Shoot and root morphology and root anatomical plasticity facilitate effective water uptake and use, making wheat more tolerant of water deficit stress than rice.
Abstract
Water scarcity and the increasing severity of water deficit stress are major challenges to sustaining irrigated rice (Oryza sativa) production. Despite the technologies developed to reduce the water requirement, rice growth is seriously constrained under water deficit stress compared with other dryland cereals such as wheat (Triticum aestivum). We exposed rice cultivars with contrasting responses to water deficit stress and wheat cultivars well adapted to water-limited conditions to the same moisture stress during vegetative growth to unravel the whole-plant (shoot and root morphology) and organ/tissue (root anatomy) responses. Wheat cultivars followed a water-conserving strategy by reducing specific leaf area and developing thicker roots and moderate tillering. In contrast, rice ‘IR64’ and ‘Apo’ adopted a rapid water acquisition strategy through thinner roots under water deficit stress. Root diameter, stele and xylem diameter, and xylem number were more responsive and varied with different positions along the nodal root under water deficit stress in wheat, whereas they were relatively conserved in rice cultivars. Increased metaxylem diameter and lower metaxylem number near the root tips and exactly the opposite phenomena at the root-shoot junction facilitated the efficient use of available soil moisture in wheat. Tolerant rice ‘Nagina 22’ had an advantage in root morphological and anatomical attributes over cultivars IR64 and Apo but lacked plasticity, unlike wheat cultivars exposed to water deficit stress. The key traits determining the adaptation of wheat to dryland conditions have been summarized and discussed.
Among cereals, rice (Oryza sativa) and wheat (Triticum aestivum) are the most important staple food crops, and they belong to the family Poaceae. These two cereals share a common ancestor and diverged about 65 million years ago (Sorrells et al., 2003). Rice eventually developed strong adaptation potential for fully flooded conditions across tropical to temperate environments, while wheat became well adapted to aerobic conditions mostly restricted to temperate environments. Rice, with a semiaquatic behavior, consumes about 30% of the total fresh water available for agricultural crops worldwide, which equates to a 2- to 3-fold higher consumption than other cereals such as wheat and maize (Zea mays; Peng et al., 2006). Despite a significantly lower water requirement, the potential yield of wheat in a favorable environment (9 tons ha−1) is comparable with the yield of fully flooded rice (9 tons ha−1) in the dry season at the International Rice Research Institute (IRRI; Fischer and Edmeades, 2010). Hence, rice records very low water productivity compared with wheat and other dryland cereals. Because of growing concerns about water scarcity and the increased frequency and magnitude of water deficit stress events under current and future climates, increasing or even sustaining rice yield under fully flooded conditions is highly challenging. To minimize the total water requirement for cultivating rice, several water-saving technologies have been developed, such as direct-seeded aerobic rice cultivation (Bindraban et al., 2006). These water-saving technologies increased water productivity substantially compared with flooded conditions but were invariably associated with a yield penalty. A major challenge that water-saving technologies including aerobic rice currently face is the lack of mechanistic understanding for further genetic improvement.
By virtue of its wider adaptation to a range of edaphic conditions, rice is considered to possess the diversity to adapt to upland or aerobic scenarios extending into water deficit conditions (Khush, 1997). Genetic differences in rice root biomass and rooting depth and variation in root morphology with water deficit stress exposure are well documented (Kato et al., 2006, 2007; Henry et al., 2011; Kano et al., 2011). But the underlying mechanisms differing across diverse germplasm that influence water uptake under water deficit stress are not fully understood (Gowda et al., 2011). A recent report has documented water deficit-tolerant genotypes recording a lower bleeding rate and narrow xylem diameter under stress (Henry et al., 2012). Contrastingly, a higher root hydraulic conductivity helped to maintain a higher photosynthetic rate (Adachi et al., 2010), with tolerant cultivars maintaining greater root hydraulic conductivity than susceptible cultivars (Matsuo et al., 2009). Furthermore, upland rice cultivars with deeper roots outperformed lowland cultivars possessing a shallow root system when encountered with water deficit stress (Uga et al., 2013). Additionally, major-effect grain yield metaquantitative trait loci under water deficit stress identified in rice were found to colocalize on the genomes of other dryland cereals such as wheat, maize, and pearl millet (Pennisetum glaucum; Swamy et al., 2011), indicating a possible common evolutionary pathway for water deficit adaptation across cereals. Despite these achievements and the relatedness among cereals, rice does not respond in a way similar to other dryland cereals to water deficit stress conditions. To bring in a revolutionary change in future breeding strategies for upland/aerobic rice and for water deficit tolerance in rice, there is a need for a fundamental understanding and identification of the key traits that determine the water deficit stress response in well-adapted dryland cereals. Hence, comparing whole-plant responses (shoot and root) of rice with those of other dryland cereals such as wheat is essential.
A comparative study between two C3 cereals (rice and wheat) will help identify the core adaptive mechanisms and/or a suite of traits that render wheat to grow with less water and more tolerance of water deficit stress. Such comparative analysis should target key morphological, physiological, anatomical, and agronomic traits throughout the crop growth cycle, as water deficit stress occurs at both early (vegetative stage) and late (reproductive stage) seasons in rice (Pandey et al., 2007). Extensive research efforts are currently ongoing to reduce the impact of water deficit stress during the reproductive stage in rice (Venuprasad et al., 2008; Verulkar et al., 2010; Vikram et al., 2011; Kumar et al., 2014) and in wheat (Olivares-Villegas et al., 2007; Lopes and Reynolds, 2010; Pinto et al., 2010). Therefore, our study focused on stress during the vegetative stage to identify key checkpoints that determine whole-plant responses of representative rice cultivars adapted to lowland, upland/aerobic, or water deficit conditions and of wheat cultivars with moderate to high water deficit tolerance. Cultivars from both species were exposed to moisture levels that resemble aerobic conditions and water deficit stress during the vegetative stage. Our study follows a previous report that has successfully demonstrated the approach to expose rice and wheat to the same moisture stress conditions (Praba et al., 2009) and is designed to address the following specific objectives: (1) to quantify the adaptive plasticity in shoot and root morphology and biomass partitioning among different plant parts (leaves, stem, and root); (2) to estimate the key supportive physiological mechanisms such as whole-plant water use efficiency (WUE) and leaf-level carbon isotope discrimination (Δ13C); and (3) to dissect root anatomical plasticity across different key zones in both rice and wheat roots exposed to water deficit stress. Finally, novel traits that benefit dryland adaptation in wheat compared with high water-requiring rice cultivars are highlighted.
