A green leaf volatile primes wheat for enhanced defense against the hemibiotrophic fungus F. graminearum by boosting jasmonate-related defenses.
Abstract
Priming refers to a mechanism whereby plants are sensitized to respond faster and/or more strongly to future pathogen attack. Here, we demonstrate that preexposure to the green leaf volatile Z-3-hexenyl acetate (Z-3-HAC) primed wheat (Triticum aestivum) for enhanced defense against subsequent infection with the hemibiotrophic fungus Fusarium graminearum. Bioassays showed that, after priming with Z-3-HAC, wheat ears accumulated up to 40% fewer necrotic spikelets. Furthermore, leaves of seedlings showed significantly smaller necrotic lesions compared with nonprimed plants, coinciding with strongly reduced fungal growth in planta. Additionally, we found that F. graminearum produced more deoxynivalenol, a mycotoxin, in the primed treatment. Expression analysis of salicylic acid (SA) and jasmonic acid (JA) biosynthesis genes and exogenous methyl salicylate and methyl jasmonate applications showed that plant defense against F. graminearum is sequentially regulated by SA and JA during the early and later stages of infection, respectively. Interestingly, analysis of the effect of Z-3-HAC pretreatment on SA- and JA-responsive gene expression in hormone-treated and pathogen-inoculated seedlings revealed that Z-3-HAC boosts JA-dependent defenses during the necrotrophic infection stage of F. graminearum but suppresses SA-regulated defense during its biotrophic phase. Together, these findings highlight the importance of temporally separated hormone changes in molding plant health and disease and support a scenario whereby the green leaf volatile Z-3-HAC protects wheat against Fusarium head blight by priming for enhanced JA-dependent defenses during the necrotrophic stages of infection.
Biogenic volatile organic compounds (BVOCs) are known regulators of communication of sedentary plants with their direct environment (Dudareva et al., 2006). Besides attracting pollinators (Pichersky and Gershenzon, 2002), repelling insect herbivores (Birkett et al., 2010), and exerting direct antimicrobial properties (Friedman et al., 2002), BVOCs can act as an alarm signal to warn neighboring plants of an imminent herbivorous or pathogen attack (Heil and Ton, 2008) or serve as an intraplant signal for the induction of resistance (Karban et al., 2006). Engelberth et al. (2004) found that maize (Zea mays) seedlings emitted the green leaf volatiles (GLVs) Z-3-hexenal, Z-3-hexenol (Z-3-HOL), and Z-3-hexenyl acetate (Z-3-HAC) after they had been infested with caterpillars of Spodoptera exigua. Neighboring uninfested seedlings that had been exposed to these GLVs subsequently showed a considerable higher production of the plant defense hormone jasmonic acid (JA) after treatment with caterpillar regurgitant. This form of induced resistance is called priming. Plants in a primed state display faster and/or stronger activation of defense pathways when challenged by microbial pathogens, herbivorous insects, or abiotic stresses (Conrath, 2009). Exposure to these priming signals does not entail a direct activation of costly defense mechanisms but rather a stronger up-regulation of defense pathways when the plant is actually under attack (van Hulten et al., 2006). Besides resulting in a stronger induction of the JA pathway, priming also has been shown to enhance defense associated with the salicylic acid (SA) pathway, which plays a critical role in plant defense against biotrophic pathogens (Conrath et al., 2006; Jung et al., 2009).
The lion’s share of attention on the use of GLVs in induced resistance has been directed to plant-insect interactions. However, the literature regarding priming by GLVs in plant-pathogen interactions remains scarce (Heil, 2014). Few studies have been performed investigating the effect of priming by GLVs on plant-fungus interactions (Scala et al., 2013a, and refs. therein). For example, hexanoic acid, a molecule with a similar structure to GLVs, has been shown to act as a priming agent in tomato (Solanum lycopersicum) plants against an infection by the necrotrophic fungus Botrytis cinerea, leading to a reduced accumulation of reactive oxygen species in primed plants (Vicedo et al., 2009; Kravchuk et al., 2011; Finiti et al., 2014). Since the GLVs E-2-hexenal (E-2-HAL), Z-3-HOL, E-2-hexenol, and Z-3-HAC also have been reported to be emitted by perennial ryegrass (Lolium perenne) after infection with Fusarium poae (Pańka et al., 2013) and by wheat (Triticum aestivum) seedlings after infection with Fusarium graminearum (Piesik et al., 2011), one may speculate that GLVs not only serve as a priming agent against the impending threat of herbivorous insects but rather constitute a general warning and priming mechanism against insects, bacteria, and fungi alike.
Fusarium head blight (FHB) is an important disease in cereals caused by a complex of Fusarium spp., of which the hemibiotroph F. graminearum is one of the most prevalent (Parry et al., 1995; Goswami and Kistler, 2004; Audenaert et al., 2009). Besides yield losses of up to 40%, FHB also confers quality losses because of the production of mycotoxins such as deoxynivalenol (DON; Parry et al., 1995; Bottalico and Perrone, 2002; Vanheule et al., 2014).
The hemiobiotrophic nature of F. graminearum entails that its lifestyle is characterized by a biotrophic phase followed by a necrotrophic phase. During the biotrophic phase, spores will germinate and hyphae will grow extracellularly and intercellularly. To counteract fungal colonization during the biotrophic phase, the host plant will accumulate hydrogen peroxide (H2O2) to induce programmed cell death. However, H2O2 acts as a signal for F. graminearum to produce DON, which in turn creates a positive feedback loop leading to increased H2O2 and DON production, clearing the way for F. graminearum to further colonize the host plant (Desmond et al., 2008). Plant defense against the biotrophic and necrotrophic phases generally has been linked to SA- and JA-related pathways, respectively (Glazebrook, 2005). This was also found in the study by Ding et al. (2011). They reported higher endogenous SA concentrations during the first hours of infection, followed by a rise in JA concentrations later on. However, plant defense against pathogens is regulated by a whole array of plant hormones, between which an intricate cross talk exists (Pieterse et al., 2012). One of the best-studied antagonistic signaling pathways is between SA and JA (Thaler et al., 2002; Pieterse et al., 2012). Research investigating the hormonal modulation of plant immunity has been done primarily in dicots. The negative relationship between SA and JA also seems to be conserved in rice (Oryza sativa), another monocot (De Vleesschauwer et al., 2013). Because of the presence of this possible antagonistic signaling and the hemibiotrophic lifestyle of F. graminearum, it is important to look more closely to the effect of priming on these two defense pathways in wheat.
Here, we show that preexposure of wheat to the GLV Z-3-HAC primes wheat plants for an enhanced defense against a future infection with F. graminearum. Furthermore, our results indicate that pretreatment with Z-3-HAC leads to a stronger activation of JA-related defense while exerting suppressive effects on SA-responsive gene expression. Lastly, we found evidence that enhanced plant defense led to increased DON production by F. graminearum.
