Protection of a homoiochlorophyllous resurrection plant against oxidative damage during dehydration involves changes in the levels and quaternary organization of photosynthetic proteins.
Abstract
During desiccation, homoiochlorophyllous resurrection plants retain most of their photosynthetic apparatus, allowing them to resume photosynthetic activity quickly upon water availability. These plants rely on various mechanisms to prevent the formation of reactive oxygen species and/or protect their tissues from the damage they inflict. In this work, we addressed the issue of how homoiochlorophyllous resurrection plants deal with the problem of excessive excitation/electron pressures during dehydration using Craterostigma pumilum as a model plant. To investigate the alterations in the supramolecular organization of photosynthetic protein complexes, we examined cryoimmobilized, freeze-fractured leaf tissues using (cryo)scanning electron microscopy. These examinations revealed rearrangements of photosystem II (PSII) complexes, including a lowered density during moderate dehydration, consistent with a lower level of PSII proteins, as shown by biochemical analyses. The latter also showed a considerable decrease in the level of cytochrome f early during dehydration, suggesting that initial regulation of the inhibition of electron transport is achieved via the cytochrome b6f complex. Upon further dehydration, PSII complexes are observed to arrange into rows and semicrystalline arrays, which correlates with the significant accumulation of sucrose and the appearance of inverted hexagonal lipid phases within the membranes. As opposed to PSII and cytochrome f, the light-harvesting antenna complexes of PSII remain stable throughout the course of dehydration. Altogether, these results, along with photosynthetic activity measurements, suggest that the protection of retained photosynthetic components is achieved, at least in part, via the structural rearrangements of PSII and (likely) light-harvesting antenna complexes into a photochemically quenched state.
Desiccation tolerance, the ability to survive absolute water contents down to approximately 0.1 g water g−1 dry weight, is a trait found in some bacteria, algae, fungi, as well as animals and plants. In the plant kingdom, desiccation tolerance is common in ferns, mosses, and most seeds and pollen of flowering plants (angiosperms). Resurrection plants, a diverse group of approximately 300 angiosperm species, possess this trait also in their vegetative tissues. These plants are able to withstand prolonged periods of dehydration and to recover within hours to a few days once water is available. A major and interesting aspect in the study of desiccation tolerance in resurrection plants is how they protect themselves against oxidative damage during dehydration, which is often accompanied by conditions of high irradiance (for review, see Bartels and Hussain, 2011; Farrant and Moore, 2011; Morse et al., 2011).
A decrease in water content quickly results in lowered leaf stomatal conductance and, consequently, decreased uptake of CO2. This hinders and ultimately blocks the Calvin cycle. The light-driven reactions, however, typically continue well after the onset of water deficiency, with intact chlorophyll-protein complexes absorbing light energy. The imbalance between the light reactions and the downward biochemical pathways results in a lack of electron sinks and in the system becoming overenergized. This, in turn, leads to enhanced generation of reactive oxygen species (ROS), which inflict damage onto photosynthetic components as well as onto other chloroplast and cellular constituents. At times, the damage may be severe and lead to irreversible impairment and finally plant death (Dinakar et al., 2012).
Resurrection plants minimize such potential ROS damage by shutting down photosynthesis during early stages of dehydration (Farrant, 2000; Farrant et al., 2007). There are two mechanisms whereby this is achieved. In poikilochlorophyllous resurrection plants, chlorophyll, along with photosynthetic protein complexes, are degraded, and thylakoids, the membranes that host the photosynthetic pigment-protein complexes, are dismantled. This straightforward mechanism prevents the formation of ROS, yet it comes at the cost of resynthesizing photosynthetic components de novo upon rehydration. On the other hand, homoiochlorophyllous species retain most of their photosynthetic complement and so must rely on other means to protect themselves from oxidative damage in the desiccated state. Some of these, such as leaf folding or curling, which minimize the exposure of inner leaves and/or of adaxial (upper) leaf surfaces to the light, and the accumulation of anthocyanins in leaf surfaces, which act as sunscreens, and the presence of reflective hairs and waxy cuticles, reduce the overall absorption of radiation and thus protect against photodamage (Sherwin and Farrant, 1998; Farrant, 2000; Bartels and Hussain, 2011; Morse et al., 2011). ROS that are generated are dealt with by antioxidants, ROS scavengers, and in some cases also by anthocyanins and other polyphenols (Moore et al., 2005; Kytridis and Manetas, 2006; Farrant et al., 2007). Nevertheless, all of these mechanisms are insufficient to completely prevent and/or detoxify all ROS that are formed, necessitating additional means to prevent or deal with possible damage that ROS may inflict during dehydration and while desiccated (Dinakar et al., 2012).
The major photoprotective mechanism in plants and algae is nonphotochemical quenching (NPQ), in which excess light energy absorbed at the antennae of PSII is dissipated as heat. NPQ has been shown to be active in desiccation-tolerant bryophytes and pteridiophytes (Eickmeier et al., 1993; Oliver, 1996), in homoiochlorophyllous angiosperms (Alamillo and Bartels, 2001; Georgieva et al., 2009; Dinakar and Bartels, 2012; Huang et al., 2012), and during the initial stages of drying in poikilochlorophyllous angiosperms (Beckett et al., 2012). Photoinhibition, when damage to PSII (mainly to its D1 subunit) exceeds the repair capacity, typically under conditions of light stress, is also observed in homoiochlorophyllous resurrection plants (e.g. Georgieva and Maslenkova, 2006). Other ways to avoid ROS-induced damage include the rerouting of reducing equivalents to alternative electron sinks, such as the water-water cycle and/or photorespiration, as well as structural rearrangements of PSII and light-harvesting antenna (LHCII) complexes into energy-dissipating states (for review, see Dekker and Boekema, 2005; Yamamoto et al., 2014). These latter processes, in particular the ones pertaining to possible changes in PSII-LHCII macrostructure, have not yet been characterized in homoiochlorophyllous resurrection plants.
To gain insight into the ways homoiochlorophyllous resurrection plants cope with dehydration while retaining most of their photosynthetic apparatus, we combined microscopic, spectroscopic, and biochemical approaches. Investigation of the supramolecular organization of photosynthetic complexes was carried out using cryoscanning electron microscopy (cryo-SEM) of high-pressure frozen, freeze-fractured leaf samples; to our knowledge, this combination of procedures has not been utilized previously to investigate thylakoid membranes within plant tissues.
The studies reveal that during dehydration, the density of PSII in grana membranes gradually decreases. Notably, in the dehydrated state, in which photosynthetic activity is halted, PSII complexes are also observed to be arranged into rows and two-dimensional arrays. These arrangements are proposed to represent quenched PSII complexes that likely minimize the generation of ROS during desiccation. Furthermore, we observe inverted hexagonal (HII) phases in this dry state, and these two structural rearrangements are correlated with the massive accumulation of Suc. Biochemical studies of thylakoid membrane fractions support the finding that the relative level of PSII proteins decreases during dehydration. These analyses also reveal that the level of the cytochrome f subunit of the cytochrome b6f complex decreases quite dramatically and early during dehydration. This provides evidence for an additional level of regulation that inhibits/shuts down the photosynthetic light reactions during desiccation.
RESULTS
Dehydration and Rehydration and Changes in Photosynthetic Activity
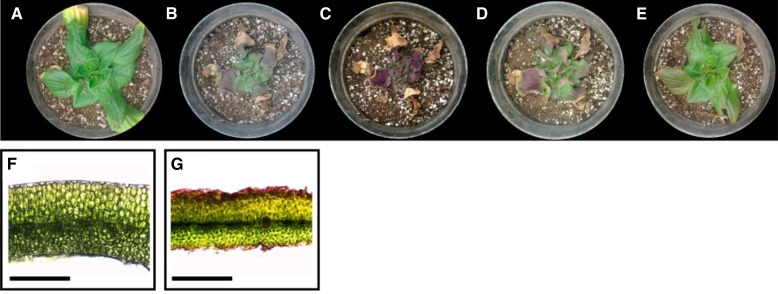
As a model for homoiochlorophyllous resurrection plants, we used Craterostigma pumilum, an herbaceous plant from East African savannas (beautifully described by Ward and Dale, 1899). A typical dehydration-rehydration cycle of C. pumilum is shown in Figure 1. Plants dehydrated to approximately 15% relative water content (RWC) have the ability to rehydrate very rapidly, returning to their state prior to dehydration within only approximately 24 h (Fig. 1, D and E). In dry C. pumilum plants of 15% to 20% RWC, roughly 75% of the chlorophyll is retained (determined by extraction from leaves; see also Christ et al., 2014). However, the amount of light absorbed by chlorophyll that is still present in this state is attenuated by anthocyanin pigments (D. Charuvi and Z. Reich, unpublished data), which accumulate at the top and bottom epidermal layers of the leaves (Fig. 1, B, C, and G).
Figure 1.
Dehydration and rehydration of C. pumilum. A typical dehydration/rehydration cycle of C. pumilum is shown. A hydrated plant (A) was dried out by withholding water. Anthocyanin pigment begins to accumulate in leaves at 40% to 50% RWC (B), and leaf surfaces are completely purple when RWC decreases further to approximately 15% (C). At 4 h (D) and 24 h (E) after watering, the plant was rehydrated and returned to its state prior to drying. Images of leaf cross sections from a hydrated plant (F) and a plant at 10% to 15% RWC (G) also are shown. Note the presence of anthocyanin in both the top and bottom epidermis of the dry leaf (G). Bars = 500 µm.