RESULTS
Shoot Morphology and Whole-Plant and Leaf-Level WUE
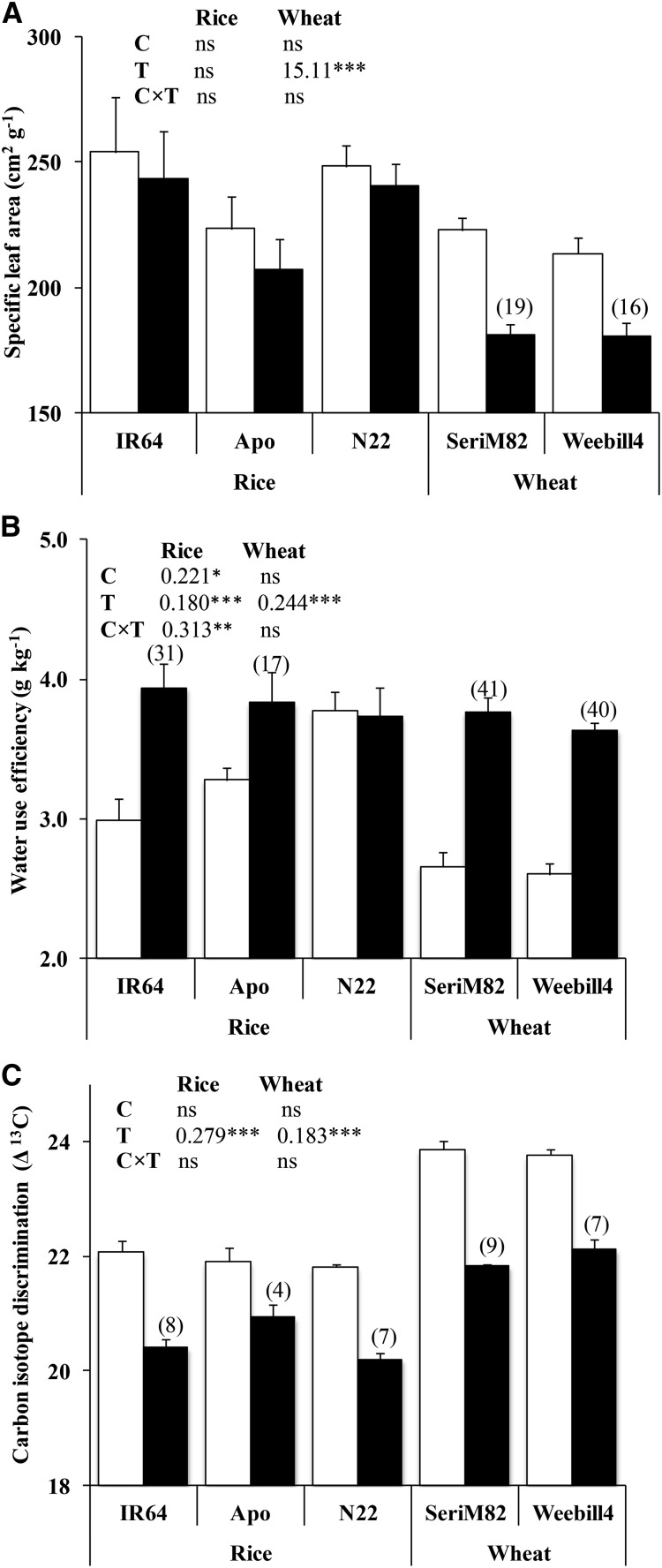
Significant reductions in total leaf area, total biomass, and cumulative water transpiration were recorded under water deficit stress in rice cultivars, with stronger reductions in the tolerant cv Nagina 22 (N22; P < 0.001) and in both wheat cultivars (P < 0.01 to P < 0.001; Supplemental Table S1). Specific leaf area and tiller number decreased under water deficit stress only in wheat cultivars (P < 0.001; Fig. 1A; Supplemental Table S1). Whole-plant WUE increased in response to water deficit stress in two out of three rice cultivars (rice ‘IR64,’ 31%; rice ‘Apo,’ 17%) and in both wheat cultivars (approximately 40%). The tolerant rice ‘N22’ recorded higher WUE than the other two cultivars in the absence of stress and was not altered by water deficit stress; hence, significant cultivar and treatment interaction (P < 0.01; Fig. 1B) was observed. Δ13C of leaf is often used as a proxy to measure WUE (lower Δ13C means higher WUE; Impa et al., 2005). In both species, water deficit stress had a strong effect on Δ13C (P < 0.001), but there were no cultivar differences (P > 0.05). On average, Δ13C decreased by 6% in rice cultivars and by 8% in wheat cultivars. The absolute value of Δ13C was higher in wheat cultivars than in rice cultivars (Fig. 1C).
Figure 1.
Specific leaf area (A), whole-plant WUE (B), and Δ13C (C) of rice and wheat (mean ± se). White columns represent control, and black columns represent water deficit stress. Values in parentheses represent the significant percentage change (increase or decrease) over the control. The ANOVA results with lsd values are given for cultivar (C), treatment (T), and cultivar-treatment interaction (C×T). Significance levels are as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Biomass Partitioning among Leaf, Stem, and Root
In general, both species recorded higher biomass partitioning to leaf and stem than to root, with a higher proportion of biomass partitioned to roots in wheat than in rice (Supplemental Table S1). Leaf weight ratio (LWR) and stem weight ratio (SWR) varied only among rice cultivars (P < 0.01), with a significant effect of water deficit stress (P < 0.05 to P < 0.01). The susceptible rice ‘IR64’ had 16% lower LWR and 24% higher SWR with water deficit stress. The tolerant rice ‘N22’ had lower LWR and higher SWR than other cultivars and was not altered by water deficit stress. In both species, root weight ratio did not differ significantly among cultivars and treatments (P > 0.05), but an increasing trend was observed with the tolerant rice ‘N22’ and both wheat cultivars in water deficit stress.
Root Morphology
Root morphological traits such as maximum root length, total root length, and root length density did not differ with either cultivars or stress treatments in rice (P > 0.05), but root volume and root biomass differed with both cultivars and treatments (P < 0.05 to P < 0.01; Supplemental Table S2). Conversely, in both wheat cultivars, the water deficit stress treatment effect was highly significant for all the above-mentioned traits (P < 0.05 to P < 0.001), but there were no cultivar differences. The maximum root length of the two wheat cultivars was increased in response to water deficit stress compared with control conditions (Supplemental Table S2).
Specific Root Length, Average Root Thickness, and Total Root Weight Density
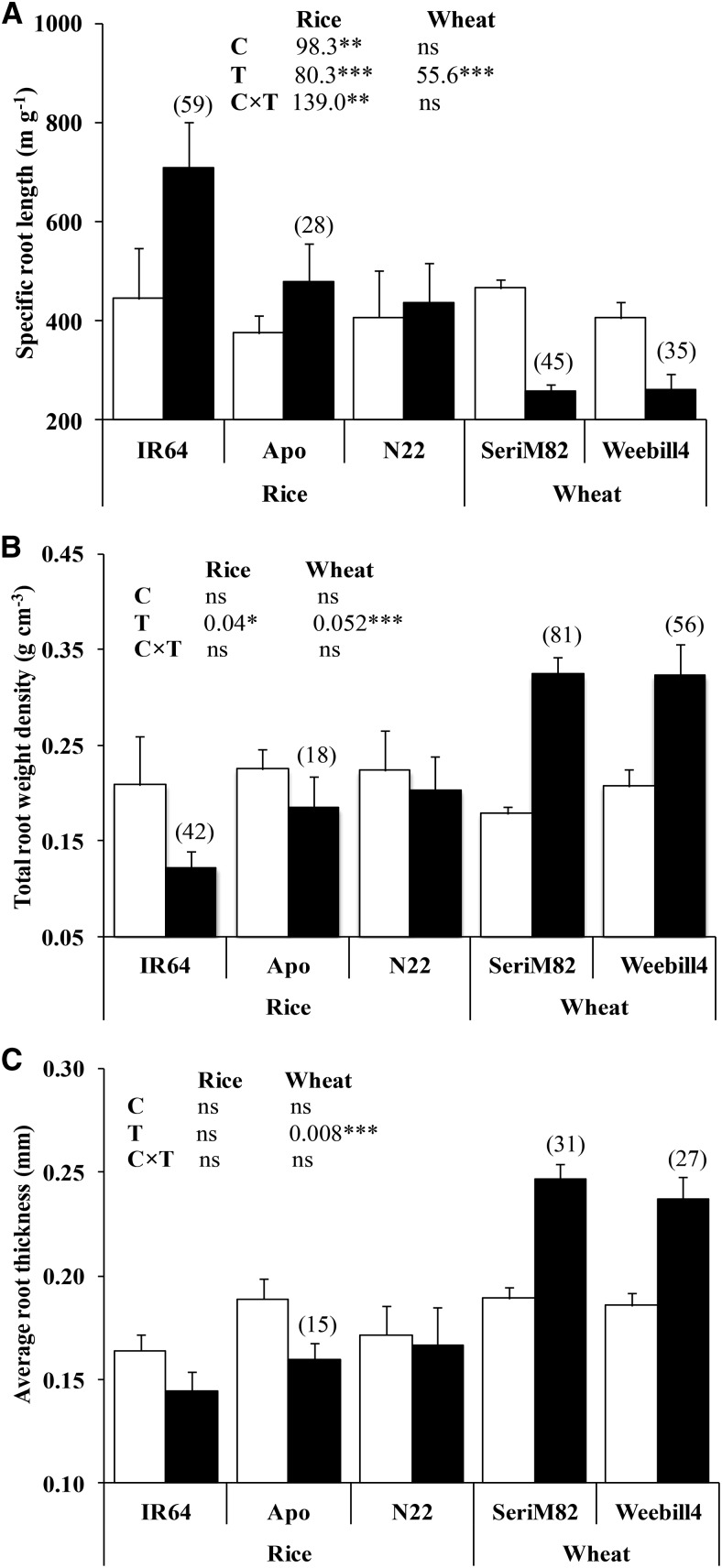
Specific root length (SRL), expressed as the ratio of root length to root biomass, is a key indicator of root thickness. In response to water deficit stress, SRL increased significantly in two of the three rice cultivars (rice ‘IR64,’ 59%; rice ‘Apo,’ 28%) but decreased in both wheat cultivars by approximately 40% (Fig. 2A). The SRL is independently controlled by two other components, root thickness and root weight density (Ostonen et al., 2007). Our results support this, with an increased SRL in rice ‘IR64’ determined mainly by reduced total root weight density (42%), while in cv Apo this was due to a reduction in both average root thickness (15%) and total root weight density (18%). On average, the lower SRL in the wheat cultivars was due to a greater increase in total root weight density (69%) than in average root thickness (29%; Fig. 2, B and C).