RESULTS
Effect of Z-3-HAC Pretreatment on the Severity of Infection by F. graminearum
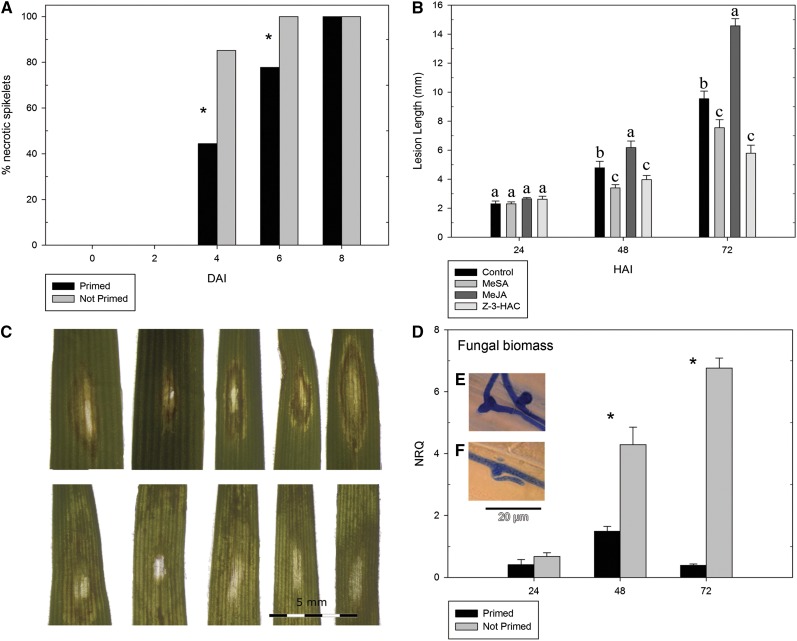
To assess whether preexposure to Z-3-HAC results in increased resistance against infection with F. graminearum, we point inoculated 27 spikelets of nine wheat ears, the target tissue of F. graminearum, with 20 µL of a conidia suspension for the preexposed and control treatments. Four days after infection, we observed the first necrotic lesions (Fig. 1A). The preexposed treatment exhibited a significantly lower infection rate than the control treatment, which persisted after 6 d. All inoculated spikelets showed necrosis 8 d after infection for both treatments.
Figure 1.
Preexposure with Z-3-HAC leads to lower fungal biomass, smaller necrotic lesions, and higher DON content in wheat seedlings. A, Percentage of spikelets (n = 27) showing necrotic lesions after pretreatment with Z-3-HAC at 0, 2 4, 6, and 8 d after infection (DAI). Significant differences between treatments are depicted with asterisks. Significance was determined using the χ2 test with a significance level of 0.05. B, Leaves of seedlings preexposed to Z-3-HAC or MeSA show smaller necrotic lesions compared with nonprimed control seedlings, while preexposure to MeJA exacerbates lesion length. Leaves were cut from the seedlings and subsequently wounded, after which a droplet of a conidia suspension of F. graminearum (5 × 104 conidia mL−1) was applied on the wound. Lesion length was monitored at 24, 48, and 72 hai. Bars represent means of 15 biological replicates. Bars depicted with different letters per time point indicate significant differences between the treatments (P < 0.05). Error bars represent se. C, Photographs depicting representative necrosis symptoms at 72 hai. The top row shows leaves that have been primed with Z-3-HAC, while the bottom row shows leaves that have not been primed. D, Normalized quantitative relative values (NRQ) of fungal biomass. Leaf sheaths were exposed overnight to Z-3-HAC. The next day, a conidia suspension of F. graminearum (5 × 105 conidia mL−1) was applied in the leaf sheaths. Biomass was determined using pre-mRNA slicing factor of F. graminearum (FGSG_01244) as a reference gene and expressed relative to the plant reference genes cell division control protein (Ta54227) and protein transport protein Sec23A (Ta35284). Bars represent means of two biological repeats of four pooled leaf sheaths each. E and F, Microscopic images illustrate the formation of infection structures at 24 hai (E) followed by invasion of the plant cell (F).
Previously, Purahong et al. (2012) reported on the high correlation between FHB resistance levels of wheat ears in field trials and those of detached leaves in a petri dish bioassay. Given the experimental tractability of the latter assays, we next tested the ability of Z-3-HAC to reduce FHB development and severity in a series of detached leaf experiments. At 24 h after infection (hai), we did not find significant differences in lesion length between preexposed and control seedlings. However, at 48 and 72 hai, lesion length was significantly larger (+20%, P < 0.05 and +72%, P < 0.01, respectively) in control seedlings compared with preexposed seedlings (Fig. 1B). Additionally, lesions of preexposed seedlings showed an easily distinguishable front of dark necrotic cells, while control seedlings showed more water-soaked lesions (Fig. 1C).
To investigate the effect of seedling preexposure to Z-3-HAC on fungal growth, we inoculated leaf sheaths with a conidia suspension using the leaf sheath bioassay. At each time point, we consistently found lower fungal biomass in the preexposed seedlings as compared with the control treatment (24 hai, −39%, P = 0.25; 48 hai, −65%, P < 0.05; and 72 hai, −94%, P < 0.05; Fig. 1D).
We inoculated leaf sheaths of wheat seedlings with a conidia suspension in order to establish the time point at which the fungal hyphae form infection structures and invade the plant cell wall. After an incubation period of 24 h, we found a large formation of appressoria-like structures and foot structures (Jansen et al., 2005; Boenisch and Schäfer, 2011; Fig. 1, E and F) and decided to take this time point as a starting point for further experiments.
Effect of Z-3-HAC, SA, and JA on Plant Defense during the Infection Process of F. graminearum
Expression of JA and SA Biosynthesis Genes after F. graminearum Infection
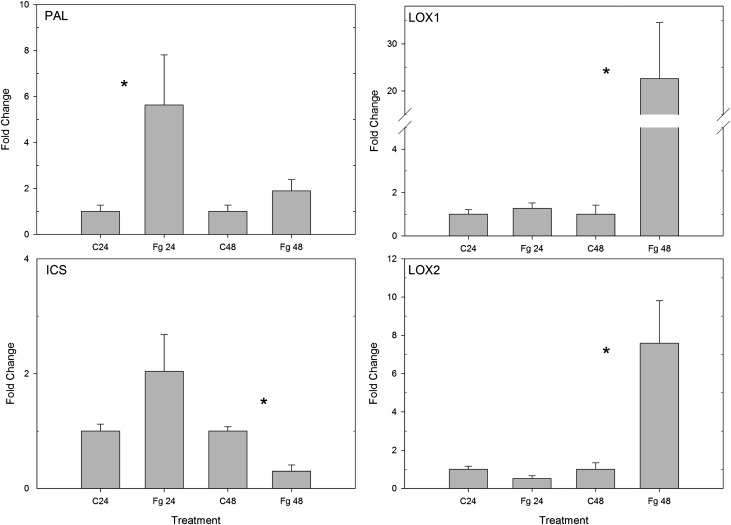
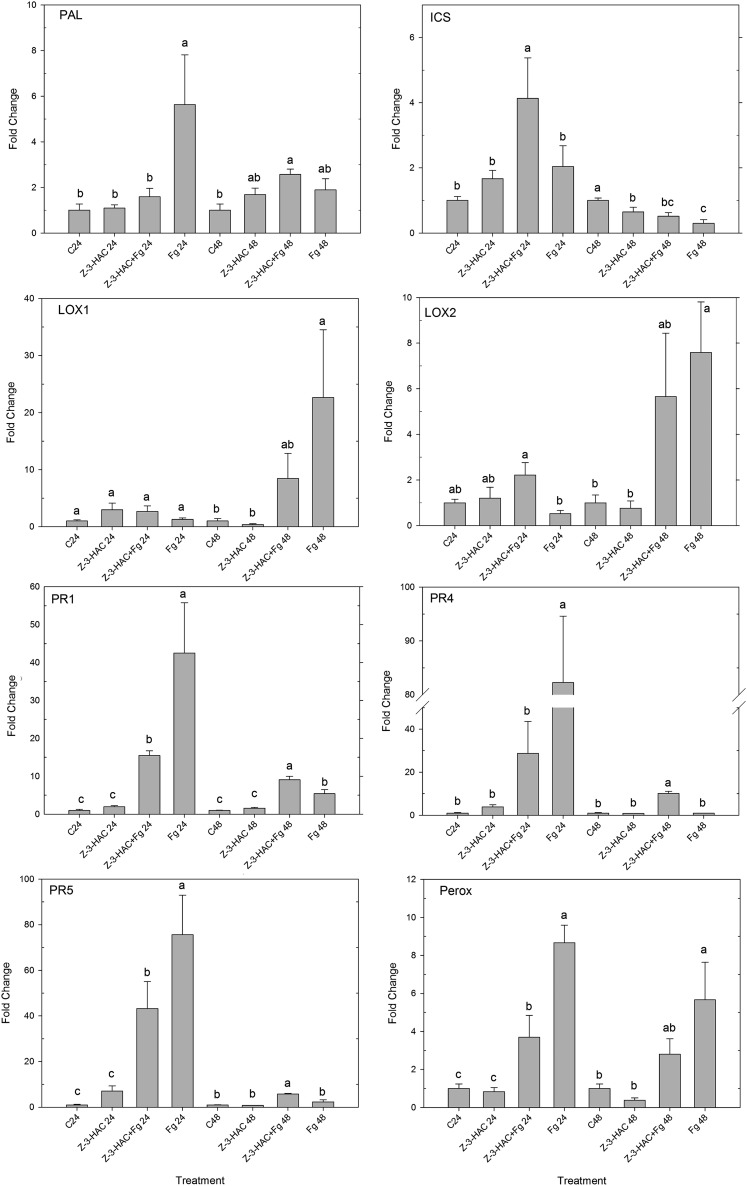
