An Arabidopsis transcription factor modulates phosphate homeostasis by regulating phosphate uptake and translocation.
Abstract
The Arabidopsis (Arabidopsis thaliana) WRKY transcription factor family has more than 70 members, and some of them have been reported to play important roles in plant response to biotic and abiotic stresses. This study shows that WRKY42 regulated phosphate homeostasis in Arabidopsis. The WRKY42-overexpressing lines, similar to the phosphate1 (pho1) mutant, were more sensitive to low-inorganic phosphate (Pi) stress and had lower shoot Pi content compared with wild-type plants. The PHO1 expression was repressed in WRKY42-overexpressing lines and enhanced in the wrky42 wrky6 double mutant. The WRKY42 protein bound to the PHO1 promoter under Pi-sufficient condition, and this binding was abrogated during Pi starvation. These data indicate that WRKY42 modulated Pi translocation by regulating PHO1 expression. Furthermore, overexpression of WRKY42 increased root Pi content and Pi uptake, whereas the wrky42 mutant had lower root Pi content and Pi uptake rate compared with wild-type plants. Under Pi-sufficient condition, WRKY42 positively regulated PHOSPHATE TRANSPORTER1;1 (PHT1;1) expression by binding to the PHT1;1 promoter, and this binding was abolished by low-Pi stress. During Pi starvation, the WRKY42 protein was degraded through the 26S proteasome pathway. Our results showed that AtWRKY42 modulated Pi homeostasis by regulating the expression of PHO1 and PHT1;1 to adapt to environmental changes in Pi availability.
Phosphorus is an essential nutrient for plant growth (Raghothama, 1999) and the main component of fertilizers to sustain modern agriculture. Approximately 70% of global cultivated land suffers from phosphate deficiency (López-Arredondo et al., 2014). Maintenance of phosphate homeostasis in plants is important for plant growth and reproduction, and it is achieved mainly by coordination of acquisition of inorganic phosphate (Pi; orthophosphate) from the soil solution, translocation of Pi from roots to shoots, and remobilization of internal Pi (Poirier and Bucher, 2002).
Pi is the only form of phosphorus that can be absorbed in plants (Chiou and Lin, 2011; López-Arredondo et al., 2014). Plants take up Pi from soil solution through phosphate transporters (PHTs) encoded by members of the PHT1 gene family. There are at least nine members (PHT1;1–PHT1;9) of the PHT1 family in Arabidopsis (Arabidopsis thaliana), and transcripts of PHT1;1 are the most abundant among nine PHT1 genes (Mudge et al., 2002). PHT1;1 and PHT1;4 play important roles in Pi uptake from soil. Under high-Pi condition, the pht1;1 mutants’ uptake rate was only 59% to 66% of the wild type, and the Pi uptake rates of pht1;4 mutants increased slightly (Shin et al., 2004), indicating that PHT1;1 plays an important role in Pi uptake under Pi-sufficient condition. During Pi starvation, the expressions of PHT1;1 and PHT1;4 are significantly induced (Muchhal et al., 1996; Karthikeyan et al., 2002; Mudge et al., 2002; Shin et al., 2004), and overexpression of PHT1;1 increases Pi uptake in Arabidopsis (Wang et al., 2014). Several transcription factors have been reported to regulate PHT1;1 expression. Under Pi-deficient condition, the transcription of PHT1;1 is positively regulated by PHOSPHATE STARVATION RESPONSE1 (PHR1; Rubio et al., 2001), WRKY75 (Devaiah et al., 2007), and WRKY45 (Wang et al., 2014) and negatively regulated by MYB DOMAIN PROTEIN62 (MYB62; Devaiah et al., 2009). However, under Pi-sufficient condition, the molecular mechanism for the regulation of PHT1;1 expression is unknown. PHT1;1 is also regulated at posttranscription level. The PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 (PHF1) protein is necessary for PHT1;1 plasma membrane localization, and mutation of PHF1 impairs the localization of PHT1;1 at the plasma membrane (González et al., 2005). Additionally, E2 conjugase PHOSPHATE2 (PHO2) modulates PHT1;1 protein degradation (Huang et al., 2013), and NITROGEN LIMITATION ADAPTATION mediates PHT1;1 and PHT1;4 degradation to maintain phosphate homeostasis (Lin et al., 2013; Park et al., 2014).
Another important pathway controlling Pi homeostasis involves PHO1, which plays an important role in Pi translocation from roots to shoots (Poirier et al., 1991; Hamburger et al., 2002; Wang et al., 2004). The pho1 mutant is deficient in loading Pi acquired by roots to the xylem vessel and only accumulates 24% to 44% as much total phosphate as wild-type plants in shoots (Poirier et al., 1991). PHO1 is located primarily in the root stelar cells and has a role in Pi efflux out of root stelar cells for xylem loading (Hamburger et al., 2002). There are 11 members of the PHO1 gene family in the Arabidopsis genome, and only PHO1 and PHO1;H1 can complement the pho1 mutant (Wang et al., 2004), indicating that PHO1 and PHO1;H1 are involved in long-distance Pi transport from roots to shoots. The increased transcript level of PHO1;H1 during Pi starvation is mainly controlled by the PHR1 transcription factor (Stefanovic et al., 2007), whereas expression of PHO1 is independent of PHR1 regulation (Stefanovic et al., 2007). The PHO1 expression is directly down-regulated by the WRKY6 transcription factor under Pi-sufficient condition (Chen et al., 2009). WRKY42, a homolog of WRKY6, could bind to the PHO1 promoter in vivo and repressed the PHO1 promoter activity in tobacco (Nicotiana benthamiana; Chen et al., 2009), indicating that WRKY42 also negatively regulates PHO1 expression. PHO2 can modulate PHO1 degradation (Liu et al., 2012).
In this article, we report that Arabidopsis WRKY42 plays important roles in modulating Pi homeostasis in Arabidopsis. WRKY42 modulates Pi uptake and translocation by directly regulating PHT1;1 and PHO1 expression under Pi-sufficient condition. During Pi starvation, WRKY42 expression is repressed, and the WRKY42 protein is degraded through a proteasome pathway; then, the binding of WRKY42 to the promoters of PHO1 and PHT1;1 is abolished.
RESULTS
WRKY42 Encodes a Phosphate Starvation-Responsive Transcription Factor
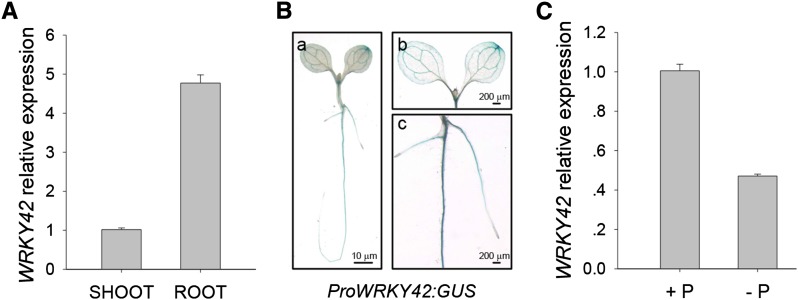
WRKY42 is a homolog of WRKY6 in Arabidopsis (Eulgem et al., 2000), and our previous results showed that WRKY6 regulated Pi translocation (Chen et al., 2009). We wonder whether WRKY42 plays a role in Arabidopsis responses to Pi starvation. The expression pattern of WRKY42 was first tested. Quantitative real-time (qRT)-PCR analysis showed that WRKY42 was mainly expressed in the roots (Fig. 1A). To further confirm the expression pattern of WRKY42, the homozygous single-copy ProWRKY42:GUS transgenic lines were generated. GUS staining was strong in roots (Fig. 1, B, a and c) and weak in leaves (Fig. 1, B, a and b). Then, the expression of WRKY42 was tested under Pi starvation. The 7-d-old wild-type seedlings were transferred to Pi-sufficient (Murashige and Skoog [MS] medium) or Pi-deficient (low-phosphate [LP] medium with 10 μm Pi) condition for 3 d, and then, the roots were harvested for qRT-PCR analysis. Transcription of WRKY42 was obviously suppressed under Pi-deficient condition (Fig. 1C), indicating that WRKY42 was involved in Arabidopsis responses to Pi starvation.
Figure 1.
Expression pattern of Arabidopsis WRKY42. A, qRT-PCR analysis of WRKY42 expression from shoots and roots of 10-d-old wild-type seedlings. Transcript level of WRKY42 was quantified relative to ACTIN2/8. The data represent the mean values of three replicates ± se. B, GUS staining of transgenic ProWRKY42:GUS. The ProWRKY42:GUS seedlings were germinated, grown on MS medium for 7 d, and then harvested for GUS staining (a). Details of the leaf and root of the ProWRKY42:GUS transgenic line are shown in b and c. C, qRT-PCR analysis of WRKY42 expression in Arabidopsis under Pi starvation. Seven-day-old wild-type seedlings were transferred to Pi-sufficient condition (MS medium; +P) or Pi-deficient condition (LP medium with 10 μm Pi; −P) for 3 d; then, the roots were harvested for RNA extraction. Transcript level of WRKY42 was quantified relative to ACTIN2/8. The data represent the mean values of three replicates ± se.