Figure 2.
Specific root length (A), total root weight density (B), and average root thickness (C) of rice and wheat (mean ± se). White columns represent control, and black columns represent water deficit stress. Values in parentheses represent the significant percentage change (increase or decrease) over the control. The ANOVA results with lsd values are given for cultivar (C), treatment (T), and cultivar-treatment interaction (C×T). Significance levels are as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Radial Root Anatomy
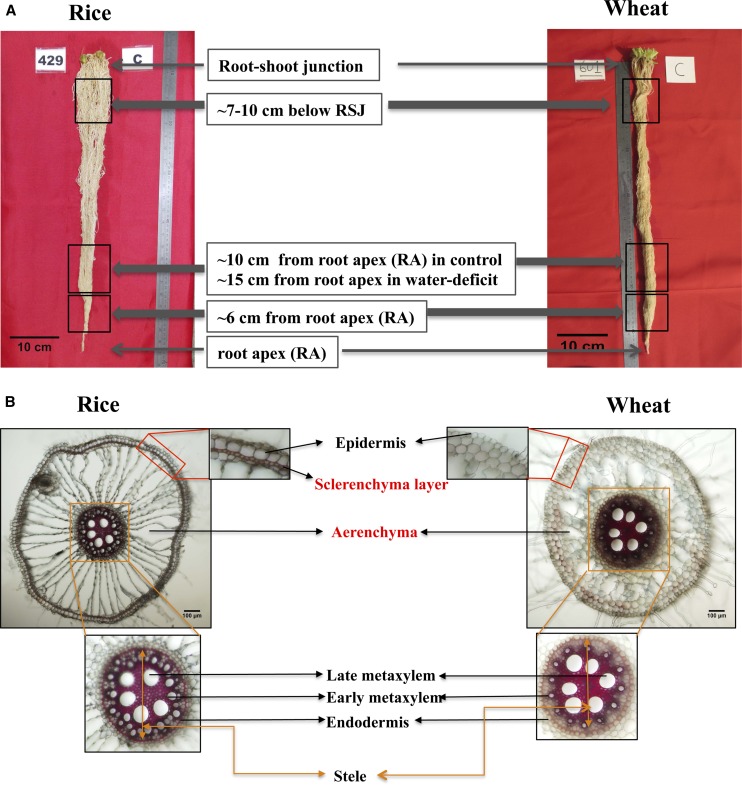
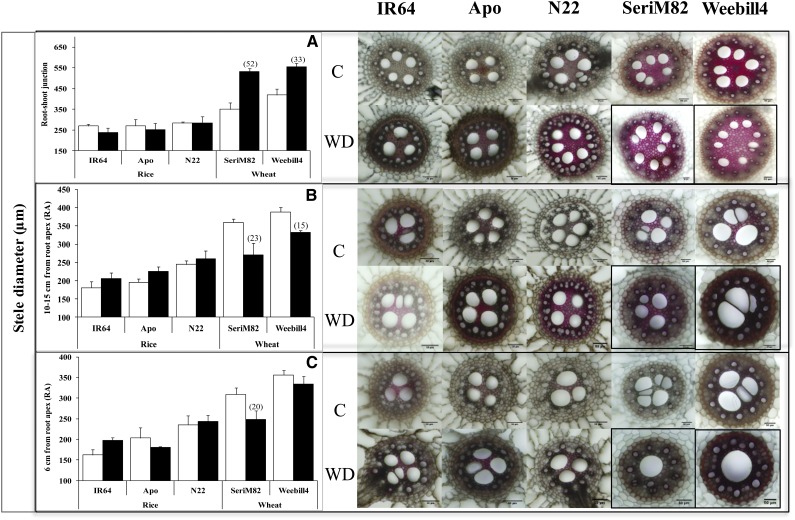
To further confirm the observed variation in root morphology, we investigated root anatomical variables at three different locations along the root length (Fig. 3). Note that root cross sections were stained with phloroglucinol to assess the secondary cell wall thickening and lignin deposition under water deficit stress. While it appears that there were changes in the staining pattern (e.g. cv Weebill4 under water deficit treatment; Fig. 4), the results were not consistent across replications in both species; thus, we will not discuss such changes.
Figure 3.
A, Root samples were collected in three different zones on nodal roots for anatomy study. B, Radial root cross sections showing anatomical variation in rice and wheat. Scale bars on root images = 10 cm (A) and 100 µm (B).
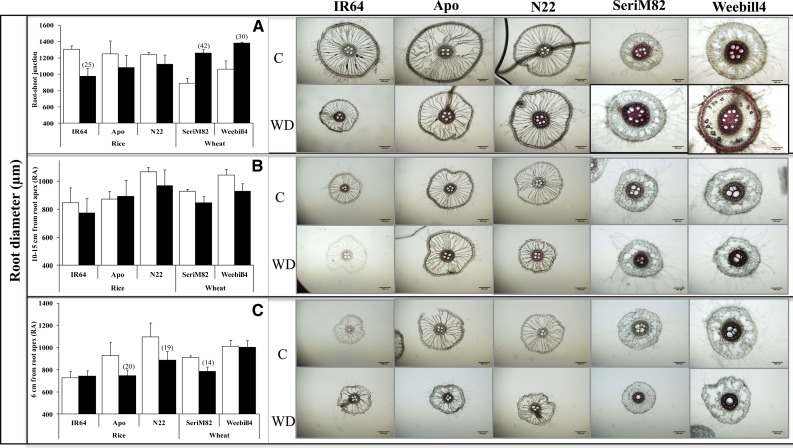
Figure 4.
Root diameter at the RSJ (A), 10 to 15 cm from the RA (B), and 6 cm from the RA (C) on nodal roots of rice and wheat cultivars (mean ± se). White columns represent control (C), and black columns represent water deficit stress (WD). Values in parentheses represent the significant percentage change (increase or decrease) over the control value. Scale bars on root images = 200 µm.
Root Diameter
Variation in root diameter is due to change in the number and size/width of cortical cells and in stele diameter. In both species, root diameter varied significantly with cultivar (P < 0.05 to P < 0.001) and position along the root (P < 0.001). A significant effect of water deficit stress on root diameter was documented with rice cultivars (P < 0.01; Supplemental Table S3). Root diameter at the root-shoot junction (RSJ; Fig. 3A) decreased with stress exposure in rice ‘IR64’ (25%), with no change in the aerobic cv Apo and the tolerant cv N22 (Fig. 4A). A clear pattern was not observed at 10 to 15 cm from the root apex (RA; Fig. 4B). However, an opposite response was observed at 6 cm from the RA, where the tolerant cultivars showed lower root diameter (cv N22, 19%; cv Apo, 20%), with no change in cv IR64 (Fig. 4C). Unlike in rice cultivars, root diameter at the RSJ increased significantly in both wheat cultivars (wheat ‘SeriM82,’ 42%; wheat ‘Weebill4,’ 30%), but at two other positions a decreasing trend was observed (i.e. 10–15 cm from the RA and 6 cm from the RA; Fig. 4).
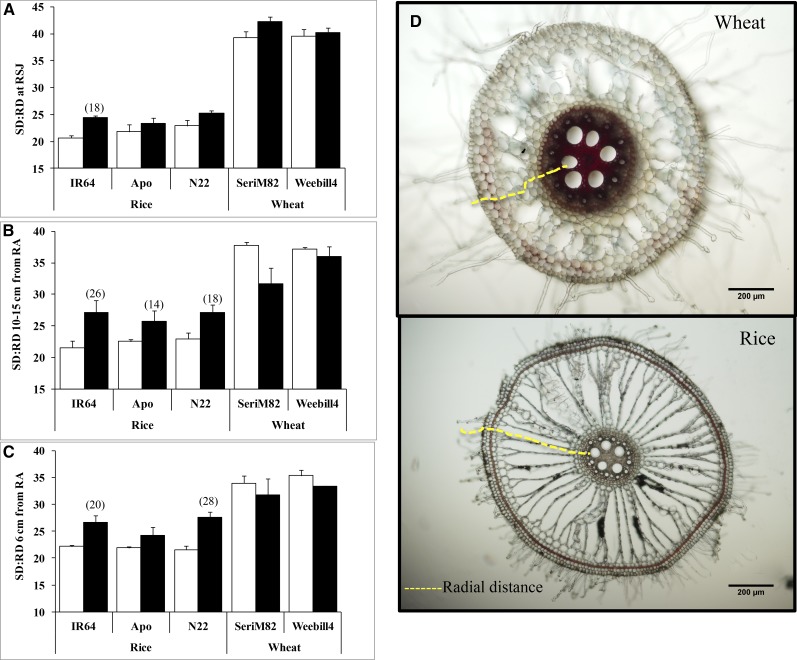
Stele Diameter and Stele Diameter in Proportion to Root Diameter
Stele is the central part of the root system that contains vascular tissue (i.e. xylem and phloem; Fig. 3B). Both cereals recorded strong cultivar and spatial (different positions along the root) variations (P < 0.001) for stele diameter (Supplemental Table S3). Stele diameter at the RSJ did not differ in any of the rice cultivars (Fig. 5A), but the tolerant cv N22 maintained a higher stele diameter at 10 to 15 cm from the RA and at 6 cm from the RA (Fig. 5, B and C). Additionally, stele diameter was more stable and was not affected by water deficit stress in rice (P > 0.05). Unlike in rice, stele diameter increased significantly under water deficit stress at the RSJ in wheat cultivars, with wheat ‘SeriM82’ (52%) showing a greater increase than wheat ‘Weebill4’ (33%; Fig. 5A). Stele diameter responded in an opposite manner, with a strong reduction at two other positions on roots (10–15 and 6 cm from the RA) in cv SeriM82 and at 10 to 15 cm from the RA only for cv Weebill4 (Fig. 5, B and C).