Because of the hemibiotrophic lifestyle of F. graminearum, we verified whether a sequential up-regulation of the biosynthesis genes for the SA and JA pathways was present. We selected PHENYLALANINE AMMONIA LYASE (PAL) and ISOCHORISMATE SYNTHASE (ICS) as marker genes for the biosynthesis of salicylate (Ding et al., 2011) and LIPOXYGENASE1 (LOX1) and LOX2 as marker genes for the biosynthesis of JA (Feng et al., 2010). Using the leaf sheath bioassay, we infected seedlings with a conidia suspension of F. graminearum. Expression analysis revealed at 24 hai a significant up-regulation of PAL (P < 0.05), while the expression of ICS, LOX1, and LOX2 was not significantly different from the control treatment (Fig. 2). However, at 48 hai, we saw a significant up-regulation of LOX1 and LOX2 (P < 0.05), while the expression of PAL was not different from the control and ICS was even significantly down-regulated (P < 0.05; Fig. 2).
Figure 2.
Expression profiles of PAL, ICS, LOX1, and LOX2 at 24 and 48 h after challenge with a conidia suspension of F. graminearum. Data represent means of four biological replicates, each consisting of four pooled leaf sheaths. Error bars represent se. Significant differences between the treatments per time point (P < 0.05) are depicted with asterisks.
Effect of Exogenous Methyl Salicylate and Methyl Jasmonate on Disease Development
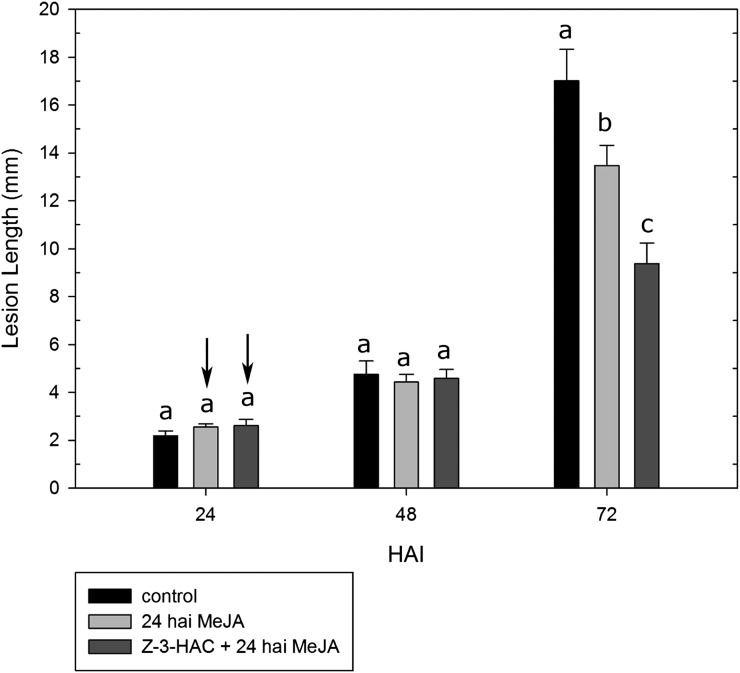
As defense against F. graminearum has been attributed to both SA- and JA-related defense pathways and following our previous results, we assessed whether preexposure to methyl salicylate (MeSA) or methyl jasmonate (MeJA) contributed to smaller lesions in leaves of infected wheat seedlings. Remarkably, while seedlings that had been preexposed to MeSA exhibited significantly smaller lesions at 48 hai (−29%, P < 0.05) and 72 hai (−21%, P < 0.05) compared with the control seedlings (Fig. 1B), preexposure to MeJA led to significantly longer lesions at 48 hai (+29%, P < 0.05) and 72 hai (+53%, P < 0.05), showing enhanced susceptibility (Fig. 1B). However, because our previous observations showed an induction of JA biosynthesis genes at 24 hai, we verified whether treating the seedlings with MeJA at 24 hai would lead to enhanced defense. At 24 and 48 hai, there were no significant differences between the treatments (Fig. 3). Nonetheless, at 72 hai, the treatment with MeJA led to lower lesion length. Additionally, lesion length for the seedlings that had been preexposed to Z-3-HAC was lower (−57%, P < 0.05) than that of nonpreexposed seedlings treated with MeJA (−20%, P < 0.05; Fig. 3).
Figure 3.
Leaves of seedlings preexposed to Z-3-HAC and treated with MeJA at 24 hai show smaller lesions compared with the control treatment. Leaves were cut from the seedlings and subsequently wounded, after which a droplet of a conidia suspension of F. graminearum (5 × 104 conidia mL−1) was applied on the wound. Lesion length was monitored at 24, 48, and 72 hai. Arrows indicate the time points at which the seedlings were treated with 10 µL of MeJA applied on a filter paper. Bars represent means of 10 to 15 biological replicates. Bars depicted with different letters per time point indicate significant differences between the treatments (P < 0.05). Error bars represent se.
In conjunction with our gene expression results, these observations support an important role of both SA and JA in plant defense against F. graminearum, with SA mainly contributing to resistance during the pathogen’s early biotrophic growth and JA conditioning plant immunity during later stages of infection.
Gene Expression of Preexposed Seedlings after Treatment with MeSA or MeJA
To elucidate whether the preexposure of Z-3-HAC leads to a direct activation of plant innate immunity or rather primes for an enhanced defense response following pathogen attack, we first investigated the impact of Z-3-HAC preexposure on MeSA- and MeJA-inducible gene expression.
We selected PAL and ICS as marker genes for the salicylate pathway (Ding et al., 2011) and LOX1 and LOX2 for the jasmonate pathway (Feng et al., 2010). Additionally, we selected different plant defense genes encoding pathogenesis-related proteins, which are known to play a role in the defense against infection with F. graminearum, namely, the pathogenesis-related proteins PR1 (Makandar et al., 2012), PR2, β-1,3-glucanase (Gao et al., 2013), PR4, shown to possess antifungal properties against Fusarium spp. (Bertini et al., 2009), and PR5 (thaumatin-like protein; Gao et al., 2013). As F. graminearum is known to interfere with the redox state of plant cells through the action of the mycotoxin DON, we also analyzed the expression of PEROXIDASE (PEROX) and NADPH OXIDASE (NADPHox; Desmond et al., 2008). Because cell wall reinforcement is a component of plant defense against fungal pathogens, we selected CINNAMOYL COENZYME A REDUCTASE3 (CCR3) and CINNAMYL ALCOHOL DEHYDROGENASE1 (CAD1) as marker genes for lignin biosynthesis (Bi et al., 2011).