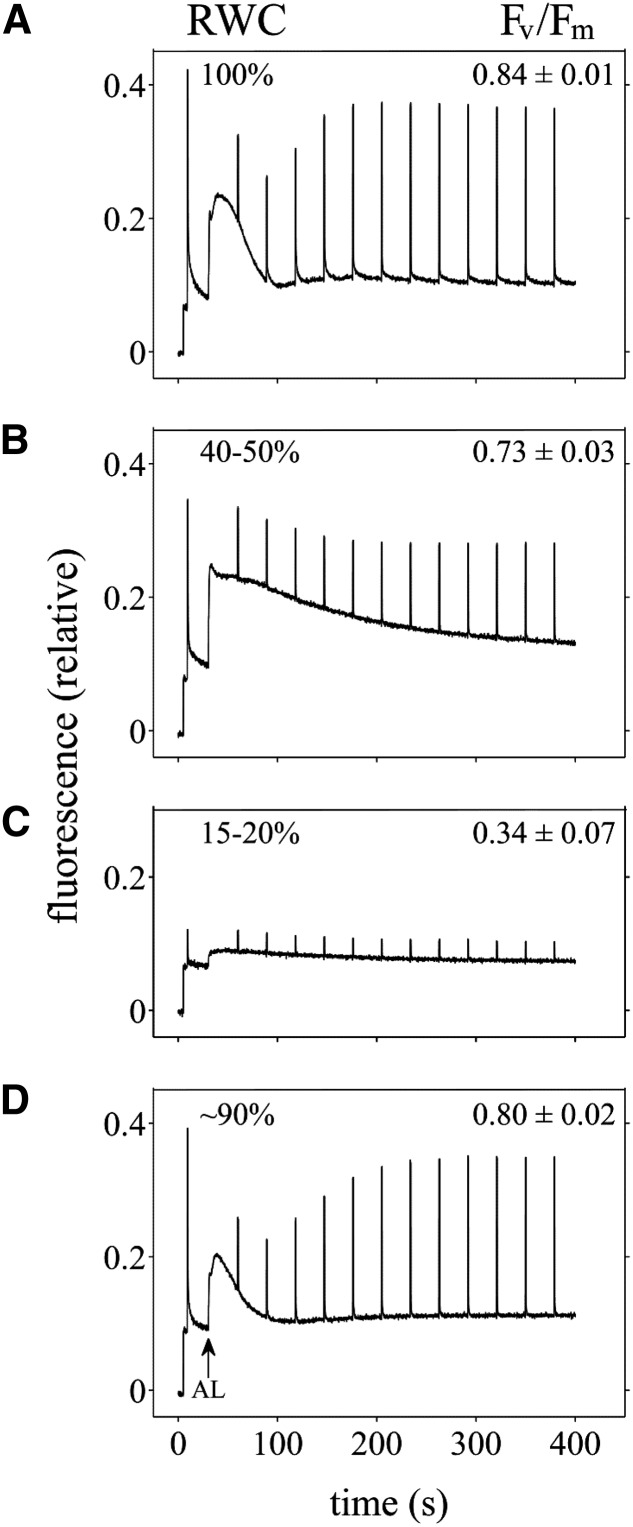
To probe photosynthetic activity during dehydration and rehydration, pulse-amplitude modulated (PAM) chlorophyll fluorescence measurements were performed on leaves of plants at different hydration states (Fig. 2). Hydrated plants show typical traces, with the value of the maximum quantum yield of PSII (Fv/Fm) being 0.84 ± 0.01. Activation of the photosynthetic system with an actinic light results in a characteristic rise followed by a decline (the Kautsky effect, indicative of the onset of the carbon [C] fixation reactions) of the fluorescence signal (Fig. 2A). In partially dehydrated plants (40%–50% RWC), which possess an Fv/Fm value of 0.73 ± 0.03, a lag in the fluorescence decline following the onset of illumination is apparent (Fig. 2B). The retarded fluorescence decline provides an indication for slowed Calvin cycle reactions, likely due to CO2 limitation resulting from stomatal closure during the water stress. In addition, we also observed an increase in the fluorescence yield and a decrease in the maximal fluorescence yield in the light (Fm′; Fig. 2B). These changes are characteristic of overly reduced PSII centers and plastoquinone (PQ) pools. A slower induction of the energy-dependent component of the NPQ response [derived from (Fm {for maximal fluorescence yield in the dark} − Fm′)/Fm′] during the onset of illumination is likewise apparent at this partially hydrated state. The attenuation of the photoprotective energy-dependent component of the NPQ response, signifying slower acidification of the thylakoid lumen, likely reflects the decrease in intracellular CO2 levels and the closure of alternative electron transport routes affected by the decrease in stomatal conductance. At 15% to 20% RWC, the steady-state fluorescence is diminished significantly and Fv/Fm is much lower (0.34 ± 0.07). Furthermore, while some Fm′ is still attainable, it is quite low (Fig. 2C). Thus, at this dry state, PSII has very little, if any, photochemical activity. Traces from rehydrated plants (18 h after watering; Fig. 2D) are indistinguishable from those of hydrated plants, with Fv/Fm (0.8 ± 0.02) and Fm′ characteristic of photosynthetically competent plants.
Figure 2.
Modulated chlorophyll fluorescence of plants at different hydration states. Representative traces recorded on leaves from plants at RWC of 100% (A), 40% to 50% (B), and 15% to 20% (C) during dehydration, and of approximately 90% (D) following rehydration for 18 h, are shown. A saturating flash was applied to dark-adapted leaves to determine Fv/Fm (denoted on graphs ± sd; n = 3), and afterward a red actinic light (AL; 635 nm, 100 µmol photons m−2 s−1) was turned on. Saturating flashes were also applied during the light treatment to determine Fm′.
Cryo-SEM Studies of Freeze-Fractured Thylakoid Membranes within Intact Leaf Tissues
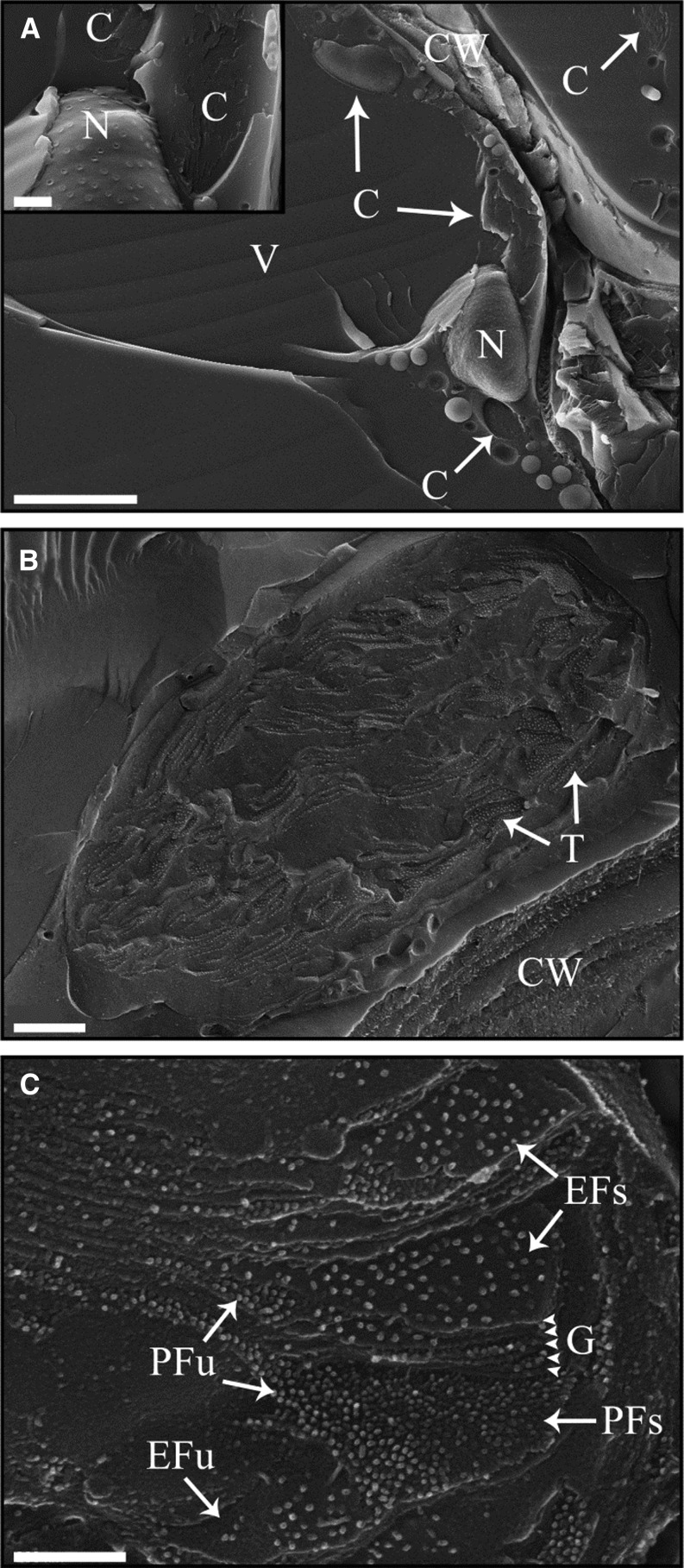
Most of the chlorophyll, and thus chlorophyll-binding proteins, remains in dry leaves of C. pumilum, yet plants at this state are not photosynthetically active. Therefore, we studied the supramolecular rearrangements that take place in the thylakoid membranes during dehydration and rehydration using freeze-fracture electron microscopy. We utilized well-established procedures known to preserve samples in their most native state, namely high-pressure freezing and freeze fracturing, and examined these physically processed samples by cryo-SEM (Walther, 2008). This is in contrast to almost all reported (yet see earlier works conducted on plant tissues, Platt-Aloia and Thomson, 1989, and refs. therein; Platt et al., 1994) freeze-fracture studies of thylakoid membranes, which were performed on isolated chloroplasts or thylakoid membranes and utilized the replica technique. The latter have the advantages that searching for the region of interest (i.e. the thylakoid membranes) is facilitated and that replicas of the samples can be relatively easily prepared and viewed using transmission electron microscopy, which provides higher resolution than scanning electron microscopy. However, this comes with the cost of alterations or artifacts in membrane structure or protein organization that likely occur during the isolation procedures. Thus, the analyses carried out in this work have the benefit that thylakoid membrane proteins are examined in the native context of intact chloroplasts, within cryopreserved cells and leaf tissues (Fig. 3, A and B).
