Abstract
Background:
The incidence of skin cancers is rising gradually. The treatment of melanoma is also necessary to prevent the spread of cancer to other body organs. Scientific literatures have not documented any evidence of the antitumor potential of Ipomoea pes-caprae on melanoma.
Aim of the Study:
Explore in vivo antitumor potential of I. pes-caprae on melanoma cancer.
Materials and Methods:
Petroleum ether (60°C–80°C), methanolic and aqueous extracts, and swaras prepared from the whole herb of I. pes-caprae were assessed for their antitumor activity. The extracts and swaras at doses of 25 and 50 mg/kg b. wt. were administered intraperitoneal along with chemo and radiotherapy for 40 days for exploring antitumor activity against melanoma cancer (B16F10) in male C57BL mice. The results obtained from tumor volume, and histopathological studies were compared with the control and dacarbazine used as a standard.
Results:
Antitumor effect of I. pes-caprae extracts and swaras on melanoma cancer was found to be significant (P < 0.01) compared to normal control. The tumor volume inhibition against tumor-bearing mice, although differed from each other, was concentration dependent. Administration of plant extracts and swaras from the day 1 since tumor inducted. The induction of tumor was found delayed by 10–15 days and the tumor volume on the day 40 was similar to the Dacarbazine treatment used as a standard.
Conclusion:
The results obtained from the tumor volume and histopathological studies clearly revealed the antitumor potential of I. pes-caprae on melanoma cancer.
Keywords: Chemo and radiotherapy, dacarbazine, enzymatic antioxidants, Ipomoea pes-caprae, melanoma cancer, tumor volume
INTRODUCTION
Herbs and their extracts are inexpensive and rich resources of active compounds that can be utilized as novel cytotoxic agents as well as can be used for the treatment of dermatological disorders associated with melanin hyperpigmentation. In vivo antitumor activity is an emerging research area in cancer biology. Research in the development of antitumor drugs, worldwide, has been focused on screening therapeutically effective and safe, synthetic and herbal molecules. There are numerous natural or synthetic drugs which were studied for their antitumor potentials, but none of them have uniqueness to meet all the desired requisites, that is, freedom from cumulative or irreversible toxicity, effective long-term protection, prolong stability and ease of administration.[1]
Ipomoea pes-caprae (Convolvulaceae) also known as do-patti-lata, railroad vine, goat's foot and morning glory, is a valuable medicinal plant, distributed in the tropics and subtropics. Traditionally, leaf juice of I. pes-caprae used as a first aid for treatment of jellyfish stings.[2] In some part of India, it is used in ritual baths to alleviate evil spirits. The plant is astringent, acrid, refrigerant, mucilaginous, somatic, laxative, diuretic and tonic and used in the treatment of skin diseases, boils, swelling, wounds, ulcer, carbuncle, dropsy, menorrhagia, hemorrhoids, colic, flatulence, dyspepsia, cramp, and burning sensation. Many activities have also been reported for I. pes-caprae revealing its antioxidant, analgesic and anti-inflammatory, antispasmodic, antinociceptive activities, antihistaminic, immunostimulant, insulinogenic, hypoglycemic,[3] antimicrobial, antifungal and antibacterial characteristics.[4] Kirtikar and Basu[5] have reported its use in the inhibition of platelet aggregation, diarrhea, vomiting, and piles.
Although enough literature is available on medicinal aspects of I. pes-caprae, but there are still few research evidence supporting its antitumor activity. Therefore, the present investigation was aimed to explore it's in vivo antitumor potential using its extracts and swaras on B16F10 melanoma cancer cell line.
MATERIALS AND METHODS
Plant material
Ipomoea pes-caprae with its leaves, stems, and flowers was collected on December 2008 from Indian Ocean from Kuttomangalam Mandaikadu, District, Kanyakumari (Tamil Nadu) and authenticated by Botanical Survey of India, (Pune) with reference number: BSI/WC/Tech/09/447 and voucher specimen (V. No. ASIP1).
Preparation of extracts
Freshly collected plant material was shade-dried, powdered, and sieved through 20 mesh size. 100 g powdered material was soxhleted with petroleum ether (60°C–80°C) followed by methanol. The aqueous extract was prepared by treating marc (obtained after methanolic extraction) with lukewarm water for 24 h and then filtering it with filter paper. The solvent from respective extracts was then recovered at low temperature (<40°C) under reduced pressure. Swaras was prepared by taking fresh plant material in a mixture cum grinder and grinding it well with double distilled water to convert it to a pasty consistency and then straining the paste with thick cotton cloth. The extract so obtained was kept overnight for sedimentation after which it was decanted and dried at room temperature to get the swaras.