Figure 5.
Stele diameter at the RSJ (A), 10 to 15 cm from the RA (B), and 6 cm from the RA (C) on nodal roots of rice and wheat (mean ± se). White columns represent control (C), and black columns represent water deficit stress (WD). Values in parentheses represent the significant percentage change (increase or decrease) over the control value. Scale bars on root images = 50 µm.
Stele diameter in proportion to root diameter (SD:RD) was strongly affected by water deficit stress in rice (P < 0.001) and lacked cultivar and tissue position variation on nodal roots. By contrast, wheat cultivars documented a significant variation along the root tissue position (P < 0.001) and its interaction with treatment (P < 0.05; Supplemental Table S3). Wheat cultivars maintained higher SD:RD (approximately 35%–40%) than rice cultivars (approximately 20%–25%) across three root positions sectioned (Fig. 6). An increasing trend with SD:RD was observed in response to water deficit stress in all three rice cultivars (Fig. 6), but this was least affected by water deficit stress in wheat, except for a noticeable reduction at 10 to 15 cm from the RA for cv SeriM82 (Fig. 6B).
Figure 6.
SD:RD (%) at the RSJ (A), 10 to 15 cm from the RA (B), and 6 cm from the RA (C) on nodal roots of rice and wheat (mean ± se). White columns represent control, and black columns represent water deficit stress. A pictorial representation of radial distance in wheat and rice is shown in D. Values in parentheses represent the significant percentage change (increase or decrease) over the control value. Scale bars on root images = 200 µm.
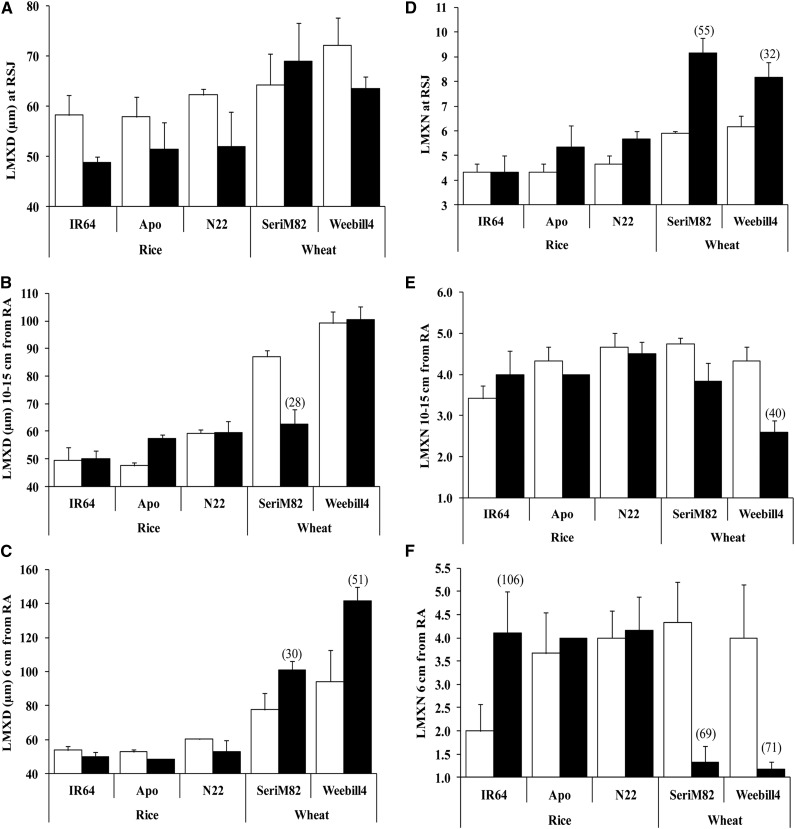
Late Metaxylem Diameter and Number
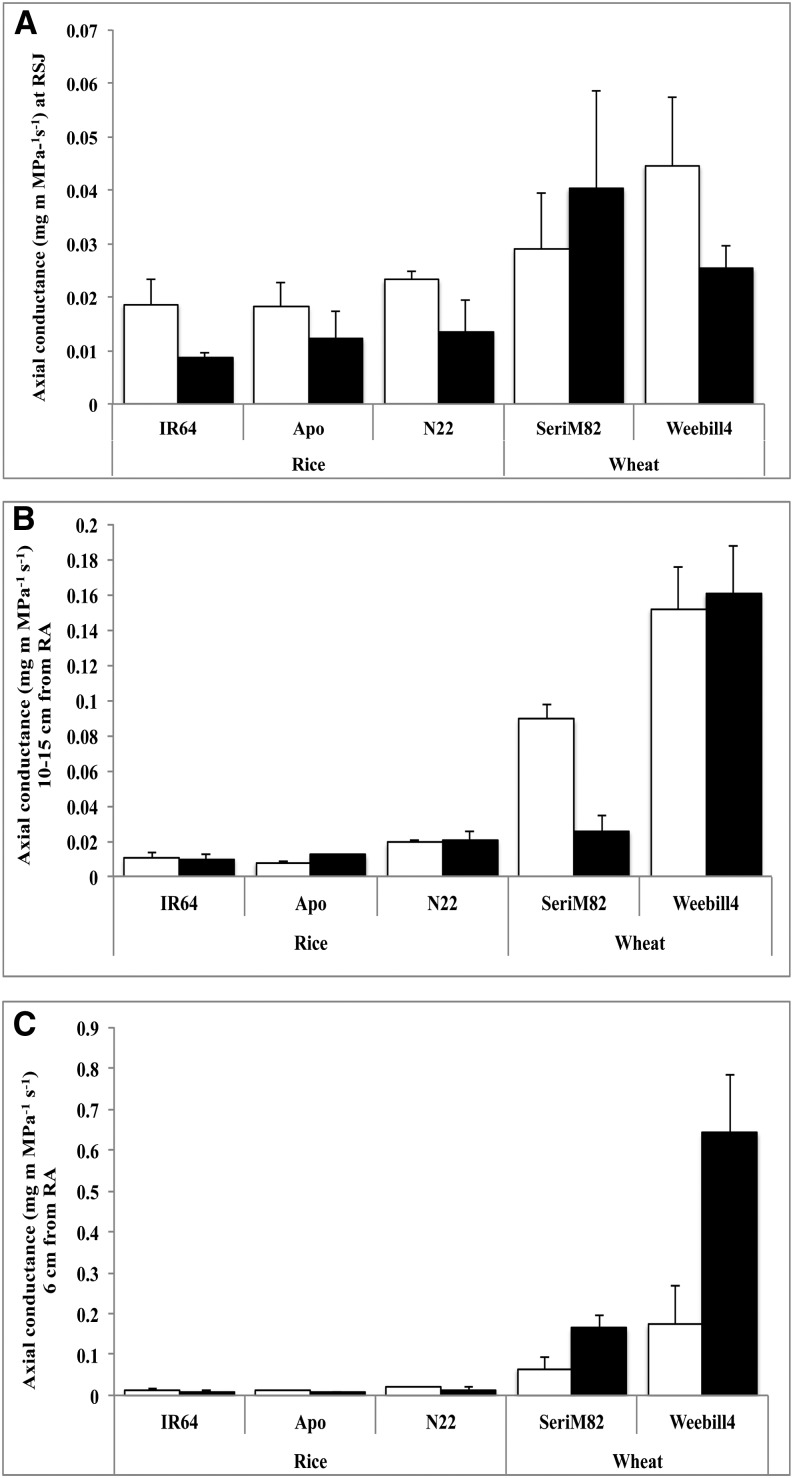
Late metaxylem diameter (LMXD) remained relatively constant in rice cultivars across different root tissue positions under both control and water deficit stress, with a narrow variation (P < 0.05) recorded among cultivars (Supplemental Table S3). The effect of water deficit stress on LMXD was not significant, but a decreasing trend was observed across all three rice cultivars near the RSJ (Fig. 7A). Late metaxylem number (LMXN) varied significantly with root tissue position (P < 0.01), cultivar, and treatment (P < 0.05) in rice. Among rice cultivars, lowland-adapted rice ‘IR64’ had lower LMXN at 6 cm from the RA in nonstress conditions, but upon exposure to stress, LMXN increased significantly and was similar to that of other cultivars (Fig. 7F). Unlike in rice, LMXD varied with cultivar and tissue position and their interaction in wheat (P < 0.001; Supplemental Table S3). In both control and water deficit stress, wheat cultivars maintained higher LMXD at 10 to 15 cm from the RA and at 6 cm from the RA compared with RSJ, except for cv SeriM82 recording a 28% lower LMXD at 10 to 15 cm from the RA under water deficit stress (Fig. 7B). LMXD increased greatly in both wheat cultivars at 6 cm from the RA under water deficit stress exposure, with the increase being higher in wheat ‘Weebill4’ (51%) than in wheat ‘SeriM82’ (30%; Fig. 7C). Additionally, LMXN displayed strong interaction between treatment and tissue position (P < 0.001). Exposure to water deficit stress resulted in an increase in LMXN at the RSJ in wheat cultivars (Fig. 7D), but this decreased at two other positions, with a highly significant reduction observed at 6 cm from the RA (Fig. 7, E and F). According to Hagen-Poisseuille’s law, the flow of water in any given conduit is the fourth power of the radius of the conduit. Theoretically calculated axial conductance by modified Hagen-Poisseuille’s law also followed a pattern similar to that of LMXD across three different positions on nodal roots in both species (Fig. 8).