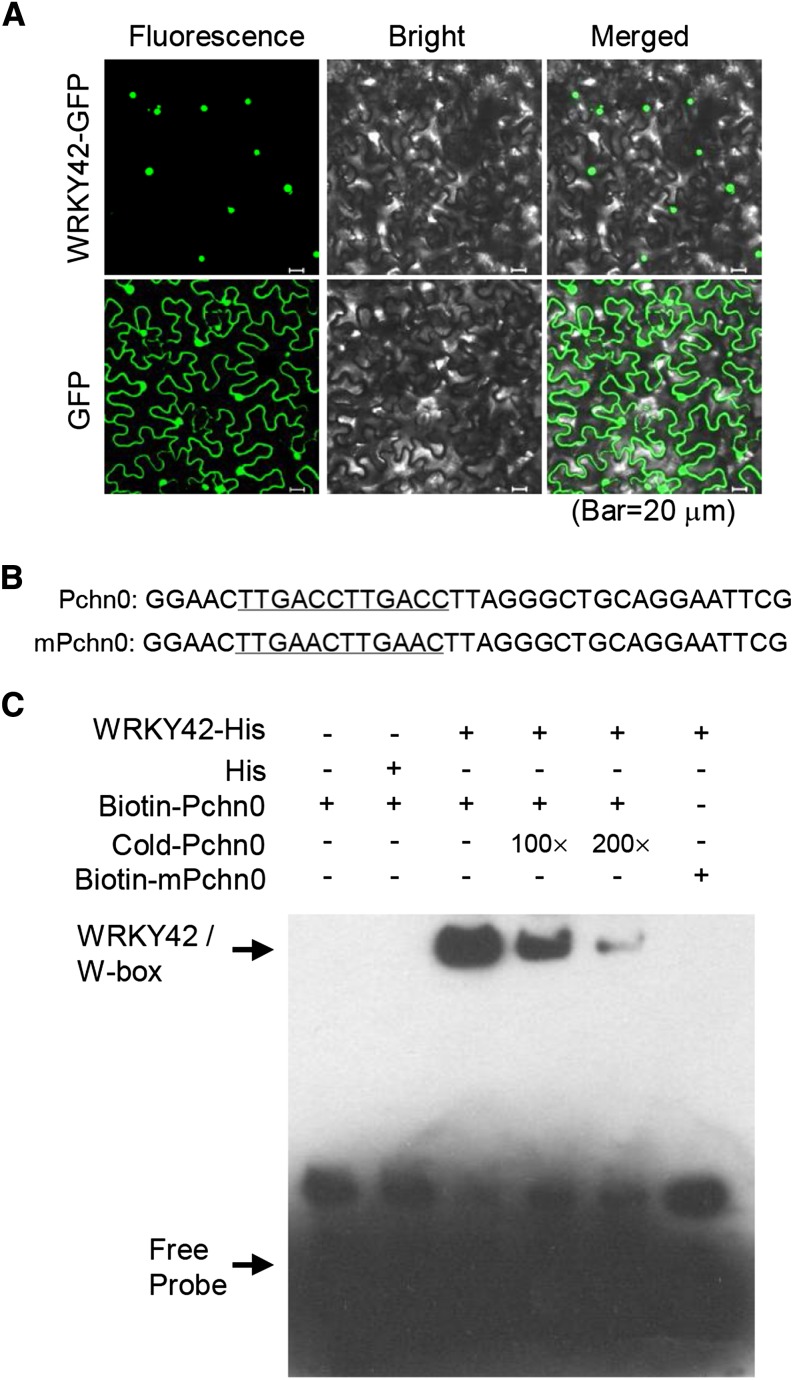
The WRKY42 protein, as a transcription factor, is likely to be localized to the nucleus. To detect this, the coding region of WRKY42 was fused with the 3′-end of the GFP reporter gene and expressed under the control of Super promoter (Li et al., 2001). The GFP gene alone under control of the Super promoter served as a control. The subcellular localization of WRKY42 was tested in a transient expression system in tobacco leaves. The WRKY42-GFP fusion protein was exclusively localized in the nucleus, and GFP alone was localized in the cytoplasm and nucleus (Fig. 2A).
Figure 2.
WRKY42 is localized in the nucleus and binds to W-box motifs. A, Subcellular localization of WRKY42-GFP fusion protein in tobacco leaves. The GFP alone was used as the control. B, Oligonucleotides used in the EMSA (C). The Pchn0 probe contains two W-box (TTGACC) sequences, and the mPchn0 probe has two mutated W-boxes (TTGAAC). The wild-type and mutated W-boxes are underlined. C, EMSA showing the binding of recombinant WRKY42 to W-box motif. The oligonucleotides (Pchn0 and mPchn0) were used as the probes. Each biotin-labeled DNA probe was incubated with recombinant WRKY42-His protein. An excess of unlabeled probe (Cold-Pchn0) was added to compete with labeled Pchn0 probe (Biotin-Pchn0). Biotin-labeled Pchn0 probe incubated with His protein served as the negative control.
As a member of the WRKY transcription factor family, WRKY42 has a highly conserved WRKYGQK motif and a characteristic Cys2His2 zinc finger motif (Eulgem et al., 2000). Both the WRKYGQK and Cys2His2 motifs are necessary for the binding affinity of WRKY proteins to the consensus sequence (C/T)TGAC(C/T), known as W-box (Eulgem et al., 2000; Rushton et al., 2010). To test whether WRKY42 protein bound to the W-box, WRKY42 was expressed in Escherichia coli as a fusion protein with His-tag, and an electrophoresis mobility shift assay (EMSA) was conducted with WRKY42-His fusion protein and the synthesized probes with two normal or mutant W-boxes (Fig. 2B; Lai et al., 2011). The WRKY42-His fusion protein could bind to the probe (Pchn0) with two normal W-boxes, and the binding was abolished by addition of increasing amounts of unlabeled competitors (Fig. 2C). In contrast, the WRKY42-His fusion protein could not bind to the probe (mPchn0), which has two mutant W-boxes, and the His protein alone showed no detectable binding to the W-boxes.
WRKY42 Negatively Modulates Pi Translocation
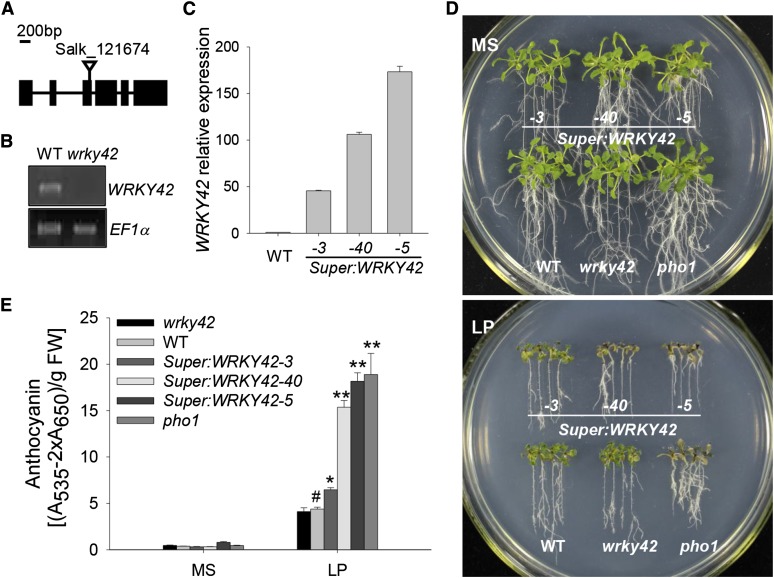
To reveal the function of WRKY42, the T-DNA insertion mutant Salk_121674 was obtained from the Arabidopsis Biological Resource Center (http://abrc.osu.edu) and named wrky42. The wrky42 mutant (Salk_121674) carried a transferred DNA (T-DNA) insertion in the third exon of WRKY42 (Fig. 3A), and reverse transcription (RT)-PCR analysis showed that WRKY42 expression was abolished in the wrky42 mutant (Fig. 3B). In addition, WRKY42-overexpressing lines were generated, and expression levels of WRKY42 in these lines were much higher than in wild-type seedlings (Fig. 3C). Of three WRKY42-overexpressing lines, Super:WRKY42-3, Super:WRKY42-40, and Super:WRKY42-5 displayed low, medium, and high WRKY42 expression, respectively (Fig. 3C).
Figure 3.
Phenotype tests of various plant materials. A, Diagram of the WRKY42 gene showing the position of the T-DNA insertion. Exons (boxes), introns (lines), and the T-DNA insertion site of Salk_121674 (triangle) are indicated. B, RT-PCR analysis of WRKY42 expression in the wrky42 mutant (Salk_121674) and wild-type (WT) seedlings. The EF1α is amplified as the control. C, qRT-PCR analysis of WRKY42 expression in the WRKY42-overexpressing lines (Super:WRKY42-3, Super:WRKY42-40, and Super:WRKY42-5) and wild-type plants. Transcript level of WRKY42 was quantified relative to ACTIN2/8. The data represent the mean values of three replicates ± se. D, Phenotype comparison of the wrky42 mutant, WRKY42-overexpressing lines, the pho1 mutant, and wild-type seedlings during Pi starvation. Seven-day-old seedlings were transferred to MS medium (Pi-sufficient medium with 1.25 mm Pi; top) or LP medium (low-Pi medium with 10 μm Pi; bottom) for another 10 d; then, photos were taken. E, Anthocyanin accumulation in the wrky42 mutant, WRKY42-overexpressing lines, the pho1 mutant, and wild-type seedlings during Pi starvation. Seven-day-old seedlings were transferred to MS or LP medium for another 10 d; then, the seedlings were harvested for anthocyanin content measurement. Data are shown as means ± se (n = 3). Asterisks indicate significant differences compared with wild-type plants (paired test). FW, Fresh weight; *, P < 0.05; **, P < 0.01; #, wild-type plants were used as a control.