Chemicals
Analytical grade dacarbazine (daczin, 200 mg) (Indon, Zydus Cadila Healthcare Ltd., Ahmedabad, India), MEM Media, Tris-HCl, Hydroxylamine hydrochloride (Hi-media Laboratories Ltd., Mumbai, India), nitroblue tetrazolium (NBT), sodium azide, ethylene diamine tetra acetic acid (EDTA), Hydrogen peroxide, Reduced glutathione, 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB), Sodium citrate, Sodium dodecyl sulfate (Sisco Research Laboratory, India), thiobarbituric acid (TBA) and trichloroacetic acid (TCA) (Merck India Ltd, Mumbai, India) were used for study.
Experimental animals
Adult male C57BL mice aged 6–8 weeks with an average weight of 25–30 g were obtained from animal house of Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal. The animals were housed in standard polypropylene cages and maintained in the air conditioned animal house (20°C–25°C; relative humidity 70–75%) in a 12 h. light-dark cycle. The animals were fed on a standard laboratory diet and water ad libitum. The studies were done in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) norms and institutional regulations and national criteria for animal experiments. Institutional approval for experimental work as per Animal Ethical Committee of Research, Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal, India with the CPCSEA Reg. No. 500/01/a/CPCSEA/2001 (2004/EC/2010-18.11.2010) was taken.
EXPERIMENTAL DESIGN
A total of 72 adult male C57BL mice aged 6–8 weeks were divided into 12 groups thus each group containing 6 animals. Animals of Group A, receiving normal saline (10 mL/kg b. wt.) treated as Normal Control. Group A1 (tumor-bearing mice) receiving normal saline (10 mL/kg) treated as Tumor Control. Group A2 and A3 animals (tumor-bearing mice) receiving I. pes-caprae extract (IPCE) (25 and 50 mg/kg), respectively, treated as sample drug. Animals of Group B receiving normal saline (10 mL/kg) + radiation (8 Gy in 4 fractions) treated as radiation control. Group B1 (tumor-bearing mice) receiving normal saline (10 mL/kg) + radiation (8 Gy in 4 fractions) treated as tumor radiation control. Group B2 and B3 (tumor-bearing mice) receiving IPCE (25 and 50 mg/kg) + radiation (8 Gy in 4 Fractions), respectively, treated as Sample Drug Adjuvant to Radiotherapy. Group C1 and C2 receiving IPCE (25 and 50 mg/kg), respectively, from day 1 of tumor induction treated as sample drug co-treatment with tumor. Group D (tumor-bearing mice) receiving dacarbazine (50 mg/kg, alternate days) served as a standard control. Group E (tumor-bearing mice) receiving dacarbazine (50 mg/kg, alternate days) + IPCE (50 mg/kg) treated as a sample drug adjuvant to chemotherapy. Dacarbazine and plant extracts were administered intraperitoneally for 40th day's duration.
Irradiation of mice
60Cobalt Theratron Teletherapy Unit 780°C (Canada) available at Department of Radiotherapy, Jawaharlal Nehru Cancer Hospital and Research Center, Bhopal, was used for irradiation of mice. Anesthetized mice were retrained in well-ventilated polypropylene box and tumor was exposed to 8 Gy in 4 fractions at a dose rate of 1 Gy/min maintain field size at 15 cm × 10 cm at a distance of 75–80 cm from the source. Tumor bearing animals were irradiated 4 times (8 Gy in 4 fractions) during the whole experiment at sufficient intervals.
Tumor induction and its assessment
Tumors were removed aseptically from the tumor-bearing animals. Cell suspension was then made from isolated tumor in MEM Media. Four lacks viable cells were injected intradermally on the hair removed dorsal skin of mice. After 8–10 days of injection, the tumor started budding. When the tumor was developed to a palpable level, two doses of the plant extracts and swaras at 25 and 50 mg/kg b. wt. of mice were injected intraperitoneally daily up to 40 days. During the treatment, the size of implanted tumor [Figure 1a] was regularly measured at given time interval, with a digital caliper and tumor volume was calculated by the formula:
Figure 1.