Figure 7.
LMXD and LMXN at the RSJ (A and D), 10 to 15 cm from the RA (B and E), and 6 cm from the RA (C and F) on nodal roots of rice and wheat (mean ± se). White columns represent control, and black columns represent water deficit stress. Values in parentheses represent the significant percentage change (increase or decrease) over the control value.
Figure 8.
Theoretically calculated axial hydraulic conductance at the RSJ (A), 10 to 15 cm from the RA (B), and 6 cm from the RA (C) on nodal roots of rice and wheat cultivars (mean ± se). White columns represents control, and black columns represent water deficit stress.
DISCUSSION
We compared rice and wheat for their adaptive responses in root morphology and anatomy to water deficit stress. The findings from our study are discussed below.
Reduced Specific Leaf Area Is a Determining Factor for Increased WUE under Stress in Wheat
Reducing specific leaf area in response to water deficit stress to conserve water has been documented across crop species (Rao and Wright, 1994; Araus et al., 1997; Craufurd et al., 1999; Nautiyal et al., 2002), and the same was observed with wheat cultivars (Fig. 1A). Increased WUE under water deficit conditions is well known (Blum, 2009). Our results also documented increased WUE with cultivars of both species except for rice ‘N22’ under water deficit stress; in wheat, this increased WUE could be due to reduced specific leaf area. Variation in Δ13C is determined by the balance between stomatal conductance and carboxylation efficiency (Farquhar et al., 1989). Both species had comparable reductions in Δ13C in water deficit stress (Fig. 1C), but possibly for different reasons. Lower specific leaf area leads to higher carboxylation rate, but under water deficit conditions, limitation of stomatal conductance also reduces photosynthesis. Therefore, reduced Δ13C under water deficit stress in wheat could be due to both lower stomatal conductance and higher carboxylation rate (Condon et al., 1990), but in rice it could be due to lower stomatal conductance only.
SRL Displays Opposing Responses among Rice and Wheat Cultivars under Water Deficit Stress
SRL captures the overall effect of both root thickness and root weight density (Fitter, 2002). In our study, SRL increased under water deficit stress in rice ‘IR64’ and ‘Apo’ because of reduced average root thickness, while lower SRL in wheat cultivars resulted from increased average root thickness and total root weight density (Fig. 2). Our results suggest that rice cultivars (except for rice ‘N22’) aimed for a rapid water-acquisition strategy, since thinner roots (higher SRL) increase overall root hydraulic conductance by exploring more soil volume for water and enabling rapid uptake of water (Reich et al., 1998; Eissenstat and Achor, 1999; Solari et al., 2006; Hernández et al., 2010). This strategy could lead to higher susceptibility to water deficit stress due to quicker water depletion (Ryser, 2006). On the other hand, the two wheat cultivars employed a conservative strategy by developing thicker roots and exploring less soil volume for water by reducing root length density. Thicker roots enhance soil-penetrating ability to access deeper layers in drying soil conditions (Davies and Bacon, 2003). This can be substantiated by our results on maximum root length. Although the full potential to express maximum root length of wheat could be influenced by limited pot size in our study, it increased in response to water deficit stress in wheat, while this was not the case with rice (Supplemental Table S2; Supplemental Fig. S1). Among the rice cultivars, tolerant rice ‘N22’ followed a conservative strategy by reducing root length density (Supplemental Table S2), but, unlike wheat, it lacked plasticity in SRL, average root thickness, and total root weight density (Fig. 2).
Stele Diameter Was More Responsive to Water Deficit Stress in Wheat Than in Rice
The SD:RD provides an indirect measure of cortex tissue area/width. The stele size and SD:RD are lower in wetland than in dryland plants (McDonald et al., 2002), and our result confirms this difference between rice and wheat (Figs. 5 and 6). This anatomical feature in wetland species aims to optimize the consumption of oxygen under waterlogging (Armstrong and Beckett, 1987; Armstrong et al., 1991; Aguilar, 1998). A distinct sclerenchyma layer as an apoplastic barrier to impede radial oxygen loss was observed in rice, even in the absence of waterlogging (Supplemental Fig. S2). These root anatomical features in rice have an advantage under flooded conditions but could affect root water uptake. An inverse relationship between overall radial hydraulic conductance and cortex width has been documented (Rieger and Litvin, 1999). Radial hydraulic conductance is lower in rice than in other cereals (Miyamoto et al., 2001), possibly because of larger aerenchyma in the cortex (Ranathunge et al., 2003).
There was a significant position effect on the stele tissue, with higher stele diameter at the RSJ than at the other two positions (10–15 and 6 cm from the RA) in both species but more conspicuously with wheat (Figs. 5 and 6). Although the exact ecophysiological significance of such a gradient in stele tissue is unclear, it could play an important role in maintaining water uptake by improving the internal aeration of roots, particularly near the root tip. The availability of oxygen is known to decrease with increasing depth of the soil, and smaller stele tissue tends to prevent oxygen deficiency to support uninterrupted xylem transport (Gibbs et al., 1998). Furthermore, water uptake by a region close to the root tip appears to be a predominant feature of all cereal roots (Greacen et al., 1976). An increased stele diameter near the RSJ in wheat may play a supportive role in water transport rather than in direct water uptake, but smaller stele diameter at the other two positions near the RA (Fig. 5) could help in maintaining water uptake under stress. Contrary to this, stele diameter did not differ significantly under water deficit in rice, but increasing trends of SD:RD were documented across all three positions along the root (Fig. 6). A similar increase in SD:RD under water deficit stress was identified previously in rice (Henry et al., 2012). The above response demonstrates the attempt of rice to reduce radial distance under water deficit stress by decreasing cortex width (i.e. an increase in SD:RD without changing stele diameter) to improve radial hydraulic conductance (for a pictorial representation of radial distance in rice and wheat, see Fig. 6D). Together, the observed variation in root diameter under water deficit in wheat was mostly due to a change in stele diameter, but in rice it was due to variation in cortex width (Fig. 4). Both wheat cultivars and the tolerant rice ‘N22’ maintained higher stele diameter, substantiating its role in water deficit stress adaptation.