Figure 3.
Cryo-SEM imaging of high-pressure frozen, freeze-fractured leaf samples. A, Low-magnification image of freeze-fractured cells within the leaf tissue. C, Chloroplast; CW, cell wall; N, nucleus; V, vacuole. The inset is a higher magnification image of the central area showing the nucleus. Nuclear pore complexes as well as fractured chloroplasts are seen. B, A fractured intact chloroplast. The thylakoid (T) membranes with their embedded protein complexes are visible. C, High-magnification image of a fractured chloroplast. The four distinct fracture faces, the exoplasmic (EF) and protoplasmic (PF) fracture faces of stacked (s) and unstacked (u) membranes, are distinguishable. PSII complexes found in stacked (grana) and unstacked (stroma lamellar) regions fracture to the EFs and EFu, respectively. LHCII fractures mostly to the PFs, PSI and ATP synthase fracture to the PFu, and cytochrome b6f fractures to both PFs and PFu. G indicates several layers of a fractured granum stack marked by arrowheads. Bars = 5 µm (A), 500 nm (inset in A and B), and 200 nm (C).
While cryo-SEM imaging of freeze-fractured tissues essentially provides a way to examine biological samples in near-native conditions (Walther, 2008), samples may suffer from beam damage during imaging. This is a critical drawback, especially when imaging at high magnifications. Here, this problem was overcome by utilizing a method called double-layer coating (Walther et al., 1995; Walther and Müller, 1997), in which the coating of fractured samples is carried out in a way similar to that used for replica preparation for transmission electron microscopy. Following the evaporation of a thin layer of platinum (Pt), used to provide contrast and conductivity, a thicker protective layer of C is deposited onto the samples. Imaging was carried out with higher acceleration voltages than those typically used for surface imaging. This enabled us to obtain high-resolution images with minimal beam damage (Fig. 3C; see “Materials and Methods”).
PSII Complexes Reorganize during Dehydration
Plant thylakoid membranes have four typical and distinct fracture faces that have been well characterized over the past several decades (Staehelin, 1976, 2003; Simpson, 1979). The membrane layer that borders the thylakoid lumen is termed the exoplasmic fracture face, EF, and it is divided into two subregions, EFs and EFu, which are the EF faces of stacked (grana) and unstacked (stroma lamellar) thylakoids, respectively. The protoplasmic fracture face, PF, is the membrane layer that borders the chloroplast stroma, and it is divided into the PFs and PFu, again from stacked and unstacked membrane regions (Fig. 3C). PSII, which is the largest and most prominent protein complex in the thylakoid membranes, fractures to the EF faces: PSII complexes in grana and stroma lamellar membrane regions fracture to the EFs and EFu, accordingly. The peripheral LHCIIs fracture to the PFs, while PSI and ATP synthase fracture to the PFu, and cytochrome b6f complexes fracture to both PF faces (Fig. 3C).
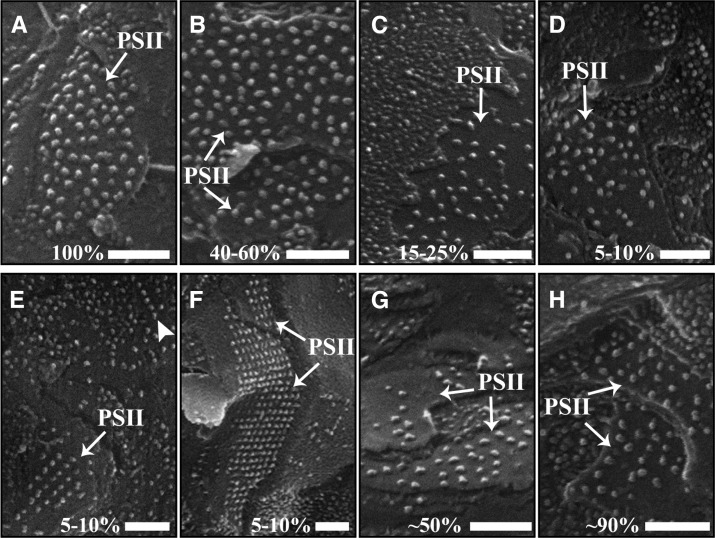
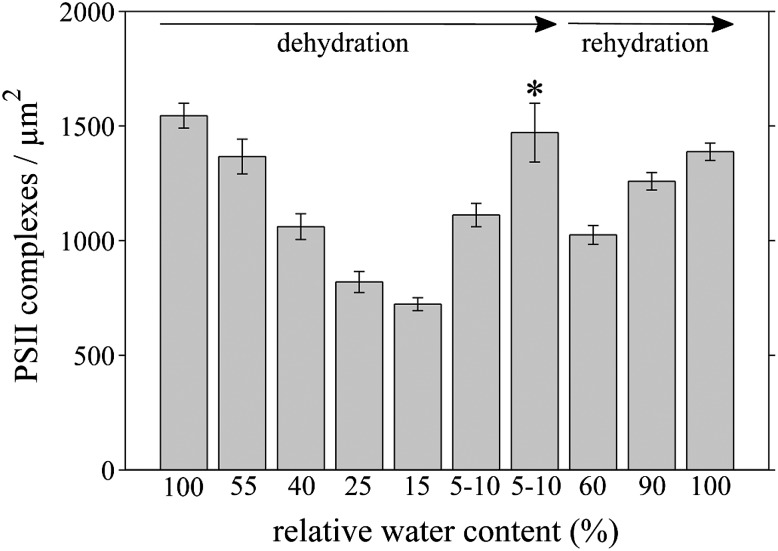
Figure 4 shows representative cryo-SEM images of the EFs (grana) of thylakoids in C. pumilum during dehydration and rehydration. It is evident that during dehydration, the density of PSII in the grana decreases (Fig. 4, A–C). Analysis of the images shows that this decrease takes place gradually during the drying process (Fig. 5). In plants at 15% to 25% RWC, PSII density is roughly half (Fig. 4C; approximately 700 complexes µm−2) of the density typical to hydrated plants (Fig. 4A; approximately 1,500 complexes µm−2). This decrease in grana regions raises the possibility that PSII complexes might relocate to stroma lamellar membrane regions (the EFu fracture face). However, analysis of PSII in the EFu face during dehydration revealed a small decrease in their density there as well (Supplemental Fig. S1). These data indicate that PSII complexes do not migrate from the grana to the stroma lamellae but rather suggest that some PSII complexes are degraded during dehydration.
Figure 4.
Changes in the density and arrangement of PSII complexes in grana thylakoid membranes during dehydration and rehydration. PSII complexes in the exoplasmic fracture face of stacked membranes (EFs) are shown in plants at different RWC during dehydration (A–F) and following 4 and 24 h of rehydration (G and H, respectively). RWC (%) is denoted at the bottom of each panel. During dehydration, the density of PSII decreases, as is clearly evident at 15% to 25% RWC (C). Remarkably, in drier plants, PSII complexes arrange into rows (E, arrowhead) and two-dimensional arrays (E and F). During rehydration, the density of PSII increases gradually (G and H). For analysis of the density of PSII at the different hydration states, see Figure 5. Bars = 100 nm.
Figure 5.
The density of PSII complexes in grana thylakoid membranes changes during dehydration and rehydration. Analysis of the density of PSII complexes per µm2 in grana membranes from cryo-SEM images of freeze-fractured leaf samples is shown. During dehydration, from 100% to 15% RWC, the density of PSII in stacked grana membranes decreases gradually. In drier plants (5%–10% RWC), the density increases, likely because of the formation of HII lipid phases in the membranes (see Fig. 6). In areas at which PSII complexes are organized into arrays (bar marked by an asterisk; see also Fig. 4, E and F), the density is even higher. During rehydration, PSII density in grana membranes increases and is restored close to the value prior to dehydration. Bars shown represent means ± se; n = 12 (100%), 9 (55%), 7 (40%), 18 (25%), 5 (15%), 19 (5%–10%), 21 (60%), 15 (90%), and 26 (100%).