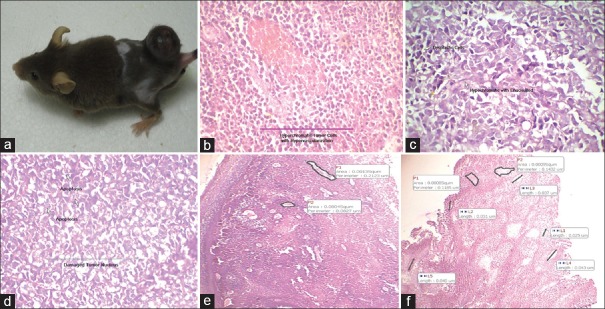
Histopathological evidence of drug treatment on tumor: (a) Figure showing tumor mice; (b)Line indicate hyperchromatic tumor cells with hypervascularization; (c) Arrow indicates dysplastic and Hypochromatic with enucleated cells; (d) Arrow indicates apoptosis and damaged tumor nucleus; (e) Marked areas indicate perimeter of blood vessels; (f) Marked areas indicate length of blood vessels
Tumor volume = (Shortest diameter)2× (Longest diameter) ×0.5.
Toxicity/safety of Ipomoea pes-caprae by evaluating enzymatic antioxidant activity
Enzymatic antioxidant activity was evaluated by sacrificing all the experimental mice were on day 41st by cervical dislocation and liver was removed.
Preparation of liver homogenate
10% liver cell homogenate was prepared by homogenizing liver with buffer containing 0.25 M sucrose and 0.1M Tris-HCl buffer (pH 7.4), in Teflon pestle and glass homogenizer and centrifuged at 1000 rpm for 10 min. The decanted supernatant was separated and used to measure thiobarbituric acid reactive substance (TBARS). The supernatant was again centrifuged at 8000 rpm for 15 min and level of antioxidant enzymes (SOD, CAT, and GSHpx) was analyzed.[6,7]
Estimation of lipid peroxidation
Lipid peroxidation in the liver cell homogenate was estimated by treating 0.2 mL of cell homogenate with 1.5 mL of 20% acetic acid, 0.2 mL of 8.1% sodium dodecyl sulfate and 1.5 mL of 0.8% TBA, used to estimate concentration of malondialdehyde. The volume of the mixture was made up to 4.0 mL with distilled water and then heated at 95°C in a water bath for 60 min. After incubation, the tube was cooled at room temperature and final volume was made up to 5.0 mL in each tube with distilled water. 5.0 mL n-butanol-pyridine (15:1) mixture was added, and the content was vortexed thoroughly for 2 min. After centrifugation at 3000 rpm for 10 min, the organic upper layer was decanted off, and its optical density was measured at 532 nm against blank without liver cell homogenate. The level of lipid peroxidation was expressed as in moles of malondialdehyde (MDA)/mg protein in liver cell homogenate.[8]
Assay of glutathione peroxidase
To 0.1 mL of the liver cell homogenate, 0.4 mL of buffer, 0.2 mL sodium azide, 0.2 mL EDTA, 0.2 mL of hydrogen peroxide, and 0.2 mL of reduced glutathione were added and volume was made up to 2 mL with distil water. The tubes were incubated at 37°C for 10 min, and 1 mL of TCA was added to terminate the reaction. The reaction mixture was centrifuged at 3000 rpm for 10 min. To 1 mL of supernatant, 2 mL of disodium hydrogen phosphate and 1 mL of TCA was added, and 1 mL of DTNB was added just prior to the estimation. The absorbance was read at 412 nm against a blank without liver homogenate. The concentration was expressed as units/mg of protein.[9,10]
Assay of catalase
The test tube containing reaction mixture of 0.1 mL of liver homogenate, 1 mL of Phosphate buffer (0.1 M) and 0.4 mL of H2O2 was incubated at 37°C for 10 min. The reaction was stopped by the addition of 2 mL of 5% of dichromate-acetic acids reagent (5% potassium dichromate and glacial acetic acids in 1:3 ratio). The blank was treated out without liver cell homogenate. The absorbance was read at 620 nm. The catalase activity was expressed as μM H2O2 consumed/min/mg of protein. The enzyme activity was expressed as units/mg protein.[11,12]
Assay of superoxide dismutase
The assay based on the reduction of NBT to water insoluble blue formazan. To 0.5 mL of liver cell homogenate, 1 mL of 50 mM sodium carbonate, 0.4 mL of 24 μM NBT, and 0.2 mL of 0.8 mM EDTA were added. The reaction was initiated by adding 0.4 mL of 1 mM hydroxylamine hydrochloride. The control was simultaneously prepared without liver cell homogenate. Absorbance of reacting mixture was measured at 560 nm. Units of SOD activity were expressed as the amount of enzymes required to inhibit the reduction of NBT by 50%. The specific activity was expressed in terms of units/mg of protein.[13,14]
Histopathological study
After the completion of drug treatment (40 days), on the day 41st mice were sacrificed by cervical dislocation. The tumor of three animals from each group was dissected out, fixed in 10% buffered formalin for 12 h and processed for histopathological examination. 4 μm-thick paraffin sections were cut and stained with hematoxylin and eosin and mounted in DPX. Sections were qualitatively assessed under the light microscope for their architecture.