Xylem Developmental Plasticity Was More Responsive to Water Deficit Stress in Wheat Than in Rice
The development of LMXD and LMXN varied strongly along the root length in wheat, with lower xylem diameter but a higher number near the RSJ and higher diameter but a lower number at 10 to 15 cm and 6 cm from the RA. Unlike in wheat, xylem diameter and number were least affected by either water deficit stress or along the three different positions in rice roots (Fig. 7). Bulk flow of water or axial conductance is known to be closely related to the cross-sectional area or diameter of xylem vessels (Niklas, 1985). Hence, wheat cultivars would have higher water uptake than rice cultivars because of higher xylem diameter (Fig. 7), which was confirmed with calculated axial conductance (Fig. 8). Recently, it has been hypothesized that combining low axial conductance (narrow xylem diameter) at the base of the root system (i.e. closer to the RSJ) with higher axial conductivity (higher xylem diameter) near the root tips in deeper soil facilitates effective water use until flowering and grain development (Wasson et al., 2012). This is a pilot report showing such a developmental gradient in xylem diameter along the root length in both wheat cultivars, and it was confirmed with calculated axial hydraulic conductance. A large proportion of the lateral roots generally are developed toward the RSJ part compared with the root tip (Bramley et al., 2009). Therefore, under water deficit increase in LMXN near the RSJ can help increase the uptake of water by lateral roots from the top soil layers but strongly decrease it at the root tip (6 cm from the RA) to conserve moisture in lower soil profiles. In summary, the response of xylem diameter and number to water deficit stress in wheat was a novel finding, and this provides additional mechanistic understanding of wheat root plasticity toward adapting to water deficit stress.
CONCLUSION
A comprehensive analysis of two diverged species, one adapted to flooded conditions and the other to aerobic conditions, allowed us to demonstrate the functional role of organ/tissue plasticity for adapting to water deficit stress. Both wheat cultivars had thicker leaves and roots and moderate tillering that help conserve soil moisture during vegetative-stage water-deficit stress (Table I). Plasticity in stele and xylem diameter and xylem number along the root length in wheat cultivars facilitates the efficient use of available moisture under water deficit stress. Therefore, future studies should aim toward establishing the relationship between root morphology, anatomy with yield, and yield components under water deficit conditions.
Table I. Summary of adaptive changes in key morphological, physiological, and root anatomical traits in response to water deficit stress of tolerant rice and wheat cultivars.
Symbols are as follows: =, no change; +, increase; −, reduction in water deficit (+/−, less than 10%; ++/−−, more than 10% to less than 20%; +++/−−−, more than 20% to less than 35%; and ++++/−−−−, more than 35%–50%).
| Parameter | Water Deficit Adaptive Traits | Root Tissue Position | Rice |
Wheat |
||
|---|---|---|---|---|---|---|
| ‘Apo’ | ‘N22’ | ‘SeriM82’ | ‘Weebill4’ | |||
| Morphology | ||||||
| Shoot | Tiller no. | = | = | −−− | −−− | |
| Specific leaf area | = | = | −− | −− | ||
| Root | Root length density | = | −−−− | −−−− | −−−− | |
| SRL | +++ | = | −−−− | −−−− | ||
| Average root thickness | −− | = | +++ | ++++ | ||
| Total root weight density | −− | = | ++++ | ++++ | ||
| Physiology | Whole-plant WUE | ++ | = | ++++ | ++++ | |
| Δ13C | − | − | − | − | ||
| Nodal root anatomy | Stele diameter | RSJ | = | = | ++++ | +++ |
| 10 to 15 cm from the RA | = | = | −−− | −− | ||
| 6 cm from the RA | = | = | −− | = | ||
| LMXD | RSJ | = | = | = | = | |
| 10 to 15 cm from the RA | = | = | −−− | = | ||
| 6 cm from the RA | = | = | +++ | ++++ | ||
| LMXN | RSJ | +++ | +++ | ++++ | +++ | |
| 10 to 15 cm from the RA | = | = | −− | −−−− | ||
| 6 cm from the RA | = | = | −−−− | −−−− | ||
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Greenhouse and controlled-environment experiments were conducted to compare the vegetative-stage water deficit stress response of rice (Oryza sativa) and wheat (Triticum aestivum) with emphasis on root morphological and anatomical plasticity. Three rice cultivars, rice ‘IR64’ (susceptible to water deficit stress), rice ‘Apo’ (aerobic/water deficit tolerant), and rice ‘N22’ (water deficit and high-temperature tolerant), were chosen for our study based on previous reports (Liu et al., 2006; Jagadish et al., 2011; Rang et al., 2011; Venuprasad et al., 2012). The two wheat cultivars selected were wheat ‘SeriM82,’ which is moderately susceptible (Pfeiffer, 1988) to tolerant of water-limited conditions (Villareal et al., 1995), and wheat ‘Weebill4,’ a highly drought-tolerant check cultivar (Reynolds et al., 2007; Praba et al., 2009). Dormancy of rice seeds was broken after exposure to 50°C for 3 d, and pregerminated seeds were sown in white-painted pots (55 cm long and 15-cm diameter) as recommended by Poorter et al. (2012) to minimize the confounding effects of increasing temperature of pot surface and soil. The pots were filled with 11 kg of clay loam soil and maintained under natural greenhouse conditions at the IRRI during the 2012 wet season (i.e. during the season when temperature in the greenhouse and pot can be controlled best). Each pot was drilled with holes on either side at the bottom for imposing controlled water deficit stress and lined with polythene covers to facilitate easier separation of roots from soil at the end of the treatment. Simultaneously, wheat seeds were directly sown in pots with the same dimensions and maintained in controlled-environment large walk-in chambers (10.6-m2 area), built as an extension to the greenhouse where the rice plants were maintained. The chambers were maintained at day/night temperatures of 21°C/18°C, 60% to 70% relative humidity, 16-h/8-h light/dark cycle, and light at 650 µmol m−2 s−1, following Praba et al. (2009). Across both cereals and the treatments imposed, three replications were maintained and placed in a completely randomized design.
Water Deficit Stress Imposition, Cumulative Water Transpiration, and Whole-Plant WUE
Both rice and wheat plants were maintained at two moisture regimes: control at 100% field capacity that is the maximum soil moisture content after drainage of excess water, resembling an aerobic condition; and water deficit stress at 55% to 60% field capacity. Water deficit stress was imposed after seedling establishment (i.e. 15 d after seedling emergence), before which all the pots were maintained uniformly at 100% field capacity. Pots with the control treatment were maintained at 100% field capacity throughout the experiment, while water deficit stress was imposed by unplugging the stoppers at the bottom of the pots. A standardized gravimetric approach of daily pot weighing (Raju et al., 2014) was followed to gradually attain 55% to 60% field capacity and thereafter maintained at the same level until the end of the experiment (for details, see Supplemental Fig. S3). Once the target stress level was reached, daily consumed water due to transpiration was replenished by adding an exact amount of water to bring back the moisture content to the desired target in each pot. The soil surface was covered with a circular polythene sheet to protect from direct evaporative loss of water, and a slit across the radius of the polythene sheet prevented heat buildup on the soil surface. In addition, a set of filled pots without a plant was also maintained to correct for evaporative loss of water from the opening created by the slit in the circle-shaped polythene sheet. Daily pot weights recorded for 30 consecutive days of the stress period were used to calculate the daily evapotranspiration. After correcting for evaporative loss from empty pots, actual transpiration was calculated. Finally, daily actual transpiration was summed for the 30-d period to calculate cumulative water transpired. Whole-plant WUE (g kg−1) was calculated as the ratio of total biomass (root and shoot) to cumulative water transpired.
Shoot Morphology
Following 30 d of stress, plants were harvested 45 d after sowing, tiller numbers were counted, and total leaf area was estimated by a leaf area meter (LI-3000; LI-COR). Leaves and stems were separately oven dried at 70°C for 72 h to compute specific leaf area and shoot biomass.
Leaf Δ13C
Top-most fully expanded leaves from four to five tillers per plant were collected from control and water deficit-stressed pots immediately before relieving stress separately for three replications, then oven dried and ground to fine powder. Samples were analyzed for carbon isotope composition by a stable isotope ratio mass spectrometer facility available in the analytical service laboratory of the IRRI (http://asl.irri.org/lims/). The analytical precision of the samples was within 0.1%. Furthermore, the Δ13C value was calculated relative to the atmospheric carbon isotope composition as follows (Farquhar et al., 1989):
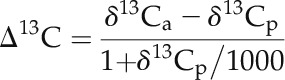 |
where δ13Ca and δ13Cp denote the carbon isotope compositions of atmosphere (−8‰) and leaf sample, respectively.