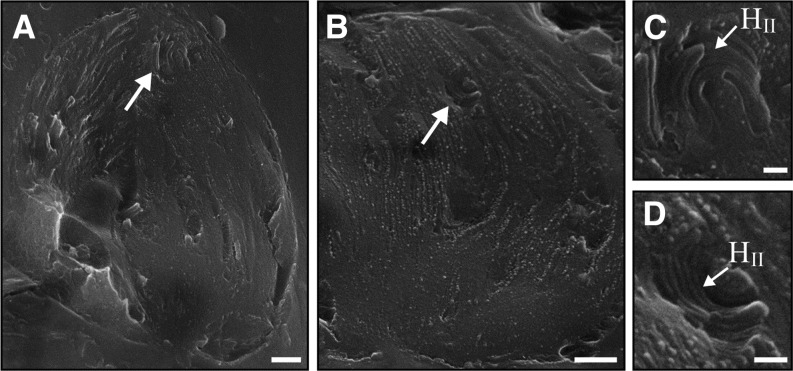
Notably, in plants dehydrated to 5% to 10% RWC (Fig. 4, D–F), some PSII complexes are observed to arrange into rows as well as into two-dimensional arrays. At this state, PSII density was higher (approximately 1,100 complexes µm−2) than in plants at 15% to 25% RWC (Figs. 4D and 5). In regions at which PSII complexes are organized into arrays, their density was even higher (approximately 1,500 complexes µm−2; Figs. 4, E and F, and 5). Remarkably, we observed HII lipid phases within fractured chloroplasts of plants at this dry state (5%–10% RWC; Fig. 6). These structures, which have a distinctive onion-like appearance, have been observed previously in thylakoid membranes of pea (Pisum sativum) and spinach (Spinacia oleracea; Williams et al., 1992; Kirchhoff et al., 2007). The formation of HII membrane phases is attributed to the separation of some of the lipids from the membrane system. As elaborated in “Discussion,” we believe that the presence of these phases accounts for the observed increase in PSII density at 5% to 10% RWC, both in areas organized into arrays and in other areas, compared with PSII density in plants at 15% to 40% RWC.
Figure 6.
Presence of HII lipid phases in chloroplasts of dehydrated plants. A and B, Low-magnification cryo-SEM images of chloroplasts in plants dehydrated to 5% to 10% RWC. C and D, Higher magnification images of the areas marked by the white arrows in A and B, showing the typical onion-like structure of HII phases. Note the lack of protein complexes within these segregated lipid phases. Bars = 200 nm (A and B) and 50 nm (C and D).
Changes in Suc Levels during Dehydration and Rehydration
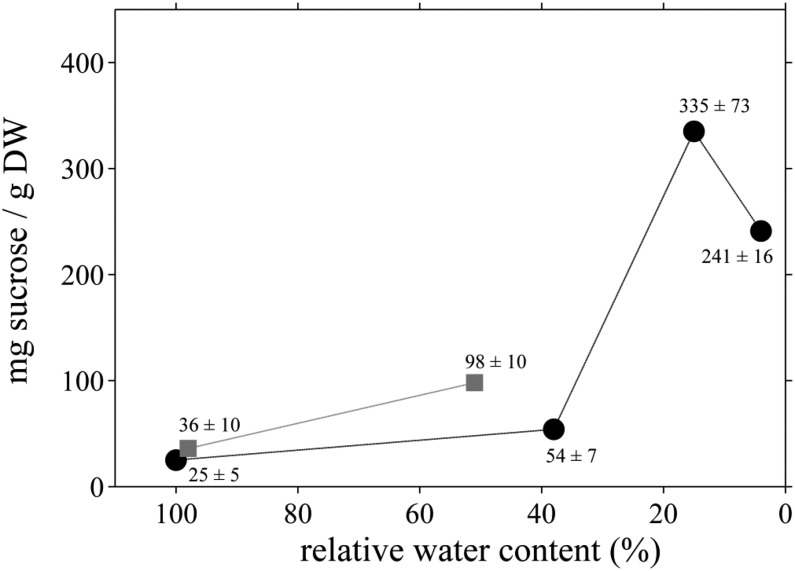
During dehydration, resurrection plants accumulate various sugars and other metabolites as well as proteins that protect their cellular components in the desiccated state. Suc is known to accumulate in all angiosperm resurrection plants during dehydration (Farrant et al., 2007, 2012; Dinakar and Bartels, 2013). Its source in the leaves of different resurrection plants is from the metabolism of different carbohydrates, such as 2-octulose in Craterostigma plantagineum (Bianchi et al., 1991; Norwood et al., 2000), whose levels decline during dehydration concomitant with the increase in the levels of Suc and/or other short-chain oligosaccharides. The accumulation of Suc is initiated at different RWC for different plants, but it usually occurs below an RWC of 50% (Farrant et al., 2007). In C. pumilum, the significant rise in Suc content is observed at RWC less than 20%, where it is 10- to 13-fold higher than the Suc content of hydrated plants (Fig. 7, black circles). Accumulation of Suc at RWC below 20% also has been reported to occur in other resurrection plants, such as Xerophyta humilis (Farrant et al., 2007) and Haberlea rhodopensis (Djilianov et al., 2011). During partial rehydration (approximately 50% RWC), the Suc content in C. pumilum declines significantly, reaching levels similar to that observed in predesiccated plants upon full rehydration (Fig. 7, gray squares).
Figure 7.
Suc content in dehydrating and rehydrating C. pumilum leaves. Suc content increases during dehydration (black circles), with massive accumulation only below approximately 20% RWC. During rehydration (gray squares), Suc levels decline back to the initial value of hydrated plants (100% RWC). Data points shown represent means ± se. DW, Dry weight.
Differential Changes in the Levels of Photosynthetic Proteins during Dehydration
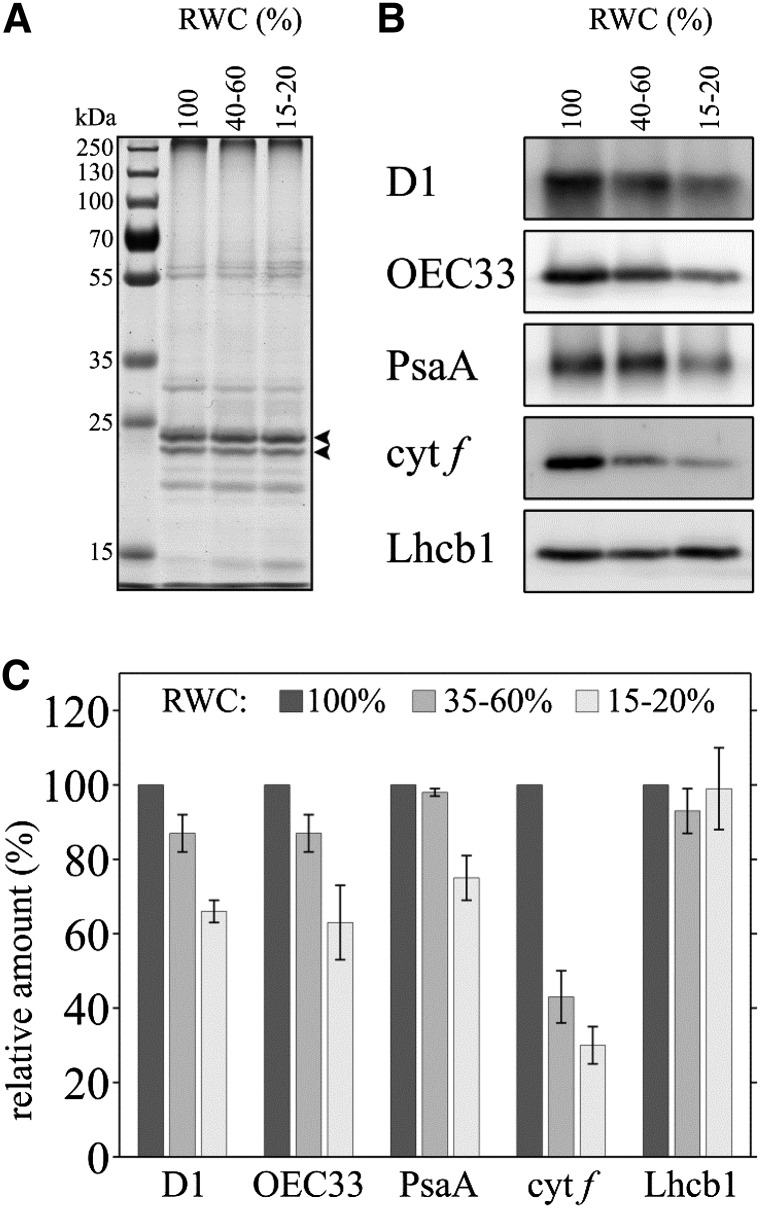
Following the finding that the density of PSII complexes decreases in the thylakoid membranes during dehydration, we used western blotting to characterize this decrease. Analysis was carried out on PSII proteins, as well as other thylakoid membrane proteins involved in the light reactions, from thylakoid membrane fractions isolated from plants at different RWC during dehydration (Fig. 8A). Representative immunoblots and quantification of several experiments are shown in Figure 8, B and C, respectively. The data show that during dehydration, the level of PSII proteins decreases, by approximately 15% in partially dehydrated plants and by approximately 35% in plants at 15% to 20% RWC, compared with hydrated plants. This is true when probing for both the PSII core protein, D1, and for the 33-kD subunit of the oxygen-evolving complex of PSII, OEC33. These results indicate that some PSII complexes are degraded during dehydration and are consistent with the cryo-SEM data, which show that the density of PSII within the thylakoid membrane decreases during drying. Probing for PSI P700 chlorophyll a apoprotein A1 (PsaA), a core subunit of PSI, showed that here, too, there is a decrease during dehydration, although slightly less pronounced (a decrease of about 25%) than in PSII, and only at 15% to 20% RWC. Notably, the level of cytochrome f, a subunit of the interchain electron mediator cytochrome b6f, decreases more dramatically, to roughly 45%, already during partial dehydration, and down to approximately 30% in air-dry plants. The significant decrease of cytochrome f at 40% to 50% RWC supports the PAM chlorophyll fluorescence measurements, which indicated an overreduced PSII and PQ pool at this state (Fig. 2B). Together, these findings suggest that regulation at the level of the cytochrome b6f complex may be a key to slowing or shutting down of photosynthetic electron transport during dehydration in these plants (see “Discussion”). In contrast to D1, OEC33, PsaA, and cytochrome f, there appears to be no significant change in Lhcb1, a subunit of LHCII, during dehydration. Indeed, in some experiments, we rather observed a slight increase in Lhcb1 in plants at 15% to 20% RWC (see Lhcb1 immunoblot shown in Fig. 8B). Coomassie Blue-stained gels support this further and show that the overall level of the LHCII does not change (Fig. 8A).