Statistical analysis
The experimental data were expressed as mean ± standard error the significance of difference among the various treated and control groups was analyzed by means of one-way ANOVA followed by Dunnett's multiple comparison tests using Graphat Instat Software (San Diego, CA, USA). The P < 0.01 were considered statistically significant.
RESULTS
Effect of Ipomoea pes-caprae extract on tumor volume
The study of tumor volume of mice [Figure 1a] shown in Tables 1 and 2 revealed that in the groups (A2 and A3) which received methanolic extract of I. pes-caprae at 25 and 50 mg/kg, b. wt. a reduction in the tumor volume was observed. On the day 40th of treatment, the reduction in the tumor volume was found to be significant (P < 0.01) for both the doses compared to tumor control group (A1), which received normal saline, 10 mL/kg, b. wt. When methanolic extract of I. pes-caprae at 25 and 50 mg/kg was used as an adjuvant to radiotherapy groups (B2 and B3) receiving radiation dose of 8 Gy in 4 fractions, there was a statistically significant (P < 0.01) reduction in tumor volume on 40th day of tumor induction as compared to tumor control radiation group (B1) as well as test drug group (A2 and A3). In groups (C1 and C2) which received methanolic extract of I. pes-caprae at 25 and 50 mg/kg, b. wt. from the day of tumor induction, the reduction in the tumor volume was found to be significant (P < 0.01) as compared to the group (A2 and A3). When dacarbazine (D) was administered alone, it caused more reduction in tumor volume as compared to all groups on 40th day of tumor induction but when administered in combination with I. pes-caprae (50 mg/kg, b. wt.) the effect was more pronounced. Aqueous extract of I. pes-caprae revealed similar activity to that of methanolic extract. Petroleum ether extract and swaras were also found effective (data were not shown) against melanoma tumor, but the activity was relatively less.
Table 1.
Tumor volume of mice subjected to methanol extract of IPCE, alone and as an adjuvant to radio and chemotherapy
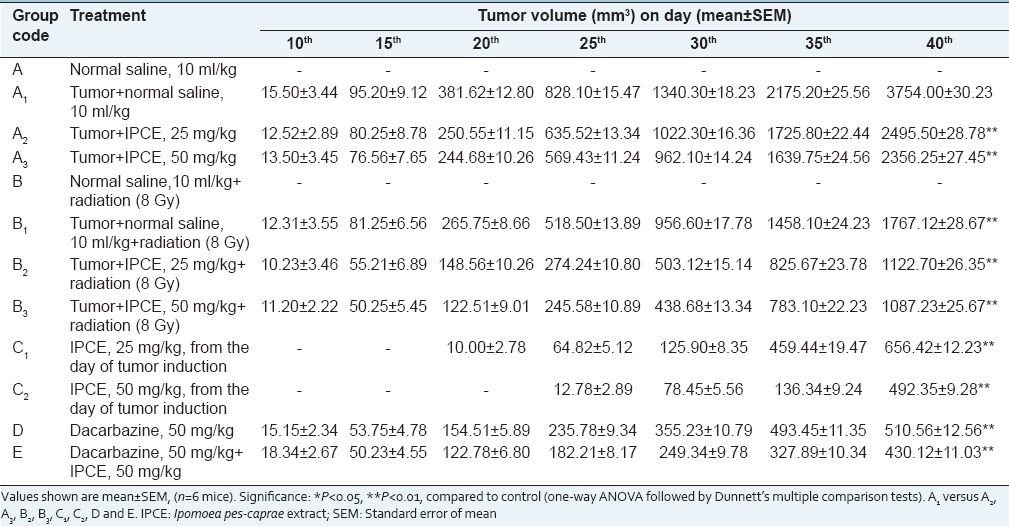
Table 2.