Anthocyanin accumulation is one of the most striking symptoms of Pi starvation in plants (Marschner, 1995). When grown in Pi-sufficient condition (MS medium with 1.25 mm Pi), all tested plants showed no obvious differences in phenotypes (Fig. 3D, top). When the 7-d-old seedlings were transferred to Pi-deficient condition (LP medium with 10 μm Pi) for 10 d, the WRKY42-overexpressing lines, particularly Super:WRKY42-40 and Super:WRKY42-5 (both had much higher WRKY42 expression than Super:WRKY42-3), turned purple, similar to the pho1 mutant, whereas the wrky42 mutant and wild-type plants remained green (Fig. 3D, bottom). During Pi starvation, the anthocyanin contents in the WRKY42-overexpressing lines (Super:WRKY42-40 and Super:WRKY42-5) and the pho1 mutant were approximately 3-fold those in wild-type seedlings (Fig. 3E).
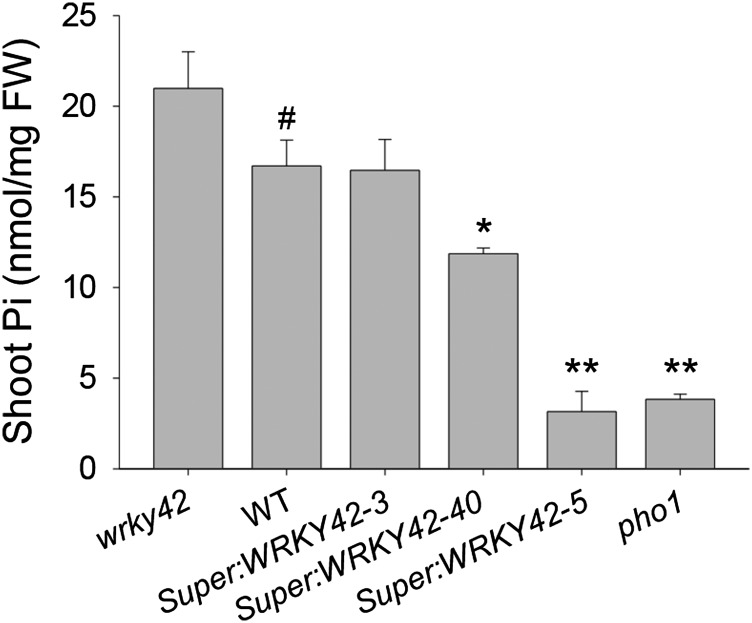
The pho1 mutant has a defect in Pi transfer from roots to shoots, which results in reduced Pi content in shoots (Poirier et al., 1991; Hamburger et al., 2002). Therefore, a role for WRKY42 in translocating Pi was hypothesized. To test this, the shoot Pi was measured in 10-d-old WRKY42-overexpressing lines, wrky42 mutant, pho1 mutant, and wild-type seedlings grown under Pi-sufficient condition. The WRKY42-overexpressing lines had similarly reduced shoot Pi contents to the pho1 mutant, and the reduced level of shoot Pi content was closely related to WRKY42 expression (Fig. 4), indicating that WRKY42 negatively modulated Pi translocation in Arabidopsis.
Figure 4.
Shoot Pi content measurement in various plant materials. The shoot Pi contents of 10-d-old wrky42 mutant, WRKY42-overexpressing lines, pho1 mutant, and wild-type (WT) seedlings grown in Pi-sufficient condition. Data are shown as means ± se (n = 4). Asterisks indicate significant differences compared with wild-type plants (paired test). FW, Fresh weight; *, P < 0.05; **, P < 0.01; #, wild-type plants were used as a control.
WRKY42 Directly Down-Regulates PHO1 Expression
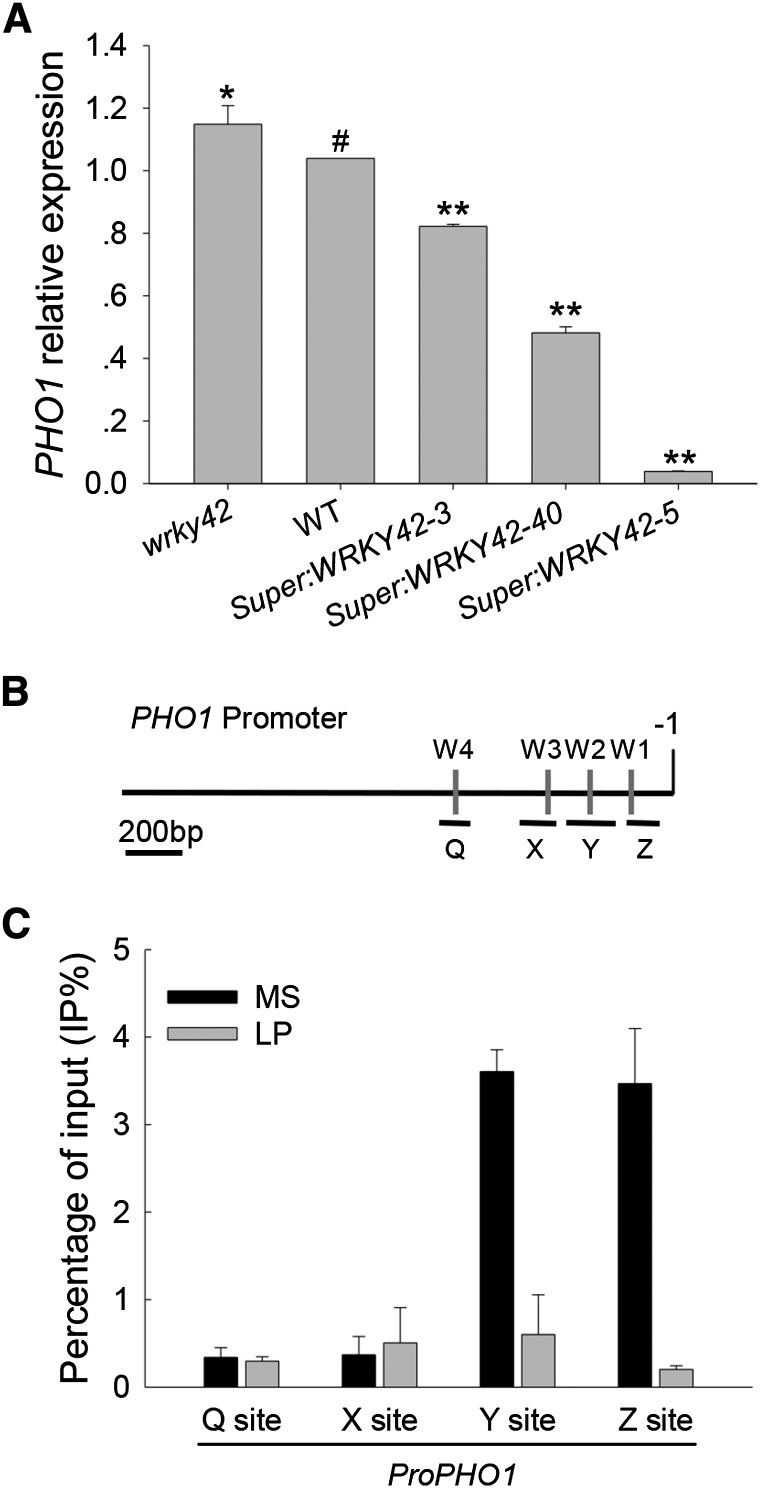
Because WRKY42-overexpressing lines and the pho1 mutant had similar low Pi-sensitive phenotypes and lower shoot Pi contents (Figs. 3 and 4), it was hypothesized that WRKY42 negatively regulated PHO1 expression. The transcription level of PHO1 gene was evaluated in the roots of WRKY42-overexpressing lines, the wrky42 mutant, and wild-type plants, because PHO1 is mainly expressed in roots (Hamburger et al., 2002). The transcription of PHO1 was repressed in the WRKY42-overexpressing lines (Fig. 5A), and the repression level of PHO1 expression was consistent with WRKY42 expression in the WRKY42-overexpressing lines, with the strongest repression in Super:WRKY42-5 and the weakest in Super:WRKY42-3.
Figure 5.
WRKY42 down-regulates PHO1 expression and binds to the PHO1 promoter. A, qRT-PCR analysis of PHO1 expression in the wrky42 mutant, WRKY42-overexpressing lines, and wild-type (WT) plants. All plants were germinated and grown on MS medium for 10 d; then, the roots were harvested for RNA extraction. Transcript level of PHO1 was quantified relative to ACTIN2/8. The data represent the mean values of three replicates ± se. Asterisks indicate significant differences compared with the wild type (paired test). *, P < 0.05; **, P < 0.01; #, wild-type plants were used as a control. B, Diagram of the PHO1 promoter showing the relative positions of the W-boxes. The adenine residue of the translational start codon ATG was assigned position +1, and the numbers flanking the sequences of the PHO1 promoter fragments were counted based on this number. The W-boxes are marked by gray rectangles, and relative positions and sizes of the different PCR-amplified fragments are indicated by black lines under the W-box. C, ChIP-qPCR assay to detect the association between WRKY42 and the PHO1 promoter. Seven-day-old seedlings were transferred to Pi-sufficient (MS) or Pi-deficient (LP) condition for another 7 d; then, the roots were harvested for ChIP-qPCR. Chromatins were immunoprecipitated with anti-WRKY42 antibody, and the amount of indicated DNA in immune complex was tested by qRT-PCR. The ratio of immunoprecipitation DNA over the input was presented as the percentage of input (IP%). The experiments were repeated three times, and three replicates were included for each sample in each experiment. The data are presented as means ± se (n = 3).