Quantitative PCR analysis of the above-mentioned genes revealed that there were no significant differences between the control treatment and the Z-3-HAC-pretreated seedlings in both bioassays (Figs. 3 and 4), suggesting that Z-3-HAC does not function as a direct activator of plant defense.
Figure 4.
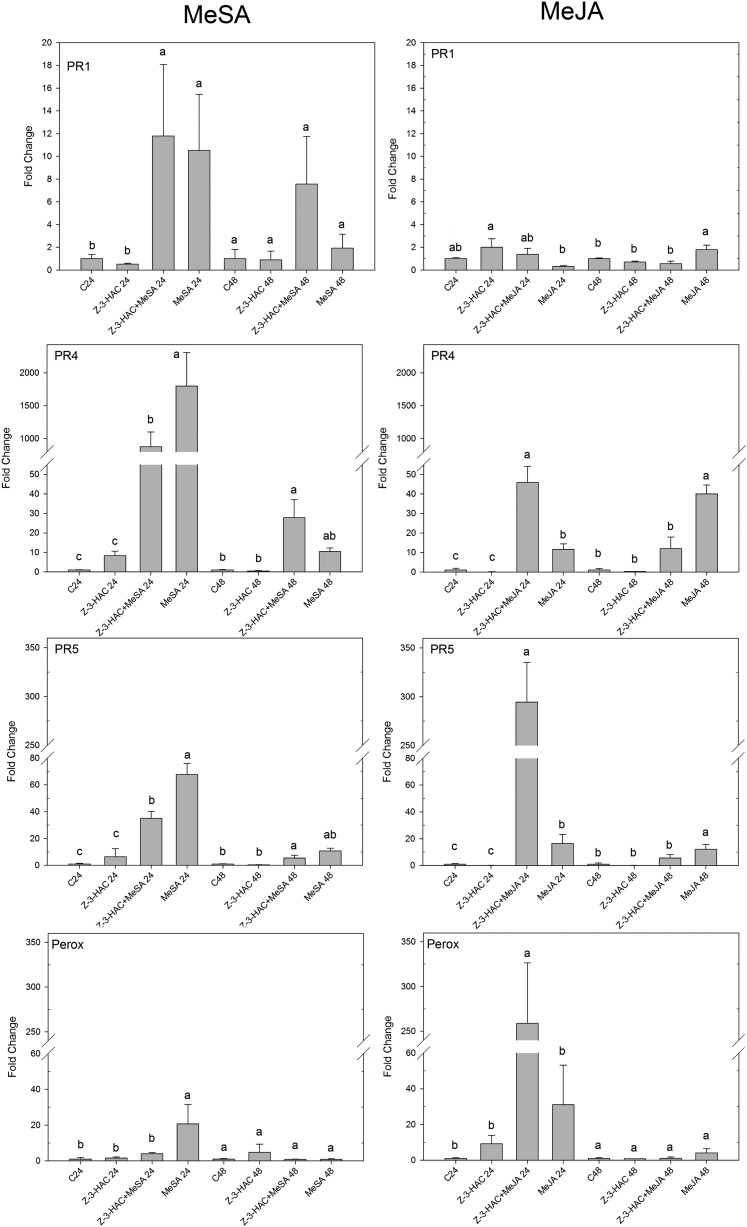
Expression profiles of PR1, PR4, PR5, and PEROX at 24 and 48 h after challenge with MeSA or MeJA. Data represent means of three biological replicates, each consisting of four pooled leaf sheaths. Error bars represent se. Different letters per time point indicate significant differences between the treatments (P < 0.05).
After treatment with MeSA, compared with the control treatment, we observed a significant up-regulation of PR1, PR4, and PR5 in both preexposed and nonpreexposed seedlings at both time points (Fig. 4). Additionally, PEROX showed a significant up-regulation in the nonpreexposed seedlings at 24 hai (20-fold, P < 0.05). Interestingly, at 24 h after challenge with MeSA, preexposed seedlings showed a significantly lower up-regulation of PR4 (877-fold versus 1,799-fold, P < 0.05) and PR5 (35-fold versus 68-fold, P < 0.05) compared with the nonpreexposed seedlings (Fig. 4). As SA- and JA-regulated defense have mainly been reported to act antagonistically (Glazebrook, 2005), we investigated whether a similar or opposite trend was present in preexposed and control seedlings after treatment with MeJA. MeJA treatment did not result in significant differences in the expression of PR1 between the different treatments, but at 24 hai, MeJA did induce a significant up-regulation of PR4, PR5, and PEROX in the preexposed treatment compared with the control (Fig. 4). Contrary to treatment with MeSA, treatment with MeJA resulted in a significantly stronger up-regulation in the preexposed seedlings compared with the nonpreexposed seedlings for PR4 (46-fold versus 12-fold, P < 0.05), PR5 (294-fold versus 17-fold, P < 0.05), and PEROX (259-fold versus 31-fold, P < 0.05; Fig. 4). For both the MeSA and MeJA treatments, PR2, NADPHox, CCR3, and CAD1 were not significantly induced (data not shown). Additionally, expression of the biosynthesis genes PAL and ICS was not affected by MeSA or MeJA treatment, suggesting that SA biosynthesis was not affected by these compounds.
Expression of Defense Genes upon Infection with F. graminearum
In order to elucidate whether the delay in disease progression of F. graminearum in the primed seedlings can be attributed to a stronger activation of JA-associated defense through priming by Z-3-HAC, we analyzed the expression of the defense genes (Fig. 5) upon infection with F. graminearum.
Figure 5.
Expression profiles of PAL, ICS, LOX1, LOX2, PR1, PR4, PR5, and PEROX at 24 and 48 h after challenge with a conidia suspension of F. graminearum. Data represent means of four biological replicates, each consisting of four pooled leaf sheaths. Error bars represent se. Different letters per time point indicate significant differences between the treatments (P < 0.05).
Both primed and nonprimed seedlings showed a stronger up-regulation of PR1, PR4, PR5, and PEROX. Consistent with our results from the MeSA experiment, at 24 hai, we saw a higher up-regulation in the nonprimed treatment compared with the primed treatment of PR1 (30-fold versus 10-fold, P < 0.05), PR4 (109-fold versus 37-fold, P = 0.059), PR5 (90-fold versus 33-fold, P < 0.05), and PEROX (9-fold versus 5-fold, P = 0.056; Fig. 5). At 48 hai, the expression pattern followed a similar trend as in MeJA-challenged seedlings at 24 hai. Namely, the expression of PR4 (10-fold versus 1-fold, P < 0.05) and PR5 (5-fold versus 2-fold, P < 0.05) was significantly higher in the primed treatment compared with the nonprimed treatment (Fig. 5). PR2, NADPHox, CCR3, and CAD1 were not significantly induced after infection with F. graminearum (data not shown).