Tumor volume of mice subjected to aqueous extract of I. pes-caprae, alone and as an adjuvant to radio and chemotherapy
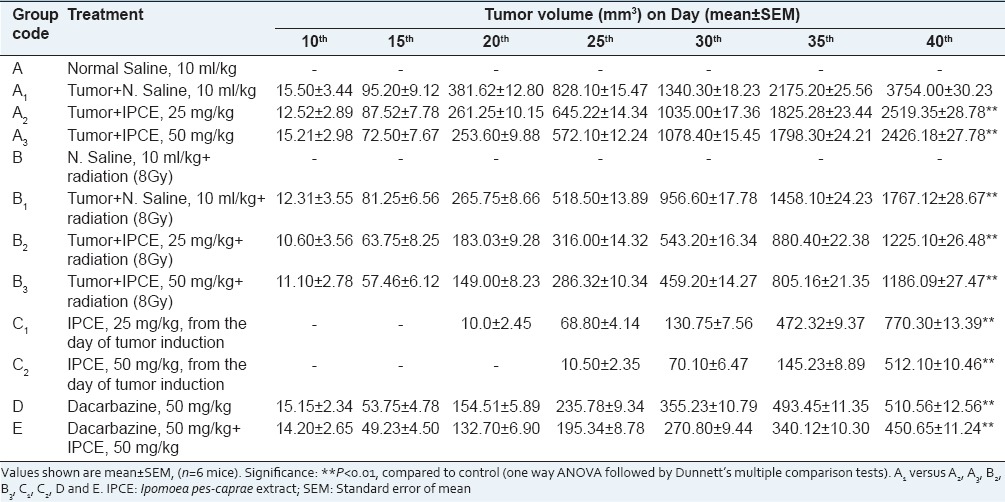
Overall study revealed that the methanolic extract of I. pes-caprae exhibited stronger antitumor effect against melanoma tumor as compared to aqueous and petroleum ether extracts and swaras.
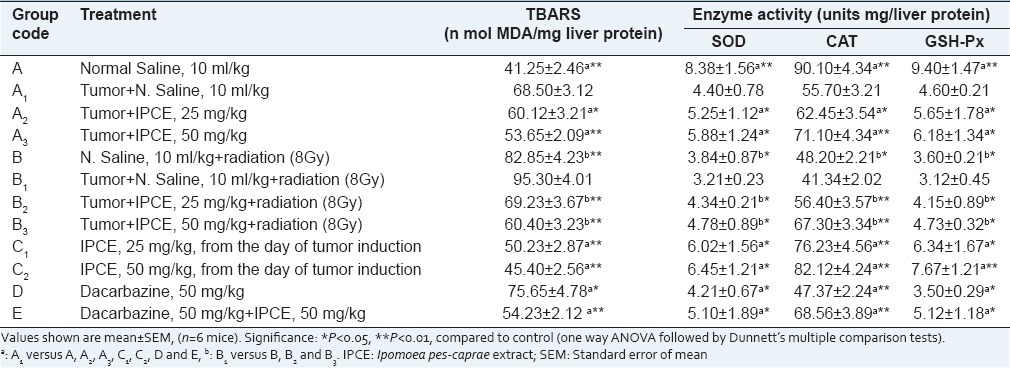
Effect of Ipomoea pes-caprae on enzymatic antioxidant
The result of enzymatic antioxidant activity of mice liver has been shown in Tables 3 and 4. Antioxidant enzymes activities of SOD, CAT and GSH-Px (units mg/protein) in the liver cell homogenate were significantly (P < 0.01) decreased and the level of MDA (n mol MDA/mg protein) was increased in tumor control, radiation and dacarbazine treated groups as compared to normal control group. Administration of methanolic extract of I. pes-caprae at the doses of 25 and 50 mg/kg, body weight. significantly (P < 0.01) restored the activities of the antioxidant enzymes and the level of glutathione peroxidase and also inhibited the formation of MDA in mice which was near to normal control group. In the groups C1 and C2, which received I. pes-caprae extract at 25 and 50 mg/kg body weight from the day of tumor initiation, the activities of SOD, CAT, GSH-Px, and MDA were much closer to normal control. A similar result was observed in aqueous, petroleum ether extracts and swaras of I. pes-caprae. Methanolic and aqueous extracts of I. pes-caprae exhibited stronger antioxidant activity as compared to petroleum ether extract and swaras at a dose of 50 mg/kg body weight.
Table 3.