Figure 8.
Changes in the levels of photosynthetic proteins during dehydration. A, Stained gel of thylakoid membrane fractions (3 µg of protein per lane) isolated from plants at 100%, 40% to 50%, and 15% to 20% RWC. Arrowheads mark the major LHCII bands. B, Representative immunoblots of thylakoid fractions probed against the PSII core protein D1, OEC33, the cytochrome f subunit of the cytochrome b6f complex, the PsaA core protein of PSI, and the Lhcb1 subunit of LHCII. C, Quantification of changes in the levels of the different thylakoid proteins shown in B during dehydration. Bars shown represent means ± se from at least three independent experiments.
DISCUSSION
When encountered with water deficit, plants employ various mechanisms in order to minimize and/or protect their tissues from damage. However, below a certain threshold of water deficiency (approximately 60%–30% total water content), most plants are usually unable to recover (Höfler et al., 1941). The uniqueness of resurrection plants is that they are able to withstand much more severe water stress conditions while retaining cellular integrity and protecting their subcellular constituents. Some of the mechanisms utilized by resurrection plants are extensive folding of the cell wall and replacement of water in vacuoles with compatible solutes in order to minimize mechanical stress; up-regulation of housekeeping and novel antioxidants to minimize free radical damage and the accumulation of sugars and protective proteins (such as small heat shock proteins and late embryogenesis abundant proteins), which have been proposed to act (inter alia) as water-replacement molecules responsible for the maintenance of macromolecular and membrane structures, as well as in vitrification of the cytoplasm in the desiccated state (for review, see Farrant et al., 2007, 2012; Moore et al., 2009; Bartels and Hussain, 2011; Mitra et al., 2013).
As water deficit stress is often accompanied by high-irradiation conditions, plants must protect themselves from oxidative damage during dehydration. Thus, there is great importance to adaptation of the photosynthetic system during water stress. The alterations that take place in the photosynthetic apparatus during dehydration in resurrection plants, especially structural ones, have been relatively less characterized. Our work provides new insight into these adaptations during the dehydration of homoiochlorophyllous resurrection plants. While these plants retain most of their chlorophyll and chlorophyll-binding proteins, several mechanisms are used to slow down and eventually shut down the system in order to minimize oxidative damage in the desiccated state. Below, we discuss our findings and relate them (mostly) to other homoiochlorophyllous resurrection plant species.
Decline in Photosynthetic Activity and Protein Levels
During the early stages of dehydration (approximately 50% RWC), we observed a lower density of PSII complexes in grana membranes by freeze-fracture cryo-SEM as well as a lower level of PSII proteins in thylakoid membrane fractions compared with hydrated plants. Functionally, as probed by PAM fluorometry, these plants exhibit characteristics of an overreduced state of PSII and the PQ pool and attenuated Calvin cycle reactions. Of particular interest is the reduction in levels of the cytochrome f protein (a decrease of approximately 60% relative to hydrated plants) at this partially dehydrated state. This reduction in cytochrome f, and thus in cytochrome b6f complex activity, would quickly result in an overreduction of PSII and the PQ pool, as indicated by the PAM measurements. Therefore, the cytochrome b6f complex is likely a key to slowing/shutting down electron transport early on during dehydration in homoiochlorophyllous resurrection plants. The finding that the level of cytochrome f decreases relatively early during dehydration is consistent with studies showing that targeting of the cytochrome b6f complex for degradation plays an important role in the modulation of photosynthetic activity during different stress conditions, such as nitrogen and sulfur starvation, in the green alga Chlamydomonas reinhardtii (Malnoë et al., 2014; Wei et al., 2014). Thus, it seems that regulation of the cytochrome b6f complex to halt electron transport (both linear and cyclic) may be a global strategy in different organisms and at different stress conditions. It was recently shown that in mature tobacco (Nicotiana tabacum) leaves, the cytochrome b6f complex has a rather long lifetime (Hojka et al., 2014). It is interesting, therefore, that this complex is targeted for degradation earlier than other proteins of the photosynthetic apparatus, which have considerably shorter lifetimes.
Upon further dehydration, PAM chlorophyll fluorescence measurements denoted very little or no activity of PSII. Immunoblotting of thylakoid membrane fractions at this level of dehydration showed additional decrease in PSII proteins, some decrease in PSI, and a further decline in cytochrome f. The freeze-fracture studies revealed a continuous decrease in the density of PSII complexes, both in grana and stroma lamellar membrane domains, which is supported by the biochemical assays. Together, these data suggest that, aside from the regulation of electron transport by modulation of the cytochrome b6f complex, some components of the system may be degraded. Elevated levels of the thylakoid protease FtsH have been observed at late stages of dehydration of the poikilochlorophyllous resurrection plant Xerophyta viscosa (Ingle et al., 2007), in which degradation of thylakoid proteins is a natural occurrence during desiccation. However, the observation of a similar phenomenon in homoiochlorophyllous types such as Selaginella tamariscina (Wang et al., 2010), H. rhodopensis (Georgieva et al., 2009; Sárvári et al., 2014), and Boea hygrometrica (Jiang et al., 2007) suggests that such an occurrence is likely to be operational, although to a lesser extent, in such species. Recently, it has been shown that Rubisco as well as some photosynthetic proteins are partially degraded in dehydrated C. pumilum (Christ et al., 2014).
In contrast to cytochrome f, PSII, and PSI, we found no significant changes in the levels of LHCII during dehydration. In fact, in some plants at approximately 15% to 20% RWC, we observed an approximately 20% to 30% increase in the protein Lhcb1. This is similar to results obtained for LHCII proteins from immunoblot analysis of desiccated H. rhodopensis (Georgieva et al., 2009) as well as from proteome analysis of the desiccation-tolerant grass Sporobolus stapfianus (Oliver et al., 2011). It has been suggested that LHCII proteins are stored in order to keep the system ready to resume function upon rehydration as well as to bind to chlorophyll catabolic enzymes in order to delay chlorophyll breakdown during desiccation (Griffiths et al., 2014). Other proposed roles for the (stable) LHCII during dehydration are discussed below.
Aside from regulation at the protein level (i.e. by the regulation of protein translation or degradation), control of gene transcription patterns also plays an important role in regulating the levels of photosynthetic proteins during dehydration (for review, see Dinakar and Bartels, 2013). Transcriptomic analyses of C. plantagineum and H. rhodopensis, both homoiochlorophyllous desiccation-tolerant species, have shown that the levels of photosynthesis-related transcripts gradually decrease during dehydration in these plants (Rodriguez et al., 2010; Gechev et al., 2013). However, results, both at the transcriptome and proteome levels, may vary between different plant species and at different light regimes as well as other environmental conditions.
The Connection between PSII Arrays, HII Lipid Phases, and Suc
In our freeze-fracture cryo-SEM studies, we observed ordered arrays of PSII complexes within dehydrated leaf tissues. We also detected HII lipid phases in the thylakoid membranes. HII regions likely contain segregated monogalactosyldiacylglycerol (MGDG), which constitutes approximately 50% of the thylakoid lipid fraction and is a nonbilayer-forming lipid (Benning, 2009; Adam et al., 2011; Boudière et al., 2014). HII phases may form in the thylakoid membranes when protein concentration goes down (e.g. due to protein degradation) and excess lipid pools are formed (Simidjiev et al., 2000). Formation of the HII phases may allow maintaining a steady protein fractional occupancy of the membrane (Garab et al., 2000). Separation of the nonbilayer lipid MGDG from the system is expected to lower the lateral pressure in the hydrophobic region of the membrane and, thus, may affect the conformation and/or microenvironment of integral membrane proteins (van den Brink-van der Laan et al., 2004). The changes in the latter properties, in turn, may drive the formation of ordered PSII arrays (Kirchhoff et al., 2007). Consistent with the above, it has been shown that a prominent decrease in MGDG is the major lipid change during the dehydration of C. plantagineum (Gasulla et al., 2013).