Because WRKY42 is a typical WRKY transcription factor that can bind to W-box motif (Fig. 2C) and sequence analysis showed that there are several W-boxes within the PHO1 promoter (Fig. 5B; Chen et al., 2009), a chromatin immunoprecipitation (ChIP) assay was used to determine whether WRKY42 could bind to the promoter of PHO1 in vivo. The 7-d-old wild-type seedlings were transferred to Pi-sufficient (MS) or Pi-deficient (LP) medium for another 7 d, and the roots were harvested for ChIP assay. Chromatin immunoprecipitated with the anti-WRKY42 antibody was enriched in the y and z sites of the PHO1 promoter (Fig. 5C) when wild-type seedlings were grown in Pi-sufficient condition (MS), consistent with a previous report (Chen et al., 2009). After Pi starvation treatment, the interactions between the WRKY42 and y or z sites of the PHO1 promoter were severely impaired (Fig. 5C). These data show that WRKY42 directly down-regulated PHO1 expression.
WRKY42 and WRKY6 Have Functional Redundancy in Down-Regulating PHO1 Expression
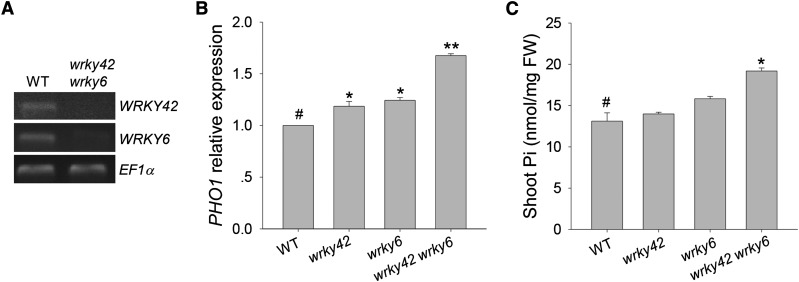
Our previous work showed that WRKY6 negatively regulated PHO1 expression, and the PHO1 expression was repressed in the WRKY6-overexpressing lines and elevated in the wrky6 mutant (Chen et al., 2009). We hypothesized that WRKY42 and WRKY6 had redundant functions in regulating PHO1 expression. To test this hypothesis, the wrky42 wrky6 double mutant was generated (Fig. 6A) that, when grown in Pi-sufficient condition, had obviously higher PHO1 expression than the wrky42 mutant, wrky6 mutant, and wild-type plants (Fig. 6B). Because overexpression of PHO1 enhances the shoot Pi content (Liu et al., 2012), the shoot Pi content was measured in the wrky42 wrky6 double mutant, wrky42 mutant, wrky6 mutant, and wild-type seedlings. The shoot Pi content of the wrky42 wrky6 double mutant was higher than that in wild-type plants (Fig. 6C). These data indicate that WRKY42 and WRKY6 had redundant functions in regulating PHO1 expression.
Figure 6.
Loss of function of WRKY42 and WRKY6 enhanced PHO1 expression and shoot Pi content. A, RT-PCR analysis of WRKY42 and WRKY6 expression in the wrky42 wrky6 double mutant and wild-type (WT) plants. The EF1α is amplified as the control. B, qRT-PCR analysis of PHO1 expression in the wrky42 mutant, wrky6 mutant, wrky42 wrky6 double mutant, and wild-type plants. Transcript level of PHO1 was quantified relative to ACTIN2/8. Each data bar represents the means ± se (n = 3). Asterisks indicate significant differences compared with the wild type (paired test). *, P < 0.05; **, P < 0.01; #, wild-type plants were used as a control. C, The shoot Pi content of 17-d-old wrky42 mutant, wrky6 mutant, wrky42 wrky6 double mutant, and wild-type seedlings grown on Pi-sufficient medium. Data are shown as means ± se (n = 3). Asterisks indicate significant differences compared with the wild type (paired test). FW, Fresh weight; *, P < 0.05; #, wild-type plants were used as a control.
WRKY42 Positively Modulates Pi Uptake
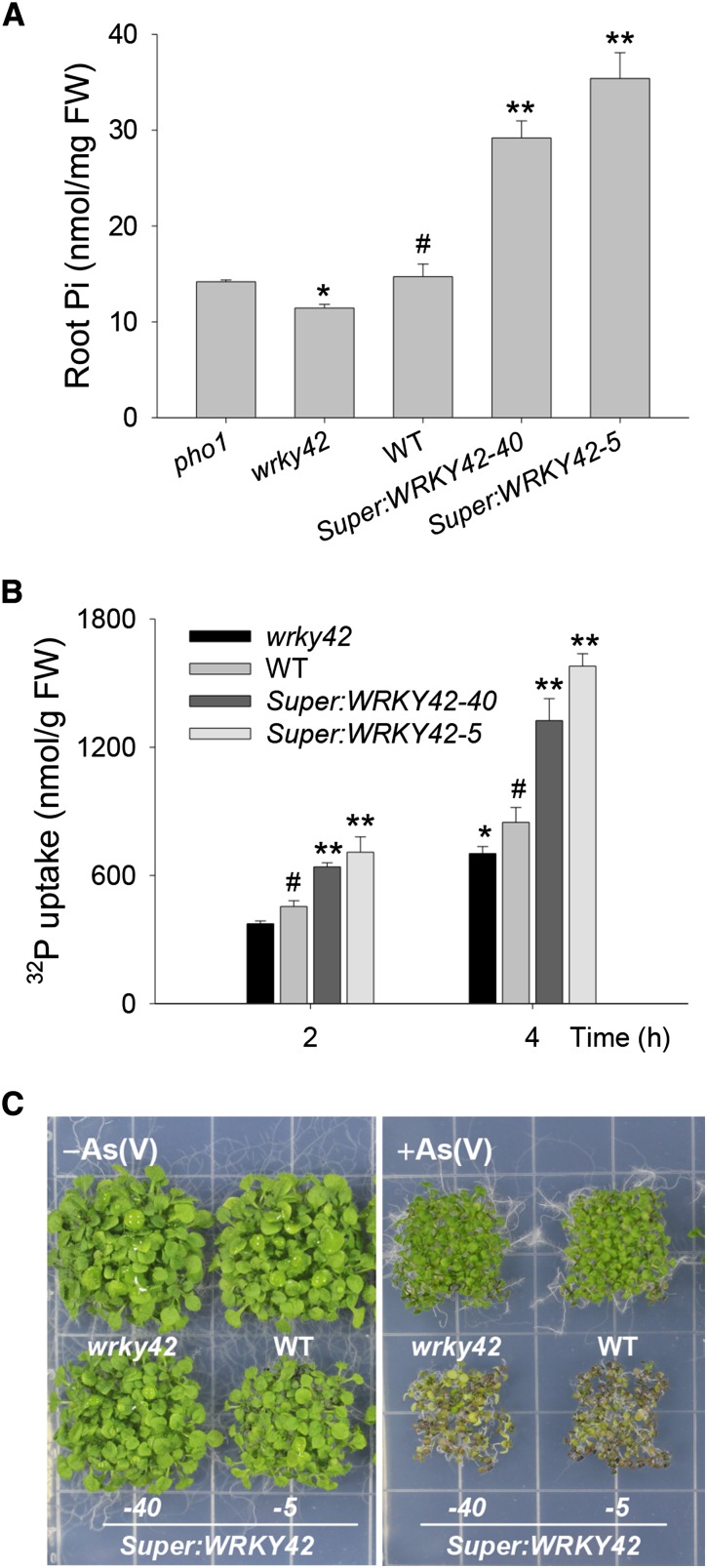
In addition to shoot Pi content, the root Pi content was also measured among various plants. When grown in Pi-sufficient condition, the WRKY42-overexpressing lines Super:WRKY42-40 and Super:WRKY42-5 (both had higher WRKY42 expression than Super:WRKY42-3) contained around 2- to 3.5-fold of the root Pi content of wild-type plants (Fig. 7A). In contrast, the wrky42 mutant had a lower root Pi content than wild-type plants (Fig. 7A), suggesting that WRKY42 may regulate Pi uptake. The root Pi content of pho1 mutant was also tested, and no obvious difference was found between pho1 mutant and wild-type plants (Fig. 7A), indicating that the induced root Pi content in WRKY42-overexpressing lines was not because of the repression of PHO1 caused by WRKY42 overexpression.
Figure 7.
Overexpression of WRKY42 enhances Pi acquisition. A, The root Pi contents of 10-d-old wrky42 mutant, WRKY42-overexpressing lines, and wild-type (WT) seedlings grown on Pi-sufficient medium. Data are shown as means ± se (n = 4). Asterisks indicate significant differences compared with the wild type (paired test). B, Pi uptake was monitored over a 4-h period in 10-d-old wrky42 mutant, WRKY42-overexpressing lines, and wild-type seedlings. Data are shown as means ± se (n = 3). Asterisks indicate significant differences compared with wild-type plants (paired test). C, As(V) tolerance phenotype of plants germinated and grown on one-half-strength MS medium with [+As(V)] or without [−As(V)] 200 μm As(V) for 20 d. FW, Fresh weight; *, P < 0.05; **, P < 0.01; #, wild-type plants were used as a control.
