION
It was observed that the extraction method alters the chemical composition of the extract, and it is possible to extract Ca and Ra from A. tibourbou's leaves using UEA. Optimization studies are important for predicting the extraction behavior of herbal compounds of interest in terms of controllable factors, such as extraction time, alcohol content, and particle size, to predict and minimize the costs involved in the production of herbal extracts.
ACKNOWLEDGMENTS
The authors would like to thank CAPES and CNPQ for their financial support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Martins FS, Borges LL, Paula JR, Conceicao EC. Impact of different extraction methods on the quality of Dipteryx alata extracts. Rev Bras Farmacognosia. 2013;23:521–6. [Google Scholar]
- 2.Martins FS, Moraes CS, Bara F, Conceição EC. Obtainment and characterization of raw material of Brosimum gaudichaudii Trecul (Moraceae) J Pharm Res. 2010;4:3. [Google Scholar]
- 3.Oliveira SE, Freitas SF, Martins SM, Couto RO, Pinto MV, Paula JR, et al. Vol. 35. Nova, São Paulo: Quím; 2012. Isolation and quantitative HPLC-PDA analysis of lupeol in phytopharmaceutical intermediate products from Vernonanthura ferruginea (Less.) H. Rob; pp. 1041–5. [Google Scholar]
- 4.Couto RO, Oliveira SE, Martins FS, de Freitas O, Bara MT, de Paula JR, et al. Processing of Apeiba tibourbou Aubl. Extract via spray drying. Lat Am J Pharm. 2012;31:7. [Google Scholar]
- 5.Filho CV, Yunes RA. Estrategies for obtaining pharmacologically active compounds from medicinal plants. concepts about structural modification for improve the activity. Quím Nova. 1998;20:6. [Google Scholar]
- 6.Jacomassi E, Moscheta S, Machado SR. Morphoanatomy and histochemistry of the reproductive organs from Brosimum gaudichaudii (Moraceae) Rev Bras Bot. 2010;33:14. [Google Scholar]
- 7.Yan X, Suzuki M, Ohnishi-Kameyama M, Sada Y, Nakanishi T, Nagata T. Extraction and identification of antioxidants in the roots of yacon (Smallanthus sonchifolius) J Agric Food Chem. 1999;47:4711–3. doi: 10.1021/jf981305o. [DOI] [PubMed] [Google Scholar]
- 8.Grigonis D, Venskutonis PR, Sivik B, Sandahl M, Eskilsson CS. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloe odorata) J Supercrit Fluid. 2005;33:223–33. [Google Scholar]
- 9.Jadhav D, Rekha BD, Gogate PR, Rathod VK. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J Food Eng. 2009;93:421–6. [Google Scholar]
- 10.Martins FS. Impact of different extraction methods on the quality of Dipteryx alata extracts. Rev Bras Farmacognosia. 2013;23:521. [Google Scholar]
- 11.Heng MY, Tan SN, Yong JW, Ong ES. Emerging green technologies for the chemical standardization of botanicals and herbal preparations. TrAC Trends Analyt Chem. 2013;50:1–10. [Google Scholar]
- 12.Ramos L. Critical overview of selected contemporary sample preparation techniques. J Chromatogr A. 2012;1221:84–98. doi: 10.1016/j.chroma.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Tang F, Zhang Q, Nie Z, Yao S, Chen B. Sample preparation for analyzing traditional Chinese medicines. TrAC Trends Analyt Chem. 2009;28:1253–62. [Google Scholar]
- 14.15th ed. Washington, DC: Association of Official Analytical Chemists; 1998. AOAC. Official methods of analysis of the Association of Official Analytical Chemists. [Google Scholar]
- 15.Roesler R, Malta LG, Carrasco LC, Holanda RB, Sousa CA. GMP. Antioxidant activity of cerrado fruits. Food Sci Technol. 2007;27:53–60. [Google Scholar]
- 16.Oliveira GD. Goiânia: Federal University of Goiás; Optimization of processes for the production and drying of rosemary extract. [Google Scholar]
- 17.Washington, DC: Pharmacopeial Convention Rockville; 2011. USP. United States Pharmacopeial Convention. U.S. [Google Scholar]
- 18.Couto RO, Conceição EC, Chaul LT, Oliveira EM, Martins FS, Bara MT, et al. Spray-dried rosemary extracts: Physicochemical and antioxidant properties. Food Chem. 2012;131:99–105. [Google Scholar]
- 19.Kostka T, Drai J, Berthouze SE, Lacour JR, Bonnefoy M. Physical activity, fitness and integrated antioxidant system in healthy active elderly women. Int J Sports Med. 1998;19:462–7. doi: 10.1055/s-2007-971945. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi ML, Antunes LM. Radicais livres e os principais antioxidantes da dieta. Rev Nutr Camp. 1999;12:7. [Google Scholar]
- 21.Akoh CC. 3rd ed. New York: CRC Press; 2008. DBM. Food Lipids: Chemistry, Nutrition and Biotechnology; p. 928. [Google Scholar]