Intriguingly, Wu et al. (2013) have shown a reduced accumulation of the cytochrome b6f complex in a tobacco mutant that has an approximately 50% lower content of MGDG. It was proposed by the authors that the reduction in cytochrome b6f is a direct structural effect of the deficiency in the MGDG lipid. This raises the possibility that control of the level of cytochrome b6f may take two forms. Early during dehydration, regulation (at least by modulation of the level of the cytochrome f subunit, as shown in this work) to inhibit electron transport may be similar to other stress conditions. Segregation of MGDG and the formation of HII phases, which occurs only later in dehydration, may be responsible for further reduction in the level of the cytochrome b6f complex.
The finding that Suc accumulation in C. pumilum occurs significantly only below leaf RWC of approximately 20% is interesting, since we also observed HII phases and ordered PSII arrays only below this value. Suc and other compatible cosolutes have been shown to induce nonbilayer phase separation in thylakoid membranes as well as to have an effect on the thermal stability of PSII (Williams et al., 1992; Tsvetkova et al., 1995). The protective effect triggered by the different solutes was shown to be most efficient in the case of Suc (Williams et al., 1992), which is also the major carbohydrate that accumulates in angiosperm resurrection plants when desiccated (for review, see Farrant et al., 2012; Dinakar and Bartels, 2013). In addition, plant cold acclimation or the subjection of isolated chloroplasts or thylakoid membranes to high Suc concentrations and/or to cold conditions have been shown to result in the formation of semicrystalline arrays of PSII (Garber and Steponkus, 1976; Semenova, 1995; Tsvetkova et al., 1995). In this work, we show that PSII arrays and HII phases are formed in dehydrated leaf tissues, which also possess high concentrations of Suc. These findings corroborate the previous observations from isolated membrane systems described above. Thus, our data link the accumulation of Suc and the rearrangements in lipid phase and macromolecular organization (HII and PSII arrays) in the thylakoid membranes during the dehydration of a homoiochlorophyllous resurrection plant.
The Physiological Significance of PSII Arrays
Semicrystalline arrays of PSII have been observed in thylakoid membranes at various, including stress, conditions as well as in mutants. These arrays, in which the PSII-LHCII supercomplexes can assume different assembly states (for review, see Dekker and Boekema, 2005), were proposed to serve different functions. For example, the ordered arrays were suggested to allow the optimization of photosynthesis by overcoming diffusion barriers in the crowded thylakoid membrane (Kirchhoff et al., 2007) and by enabling efficient energy transfer between PSII complexes under limiting light (Kouřil et al., 2013). In the latter work, it was proposed that the formation of PSII arrays requires a locally homogenous population of PSII-LHCII supercomplexes. Interestingly, arrays of PSII were observed in the thylakoids of a PSI-less barley (Hordeum vulgare) mutant (Marr et al., 1996; Morosinotto et al., 2006), in which PSII and the PQ pool are chronically overreduced. Analysis of the PSII particles by Morosinotto et al. (2006) showed that they contained a low level of bound LHCII per reaction center. The reduction of antenna size was proposed to facilitate array formation, as the resulting PSII particles are of uniform size and composition. Functionally, arrays were suggested to allow more efficient energy transfer between reaction centers and thus to lower the chance of ROS generation under excess light conditions as well as to allow for energy dissipation far away from where light had been absorbed (Morosinotto et al., 2006). Subsequently, the same type of arrays also had been observed in an Arabidopsis (Arabidopsis thaliana) mutant lacking the minor antenna protein CP24 (Lhcb6; de Bianchi et al., 2008). More recently, Goral et al. (2012) showed that PSII semicrystalline arrays are more abundant in several Lhcb mutants compared with wild-type Arabidopsis, providing additional support that PSII arrays have a higher tendency to form when lacking their cognate antenna proteins.
In this work, we observed PSII rows and arrays in a homoiochlorophyllous resurrection species in the dehydrated state. Under these conditions, the photosynthetic system is not functional and is in an overenergized state. In line with the suggestions of Morosinotto et al. (2006), we propose that LHCII detach from PSII reaction centers and, along with the formation of HII phases (see above), facilitate the organization of some PSII complexes into ordered arrays. PSII complexes found in these arrays may function in energy dissipation. While some PSII complexes are probably degraded during dehydration, our biochemical analysis reveals that the level of LHCII remains high, further supporting the notion that the antenna complexes disconnect from PSII during this phase. Such unbound LHCII may form aggregates that serve as photoprotective energy quenchers during dehydration, as has been shown for high-light conditions and during state transitions in higher plants and in C. reinhardtii (Betterle et al., 2009; Iwai et al., 2010; Johnson et al., 2011; Yamamoto et al., 2013, 2014). A quenching state of LHCII also has been proposed during dehydration of the homoiochlorophyllous resurrection plant H. rhodopensis (Sárvári et al., 2014). Thus, rearrangements in PSII-LHCII macrostructure likely play a key role in minimizing ROS formation and, therefore, photodamage in homoiochlorophyllous resurrection plants in the desiccated state.
Highly ordered intracellular assemblies are observed to form in various organisms when subjected to prolonged stress conditions (e.g. DNA-protein assemblies, ribosome crystallization). This biocrystallization of cellular components has been proposed to enable the survival of cells/organisms when perturbation of the energy balance is such that dynamic order cannot be maintained. The reversible formation of highly ordered, tightly packed structures protects essential components by their physical sequestration (Minsky et al., 2002, and refs. therein). In the context of the thylakoid membrane system, ordered PSII (and perhaps LHCII) complexes, which have been observed under various conditions, may in fact all be manifestations of the energetic imbalance of the photosynthetic apparatus.
MATERIALS AND METHODS
Plant Material
Craterostigma pumilum seeds were germinated on Murashige and Skoog (1962) agar plates supplemented with 1% (w/v) Suc and, after 3 to 4 weeks, transferred to 2-L pots. Plants were grown at approximately 300 μmol photons m−2 s−1 at 24°C during the light period (10 h) and at 18°C during the dark period (14 h). For all experiments, plants were dehydrated by withholding water, and rehydration was carried out by soaking pots in water for approximately 30 min as well as by spraying the soil from above with water. RWC of excised leaves was determined gravimetrically, calculated as (fresh weight – dry weight)/(turgid weight – dry weight), and expressed as percentage of the RWC of full turgor hydrated plants (approximately 0.92).
Chlorophyll
To determine chlorophyll content, leaf pieces (from at least five different plants for each hydration state) were ground in liquid nitrogen and chlorophyll was extracted using 80% (v/v) acetone. Chlorophyll concentration was calculated according to Porra et al. (1989). PAM chlorophyll fluorescence of intact leaves was measured with a Dual-PAM-100 (Heinz Walz) using an integrated 635-nm light-emitting diode array for actinic illumination.
High-Pressure Freezing, Freeze Fracture, and Cryo-SEM Imaging
Leaf samples were infiltrated with 1-hexadecene, and small pieces were mounted between the 100-µm cavities of two aluminum planchettes (Engineering Office M. Wohlwend). Samples were high-pressure frozen using an HPM 010 machine (Bal-Tec). Frozen samples were kept in liquid nitrogen and transferred into a BAF 060 (Leica Microsystems) for freeze fracture using a VCT 100 vacuum cryotransfer shuttle (Leica Microsystems). Freeze fracture was carried out at −140°C and immediately followed by sample shadowing/coating. Initially, conventional shadowing was used: a 1.5-nm layer of Pt/C was evaporated onto the sample at 45°, followed by an additional 1.5-nm Pt/C layer of double-axis rotary shadowing (rotation of both the sample and the electron beam gun used for Pt/C evaporation; Shibata et al., 1984; Hermann et al., 1988). Samples were then cryotransferred using the VCT 100 shuttle into an Ultra 55 FEG scanning electron microscope (Zeiss). Imaging was performed at −120°C at several accelerating voltages (1–5 kV) using an in-lens secondary electron detector to collect surface information. Working at high magnifications, however, resulted in substantial beam damage to the samples; thus, obtaining high-resolution information was not practical. Eventually (for images shown in this work), samples were evaporated with a 2-nm layer of Pt/C at 45° and an additional 6-nm layer of carbon at 90° (double-layer coating; adopted from Walther and Müller, 1997). Imaging in this case was carried out at an accelerating voltage of 10 kV and a working distance of 2 mm using the in-lens secondary electron detector.
Image Analysis
The Fiji image-processing package (Schindelin et al., 2012) was used for adjusting the brightness/contrast of the cryo-SEM images, thresholding, segmentation, and measurement of particles.
Suc
Suc quantitative assays were done on frozen leaf material collected from various dehydrating/rehydrating stages using a commercial Suc assay kit (Sigma-Aldrich). For sample preparation, the ground leaf material was weighed, mixed, heated with water at approximately 60°C to aid extraction, and finally filtered. The A340 was recorded with a Hitachi U3900 spectrometer.