The Pi uptake rate was measured to determine the effect of WRKY42 on Pi acquisition. The 10-d-old seedlings were transferred into a Pi uptake solution containing 500 μm Pi supplemented with 32P orthophosphate, and Pi uptake was measured over a 4-h period. Consistent with the root Pi content, the WRKY42-overexpressing lines had a significantly (P < 0.05) higher Pi uptake rate compared with wild-type seedlings, and that of the wrky42 mutant was lower than that of the wild type (Fig. 7B). Arsenate [As(V)] is an oxyanion structurally analogous to phosphate (Asher and Reay, 1979) and taken up mainly through Pi transporter PHT1;1 (Catarecha et al., 2007). When grown on medium containing As(V), the pht1;1 mutant showed an As(V)-tolerant phenotype, and the PHT1;1-overexpressing line was more sensitive to As(V) than wild-type plants (Supplemental Fig. S1; Catarecha et al., 2007; Wang et al., 2014). To gain additional insight into the role of WRKY42 in Pi acquisition, the phenotypes of WRKY42-overexpressing lines, the wrky42 mutant, and wild-type seedlings were tested with As(V). When grown on Pi-sufficient medium without As(V) [−As(V)], there were no obvious phenotypic differences among the WRKY42-overexpressing lines, the wrky42 mutant, and wild-type seedlings (Fig. 7C; Supplemental Fig. S1). When grown on Pi-sufficient medium with 200 μm As(V) [+As(V)], although the toxic effect of As(V) was evident in the growth of WRKY42-overexpressing lines, the wrky42 mutant, and wild-type plants, their degree of sensitivity varied. The WRKY42-overexpressing lines had a much more As(V)-sensitive phenotype, similar to the phenotype of PHT1;1-overexpressing lines, compared with wild-type seedlings (Fig. 7C; Supplemental Fig. S1). There were no obvious differences between the wrky42 mutant and wild-type seedlings when grown on Pi-sufficient medium with 200 μm As(V) (Fig. 7C; Supplemental Fig. S1). Together, these data indicate that overexpression of WRKY42 enhanced Arabidopsis Pi accumulation.
WRKY42 Directly Up-Regulates PHT1;1 Expression
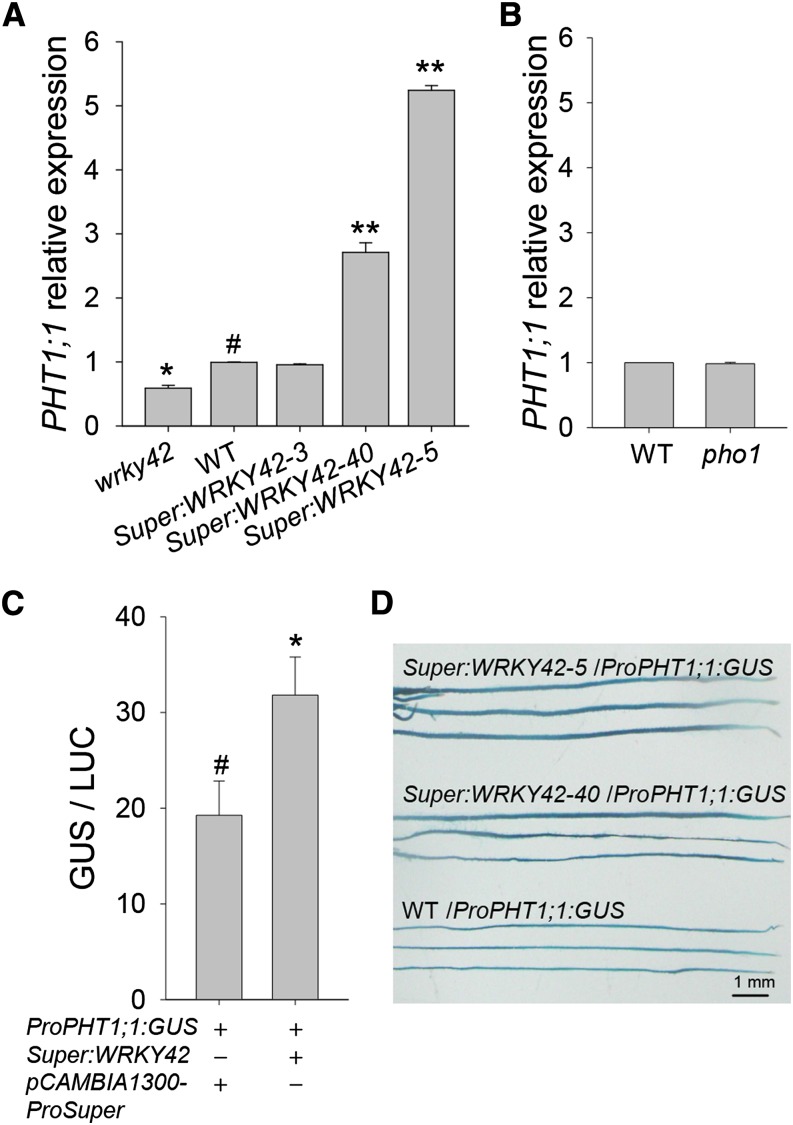
There are nine PHTs (PHT1;1–PHT1;9) in Arabidopsis (Mudge et al., 2002); of these, expression of PHT1;1 is most highly expressed in roots when wild-type plants are grown in Pi-sufficient condition (Mudge et al., 2002), and overexpression of PHT1;1 enhances Arabidopsis Pi uptake (Wang et al., 2014). Therefore, we examined expression of PHT1;1 in roots of WRKY42-overexpressing lines, the wrky42 mutant, and wild-type plants under Pi-sufficient condition. Transcription of PHT1;1 was obviously elevated in the WRKY42-overexpressing lines (Super:WRKY42-40 and Super:WRKY42-5) and repressed in the wrky42 mutant compared with wild-type plants (Fig. 8A). The PHT1;1 expression was also tested in the pho1 mutant. The expression level of PHT1;1 in the pho1 mutant was similar to that in wild-type plants (Fig. 8B), indicating that the PHT1;1 induction in WRKY42-overexpressing lines was not caused by the PHO1 repression caused by WRKY42 overexpression. To further test whether WRKY42 protein directly regulated PHT1;1 expression, transient expression experiments in tobacco leaves were performed. The cotransformation of Super:WRKY42 with PHT1;1 promoter-driving GUS reporter gene (ProPHT1;1:GUS; Wang et al., 2014) resulted in enhanced GUS activity (Fig. 8C), indicating that WRKY42 positively regulated PHT1;1 expression in vivo. In addition, we crossed the ProPHT1;1:GUS line (Wang et al., 2014) with the WRKY42-overexpressing lines (Super:WRKY42-5 and Super:WRKY42-40) and wild-type plants and obtained the Super:WRKY42-5/ProPHT1;1:GUS, Super:WRKY42-40/ProPHT1;1:GUS, and wild-type/ProPHT1;1:GUS plants, respectively. The root GUS staining showed that the PHT1;1 expression was promoted in the WRKY42-overexpressing lines (Super:WRKY42-5/ProPHT1;1:GUS and Super:WRKY42-40/ProPHT1;1:GUS) compared with that in the wild type (the wild type/ProPHT1;1:GUS) under Pi-sufficient condition (Fig. 8D). These data indicated that WRKY42 positively regulated PHT1;1 expression.
Figure 8.
WRKY42 positively regulates PHT1;1 expression. A, qRT-PCR analysis of PHT1;1 expression in the roots of the WRKY42-overexpressing lines, wrky42 mutant, and wild-type (WT) plants. The plants were germinated and grown on MS medium for 10 d; then, the roots were harvested for RNA extraction. Transcript level of PHT1;1 was quantified relative to ACTIN2/8. Each data bar represents the means ± se (n = 3). Asterisks indicate significant differences compared with wild-type plants (paired test). B, qRT-PCR analysis of PHT1;1 expression in the roots of the pho1 mutant and wild-type plants. Transcript level of PHT1;1 was quantified relative to ACTIN2/8. Each data bar represents the means ± se (n = 3). C, Transient overexpression of WRKY42 fused to ProPHT1;1:GUS in tobacco leaves. Each data bar represents the means ± se (n = 5). Asterisks indicate significant differences. D, GUS staining showing the expression patterns of PHT1;1 in the WRKY42-overexpression lines and wild-type plants. The plants were germinated, grown on MS medium for 7 d; then, harvested for GUS staining. *, P < 0.05; **, P < 0.01; #, wild-type plants were used as a control.
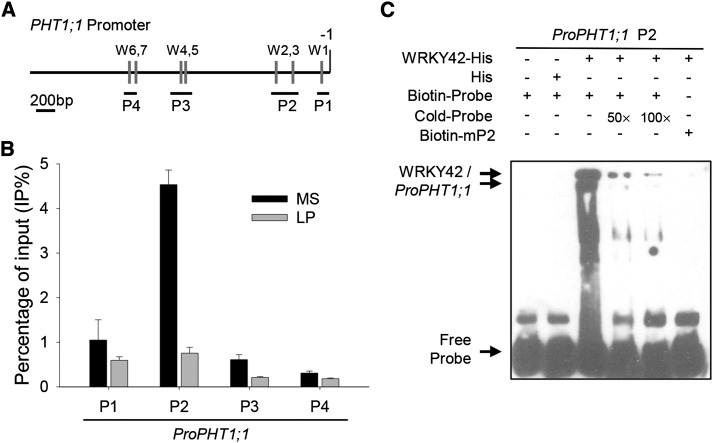
Promoter sequence analysis showed that there were several W-boxes within the PHT1;1 promoter (Fig. 9A; Martín et al., 2000; Wang et al., 2014); thus, we hypothesized that WRKY42 directly regulates PHT1;1 expression by binding to the W-box within the PHT1;1 promoter. The in vivo interaction between WRKY42 and the W-box motifs within the PHT1;1 promoter was investigated using ChIP-quantitative PCR (qPCR) analysis. The 7-d-old wild-type seedlings were transferred to Pi-sufficient (+phosphorus) or Pi-deficient (−phosphorus) medium for another 7 d, and then, the roots were harvested for ChIP-qPCR assay. When wild-type plants were grown in Pi-sufficient condition, the chromatin immunoprecipitated with the anti-WRKY42 antibody was enriched in the P2 fragment of the PHT1;1 promoter, whereas no interaction was observed between WRKY42 and the PHT1;1 promoter containing P1, P3, or P4 fragments (Fig. 9B). During Pi starvation, the interaction between WRKY42 and the P2 fragment within the PHT1;1 promoter was abolished (Fig. 9B). Furthermore, the EMSA was also performed to detect whether WRKY42 could bind to the P2 fragment of the PHT1;1 promoter in vitro. The WRKY42-His fusion protein could bind to P2 within the PHT1;1 promoter, and the binding was effectively reduced by adding increasing amounts of unlabeled competitors with the same P2 sequence (Fig. 9C). In contrast, the WRKY42-His fusion protein could not bind to the mutation probe (mP2), which has two mutated W-boxes (Fig. 9C). As the negative control, the His protein alone did not bind to the PHT1;1 promoter (Fig. 9C). These data show that WRKY42 positively regulated PHT1;1 expression.