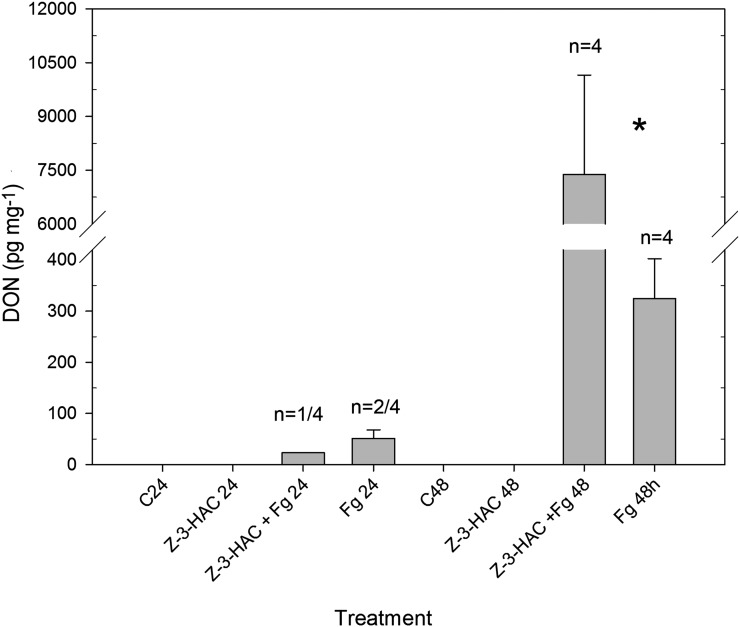
DON Analysis
Owing to its hemibiotrophic character, F. graminearum is able to switch from a biotrophic to a necrotrophic lifestyle through the production of DON. As we intended to clarify if the enhanced defense through priming resulted in a higher production of DON, we quantified DON content using ultra-high-performance liquid chromatography-mass spectrometry (U-HPLC-MS).
At 24 hai, DON was present in only one of the four biological repeats of the primed treatment (23.4 pg mg−1 plant dry weight), whereas in the nonprimed treatment, DON was present in two out of four biological repeats (51.5 pg mg−1 plant dry weight; Fig. 6). Remarkably, at 48 hai, DON content was significantly higher in the primed seedlings than in the nonprimed seedlings (7,838.4 versus 324.5 pg mg−1 plant dry weight, P < 0.05).
Figure 6.
DON concentrations (pg mg−1 plant dry weight) at 24 and 48 h after challenge with a conidia suspension of F. graminearum. Data represent means of four biological replicates, each consisting of six to eight pooled leaf sheaths. Error bars represent se. A significant difference between treatments per time point (P < 0.05) is depicted with an asterisk.
DISCUSSION
Exposure to Z-3-HAC Resulted in Enhanced Resistance against Infection by F. graminearum
Priming by GLVs has already been shown for maize (Engelberth et al., 2004), lima bean (Phaseolus lunatus; Heil and Kost, 2006), poplar (Populus spp.; Frost et al., 2008), and tomato (Finiti et al., 2014). However, despite the fact that wheat is one of the most produced cereals in the world, no studies exist that investigate priming by GLVs in wheat. Additionally, the above-mentioned studies mainly investigated the effect of priming by GLVs on defense against herbivore insects, while little research has been done on the potential of GLVs to prime crops against fungal pathogens. Therefore, expanding our knowledge on priming in a plant-fungus interaction is of paramount importance to obtain a better understanding of the mechanisms and potential of plant priming by GLVs. To our knowledge, this study is the first to investigate whether GLVs can act as a priming agent for wheat against a fungal infection. We found that exposure of wheat to the GLV Z-3-HAC caused wheat seedlings and ears to become primed against a subsequent infection of F. graminearum. We observed for the primed wheat plants a delay in infection in both ears (Fig. 1A) and seedlings (Fig. 1B) as well as lower fungal biomass accumulation (Fig. 1D). As F. graminearum is a fungus that mainly thrives on the floral organs of wheat instead of the leaves (Walter et al., 2010), it can be expected that it is better adapted to resist plant defense in spikelets compared with the leaf sheaths, possibly explaining the larger effect of priming we saw in the seedlings compared with the spikelets.
SA and JA Contribute to Defense against F. graminearum
The resistance of wheat against infection by F. graminearum has primarily been attributed to SA-mediated (Makandar et al., 2012) and JA-mediated (Li and Yen, 2008; Qi et al., 2012) defense pathways. Because of the hemibiotrophic lifestyle of F. graminearum, it can be expected that SA and JA will play a sequential role in the plant defense against F. graminearum. This biphasic defense response has already been shown by Ding et al. (2011), who reported a peak in SA content followed by a peak in JA content. At the gene level, this was confirmed in our study. At 24 hai, biosynthesis genes of the SA pathway were more strongly up-regulated, indicating an activation of SA signaling (Fig. 2). However, at 48 hai, the up-regulation of SA biosynthesis genes was diminished, and ICS expression was even down-regulated. This coincided with a stronger up-regulation at 48 hai of LOX1 and LOX2, which are indicators of JA biosynthesis. Additionally, preexposure to MeSA resulted in smaller lesions, while preexposure to MeJA exacerbated the infection by F. graminearum (Fig. 1B), suggesting that during the early infection stages, defense is regulated mainly by SA. The negative effect of MeJA preexposure on disease development may be attributed to a negative cross talk between the SA and JA pathways (Robert-Seilaniantz et al., 2011; Pieterse et al., 2012), in which activation of the JA defense pathway would suppress the SA defense pathway, which is critical in the early defense response against F. graminearum. The importance of SA-related defense contradicts the study of Li and Yen (2008), which found at 24 hai a significant up-regulation of LOX and ALLENE OXIDE SYNTHASE (AOS), an enzyme more upstream of JA biosynthesis. In addition, they reported an up-regulation of ethylene-responsive genes that led them to conclude that FHB tolerance in wheat is primarily mediated by JA and ethylene signaling, while SA mediated resistance is insignificant. However, it should be remarked here that, as the above-mentioned study investigated gene expression at 24 hai, it is possible that, at this time point, F. graminearum had already entered into the necrotrophic phase of its life cycle (Walter et al., 2010), which consequently would result in augmented JA-related defense of the plant. This was confirmed in our experiment. The addition of exogenous MeJA 24 hai rendered the seedlings more resistant (Fig. 3). These data stress the importance of timing in studying the mechanism of plant-pathogen interaction, especially if the pathogen has a hemibiotrophic character. The importance of the jasmonate-mediated defense against infection by Fusarium verticillioides also has been shown for another monocot, maize. Christensen et al. (2014) characterized the 9-LOX gene, ZmLOX12, and showed that mutants exhibited increased susceptibility toward infection accompanied by diminished levels of JA. Our results indicate that SA plays an important role during the early stages of infection, while JA contributes to resistance during the necrotrophic stage. By precisely switching from SA- to JA-related defense at the onset of the necrotrophic phase of the pathogen, the plant can defend itself more effectively against infection by F. graminearum.
Z-3-HAC Primes for a Stronger Activation of JA-Related Defense
Priming does not directly activate costly defense mechanisms but entails a stronger plant defense upon infection (van Hulten et al., 2006; Conrath, 2009). We did not observe significant effects on gene expression by a preexposure to Z-3-HAC (Figs. 4 and 5), thus showing that Z-3-HAC did not act as a direct activator of plant defense. Furthermore, as preexposure to Z-3-HAC led to enhanced defense after treatment with MeJA or F. graminearum, we can conclude that exposure to Z-3-HAC rendered wheat seedlings in a primed state.
Defense against F. graminearum is a sequential and meticulously regulated mechanism in which the plant will consecutively employ SA- and JA-mediated defense against the biotrophic and necrotrophic phases, respectively, between which a negative cross talk exists (Glazebrook, 2005; Robert-Seilaniantz et al., 2011).