Thylakoid Membrane Preparation and Immunoblot Analysis
Thylakoid membranes were prepared according to Kapri-Pardes et al. (2007) with some modifications: Leaf material (150–200 mg fresh weight of hydrated tissue or 30–40 mg fresh weight of dry tissue) was ground in liquid nitrogen to a fine powder and resuspended in 1 mL of buffer (10 mm HEPES-KOH and 2 mm EDTA, pH 8). The homogenates were filtered through four layers of gauze and centrifuged at 10,000 rpm for 5 min at 4°C. The pellets were resuspended in the same buffer, loaded onto 40% (v/v) Percoll/buffer, and centrifuged at 10,000 rpm for 10 min at 4°C. The thylakoid fraction was collected from the interphase of the buffer and the Percoll cushion, washed once in the same buffer, and resuspended in 50 µL of buffer. Protein concentration was determined by a standard Bradford assay (Sigma-Aldrich). Samples were then centrifuged at 6,000 rpm for 15 min at 4°C and resuspended in 4× sample buffer (0.2 m Tris-HCl, pH 6.8, 5 m urea, 10% [v/v] glycerol, 8% [w/v] SDS, and 20% [v/v] β-mercaptoethanol). Samples (2.5–7.5 µg protein per lane) were resolved on 12% (w/v) SDS-PAGE gels and either stained with InstantBlue (Expedeon) or blotted onto polyvinylidene difluoride membranes. Blots were probed with primary antibodies against OEC33 (1:10,000; Itzhaki et al., 1998) and Agrisera antibodies against cytochrome f (AS06-119; 1:2,000), D1 (AS05-084; 1:5,000), Lhcb1 (AS01-004; 1:2,000), and PsaA (AS06-172; 1:1,000). Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was used (1:10,000; Jackson ImmunoResearch Laboratories), and blots were developed using the EZ-ECL chemiluminescence detection kit (Biological Industries).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Density of PSII complexes in stroma lamellar thylakoid membranes during dehydration.
Supplementary Material
Acknowledgments
We thank Zohar Hajbi and Neora Swid (Weizmann Institute of Science) and Keren Cooper (University of Capetown) for help with plant growth, Onie Tsabari (Weizmann Institute of Science) for help with the PAM measurements, and Andres Kaech (University of Zurich) for helpful advice on scanning electron microscopy imaging. The electron microscopy studies were conducted at the Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging at the Weizmann Institute of Science.
Glossary
- ROS
reactive oxygen species
- NPQ
nonphotochemical quenching
- LHCII
light-harvesting antenna complex
- cryo-SEM
cryoscanning electron microscopy
- RWC
relative water content
- PAM
pulse-amplitude modulated
- Fv/Fm
maximum quantum yield of PSII
- Fm′
maximal fluorescence yield in the light
- PQ
plastoquinone
- HII
inverted hexagonal
- MGDG
monogalactosyldiacylglycerol
- Pt/C
platinum/carbon
Footnotes
This work was supported by the United States-Israel Binational Agricultural Research and Development Fund (grant no. US–4334–10 to H.K. and Z.R.) and the Israel Science Foundation (grant no. 1034/12 to Z.R.).
References
- Adam Z, Charuvi D, Tsabari O, Knopf RR, Reich Z (2011) Biogenesis of thylakoid networks in angiosperms: knowns and unknowns. Plant Mol Biol 76: 221–234 [DOI] [PubMed] [Google Scholar]
- Alamillo JM, Bartels D (2001) Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci 160: 1161–1170 [DOI] [PubMed] [Google Scholar]
- Bartels D, Hussain SS (2011) Resurrection plants: physiology and molecular biology. InLüttge U, Beck E, Bartels D, eds, Plant Desiccation Tolerance: Ecological Studies, Vol 215 Springer-Verlag, Berlin, pp 339–364 [Google Scholar]
- Beckett M, Loreto F, Velikova V, Brunetti C, Di Ferdinando M, Tattini M, Calfapietra C, Farrant JM (2012) Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilis during dehydration and rehydration. Plant Cell Environ 35: 2061–2074 [DOI] [PubMed] [Google Scholar]
- Benning C. (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol 25: 71–91 [DOI] [PubMed] [Google Scholar]
- Betterle N, Ballottari M, Zorzan S, de Bianchi S, Cazzaniga S, Dall’osto L, Morosinotto T, Bassi R (2009) Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284: 15255–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi G, Gamba A, Murelli C, Salamini F, Bartels D (1991) Novel carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. Plant J 1: 355–359 [DOI] [PubMed] [Google Scholar]
- Boudière L, Michaud M, Petroutsos D, Rébeillé F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J, et al. (2014) Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta 1837: 470–480 [DOI] [PubMed] [Google Scholar]
- Christ B, Egert A, Süssenbacher I, Kräutler B, Bartels D, Peters S, Hörtensteiner S (2014) Water deficit induces chlorophyll degradation via the ‘PAO/phyllobilin’ pathway in leaves of homoio- (Craterostigma pumilum) and poikilochlorophyllous (Xerophyta viscosa) resurrection plants. Plant Cell Environ 37: 2521–2531 [DOI] [PubMed] [Google Scholar]
- de Bianchi S, Dall’Osto L, Tognon G, Morosinotto T, Bassi R (2008) Minor antenna proteins CP24 and CP26 affect the interactions between photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 20: 1012–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker JP, Boekema EJ (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706: 12–39 [DOI] [PubMed] [Google Scholar]
- Dinakar C, Bartels D (2012) Light response, oxidative stress management and nucleic acid stability in closely related Linderniaceae species differing in desiccation tolerance. Planta 236: 541–555 [DOI] [PubMed] [Google Scholar]
- Dinakar C, Bartels D (2013) Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome and metabolome analysis. Front Plant Sci 4: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakar C, Djilianov D, Bartels D (2012) Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense. Plant Sci 182: 29–41 [DOI] [PubMed] [Google Scholar]
- Djilianov D, Ivanov S, Moyankova D, Miteva L, Kirova E, Alexieva V, Joudi M, Peshev D, Van den Ende W (2011) Sugar ratios, glutathione redox status and phenols in the resurrection species Haberlea rhodopensis and the closely related non-resurrection species Chirita eberhardtii. Plant Biol (Stuttg) 13: 767–776 [DOI] [PubMed] [Google Scholar]
- Eickmeier WG, Casper C, Osmond CB (1993) Chlorophyll fluorescence in the resurrection plant Selaginella lepidophylla (Hook and Grev) Spring during high-light and desiccation stress, and evidence for zeaxanthin-associated photoprotection. Planta 189: 30–38 [Google Scholar]
- Farrant JM. (2000) A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol 151: 29–39 [Google Scholar]
- Farrant JM, Brandt W, Lindsey GG (2007) An overview of mechanisms of desiccation tolerance in selected angiosperm resurrection plants. Plant Stress 1: 72–84 [Google Scholar]
- Farrant JM, Cooper K, Nell H (2012) Desiccation tolerance. InShabala S, ed, Plant Stress Physiology. CAB International, Wallingford, UK, pp 238–265 [Google Scholar]
- Farrant JM, Moore JP (2011) Programming desiccation-tolerance: from plants to seeds to resurrection plants. Curr Opin Plant Biol 14: 340–345 [DOI] [PubMed] [Google Scholar]
- Garab G, Lohner K, Laggner P, Farkas T (2000) Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis. Trends Plant Sci 5: 489–494 [DOI] [PubMed] [Google Scholar]
- Garber MP, Steponkus PL (1976) Alterations in chloroplast thylakoids during cold acclimation. Plant Physiol 57: 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasulla F, Vom Dorp K, Dombrink I, Zähringer U, Gisch N, Dörmann P, Bartels D (2013) The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J 75: 726–741 [DOI] [PubMed] [Google Scholar]
- Gechev TS, Benina M, Obata T, Tohge T, Sujeeth N, Minkov I, Hille J, Temanni MR, Marriott AS, Bergström E, et al. (2013) Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell Mol Life Sci 70: 689–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva K, Maslenkova L (2006) Thermostability and photostability of photosystem II of the resurrection plant Haberlea rhodopensis studied by chlorophyll fluorescence. Z Naturforsch C 61: 234–240 [DOI] [PubMed] [Google Scholar]
- Georgieva K, Röding A, Büchel C (2009) Changes in some thylakoid membrane proteins and pigments upon desiccation of the resurrection plant Haberlea rhodopensis. J Plant Physiol 166: 1520–1528 [DOI] [PubMed] [Google Scholar]
- Goral TK, Johnson MP, Duffy CD, Brain AP, Ruban AV, Mullineaux CW (2012) Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J 69: 289–301 [DOI] [PubMed] [Google Scholar]
- Griffiths CA, Gaff DF, Neale AD (2014) Drying without senescence in resurrection plants. Front Plant Sci 5: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann R, Pawley J, Nagatani T, Müller M (1988) Double-axis rotary shadowing for high-resolution scanning electron-microscopy. Scanning Microsc 2: 1215–1230 [Google Scholar]
- Höfler K, Migsch H, Rottenburg W (1941) Über die Austrocknungresistenz landwirtschaftlicher Kulturpflanzen. Forschungsdienst 12: 51–60 [Google Scholar]
- Hojka M, Thiele W, Tóth SZ, Lein W, Bock R, Schöttler MA (2014) Inducible repression of nuclear-encoded subunits of the cytochrome b6f complex in tobacco reveals an extraordinarily long lifetime of the complex. Plant Physiol 165: 1632–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yang SJ, Zhang SB, Zhang JL, Cao KF (2012) Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 235: 819–828 [DOI] [PubMed] [Google Scholar]