Figure 9.
WRKY42 binds to the PHT1;1 promoter. A, Diagram of the PHT1;1 promoter region showing the relative positions of the W-boxes (gray rectangles) and the relative positions and sizes of the different PCR-amplified fragments (black lines under the W-boxes). The adenine residue of the translational start codon ATG was assigned position +1, and the numbers flanking the sequences of the PHT1;1 promoter fragments were counted based on this number. B, ChIP-qPCR assay to detect the association between WRKY42 and the PHT1;1 promoter. Seven-day-old seedlings were transferred to Pi-sufficient (MS) or Pi-deficient (LP) condition for another 7 d; then, the roots were harvested for ChIP-qPCR assay with anti-WRKY42. The ratio of immunoprecipitation DNA to the input was presented as the percentage of input (IP%). The data are presented as means ± se (n = 3). C, EMSA to analyze the binding of WRKY42 to P2 fragment of the PHT1;1 promoter. Each biotin-labeled DNA probe was incubated with His-WRKY42 protein. An excess of unlabeled probe was added to compete with labeled promoter sequence. Biotin-labeled probe incubated with His protein served as the negative control.
WRKY42 Is Degraded during Phosphate Starvation
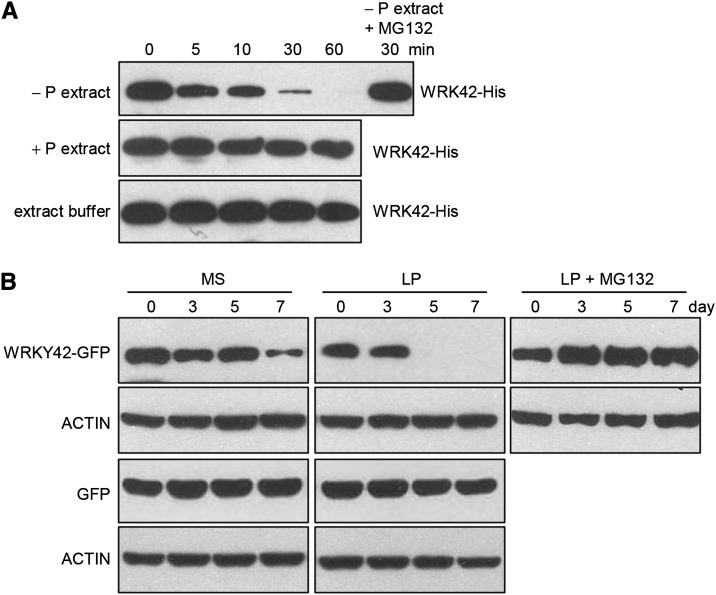
Because the interaction between WRKY42 and the promoters of PHO1 or PHT1;1 was abolished during Pi starvation (Figs. 5C and 9B), it was proposed that the WRKY42 protein was degraded under Pi-deficient stress. To determine the relationship between the WRKY42 degradation and Pi status, the cell-free degradation analysis was conducted. The recombinant WRKY42-His protein was purified from E. coli and incubated with the total protein extracts from the 7-d-old wild-type seedlings cultured under Pi-sufficient (MS medium with 1.25 mm Pi; +phosphorus) or Pi-deficient (LP medium with 10 μm Pi; −phosphorus) condition for another 5 d. When incubated with +phosphorus total protein extract, the WRKY42 protein showed very faint degradation (Fig. 10A). When the WRKY42 protein was incubated with −phosphorus total protein extract, the WRKY42 protein was obviously degraded. This degradation of WRKY42 was inhibited by the carbobenzoxyl-leucinyl-leucinyl-leucinal (MG132), a 26S proteasome inhibitor (Fig. 10A), indicating that Pi starvation induced the proteasome-dependent degradation of WRKY42.
Figure 10.
WRKY42 is degraded during Pi starvation. A, Cell-free degradation assay. Seven-day-old wild-type seedlings were transferred to Pi-sufficient medium (+P) or Pi-deficient medium (−P) for another 5 d; then, the seedlings were harvested for protein extraction. The plant protein extracts were incubated with recombinant WRKY42-His for the indicated time; then, WRKY42 abundance was determined by immunoblotting with anti-His. B, Immunoblot analysis of WRKY42 protein. Seven-day-old Super:WRKY42-GFP and Super:GFP transgenic seedlings were transferred to MS medium, LP medium, or LP medium with 10 μm MG132 (LP + MG132), and the seedlings were harvested at the indicated time for protein extraction. Protein extracts were analyzed by immunoblots using anti-GFP. Actin was used as the loading control.
To further confirm the degradation of WRKY42 during Pi starvation in vivo, the Super:WRKY42-GFP and Super:GFP transgenic lines were generated. The 7-d-old Super:WRKY42-GFP and Super:GFP seedlings were transferred to Pi-sufficient (MS) or Pi-deficient (LP) medium and then harvested at the indicated time for protein gel-blot analysis using anti-GFP. The WRKY42 protein decreased much more rapidly in Super:WRKY42-GFP exposed to Pi starvation compared with Pi-sufficient condition (Fig. 10B). To further confirm that reduction of WRKY42 protein level was caused by the proteasome-dependent degradation in vivo, the 7-d-old Super:WRKY42-GFP seedlings were also transferred to LP medium with 10 μm MG132. The addition of MG132 clearly inhibited WRKY42 degradation under Pi starvation condition (Fig. 10B). Super:GFP was used as a control, and no GFP degradation was detected in Pi-deficient or -sufficient condition (Fig. 10B). Taken together, these data showed that the WRKY42 protein was degraded through the proteasome pathway during Pi starvation and stabilized by abundant Pi.
DISCUSSION
WRKY42 Is a Key Regulator in Phosphate Homeostasis in Plants
Phosphate plays important roles in regulation of many biochemical and physiological processes and is an essential building block of cell components. The intracellular concentration of Pi in plants is tightly regulated to maintain Pi homeostasis. To achieve this, plants have evolved a series of strategies, such as enhancing Pi acquisition and remobilizing internal Pi (Raghothama, 1999; Vance et al., 2003). Arabidopsis PHO1 encodes a membrane protein and is involved in Pi loading from roots to shoots (Hamburger et al., 2002). The pho1 mutant has lower shoot Pi (Poirier et al., 1991) and shows a low Pi-sensitive phenotype caused by defective Pi loading in the xylem (Poirier et al., 1991; Chen et al., 2009). In this study, the WRKY42-overexpressing lines showed a reduced shoot Pi and low Pi-sensitive phenotype, similar to the pho1 mutant (Figs. 3 and 4), suggesting that WRKY42 played a role in regulating Pi translocation. As a typical WRKY transcription factor, WRKY42 directly bound to the W-boxes within the PHO1 promoter and repressed PHO1 expression under Pi-sufficient condition (Fig. 5). These data show that the WRKY42 transcription factor negatively regulated Pi translocation.
Interestingly, our data also showed that WRKY42 positively regulated Pi acquisition. Overexpression of WRKY42 enhanced Pi uptake and root Pi content, and WRKY42-overexpressing lines showed an As(V)-sensitive phenotype, similar to the PHT1;1-overexpressing line (Fig. 7; Supplemental Fig. S1). Additional molecular results showed that WRKY42 up-regulated PHT1;1 expression by binding to the promoter of PHT1;1 (Figs. 8 and 9), and overexpression of PHT1;1 significantly enhanced plant Pi uptake (Wang et al., 2014), showing that WRKY42 modulated Pi uptake by directly up-regulating PHT1;1 expression. There are nine PHT1 family genes in Arabidopsis (Mudge et al., 2002), and PHTs PHT1;1 and PHT1;4 play significant roles in Pi acquisition from both low- and high-Pi environments (Shin et al., 2004). During growth under high-Pi condition, the pht1:1Δ4Δ double mutant shows a 75% reduction in Pi uptake capacity relative to wild-type plants and results in significantly reduced shoot Pi contents (Shin et al., 2004). Similar to the pht1:1Δ4Δ double mutant, the pht1;1-1 mutant showed a reduction in shoot Pi content compared with wild-type plants, whereas the shoot Pi content of the pht1;4 mutant was not significantly different from the wild type (Shin et al., 2004), indicating that PHT1;1 is the main Pi transporter under high-Pi condition. Among nine PHT1 genes, PHT1;1 has the highest transcription level (Mudge et al., 2002), and PHT1;1-overexpressing lines show a high-Pi uptake rate (Wang et al., 2014), suggesting that the transcription regulation of PHT1;1 is an important mechanism for Pi acquisition in a high-Pi environment; this regulation of PHT1;1 expression is at least partially by WRKY42.