Effect of methanol extract of I. pes-caprae on activities of SOD, CAT, GSH-Px and levels of MDA in mice liver
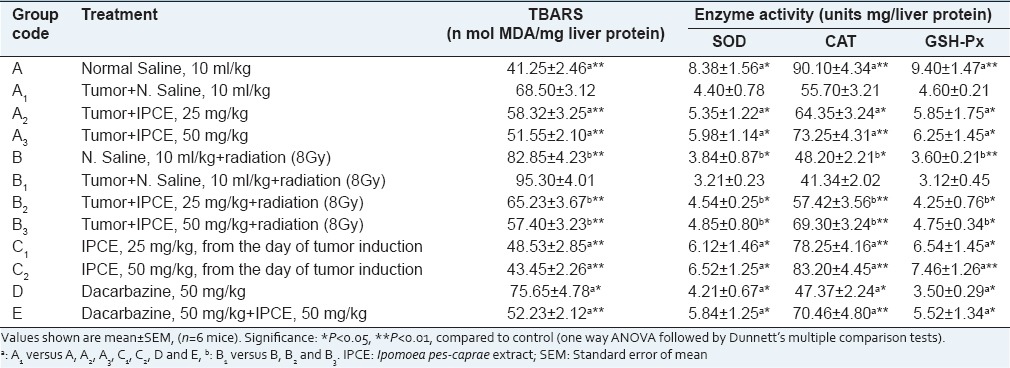
Table 4.
Effect of aqueous extract of I. pes-caprae on activities of SOD, CAT, GSH-Px and levels of MDA in mice liver
Histopathological analysis (effect of Ipomoea pes-caprae on melanoma tumor)
We have evaluated the efficacy of I. pes-caprae on B16F10 mice melanoma tumors implanted subcutaneously in C57BL mice. The observation for histopathology was made on blood vessels, proliferating tumor cells, area of tumor regression, apoptosis and reduction in tumor mass. Histology of the tumor control group (A1) revealed the presences of hypervascularization where the perimeter of blood vessels was large with the presence of hyperchromic tumor cells [Figure 1b]. The groups (A2 and A3), treated with methanolic and aqueous extracts of I. pes-caprae at a dose of 25 and 50 mg/kg, body weight revealed the presence of hypochromic with enucleated tumor cells with apoptosis [Figure 1c]. Tumor bearing mice group (B1) which received normal saline (10 mL/kg, b. wt.) and groups (B2 and B3) that received both the doses of methanolic and aqueous extract, along with radiation (8 Gy in 4 fractions) revealed the presences of damaged tumor nucleus and apoptosis [Figure 1d]. Analysis of the melanoma tumor sections on groups (C1 and C2) which received methanolic and aqueous extracts of I. pes-caprae at 25 and 50 mg/kg, body weight from the day of tumor induction showed a progressive increase of apoptotic cells and perimeter of blood vessels and vascular supply was less [Figure 1e]. The apoptosis and dysplastic cells were significantly higher in tumors treated with group D and E. Hypovascularization with small perimeter (length) of blood vessels and enucleated cells was also observed [Figure 1f]. The incidence of dysplastic cells and apoptosis in the tumor almost corresponded to the effect of tumor growth inhibition, suggesting that treatment resulted in tumor regression by significant augmentation of apoptosis. However, petroleum ether extract and swaras of I. pes-caprae were found less effective as compared to methanolic and aqueous extract.
DISCUSSION
Cancer is a growing health problem worldwide, with the introduction of 6 million new cases every year. Many approaches are being tried through modulation of antitumor immune response, apoptosis, and antitumor proteins for cancer treatment.[15] As tumor cells are normally resistant to apoptosis, therefore, selective killing of tumor cells by promoting apoptosis is an effective approach for cancer therapy. Treating cancer with herbals has been an easy and effective approach, and there are evidences suggesting that herbal medicines can favorably modify and improve the conventional approach of cancer treatment.
In the current study, we have selected I. pes-caprae extracts and swaras to treat the melanoma tumor. The mice were treated with I. pes-caprae extracts (methanolic, aqueous, and petroleum ether) and swaras at a dose of 25 and 50 mg/kg, body weight. In the control group receiving normal saline, initially, a slow and steady growth in tumor volume was observed with a drastic increase in tumor size after few days.
In the present finding, the cancer treatment through radiotherapy with the combination of I. pes-caprae was found more effective as compared to the extract alone. Earlier studies on herbs were reported that in cancer therapy many drugs when given alone give good results, however, when combined with other drugs exerting synergistic effect, the results are better. The objective of such approach is to minimize drug resistance and drug toxicity.