- Ingle RA, Schmidt UG, Farrant JM, Thomson JA, Mundree SG (2007) Proteomic analysis of leaf proteins during dehydration of the resurrection plant Xerophyta viscosa. Plant Cell Environ 30: 435–446 [DOI] [PubMed] [Google Scholar]
- Itzhaki H, Naveh L, Lindahl M, Cook M, Adam Z (1998) Identification and characterization of DegP, a serine protease associated with the luminal side of the thylakoid membrane. J Biol Chem 273: 7094–7098 [DOI] [PubMed] [Google Scholar]
- Iwai M, Yokono M, Inada N, Minagawa J (2010) Live-cell imaging of photosystem II antenna dissociation during state transitions. Proc Natl Acad Sci USA 107: 2337–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Wang Z, Shang H, Yang W, Hu Z, Phillips J, Deng X (2007) Proteome analysis of leaves from the resurrection plant Boea hygrometrica in response to dehydration and rehydration. Planta 225: 1405–1420 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Goral TK, Duffy CDP, Brain APR, Mullineaux CW, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell 23: 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapri-Pardes E, Naveh L, Adam Z (2007) The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell 19: 1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H, Haase W, Wegner S, Danielsson R, Ackermann R, Albertsson PA (2007) Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry 46: 11169–11176 [DOI] [PubMed] [Google Scholar]
- Kouřil R, Wientjes E, Bultema JB, Croce R, Boekema EJ (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta 1827: 411–419 [DOI] [PubMed] [Google Scholar]
- Kytridis VP, Manetas Y (2006) Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. J Exp Bot 57: 2203–2210 [DOI] [PubMed] [Google Scholar]
- Malnoë A, Wang F, Girard-Bascou J, Wollman FA, de Vitry C (2014) Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions. Plant Cell 26: 373–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KM, McFeeters RL, Lyon MK (1996) Isolation and structural analysis of two-dimensional crystals of photosystem II from Hordeum vulgare viridis zb63. J Struct Biol 117: 86–98 [DOI] [PubMed] [Google Scholar]
- Minsky A, Shimoni E, Frenkiel-Krispin D (2002) Stress, order and survival. Nat Rev Mol Cell Biol 3: 50–60 [DOI] [PubMed] [Google Scholar]
- Mitra J, Xu G, Wang B, Li M, Deng X (2013) Understanding desiccation tolerance using the resurrection plant Boea hygrometrica as a model system. Front Plant Sci 4: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Le NT, Brandt WF, Driouich A, Farrant JM (2009) Towards a systems-based understanding of plant desiccation tolerance. Trends Plant Sci 14: 110–117 [DOI] [PubMed] [Google Scholar]
- Moore JP, Westall KL, Ravenscroft N, Farrant JM, Lindsey GG, Brandt WF (2005) The predominant polyphenol in the leaves of the resurrection plant Myrothamnus flabellifolius, 3,4,5 tri-O-galloylquinic acid, protects membranes against desiccation and free radical-induced oxidation. Biochem J 385: 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosinotto T, Bassi R, Frigerio S, Finazzi G, Morris E, Barber J (2006) Biochemical and structural analyses of a higher plant photosystem II supercomplex of a photosystem I-less mutant of barley: consequences of a chronic over-reduction of the plastoquinone pool. FEBS J 273: 4616–4630 [DOI] [PubMed] [Google Scholar]
- Morse M, Rafudeen MS, Farrant JM (2011) An overview of the current understanding of desiccation tolerance in the vegetative tissues of higher plants. Adv Bot Res 57: 319–347 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Norwood M, Truesdale MR, Richter A, Scott P (2000) Photosynthetic carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. J Exp Bot 51: 159–165 [DOI] [PubMed] [Google Scholar]
- Oliver MJ. (1996) Desiccation tolerance in vegetative plant cells. Physiol Plant 97: 779–787 [Google Scholar]
- Oliver MJ, Jain R, Balbuena TS, Agrawal G, Gasulla F, Thelen JJ (2011) Proteome analysis of leaves of the desiccation-tolerant grass, Sporobolus stapfianus, in response to dehydration. Phytochemistry 72: 1273–1284 [DOI] [PubMed] [Google Scholar]
- Platt KA, Oliver MJ, Thomson WW (1994) Membranes and organelles of dehydrated Selaginella and Tortula retain their normal configuration and structural integrity: freeze-fracture evidence. Protoplasma 178: 57–65 [Google Scholar]
- Platt-Aloia KA, Thomson WW (1989) Advantages of the use of intact plant tissues in freeze-fracture electron microscopy. J Electron Microsc Tech 13: 288–299 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rodriguez MC, Edsgärd D, Hussain SS, Alquezar D, Rasmussen M, Gilbert T, Nielsen BH, Bartels D, Mundy J (2010) Transcriptomes of the desiccation-tolerant resurrection plant Craterostigma plantagineum. Plant J 63: 212–228 [DOI] [PubMed] [Google Scholar]
- Sárvári E, Mihailova G, Solti A, Keresztes A, Velitchkova M, Georgieva K (2014) Comparison of thylakoid structure and organization in sun and shade Haberlea rhodopensis populations under desiccation and rehydration. J Plant Physiol 171: 1591–1600 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova GA. (1995) Particle regularity on thylakoid fracture faces is influenced by storage conditions. Can J Bot 73: 1676–1682 [Google Scholar]
- Sherwin HW, Farrant JM (1998) Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul 24: 203–210 [Google Scholar]
- Shibata Y, Arima T, Yamamoto T (1984) Double-axis rotary replication for deep-etching. J Microsc 136: 121–123 [DOI] [PubMed] [Google Scholar]
- Simidjiev I, Stoylova S, Amenitsch H, Javorfi T, Mustardy L, Laggner P, Holzenburg A, Garab G (2000) Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci USA 97: 1473–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DJ. (1979) Freeze-fracture studies on barley plastid membranes. 3. Location of the light-harvesting chlorophyll-protein. Carlsberg Res Commun 44: 305–336 [Google Scholar]
- Staehelin LA. (1976) Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol 71: 136–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA. (2003) Chloroplast structure: from chlorophyll granules to supra-molecular architecture of thylakoid membranes. Photosynth Res 76: 185–196 [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Apostolova EL, Brain APR, Williams WP, Quinn PJ (1995) Factors influencing PS-II particle array formation in Arabidopsis thaliana chloroplasts and the relationship of such arrays to the thermostability of PS-II. Biochim Biophys Acta 1228: 201–210 [Google Scholar]
- van den Brink-van der Laan E, Killian JA, de Kruijff B (2004) Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666: 275–288 [DOI] [PubMed] [Google Scholar]
- Walther P. (2008) High-resolution cryoscanning electron microscopy of biological samples. In Schatten H, Pawley J B, eds, Biological Low-Voltage Scanning Electron Microscopy. Springer, New York, pp 245–261 [Google Scholar]
- Walther P, Müller M (1997) Double-layer coating for field-emission cryo-scanning electron microscopy: present state and applications. Scanning 19: 343–348 [DOI] [PubMed] [Google Scholar]
- Walther P, Wehrli E, Hermann R, Müller M (1995) Double-layer coating for high-resolution low-temperature scanning electron microscopy. J Microsc 179: 229–237 [DOI] [PubMed] [Google Scholar]
- Wang X, Chen S, Zhang H, Shi L, Cao F, Guo L, Xie Y, Wang T, Yan X, Dai S (2010) Desiccation tolerance mechanism in resurrection fern-ally Selaginella tamariscina revealed by physiological and proteomic analysis. J Proteome Res 9: 6561–6577 [DOI] [PubMed] [Google Scholar]
- Ward HM, Dale E (1899) X. On Craterostigma pumilum, Hochst., a rare plant from Somali-Land. Transactions of the Linnean Society of London 2nd Series Botany 5: 343–355 [Google Scholar]
- Wei L, Derrien B, Gautier A, Houille-Vernes L, Boulouis A, Saint-Marcoux D, Malnoë A, Rappaport F, de Vitry C, Vallon O, et al. (2014) Nitric oxide-triggered remodeling of chloroplast bioenergetics and thylakoid proteins upon nitrogen starvation in Chlamydomonas reinhardtii. Plant Cell 26: 353–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WP, Brain APR, Dominy PJ (1992) Induction of nonbilayer lipid phase separations in chloroplast thylakoid membranes by compatible co-solutes and its relation to the thermal-stability of photosystem-II. Biochim Biophys Acta 1099: 137–144 [Google Scholar]
- Wu W, Ping W, Wu H, Li M, Gu D, Xu Y (2013) Monogalactosyldiacylglycerol deficiency in tobacco inhibits the cytochrome b6f-mediated intersystem electron transport process and affects the photostability of the photosystem II apparatus. Biochim Biophys Acta 1827: 709–722 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hori H, Kai S, Ishikawa T, Ohnishi A, Tsumura N, Morita N (2013) Quality control of photosystem II: reversible and irreversible protein aggregation decides the fate of photosystem II under excessive illumination. Front Plant Sci 4: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kai S, Ohnishi A, Tsumura N, Ishikawa T, Hori H, Morita N, Ishikawa Y (2014) Quality control of PSII: behavior of PSII in the highly crowded grana thylakoids under excessive light. Plant Cell Physiol 55: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.