It was also hypothesized that the enhanced PHT1;1 expression in WRKY42-overexpressing lines was partially caused by the Pi depletion in the aerial part because of the repression of PHO1 by WRKY42 overexpression. The root Pi content results showed that the root Pi contents of WRKY42-overexpressing lines were higher than those of wild-type plants, whereas the pho1 mutant had similar root Pi content with wild-type plants (Fig. 7A). Also, the rates of root Pi uptake were similar between the pho1 mutant and wild-type plants (Poirier et al., 1991). These data indicated that the disruption of PHO1 could not enhance plant Pi uptake. The expression of PHT1;1 in WRKY42-overexpressing lines was obviously higher than that in wild-type plants (Fig. 8A), and the expression level of PHT1;1 was similar between the pho1 mutant and wild-type plants (Fig. 8B), suggesting that the enhanced PHT1;1 expression in WRKY42-overexpressing lines was independent of PHO1 disruption.
During Pi starvation, transcription of WRKY42 was repressed (Fig. 1C), and the WRKY42 protein was degraded in a proteasome-dependent manner (Fig. 10), indicating that WRKY42 regulated the expression of PHO1 and PHT1;1 under Pi-sufficient condition. The expression of PHT1;1 was obviously induced during Pi starvation (Muchhal et al., 1996; Karthikeyan et al., 2002; Mudge et al., 2002; Shin et al., 2004), suggesting that other transcription factor(s) up-regulated PHT1;1 expression under low-Pi stress. Previous reports showed that the MYB transcription factor PHR1 and the WRKY transcription factor WRKY45 modulated the increased expression of PHT1;1 during Pi starvation (Rubio et al., 2001; Wang et al., 2014), indicating that the expression level of PHT1;1 was precisely regulated by different transcription factors according to Pi availability. During Pi starvation, the PHO1 expression was induced, the WRKY42 and WRKY6 were degraded, and the repression of PHO1 by WRKY42 and WRKY6 was abolished (Figs. 6B and 10; Chen et al., 2009), suggesting that the induced expression of PHO1 during Pi starvation was at least partially dependent on the degradation of WRKY42 and WRKY6.
WRKY42 and WRKY6 Have Redundant and Nonredundant Functions during Different Arabidopsis Physiological Processes
WRKY proteins are plant-specific transcription factors, with over 70 members in the Arabidopsis WRKY family. Previous reports showed that WRKY transcription factors have redundant functions, such as WRKY18, WRKY40, and WRKY60, in response to microbial pathogens (Xu et al., 2006) as well as abscisic acid signaling (Shang et al., 2010) and WRKY3 and WRKY6 responses to herbivory (Skibbe et al., 2008). A previous report showed that WRKY6 can directly down-regulate PHO1 expression by binding to the y and z sites within the PHO1 promoter (Chen et al., 2009). In this study, WRKY42 was a negative regulator of PHO1 expression. Overexpression of WRKY42 repressed PHO1 expression, and WRKY42 bound to the y and z sites within the PHO1 promoter (Fig. 5C), showing that WRKY42 directly down-regulated PHO1 expression. The PHO1 expression was enhanced in the wrky42 or wrky6 single mutants compared with wild-type plants (Fig. 6B). Additionally, the expression level of PHO1 in the wrky42 wrky6 double mutant was much higher than that in the wild type or single mutant (Fig. 6B), and the shoot Pi content of the wrky42 wrky6 double mutant was also elevated (Fig. 6C), similar to PHO1-overexpressing lines (Liu et al., 2012). Thus, the two WRKY transcription factors, WRKY42 and WRKY6, have redundant roles in Arabidopsis Pi translocation by down-regulating PHO1 expression. In addition to negative regulation of PHO1 expression, both WRKY42 and WRKY6 activate Senescence-Induced Receptor-Like Kinase (SIRK) expression during plant senescence and pathogen defense (Robatzek and Somssich, 2002), indicating that SIRK regulation involves these two functionally redundant WRKY transcription factors, WRKY42 and WRKY6.
WRKY42 and WRKY6 have nonredundant functions in Arabidopsis Pi acquisition. In this study, PHT1;1 expression was elevated in the WRKY42-overexpressing lines and repressed in the wrky42 mutant compared with wild-type plants (Fig. 8), and WRKY42 could bind to the PHT1;1 promoter (Fig. 9), showing that WRKY42 directly up-regulated PHT1;1 expression. In contrast, expression of PHT1;1 in WRKY6-overexpressing lines was similar to that of wild-type plants (data not shown). Although WRKY6 does not modulate PHT1;1 expression under Pi-sufficient condition, WRKY6 is responsible for PHT1;1 repression under As(V) stress (Castrillo et al., 2013). When grown in the presence of As(V), WRKY6-GFP-overexpressing lines show an As(V)-tolerant phenotype compared with wild-type plants, and expression of PHT1;1 is repressed relative to the wild type (Castrillo et al., 2013). However, when grown on medium with As(V), WRKY42-overexpressing lines showed an As(V)-sensitive phenotype, similar to the PHT1;1-overexpressing line (Fig. 7C; Supplemental Fig. S1), indicating that WRKY42 was not involved in repressing PHT1;1 expression under As(V) stress. Together, although both WRKY42 and WRKY6 can regulate PHT1;1 expression, their mechanisms are different. WRKY42 activated PHT1;1 expression under Pi-sufficient condition, and WRKY6 repressed PHT1;1 transcription under As(V) stress.
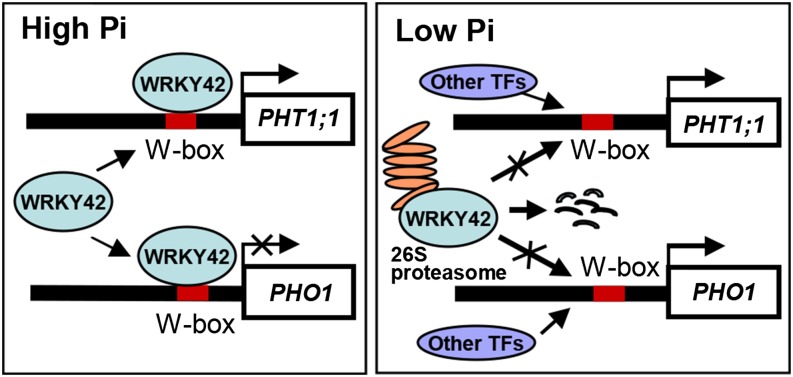
In conclusion, our genetic, physiological, and biochemical approaches showed that WRKY42 played important roles in phosphate homeostasis. The WRKY42 transcription factor regulated the expression of PHT1;1 and PHO1 to adapt environmental changes in Pi availability (Fig. 11). Under Pi-sufficient condition, WRKY42 repressed the PHO1 expression and positively regulated PHT1;1 expression. During Pi starvation, WRKY42 was degraded, and then, regulation of PHO1 and PHT1;1 by WRKY42 ceased.
Figure 11.
Hypothetical model of the WRKY42/PHO1/PHT1;1-regulatory pathway in plants regulating Pi homeostasis. Under high-Pi condition, the WRKY42 directly represses PHO1 expression and activates PHT1;1 expression by binding to the W-box motifs within the promoters of PHO1 and PHT1;1 to maintain phosphate homeostasis. Under low-Pi stress, the WRKY42 protein is degraded; then, the regulation of PHO1 and PHT1;1 by WRKY42 ceased. TF, Transcription factor.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The wild-type plants were the Columbia-0 ecotype. The Super:PHT1;1, pho1, and pht1;1 plants used in the study were described previously (Chen et al., 2009; Wang et al., 2014). The WRKY42 T-DNA insertion mutant Salk_121674 (referred to as the wrky42 mutant) and the WRKY6 T-DNA insertion mutant Salk_012997 (the wrky6 mutant) were obtained from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/abrc).
The Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized and cold treated at 4°C for 3 d. Then, the seeds were plated on MS medium containing 1.25 mm Pi, 3% (w/v) Suc, and 0.8% (w/v) agar and grown at 22°C with illumination of 100 μmol m−2 s−1 for a 16-h daily light period unless otherwise indicated.
For Pi starvation treatment, 7-d-old seedlings were transferred to MS or LP medium. The LP medium was made by modifying MS medium to contain 10 μm Pi, and the agar was replaced by agarose (Promega).
For As(V) treatment, the sterilized seeds were plated on one-half-strength MS medium or one-half-strength MS medium with 200 μm As(V).
Phosphate Content and Phosphate Uptake Assay
The Arabidopsis plants were germinated and grown on MS medium for 10 d; then, the shoots and roots were harvested for Pi content measurement. The Pi content in the samples was quantified as described previously (Ames, 1966; Chiou et al., 2006). For the Pi uptake assay, 10-d-old seedlings grown on MS medium were transferred to the Pi uptake solution containing 500 μm Pi supplemented with 0.2 μCi 32P orthophosphate. A group of 15 seedlings was used as one biological sample.
Anthocyanin Measurement
The 7-d-old seedlings were transferred to MS or LP medium for another 10 d; then, the seedlings were harvested for anthocyanin measurement. Anthocyanin was determined as described by Lu et al. (2014).
Plasmid Construction and Plant Transformation
To construct Super:WRKY42, the coding sequence of WRKY42 was cloned into the modified pCAMBIA1300-ProSuper vector under the control of the Super promoter (Li et al., 2001). To construct Super:WRKY42-GFP, the coding sequence of WRKY42 was fused in frame to the GFP in the modified pCAMBIA1300-GFP plasmid. To construct ProWRKY42:GUS, a 1,132-bp DNA fragment of the region upstream from the WRKY42 coding sequence was cloned into the pCAMBIA1381 vector. All constructs were introduced into Arabidopsis by Agrobacterium sp.-mediated transformation (Agrobacterium sp. strain GV3101) using the floral dip method (Clough and Bent, 1998); then, the single-copy transgenic lines were obtained.
qRT-PCR and RT-PCR
qRT-PCR was performed using SYBR Green PCR Master Mix (Life Technologies) on a 7500 Real Time PCR System (Applied Biosystems) following the manufacturer’s protocol. Relative quantitative results were calculated by normalization to Actin2/8.