In the mice group which received I. pes-caprae (extracts and swaras) at 25 and 50 mg/kg, body weight from the day of tumor induction, the reduction was observed in the tumor volume as compared to tumor control group and the growth of tumor induction was also delayed by 10–15 days. The positive control, dacarbazine was found to be effective in a reduction of tumor volume. However, when dacarbazine was given in combination with I. pes-caprae the effectiveness of treatment was augmented. Recent researches confirm the utility of herbs to both control cancer growth and to reduce side effects of chemotherapy. In addition, some herbs can reverse multidrug resistance.[16]
Elevated level of lipid peroxidation is the indication of cellular damage and loss of functional integrity of the cell membrane in liver. Damage to the liver cells causes release of cellular enzymes into the serum. Decrease in the activity of SOD in the liver of chemo and radiotherapy-treated mice may be due to the increased lipid peroxidation or inactivation of the enzyme by cross-linking with malondialdehyde.[17] Dacarbazine and irradiation increase the hepatic activation, leading to the formation of toxic metabolites which damage to the liver tissues as reflected by increased level of lipid peroxidation. Measurement of plasma TBARS helps to assess the extent of tissue damage in pathological conditions.[18]
The major antioxidant enzymes including SOD, GPx, and CAT are regarded as the first line of the antioxidant defense against reactive oxygen species generated in vivo during oxidative stress. Superoxide dismutates radicals form hydrogen peroxide, which in turn is decomposed to water and oxygen by GSH-Px and CAT, thereby preventing the formation of hydroxyl radicals.[19] These enzymes protect the red cells against O2 and H2O2 radical mediated lipid peroxidation.[20]
On the basis of the present study, it can be suggested that the I. pes-caprae-treated mice restores the changes in the activity of the antioxidant enzymes and the level of glutathione. It was also observed that the group receiving extracts and swaras of I. pes-caprae from the day of tumor induction reduced the level of lipid peroxidation and restored the enzymatic changes similar to normal control group. Drugs with multiple protective mechanisms including antioxidant activity may be one way of minimizing the tissue injury.[21]
A numbers of drugs are used in cancer chemo and radiotherapy, and most of them exhibit cell toxicity and can induce genotoxic, carcinogenic, and teratogenic effects in non-tumor cells.[22] These side effects limit the use of conventional chemotherapeutic agents despite their high efficacy in treating cancerous cells. Therefore, the search for alternative drugs/molecules that are effective and non-toxic in the treatment of cancers is an important research area.[23] In fact, sincere efforts are being made to isolate bio-actives from medicinal plants for their potential in cancer treatment.[24]
There are emerging scientific evidences of herbal medicines playing an important role in the supportive care of cancer therapy. Betulinic acid isolated from various plants including I. pes-caprae was identified as a highly selective growth inhibitor of human melanoma and non-melanoma,[25] neuroectodermal and malignant tumor cells and was reported to induce apoptosis in numerous cancer cell lines in vivo and in vitro.[26] It strongly and consistently suppresses the growth of all human melanoma cell lines. Apart, essential oils such as limonene, α-terpineol, α-humulene, α-thujene, α-pinene, eugenol, and β-myrcene present in many plants have been reported for their cytotoxic activity.[27,28,29] Although these essential oil components have been reported in the leaves of I. pes-caprae,[30] but so for no such report that the antitumor effect of I. pes-caprae is due to its essential oil component is available. However, it cannot be ignored that partial antitumor effect of I. pes-caprae may be attributed to the presences of these essential oil components present in its leaves.
CONCLUSION
In the present study, the in vivo antitumor activity of I. pes-caprae against mice melanoma (B16F10) cancer cells has been explored. Petroleum ether extract and swaras of I. pes-caprae also showed antitumor activity but compared to methanolic and aqueous the effect was found less. The activity of I. pes-caprae was found in following order methanolic extract > aqueous extract > swaras > petroleum ether extract. Further, the antitumor activity of I. pes-caprae in mice may be attributed to the presence of polar phytoconstituents such as alkaloids, flavonoids, tannins, terpenoids, and glycosides present in the crude extract (maximum in methanolic and aqueous) of I. pes-caprae. Further studies are needed to elucidate the molecular mechanism of action and potential usefulness of I. pes-caprae as an agent for cancer therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Arora R, Chawla R, Singh S, Sagar RK, Kumar R, Sharma A, et al. Bioprospection for radioprotective molecules from indigenous plants. In: Govil JN, editor. Recent Progress in Medicinal Plants. Phytomedicine. Vol. 16. Houston, Texas, USA: Published by Studium Press LLC; 2006. pp. 179–219. [Google Scholar]