Root Sample Processing
The entire column of soil along with the roots was placed on a 1-mm sieve and meticulously washed using a gentle stream of water to minimize the loss of small roots and root hairs. The rice root system is mainly composed of nodal roots and only one radicle or seminal root (primary root), with the latter growing to a maximum length of 15 cm and being viable until the seven-leaf stage. On the contrary, wheat develops and maintains several seminal roots until maturity (Yoshida and Hasegawa, 1982). To make a meaningful comparison between rice and wheat, the nodal root was investigated in our study. Across both rice and wheat cultivars, three replicate root sections (2–3 cm) were collected from three different positions along the nodal root for root anatomy study: (1) near the RSJ; (2) approximately 15 cm from the RA from water deficit-stressed samples and approximately 10 cm from the RA on control samples, following Henry et al. (2012); and (3) at 6 cm from the RA in both treatments (Fig. 3A). Collected samples were stored in 40% (v/v) alcohol to study root anatomy. The remaining whole-plant root samples were placed in 20% (v/v) alcohol and stored at 4°C for root scanning and image analysis.
Root Image Acquisition and Root Morphology Traits
Root samples stored in 20% (v/v) alcohol were cut meticulously to fit the scanner tray and aligned vertically on plates to avoid overlapping. An 8-bit grayscale image was acquired by scanning with an Epson Perfection 7000 scanner at a resolution of 600 dots per inch next to a ruler. After capturing the image, root samples were oven dried at 70°C for 72 h to record the total root biomass. Morphological attributes such as total root length, average root thickness, and root volume were computed by analyzing images with WinRHIZO Reg 2012b software (http://www.regent.qc.ca/assets/winrhizo_software.html). To avoid underestimation of fine root lengths during image processing, the threshold pixel was adjusted to automatic mode (Kato et al., 2010; Kato and Okami, 2011).
Derived Shoot and Root Growth Parameters
LWR, SWR, and root weight ratio were calculated as ratios of leaf, stem, and root weight to total biomass. Average specific leaf area was calculated as the ratio of total leaf area to leaf dry weight. Root length density was calculated as the ratio of total root length to the volume of soil in the pot, and total root weight density was calculated as the ratio of root length density to root biomass. Specific root length was calculated as the ratio of total root length to root biomass.
Root Anatomy
To investigate root anatomical features, samples stored in 40% (v/v) ethanol obtained from three different positions along the root (Fig. 3A) were hand sectioned with a razor blade using a dissection microscope. Root sections were stained with 0.5% (w/v) phloroglucinol in water followed by 20% (v/v) HCl (Jensen, 1962) for lignin staining. Images of the root sections were acquired with a Zeiss Axioplan 2 compound microscope with 50× and 100× magnification. At least three to five root images per replicate and tissue position were considered for measuring anatomical traits such as root cross section diameter, stele diameter, LMXD, and sclerenchyma with ImageJ software (for details, see Abramoff et al., 2004). A schematic sketch of the different root anatomical traits measured using ImageJ is provided in Figure 3B.
Theoretically Calculated Axial Conductance
If the number of xylem vessels is n, their overall theoretical axial conductance (Kh; mg m MPa−1 s−1) was calculated with the modified Hagen-Poisseuille’s law described by Tyree and Ewers (1991) and Tombesi et al. (2010):
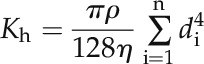 |
where di is the radius of the ith vessel in meters, ρ is the fluid density (assumed to be 1 × 109 mg m−3), and η is the viscosity (assumed to be 1 × 10−9 MPs·s).
Statistical Analysis
The shoot and root morphological data were analyzed to check the significance level through ANOVA in GenStat Release13 (https://www.vsni.co.uk/genstat), with cultivar and treatment as main factors. However, for root anatomy data, root tissue position was included in the analysis as a factor along with cultivar and treatment.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Maximum root length of rice and wheat cultivars.
Supplemental Figure S2. Variation in the sclerenchyma layer of rice and wheat cultivars.
Supplemental Figure S3. Rate of water depletion in percentage field capacity.
Supplemental Table S1. ANOVA for shoot morphological-, physiological-, and biomass-partitioning traits.
Supplemental Table S2. ANOVA for root morphological attributes.
Supplemental Table S3. ANOVA for root anatomical traits.
Supplementary Material
Glossary
- IRRI
International Rice Research Institute
- WUE
water use efficiency
- Δ13C
carbon isotope discrimination
- LWR
leaf weight ratio
- SWR
stem weight ratio
- SRL
specific root length
- RSJ
root-shoot junction
- RA
root apex
- SD:RD
stele diameter in proportion to root diameter
- LMXD
late metaxylem diameter
- LMXN
late metaxylem number
- N22
Nagina 22
Footnotes
This work was supported by the Wageningen University Fund (Ph.D. stipend and field work at the International Rice Research Institute to N.K.); by the Federal Ministry for Economic Cooperation and Development, Germany; and by the Cereal Systems Initiative for South Asia (U.S. Agency for International Development-Bill & Melinda Gates Foundation).
Articles can be viewed without a subscription.
References
- Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics International 11: 36–42 [Google Scholar]
- Adachi S, Tsuru Y, Kondo M, Yamamoto T, Arai-Sanoh Y, Ando T, Ookawa T, Yano M, Hirasawa T (2010) Characterization of a rice variety with high hydraulic conductance and identification of the chromosome region responsible using chromosome segment substitution lines. Ann Bot (Lond) 106: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar EA (1998) Responses of banana roots to oxygen deficiency and its implications for Fusarium wilt. PhD thesis. University of Western Australia, Perth, Australia [Google Scholar]
- Araus JL, Amaro T, Zuhair Y, Nachit MM (1997) Effect of leaf structure and water status on carbon isotope discrimination in field‐grown durum wheat. Plant Cell Environ 20: 1484–1494 [Google Scholar]
- Armstrong W, Beckett PM (1987) Internal aeration and the development of stellar anoxia in submerged roots: a multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol 105: 221–245 [Google Scholar]
- Armstrong W, Beckett PM, Justin SH, Lythe S (1991) Modelling, and other aspects of root aeration by diffusion. InJackson MB, Davies DD, Lambers H, eds, Plant Life under Oxygen Deprivation. SPB Academic Publishing, The Hague, The Netherlands, pp 267–282 [Google Scholar]
- Bindraban PS, Hengsdijk H, Cao W, Shi Q, Thiyagarajan TM, Van der Krogt W, Wardana IP (2006) Transforming inundated rice cultivation. Water Resources Dev 22: 87–100 [Google Scholar]
- Blum A. (2009) Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res 112: 119–123 [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol 150: 348–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Farquhar GD, Richards RA (1990) Genotypic variation in carbon isotope discrimination and transpiration efficiency in wheat: leaf gas exchange and whole plant studies. Funct Plant Biol 17: 9–22 [Google Scholar]
- Craufurd PQ, Wheeler TR, Ellis RH, Summerfield RJ, Williams JH (1999) Effect of temperature and water deficit on water-use efficiency, carbon isotope discrimination, and specific leaf area in peanut. Crop Sci 39: 136–142 [Google Scholar]
- Davies WJ, Bacon MA (2003) Adaptation of roots to drought. InKroon H, Visser EJW, eds, Root Ecology, Vol 168 Springer, Berlin, pp 173–192 [Google Scholar]
- Eissenstat DM, Achor DS (1999) Anatomical characteristics of roots of citrus rootstocks that vary in specific root length. New Phytol 141: 309–321 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Biol 40: 503–537 [Google Scholar]
- Fischer RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Sci 50: 85–98 [Google Scholar]
- Fitter A. (2002) Characteristics and functions of root systems. InWaisel Y, Eshel A, Beeckman T, Kafkaf U, eds, Plant Roots: The Hidden Half, Ed 3 Marcel Dekker, New York, pp 15–32 [Google Scholar]
- Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H (1998) Response to oxygen deficiency in primary maize roots: I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Funct Plant Biol 25: 745–758 [Google Scholar]
- Gowda VR, Henry A, Yamauchi A, Shashidhar HE, Serraj R (2011) Root biology and genetic improvement for drought avoidance in rice. Field Crops Res 122: 1–13 [Google Scholar]
- Greacen EL, Ponsana P, Barley KP (1976) Resistance to water flow in the roots of cereals. InLango OL, Kappen L, Schulze ED, eds, Water and Plant Life, Ed 1, Vol 19 Springer, Berlin, pp 86–100 [Google Scholar]