For RT-PCR assay, the total RNA was extracted from the wrky42 mutant, wrky42 wrky6 double mutant, and wild-type plants, and then, the expression of WRKY42 or WRKY6 was determined by RT-PCR as described by Chen et al. (2009). Elongation Factor EF1α (EF1α) was used as a quantitative control.
The primers used are listed in Supplemental Table S1.
Subcellular Localization
For the subcellular localization assay, WRKY42 fused to GFP was cloned into a modified pCAMBIA1300:GFP vector, resulting in a WRKY42:GFP construct. The plasmids were transformed into Agrobacterium sp. GV1301. The transient expression assays were conducted as described by Chen et al. (2009). Fluorescence of GFP in the transformed leaves was imaged using a confocal laser-scanning microscope (LSM510; Carl Zeiss).
Transient Expression Assays in Tobacco
The transient GUS expression assays were performed as described (Chen et al., 2009). The constructs ProPHT1;1:GUS, Super:WRKY42, and pCAMBIA1300-ProSuper were transformed into Agrobacterium sp. strain GV3101 separately. For every infiltration sample, Super:Luciferase (LUC) was added as an internal control. Agrobacterium sp. cells were harvested by centrifugation and suspended in induction buffer to an optical density at 600 nm of 0.4. After 2 h at 22°C, Agrobacterium sp. cells were infiltrated into 7-week-old tobacco (Nicotiana benthamiana) leaves, and the infiltration ratio of Super:WRKY42 to ProPHT1;1 or pCAMBIA1300-ProSuper to ProPHT1;1 was 9:1 (v/v). After infiltration for 36 h, leaf discs were harvested for GUS and LUC proteins extraction. The GUS and LUC activities of the infiltrated leaves were quantitatively determined, and the GUS to LUC ratio was used to quantify the promoter activity.
ChIP-qPCR Assay
To generate the anti-WRKY42 antibody, the whole coding sequence of WRKY42 was cloned into the pET30a vector. The recombinant WRKY42-His protein was expressed in Escherichia coli and purified. The polyclonal anti-WRKY42 antibody was generated by inoculating a mouse with the recombinant WRKY42. For ChIP-qPCR assay, 7-d-old seedlings were transferred to MS or LP medium for another 7 d; then, the roots were harvested for ChIP assay. The ChIP-qPCR assay was conducted as previously described (Chen et al., 2009; Feng et al., 2014), and the primers used are listed in Supplemental Table S1. Three independent experiments were performed with similar results. Data are mean values of three replicates ± se from one experiment.
EMSA
The EMSA was conducted using a LightShift Chemiluminescent EMSA Kit (Pierce) following the manufacturer’s protocol. The recombinant WRKY42-His protein and His protein were purified from E. coli. The fragments of the PHT1;1 promoters were obtained by PCR using biotin-labeled or unlabeled primers (Supplemental Table S1). Biotin-unlabeled fragments of the same sequences were used as competitors, and the His protein alone was used as the negative control.
Protein Extraction and Cell-Free Degradation
Seven-day-old Arabidopsis seedlings were transferred to MS (+phosphorus) or LP (−phosphorus) medium for 5 d; then, the seedlings were harvested and ground into fine powder in liquid nitrogen. Total proteins were extracted in degradation buffer containing 25 mm Tris-HCl, pH 7.5, 10 mm NaCl, 10 mm MgCl2, 4 mm phenylmethylsulfonyl fluoride, 5 mm dithiothreitol, and 10 mm ATP as described by Wang et al. (2009). The total protein concentration was determined by Bio-Rad protein assay. The total protein extracts prepared were adjusted to equal concentrations in the degradation buffer for each assay. Then, exogenous MG132 was added to the total proteins extracted from −phosphorus plants, and the final concentration was 10 μm; 250 ng of recombinant WRKY42-His protein was incubated in 20-μL extracts (containing 50 μg of total proteins) for the individual assays. The extracts were incubated at 22°C, and samples were taken at indicated times for determination of WRKY42 protein abundance by immunoblots with anti-His.
Immunoblot Analysis
Total proteins were extracted according to Saleh et al. (2008), and 80 μg of proteins of each sample was separated on a 10% (w/v) SDS-PAGE and transferred to polyvinylidene fluoride membranes. MG132 treatment was conducted as described by Chen et al. (2009). WRKY42-GFP and GFP proteins were detected by anti-GFP at 1:5,000 dilution (Miltenyi).
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: WRKY42 (At4g04450), PHO1 (At3g23430), PHT1;1 (At5g43350), WRKY6 (At1g62300), ACT2 (At3g18780), ACT8 (At1g49240), and EF1α (At5g60390).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. As(V) tolerance phenotype test.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Glossary
- As(V)
arsenate
- ChIP
chromatin immunoprecipitation
- EMSA
electrophoresis mobility shift assay
- LP
low phosphate
- MS
Murashige and Skoog
- Pi
inorganic phosphate
- qPCR
quantitative PCR
- qRT
quantitative real time
- RT
reverse transcription
Footnotes
This work was supported by the 973 Project of China (grant nos. 2012CB114203 to W.-H.W. and 2011CB100305 to Y.-F.C.), the National Natural Science Foundation of China (grant nos. 30380013 and 31121002 to W.-H.W. and grant nos. 30970220 and 31170248 to Y.-F.C.), and the 111 Project of China (grant no. B06003 to W.-H.W. and Y.-F.C.).
Articles can be viewed without a subscription.
References
- Ames BN. (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8: 115–118 [Google Scholar]
- Asher CJ, Reay PF (1979) Arsenic uptake by barley seedlings. Aust J Plant Physiol 6: 459–466 [Google Scholar]
- Castrillo G, Sánchez-Bermejo E, de Lorenzo L, Crevillén P, Fraile-Escanciano A, Tc M, Mouriz A, Catarecha P, Sobrino-Plata J, Olsson S, et al. (2013) WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 25: 2944–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarecha P, Segura MD, Franco-Zorrilla JM, García-Ponce B, Lanza M, Solano R, Paz-Ares J, Leyva A (2007) A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell 19: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21: 3554–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by MicroRNA in Arabidopsis. Plant Cell 18: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62: 185–206 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG (2007) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143: 1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Feng CZ, Chen Y, Wang C, Kong YH, Wu WH, Chen YF (2014) Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. Plant J 80: 654–668 [DOI] [PubMed] [Google Scholar]
- González E, Solano R, Rubio V, Leyva A, Paz-Ares J (2005) PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17: 3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14: 889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TK, Han CL, Lin SI, Chen YJ, Tsai YC, Chen YR, Chen JW, Lin WY, Chen PM, Liu TY, et al. (2013) Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25: 4044–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D’Urzo MP, Damsz B, Raghothama KG (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z, Li Y, Wang F, Cheng Y, Fan B, Yu JQ, Chen Z (2011) Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 23: 3824–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gong Z, Koiwa H, Niu X, Espartero J, Zhu X, Veronese P, Ruggiero B, Bressan RA, Weller SC, et al. (2001) Bar-expressing peppermint (Mentha x Piperita L. var. Black Mitcham) plants are highly resistant to the glufosinate herbicide liberty. Mol Breed 8: 109–118 [Google Scholar]
- Lin WY, Huang TK, Chiou TJ (2013) Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25: 4061–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Tseng CY, Lai YS, Lin SI, Lin WY, Chen JW, Chiou TJ (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L (2014) Phosphate nutrition: improving low-phosphate tolerance in crops. Annu Rev Plant Biol 65: 95–123 [DOI] [PubMed] [Google Scholar]
- Lu YT, Li MY, Cheng KT, Tan CM, Su LW, Lin WY, Shih HT, Chiou TJ, Yang JY (2014) Transgenic plants that express the phytoplasma effector SAP11 show altered phosphate starvation and defense responses. Plant Physiol 164: 1456–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (1995) Mineral Nutrition of Higher Plants. Academic Press, London [Google Scholar]
- Martín AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de La Peña A, Leyva A, Paz-Ares J (2000) Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J 24: 559–567 [DOI] [PubMed] [Google Scholar]
- Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 10519–10523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Park BS, Seo JS, Chua NH (2014) NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26: 454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Bucher M (2002) Phosphate transport and homeostasis in Arabidopsis. Arabidopsis Book 1: e0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J (1991) Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol 97: 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Robatzek S, Somssich IE (2002) Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev 16: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258 [DOI] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu ZQ, Cao Z, Mei C, Xin Q, Wu FQ, Wang XF, Du SY, Jiang T, et al. (2010) The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell 22: 1909–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Skibbe M, Qu N, Galis I, Baldwin IT (2008) Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 20: 1984–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic A, Ribot C, Rouached H, Wang Y, Chong J, Belbahri L, Delessert S, Poirier Y (2007) Members of the PHO1 gene family show limited functional redundancy in phosphate transfer to the shoot, and are regulated by phosphate deficiency via distinct pathways. Plant J 50: 982–994 [DOI] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu D, Huang X, Li S, Gong Y, Yao Q, Fu X, Fan LM, Deng XW (2009) Biochemical insights on degradation of Arabidopsis DELLA proteins gained from a cell-free assay system. Plant Cell 21: 2378–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu Q, Kong YH, Chen Y, Duan JY, Wu WH, Chen YF (2014) Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1;1 expression in response to phosphate starvation. Plant Physiol 164: 2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ribot C, Rezzonico E, Poirier Y (2004) Structure and expression profile of the Arabidopsis PHO1 gene family indicates a broad role in inorganic phosphate homeostasis. Plant Physiol 135: 400–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen C, Fan B, Chen Z (2006) Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18: 1310–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.