To elucidate whether the GLV Z-3-HAC targets SA- or JA-related defense, we analyzed the expression of defense genes of primed and nonprimed seedlings after challenge with MeSA and MeJA, respectively. Our results demonstrated that priming of wheat seedlings by Z-3-HAC resulted in a stronger up-regulation of PR4, PR5, and PEROX after challenge with MeJA, while expression of these genes was suppressed in the primed seedlings after challenge with MeSA, both compared with the nonprimed seedlings (Fig. 4). These results strongly suggest that Z-3-HAC promotes JA-related defense pathways but antagonizes SA-related immunity. It remains unclear whether Z-3-HAC acts a direct repressor of SA-regulated defense or as an indirect repressor through a stronger activation of JA-related defense. Although we failed to see a priming effect of Z-3-HAC on MeJA-induced LOX1 or LOX2 expression, it cannot be ruled out that Z-3-HAC indirectly suppresses SA action by stimulating the JA pathway downstream of JA biosynthesis. Elucidating the exact mechanism(s) by which Z-3-HAC taps into the SA and JA pathways is a key challenge for future research.
A stronger defense against insects also has been shown in several studies that investigate the effect of BVOCs (Karban et al., 2006; Kessler et al., 2006; Ton et al., 2007) and GLVs in particular (Engelberth et al., 2004; Kost and Heil, 2006; Frost et al., 2008). Engelberth et al. (2007) also showed that maize plants that were preexposed to Z-3-HAC had higher production of JA and 12-oxophytodienoic acid after application with caterpillar regurgitant. This is in accordance with a similar study of Ton et al. (2007), where maize plants were exposed to the BVOCs of Spodoptera littoralis-infested plants seedlings. They reported a higher defense gene expression after these exposed seedlings were subsequently infested with S. littoralis. Similar to our study, these BVOCs contained several GLVs and enhanced a specific subset of JA-inducible genes. Therefore, it is conceivable that pretreatment with GLVs confers increased resistance against insects and pathogens that are susceptible to JA-related plant defense. Hence, we want to remark here that, since it is known that most plant tissue biting-chewing insects such as caterpillars activate the JA-related defense while piercing-sucking herbivores such as aphids induce the SA-related defense (Heil, 2008; Smith et al., 2010; Liu et al., 2011), it would be interesting to investigate the priming potential of GLVs against insect herbivores with different modes of feeding.
A study by Scala et al. (2013b) investigated the mode of action of another GLV, E-2-HAL, in the defense of Arabidopsis (Arabidopsis thaliana) against the biotrophic bacterium Pseudomonas syringae. They found that plant mutants impaired in the production of GLVs were more resistant against P. syringae and exhibited higher SA and lower JA levels. They also showed that expression of MYC2 was not influenced by E-2-HAL but expression of the transcription factor AP2/ERF domain OCTADECANOID-RESPONSIVE ARABIDOPSIS (ORA59) was. Both genes are important players in the JA signaling pathway, the former contributing to defense against herbivorous insects and the latter promoting resistance to necrotrophic pathogens by integrating the ethylene and jasmonate pathways (Pieterse et al., 2012). Ethylene also plays an important role in the defense of the monocot rice against both (hemi)biotrophic and necrotrophic fungi, contributing to both increased resistance and susceptibility (De Vleesschauwer et al., 2013). More research is needed to elucidate whether Z-3-HAC also interacts with the ethylene signaling pathway or other plant defense hormones in wheat.
Nonprimed Seedlings Exhibit a Stronger Up-Regulation of Defense Genes upon F. graminearum Infection
Our finding that priming by Z-3-HAC activates a stronger JA-related defense response concurs with the gene expression of seedlings after F. graminearum infection. At 24 hai, we found a significantly stronger up-regulation of defense genes of the nonprimed seedlings compared with the primed seedlings, following the pattern after MeSA challenge. Nevertheless, primed seedlings were able to slow the infection process of F. graminearum (Fig. 1, A and B), suggesting that another mechanism was influenced by Z-3-HAC. At 48 hai, the expression pattern of PR1, PR4, PR5, and PEROX (Fig. 5) followed the expression pattern of the seedlings at 24 h after treatment with MeJA (Fig. 4). Even though at 24 hai, the expression of these defense genes was higher in the nonprimed seedlings, expression might still have been high enough to contribute to defense in both primed and nonprimed seedlings. However, as F. graminearum switched to a necrotrophic phase, the higher gene expression in primed seedlings at 48 hai might have contributed to the enhanced defense of primed seedlings we saw in our infection experiments (Fig. 1).
The molecular mechanisms for priming remain largely elusive. Recent reviews have attributed the enhanced defense to the accumulation of dormant mitogen-activated protein kinases, chromatin modifications, modifications of primary metabolism, accumulation of inactive defense metabolite conjugates, and activation of a second reactive oxygen species burst (Conrath, 2011; Pastor et al., 2013). The direct effect of BVOCs as defense signals in plant-insect interactions has already been shown in different studies (Gatehouse, 2002; Arimura et al., 2005; Kessler et al., 2006). Other studies found minor inductions of gene expression after treatment with GLVs. For example, Bate and Rothstein (1998) exposed Arabidopsis seedlings to the GLV E-2-HAL. They found induction of PAL, LOX, and AOS but no induction of PR1 and PR2. Additionally, the effect of E-2-HAL was only moderate compared with treatment with the volatile MeJA. Engelberth et al. (2013) performed a microarray analysis of maize seedlings at 20 and 60 min after exposure to the GLV Z-3-HOL. They found a significant expression of genes involved in transcriptional regulation and signaling (AOS, WRKY12, and MYC7, an ortholog of MYC2 in Arabidopsis). Furthermore, they suggest that these early regulators serve as a main switch for the subsequent remodeling through the activation of a second-tier level of genes. Thus, these early responses might play a role in the underlying mechanism of defense priming. Contrary to Bate and Rothstein (1998), they found Z-3-HOL to be a more potent inducer of defense genes than MeJA, MeSA, and ethylene. In our study, we did not observe a significantly differential gene response between the control treatment and the wheat seedlings exposed to Z-3-HAC but not infected at the two time points (Fig. 4). This shows that overnight exposure to Z-3-HAC had no direct effect on gene expression.