- Henry A, Cal AJ, Batoto TC, Torres RO, Serraj R (2012) Root attributes affecting water uptake of rice (Oryza sativa) under drought. J Exp Bot 63: 4751–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A, Gowda VR, Torres RO, McNally KL, Serraj R (2011) Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Res 120: 205–214 [Google Scholar]
- Hernández EI, Vilagrosa A, Pausas JG, Bellot J (2010) Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol 207: 233–244 [Google Scholar]
- Impa SM, Nadaradjan S, Boominathan P, Shashidhar G, Bindumadhava HY, Sheshshayee MS (2005) Carbon isotope discrimination accurately reflects variability in WUE measured at a whole plant level in rice. Crop Sci 45: 2517–2522 [Google Scholar]
- Jagadish SK, Muthurajan R, Rang ZW, Malo R, Heuer S, Bennett J, Craufurd PQ (2011) Spikelet proteomic response to combined water deficit and heat stress in rice (Oryza sativa cv. N22). Rice 4: 1–11 [Google Scholar]
- Jensen WA. (1962) Botanical Histochemistry: Principles and Practice. WH Freeman, San Francisco [Google Scholar]
- Kano M, Inukai Y, Kitano H, Yamauchi A (2011) Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil 342: 117–128 [Google Scholar]
- Kato Y, Abe J, Kamoshita A, Yamagishi J (2006) Genotypic variation in root growth angle in rice (Oryza sativa L.) and its association with deep root development in upland fields with different water regimes. Plant Soil 287: 117–129 [Google Scholar]
- Kato Y, Kamoshita A, Yamagishi J, Imoto H, Abe J (2007) Growth of rice (Oryza sativa L.) cultivars under upland conditions with different levels of water supply. 3. Root system development, soil moisture change and plant water status. Plant Prod Sci 10: 3–13 [Google Scholar]
- Kato Y, Okami M (2011) Root morphology, hydraulic conductivity and plant water relations of high-yielding rice grown under aerobic conditions. Ann Bot (Lond) 108: 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Okami M, Tajima R, Fujita D, Kobayashi N (2010) Root response to aerobic conditions in rice, estimated by Comair root length scanner and scanner-based image analysis. Field Crops Res 118: 194–198 [Google Scholar]
- Khush GS. (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35: 25–34 [PubMed] [Google Scholar]
- Kumar A, Dixit S, Ram T, Yadaw RB, Mishra KK, Mandal NP (2014) Breeding high-yielding drought-tolerant rice: genetic variations and conventional and molecular approaches. J Exp Bot 65: 6265–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Liao DQ, Oane R, Estenor L, Yang XE, Li ZC, Bennett J (2006) Genetic variation in the sensitivity of anther dehiscence to drought stress in rice. Field Crops Res 97: 87–100 [Google Scholar]
- Lopes MS, Reynolds MP (2010) Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct Plant Biol 37: 147–156 [Google Scholar]
- Matsuo N, Ozawa K, Mochizuki T (2009) Genotypic differences in root hydraulic conductance of rice (Oryza sativa L.) in response to water regimes. Plant Soil 316: 25–34 [Google Scholar]
- McDonald MP, Galwey NW, Colmer TD (2002) Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant Cell Environ 25: 441–451 [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R (2001) Hydraulic conductivity of rice roots. J Exp Bot 52: 1835–1846 [DOI] [PubMed] [Google Scholar]
- Nautiyal PC, Rachaputi NR, Joshi YC (2002) Moisture-deficit-induced changes in leaf-water content, leaf carbon exchange rate and biomass production in groundnut cultivars differing in specific leaf area. Field Crops Res 74: 67–79 [Google Scholar]
- Niklas KJ. (1985) The evolution of tracheid diameter in early vascular plants and its implications on the hydraulic conductance of the primary xylem strand. Evolution 39: 1110–1122 [DOI] [PubMed] [Google Scholar]
- Olivares-Villegas JJ, Reynolds MP, McDonald GK (2007) Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Funct Plant Biol 34: 189–203 [DOI] [PubMed] [Google Scholar]
- Ostonen I, Puttsepp U, Biel C, Alberton O, Bakker MR, Lohmus K, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biosyst 141: 426–442 [Google Scholar]
- Pandey S, Bhandari H, Hardy B. , editors (2007) Economic Costs of Drought and Rice Farmers Coping Mechanisms: A Cross-Country Comparative Analysis. International Rice Research Institute, Manila, Philippines, p 203 [Google Scholar]
- Peng S, Bouman B, Visperas RM, Castañeda A, Nie L, Park HK (2006) Comparison between aerobic and flooded rice in the tropics: agronomic performance in an eight-season experiment. Field Crops Res 96: 252–259 [Google Scholar]
- Pfeiffer WH. (1988) Drought tolerance in bread wheat: analysis of yield improvement over the years in CIMMYT germplasm. InKlatt AR, ed, Wheat Production Constraints in Tropical Environments: Proceedings of the International Conference. International Maize and Wheat Improvement Center, Mexico City, pp 274–284 [Google Scholar]
- Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas JJ, Chapman SC (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor Appl Genet 121: 1001–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Fiorani F, Stitt M, Schurr U, Finck A, Gibon Y, Usadel B, Munns R, Atkin OK, Tardieu F, et al. (2012) The art of growing plants for experimental purposes: a practical guide for the plant biologist. Funct Plant Biol 39: 821–838 [DOI] [PubMed] [Google Scholar]
- Praba ML, Cairns JE, Babu RC, Lafitte HR (2009) Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J Agron Crop Sci 195: 30–46 [Google Scholar]
- Raju BR, Narayanaswamy BR, Mohankumar MV, Sumanth KK, Rajanna MP, Mohanraju B, Udaykumar M, Sheshshayee MS (2014) Root traits and cellular level tolerance hold the key in maintaining higher spikelet fertility of rice under water limited conditions. Funct Plant Biol 41: 930–939 [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R (2003) Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta 217: 193–205 [DOI] [PubMed] [Google Scholar]
- Rang ZW, Jagadish SVK, Zhou QM, Craufurd PQ, Heuer S (2011) Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ Exp Bot 70: 58–65 [Google Scholar]
- Rao RC, Wright GC (1994) Stability of the relationship between specific leaf area and carbon isotope discrimination across environments in peanut. Crop Sci 34: 98–103 [Google Scholar]
- Reich PB, Walters MB, Tjoelker MG, Vanderklein D, Buschena C (1998) Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct Ecol 12: 395–405 [Google Scholar]
- Reynolds M, Dreccer F, Trethowan R (2007) Drought-adaptive traits derived from wheat wild relatives and landraces. J Exp Bot 58: 177–186 [DOI] [PubMed] [Google Scholar]
- Rieger M, Litvin P (1999) Root system hydraulic conductivity in species with contrasting root anatomy. J Exp Bot 50: 201–209 [Google Scholar]
- Ryser P. (2006) The mysterious root length. Plant Soil 286: 1–6 [Google Scholar]
- Solari LI, Pernice F, DeJong TM (2006) The relationship of hydraulic conductance to root system characteristics of peach (Prunus persica) rootstocks. Physiol Plant 128: 324–333 [Google Scholar]
- Sorrells ME, La Rota M, Bermudez-Kandianis CE, Greene RA, Kantety R, Munkvold JD, Miftahudin, Mahmoud A, Ma X, Gustafson PJ, et al. (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Res 13: 1818–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BP, Vikram P, Dixit S, Ahmed HU, Kumar A (2011) Meta-analysis of grain yield QTL identified during agricultural drought in grasses showed consensus. BMC Genomics 12: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombesi S, Johnson RS, Day KR, DeJong TM (2010) Relationships between xylem vessel characteristics, calculated axial hydraulic conductance and size-controlling capacity of peach rootstocks. Ann Bot (Lond) 105: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody plants. New Phytol 119: 345–360 [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Venuprasad R, Bool ME, Quiatchon L, Cruz MS, Amante M, Atlin GN (2012) A large-effect QTL for rice grain yield under upland drought stress on chromosome 1. Mol Breed 30: 535–547 [Google Scholar]
- Venuprasad R, Sta Cruz MT, Amante M, Magbanua R, Kumar A, Atlin GN (2008) Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crops Res 107: 232–244 [Google Scholar]
- Verulkar SB, Mandal NP, Dwivedi JL, Singh BN, Sinha PK, Mahato RN, Dongre P, Singh ON, Bose LK, Swain P, et al. (2010) Breeding resilient and productive genotypes adapted to drought-prone rainfed ecosystem of India. Field Crops Res 117: 197–208 [Google Scholar]
- Vikram P, Swamy BP, Dixit S, Ahmed HU, Sta Cruz MT, Singh AK, Kumar A (2011) qDTY₁.₁, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet 12: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal RL, del Toro E, Mujeeb-Kazi A, Rajaram S (1995) The 1BL/1RS chromosome translocation effect on yield characteristics in a Triticum aestivum L. cross. Plant Breed 114: 497–500 [Google Scholar]
- Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63: 3485–3498 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Hasegawa S (1982) The rice root system: its development and function. InDrought Resistance in Crops with Emphasis on Rice. International Rice Research Institute, Manila, Philippines, pp 97–134 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.