- 2.Pongprayoon U, Bohlin L, Wasuwat S. Neutralization of toxic effects of different crude jellyfish venoms by an extract of Ipomoea pes-caprae (L.) R. Br. J Ethnopharmacol. 1991;35:65–9. doi: 10.1016/0378-8741(91)90133-x. [DOI] [PubMed] [Google Scholar]
- 3.Manigauha A, Ganesh N, Kharya MD. Morning glory: A new thrust in-search of de-novo therapeutic approach. Int J Phytomed. 2010;2:18–21. [Google Scholar]
- 4.Bragadeeswaran S, Prabhu K, Rani SS, Priyadharsini S, Vembu N. Biomedical application of beach morning glory Ipomoea pes-caprae. Int J Trop Med. 2010;5:81–5. [Google Scholar]
- 5.Basu K. Fourth Reprint. 2nd ed. Vol. 3. Allahabad, India: Published by Lalit Mohan Basu; 2006. A Text Book of Indian Medicinal Plant; p. 1726. [Google Scholar]
- 6.Kaur G, Alam MS, Jabbar Z, Javed K, Athar M. Evaluation of antioxidant activity of Cassia siamea flowers. J Ethnopharmacol. 2006;108:340–8. doi: 10.1016/j.jep.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 8.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 9.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–21. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 10.Manigauha A, Ganesh N, Kharya MD. Hepatoprotection by Kaempferia galanga against carbon tetrachloride induced liver damage in rats. Indian Drugs. 2010;47:55–60. [Google Scholar]
- 11.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 12.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 13.Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 14.Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–5. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 15.Tascilar M, de Jong FA, Verweij J, Mathijssen RH. Complementary and alternative medicine during cancer treatment: Beyond innocence. Oncologist. 2006;11:732–41. doi: 10.1634/theoncologist.11-7-732. [DOI] [PubMed] [Google Scholar]
- 16.Shu X, McCulloch M, Xiao H, Broffman M, Gao J. Chinese herbal medicine and chemotherapy in the treatment of hepatocellular carcinoma: A meta-analysis of randomized controlled trials. Integr Cancer Ther. 2005;4:219–29. doi: 10.1177/1534735405279927. [DOI] [PubMed] [Google Scholar]
- 17.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–27. [PubMed] [Google Scholar]
- 18.Dasgupta T, Rao AR, Yadava PK. Chemomodulatory action of curry leaf (Murraya koenigii) extract on hepatic and extrahepatic xenobiotic metabolising enzymes, antioxidant levels, lipid peroxidation, skin and forestomach papillomagenesis. Nutr Res. 2003;23:1427–46. [Google Scholar]
- 19.Yao D, Shi W, Gou Y, Zhou X, Yee Aw T, Zhou Y, et al. Fatty acid-mediated intracellular iron translocation: A synergistic mechanism of oxidative injury. Free Radic Biol Med. 2005;39:1385–98. doi: 10.1016/j.freeradbiomed.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Scott MD, Lubin BH, Zuo L, Kuypers FA. Erythrocyte defense against hydrogen peroxide: Preeminent importance of catalase. J Lab Clin Med. 1991;118:7–16. [PubMed] [Google Scholar]
- 21.Halliwell B. Drug antioxidant effects. A basis for drug selection? Drugs. 1991;42:569–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philip PA. Experience with docetaxel in the treatment of gastric cancer. Semin Oncol. 2005;32:S24–38. doi: 10.1053/j.seminoncol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Tang W, Hemm I, Bertram B. Recent development of antitumor agents from Chinese herbal medicines. Part II. High molecular compounds (3) Planta Med. 2003;69:193–201. doi: 10.1055/s-2003-38494. [DOI] [PubMed] [Google Scholar]
- 24.Kinghorn AD, Su BN, Jang DS, Chang LC, Lee D, Gu JQ, et al. Natural inhibitors of carcinogenesis. Planta Med. 2004;70:691–705. doi: 10.1055/s-2004-827198. [DOI] [PubMed] [Google Scholar]
- 25.Selzer E, Pimentel E, Wacheck V, Schlegel W, Pehamberger H, Jansen B, et al. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J Invest Dermatol. 2000;114:935–40. doi: 10.1046/j.1523-1747.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- 26.Fisher DE. Apoptosis in cancer therapy: Crossing the threshold. Cell. 1994;78:539–42. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 27.Jessica AM, Patricia AT, Iman AH, Sherry Chow HH, Cynthia AT. d-Limonene: A bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol Rev. 2011;5:31–42. [Google Scholar]
- 28.Hassan SB, Gali-Muhtasib H, Göransson H, Larsson R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 2010;30:1911–9. [PubMed] [Google Scholar]
- 29.Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, et al. Helichrysum gymnocephalum essential oil: Chemical composition and cytotoxic, antimalarial and antioxidant activities, attribution of the activity origin by correlations. Molecules. 2011;16:8273–91. doi: 10.3390/molecules16108273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniel EP, Brkic D, Joelle QL. GC-MS Analysis of the leaf essential oil of Ipomea pes-caprae, a traditional herbal medicine in mauritius. Nat Prod Commun. 2007;2:1225–8. [Google Scholar]