F. graminearum Produces More DON in Primed Seedlings
Pathogens have evolved different mechanisms to evade or hijack plant defenses in order to successfully infect plant tissue. It has generally been accepted that SA-mediated defense provides protection against biotrophic pathogens and JA-mediated defense provides protection against necrotrophic pathogens (Thaler et al., 2012). However, besides SA and JA, plant defense against pathogens is regulated by an intricate network of different plant hormones between which a complicated cross talk exists (López et al., 2008; Robert-Seilaniantz et al., 2011; De Vleesschauwer et al., 2013). By manipulating this cross talk, pathogens can use the host’s own defense to their own benefit and successfully infect the plant. This phenomenon also has been described for pathogenic fungi. The necrotrophic fungus Alternaria alternata is known to produce different host-specific toxins and cause disease on different host plants (Ito et al., 2004). Prasad and Upadhyay (2010) showed that the toxin produced by A. alternata f. sp. lycopersici triggers the production of H2O2 and ethylene in tomato leaves. The induced production of ethylene is known to further potentiate H2O2 production (de Jong et al., 2002), leading to programmed cell death, thus making the host plant more vulnerable for necrotrophic pathogens. F. graminearum also interacts with plant defense through the production of the mycotoxin DON. Namely, after hyphal growth in the apoplast, plants accumulate H2O2 to induce programmed cell death in order to counteract the biotrophic phase of Fusarium spp. However, H2O2 acts as a signal for F. graminearum to produce DON, which in turn creates a positive feedback loop leading to increased H2O2 and DON production, thus successfully hijacking the plant defense system and clearing the path for the necrotrophic phase of F. graminearum (Walter et al., 2010; Audenaert et al., 2013). Because of the health risks associated with DON (Rotter et al., 1996), it is of paramount importance to investigate whether the enhanced plant defense impacts fungal DON production. We found at 48 hai that the DON content in the primed seedlings was up to 22 times higher than in the nonprimed seedlings (Fig. 6). In contrast, a recent study by Christensen et al. (2014) reported an increased growth of the fungus F. verticillioides together with increased production of the mycotoxin fumonisin in maize mutants that had been compromised in JA-mediated defense. However, fumonisin does not play a role in the virulence of F. verticillioides, contrary to DON in the pathogenicity of F. graminearum (Proctor et al., 2002). We found lower fungal biomass at 48 hai (Fig. 1D) and a stronger up-regulation of defense genes in primed seedlings coinciding with a massive increase in DON (Figs. 5 and 6), supporting the hypothesis that F. graminearum produced more DON in an attempt to circumvent the enhanced defense. A similar phenomenon also has been reported by Audenaert et al. (2010). They showed that treating F. graminearum with sublethal concentrations of fungicides triggered DON biosynthesis. This and our results illustrate that a visible reduction in symptoms does not always result in lower DON concentrations. Because of the health risks associated with mycotoxins (Bennett and Klich, 2003), future research efforts should be focused on exploring the impact of enhanced plant defense on mycotoxin production by different fungi.
CONCLUSION
In summary, we have found that the GLV Z-3-HAC primes wheat for enhanced defense against the hemibiotrophic fungus F. graminearum, resulting in slower disease progress, reduced symptom development, lower fungal growth, and higher DON production in planta. Furthermore, we show that defense against F. graminearum is sequentially regulated by SA and JA and propose a model whereby Z-3-HAC treatment boosts JA-dependent defenses to block the pathogen during its necrotrophic growth stage.
MATERIALS AND METHODS
Fusarium graminearum and Conidia Spore Suspension
A GFP transformant of F. graminearum strain 8/1 (Jansen et al., 2005; kindly provided by Dr. Karl Heinz-Kogel) was grown on potato dextrose agar for 7 to 10 d at 20°C under a regime of 12 h of dark and 12 h of combined UVC and UVA light (2x TUV 8W T5 and 1x TL 8W BLB; Philips). Macronidia were harvested by adding a solution of 0.01% (v/v) Tween 80 to the potato dextrose agar plates and rubbing the mycelium with a Drigalski spatula. Subsequently, the suspension was diluted to a final concentration of 5 × 105 conidia mL−1.
Plant Material
Six seeds of wheat (Triticum aestivum var Sahara) were germinated and grown in pots (8.5-cm diameter × 6.5-cm height) in a growth chamber (18°C, 16-h-light/8-h-dark regime) for 2 weeks. When the seedlings reached wheat GS 12 (Lancashire et al., 1991), plants were selected for the leaf sheath bioassay.
Experimental Design
Treatments and Cuvette System
Throughout this study, the same methodology was used. We designate primed plants as wheat plants that have been preexposed to Z-3-HAC. Unless stated otherwise, four different treatments were used: (1) a control treatment; (2) a priming treatment in which wheat plants were primed with Z-3-HAC; (3) a treatment in which primed wheat plants were subsequently challenged with a conidia suspension of F. graminearum, MeSA, or MeJA; and (4) a treatment in which nonprimed plants were challenged with a conidia suspension of F. graminearum, MeSA, or MeJA.
To expose wheat to Z-3-HAC, a dynamic push-pull cuvette system was used (Tholl et al., 2006). Wheat plants were placed in one of four nalophan (Foodpack) cuvettes, each of which was assigned to one of the four above-mentioned treatments.
We applied 70 µL of Z-3-HAC (Sigma-Aldrich) on a piece of filter paper inside the two cuvettes that were assigned to the priming treatment (treatments 2 and 3; 5 pm). To eliminate a direct effect of Z-3-HAC on F. graminearum, the filter paper was removed the following day (8 am) prior to infection. Additionally, the cuvettes were allowed to flush in order to eliminate trace amounts of Z-3-HAC. Subsequently, the plants were challenged with a suspension of F. graminearum, MeSA, or MeJA according to the respective treatments (treatments 3 and 4) and experiments described below.
To supply the cuvettes with sufficient air, we used a slightly modified design by Joo et al. (2010). A membrane pump (N035AN.18; KNF Neuberger), which was installed after a dust filter (2-µm pore size Zefluor PTFE Membrane Filter), provided a continuous air stream of 600 mL min−1 (GF40; Brooks Instruments). In order to purify the incoming air of pollutants and ozone, air passed through two active carbon filters (Airpel 10; Desotec) and an ozone filter (ETO342FC002A; Ansyco), respectively. Tubing and connections were made out of perfluoroalkoxy (Swagelok).
Ear Infection Experiment
To evaluate the effect of preexposure to the GLV Z-3-HAC on infection of wheat ears by F. graminearum, we performed an infection assay. We placed a total of nine wheat ears (var Sahara) for each treatment in cuvettes made out of nalophan (Foodpack). We applied 70 µL of Z-3-HAC (Sigma-Aldrich) on a piece of filter paper inside the cuvette that was assigned to the priming treatment and removed it the following day. Subsequently, we point inoculated three spikelets of each ear with 20 µL of a conidia suspension of 5 × 105 conidia mL−1. Each 2 d, we evaluated the inoculated spikelets for signs of necrosis.
Detached Leaf Assay
To investigate whether preexposure of wheat seedlings to Z-3-HAC, MeSA, or MeJA (Sigma-Aldrich) leads to enhanced defense against a subsequent infection by F. graminearum, we performed a detached leaf assay experiment following Imathiu et al. (2009). In short, after seedlings were preexposed to 70 µL of Z-3-HAC, MeSA, or MeJA inside the cuvettes as described previously, 4-cm leaf segments were cut from the tip of the leaves of seedlings at GS 12. These leaves were placed on their abaxial surface in petri dishes containing 0.5% (w/v) bacteriological water agar amended with 40 mg L−1 benzimidazole. The center of the leaf segment was wounded using a sterile inoculation needle, after which a droplet of conidia suspension of 5 × 105 conidia mL−1 was placed on the wound. Lesion length was measured the following day using Cell^F (Olympus).
Leaf Sheath Bioassay
To evaluate the expression of defense genes of wheat after F. graminearum infection, a leaf sheath bioassay, based on Koga et al. (2004), was used. This allows for a minimal wound response on gene expression. In short, after overnight priming in the cuvettes, the leaf sheath of the first leaf was carefully peeled off while still remaining attached to the plant. Afterward, the curved cavity was filled with the conidia suspension of 5 × 105 conidia mL−1 or water, according to the respective treatments. The inoculated seedlings were subsequently placed back inside the cuvette. After an incubation period of 24 and 48 h, leaf sheaths from the different treatments were excised and flash frozen in liquid nitrogen and stored at −80°C for later RNA extraction.
To establish a time point for fungal penetration of the plant cell wall, microscopic images were taken at different time points from inoculated (5 × 105 conidia mL−1) leaf sheaths after they had been submerged in 0.05% (w/v) Aniline Blue dye prepared in lactic acid for 30 min (Audenaert et al., 2010).
RNA Extraction and Quantitative Reverse Transcription-PCR
RNA from the leaf sheaths was extracted using TRI reagent (Sigma-Aldrich) according to the manufacturer’s specifications and quantified with a spectrophotometer (ND1000; Nanodrop). For each sample, four leaf sheaths were pooled to a total of three to four biological repeats. First strand complementary DNA was synthesized from 500 ng of total RNA using the SuperScript III First-Strand Synthesis Supermix kit (Life Technologies). The presence of genomic DNA was checked using gel electrophoresis. The primers used for quantitative reverse transcription (qRT)-PCR analysis are listed in Table I. qRT-PCR analysis was performed using a CFX96 system (Bio-Rad). The thermal profile consisted of an initial denaturation step for 3 min at 95°C followed by 40 cycles of 95°C for 30 s and 60°C for 60 s. Finally, melting curve analysis was performed using a temperature profile of 95°C for 10 s, cooling to 65°C for 5 s, and subsequently heating to 95°C at a rate of 0.5°C per 10 s.
Table I. Primers used for qRT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Reference |
|---|---|---|---|
| FGSG_01244 | CTAGCAACTTTCCGCGATGC | CCGTCCACAAGTCGACAGAA | This article |
| LOX1 | GGCACGCCATCGAGCAGTACG | TACTGCCCGAAGTTGACCGCC | Feng et al. (2010) |
| LOX2 | AACAAGTTCGCCGTCACCTT | TTGTCGAGGGTGATGGTCTT | Beccari et al. (2011) |
| PR1 | CGTCTTCATCACCTGCAACTA | CAAACATAAACACACGCACGTA | Gao et al. (2013) |
| PR2 | CCGCACAAGACACCTCAAGATA | CGATGCCCTTGGTTTGGTAGA | Gao et al. (2013) |
| PR4 | ACACCGTCTTCACCAAGATCGACA | AGCATGGATCAGTCTCAGTGCTCA | Qi et al. (2012) |
| PR5 | ACAGCTACGCCAAGGACGAC | CGCGTCCTAATCTAAGGGCAG | Gao et al. (2013) |
| Ta54227 | CAAATACGCCATCAGGGAGAACATC | CGCTGCCGAAACCACGAGAC | Paolacci et al. (2009) |
| Ta35284 | AGCAATTCGCACAATTATTACAAG | CTCACAGAAGACCTGGAAGC | Paolacci et al. (2009) |
| ICS | AGAAATGAGGACGACGAGTTTGAC | CCAAGTAGTGCTGATCTAATCCCAA | Ding et al. (2011) |
| PAL | TTGATGAAGCCGAAGCAGGACC | ATGGGGGTGCCTTGGAAGTTGC | Ding et al. (2011) |
| PEROX | GAGATTCCACAGATGCAAACGAG | GGAGGCCCTTGTTTCTGAATG | Desmond et al. (2005) |
| NADPHox | ATGCTCCAGTCCCTCAACCAT | TTCTCCTTGTGGAACTCGAATTT | Ding et al. (2011) |
| CAD1 | AGATACCGCTTCGTCATCG | GAATCGCACGCACCAACC | Bi et al. (2011) |
| CCR3 | CTGTCGGCTAGTTAATTCTATG | ATATGATCGCCAACCAACC | Bi et al. (2011) |
Fungal biomass was quantified using a pre-mRNA slicing factor of F. graminearum (FGSG_01244; Becher et al., 2011). Normalization of wheat defense genes was carried out using cell division control protein (Ta54227) and protein transport protein Sec23A (Ta35284) as reference genes (Paolacci et al., 2009). All calculations and analysis of the quality of the reference genes were performed using qBase+ software (Biogazelle).
DON Quantification
Sample Preparation
To investigate whether preexposure of wheat seedlings to Z-3-HAC will impact DON production by F. graminearum, DON concentration levels were measured using U-HPLC-MS based on a method described by Van Meulebroek et al. (2012). In short, 200 mg of six to eight pooled leaf sheaths was crushed using liquid nitrogen. Afterward, 1 mL of cold-modified Bieleski extraction buffer (−20°C), consisting of methanol, ultrapure water, and formic acid (75:20:5, v/v/v), was added. Additionally, the suspension was amended with a deuterium-labeled internal standard of 100 pg µL−1 d6-abscisic acid (OlChemIm). Subsequently, the samples were vortexed and placed at −20°C for 12 h of cold extraction. The samples were centrifuged, and 500 µL of the supernatant was transferred to a 30-kD Amicon Ultra centrifugal filter unit (Merck, Millipore). The purified extract was subsequently reduced under vacuum at 35°C to one-fourth of the original volume (Gyrovap). Finally, the extract was transferred to an HPLC vial, and 10 µL was injected directly on column.
U-HPLC-MS
The U-HPLC-MS system consisted of an Accela U-HPLC pumping system (Thermo Fisher Scientific) coupled to an Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific) and equipped with a heated electrospray ionization source, operating in both the positive and negative modes (switching polarity mode). Chromatographic separation of the compounds was achieved with a gradient elution program using a reverse-phase Nucleodur Gravity C18 column (1.8 µm, 50-mm × 2.1-mm i.d.; Macherey-Nagel). The column oven temperature was set at 30°C. The mobile phase consisted of a binary solvent system: 0.1% (v/v) formic acid in ultrapure water (solvent A) and methanol (solvent B) at a constant flow rate of 300 µL min−1. A linear gradient profile with the following proportions (v/v) of solvent A was applied: 0 to 1 min at 98%, 1 to 2.5 min from 98% to 60%, 2.5 to 4 min from 60% to 50%, 4 to 5 min from 50% to 20%, 5 to 7 min at 20%, 7 to 7.1 min from 20% to 0%, 7.1 to 8 min at 0%, and 8 to 8.01 min from 0% to 98%, followed by 2 min of reequilibration. The instrumental parameters for heated electrospray ionization can be found in Van Meulebroek et al. (2012). DON was identified based on both the retention time relative to the internal standard and the accurate mass (mass-to-charge ratio of 297.1337). After identification, concentrations were calculated by fitting the area ratios into a seven-point calibration curve set up in a leaf sheath matrix. DON was kindly provided by Dr. Marc Lemmens.
Data Analysis
Gene expression data were checked for normality using the Kolmogorov-Smirnov test, and statistical comparisons between different treatments were calculated using proc mixed (SAS 9.0). Statistical differences between the primed and nonprimed treatments in the spikelet infection experiment were calculated using the χ2 test (SPSS 20; IBM).
Acknowledgments
We thank Philip Deman and Erik Moerman for aid in constructing the dynamic capture system.
Glossary
- BVOCs
biogenic volatile organic compounds
- GLV
green leaf volatile
- Z-3-HOL
Z-3-hexenol
- Z-3-HAC
Z-3-hexenyl acetate
- JA
jasmonic acid
- SA
salicylic acid
- E-2-HAL
E-2-hexenal
- FHB
Fusarium head blight
- DON
deoxynivalenol
- H2O2
hydrogen peroxide
- hai
hours after infection
- MeSA
methyl salicylate
- MeJA
methyl jasmonate
- U-HPLC-MS
ultra-high-performance liquid chromatography-mass spectrometry
- qRT
quantitative reverse transcription
Footnotes
This work was supported by the Special Research Fund of Ghent University, the Fund for Scientific Research-Flanders, and the Institute for the Promotion of Innovation by Science and Technology in Flanders.
References
- Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta 1734: 91–111 [DOI] [PubMed] [Google Scholar]
- Audenaert K, Callewaert E, Höfte M, De Saeger S, Haesaert G (2010) Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum. BMC Microbiol 10: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, Van Broeck R, Bekaert B, De Witte F, Heremans B, Messens K, Hofte M, Haesaert G (2009) Fusarium head blight (FHB) in Flanders: population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. Eur J Plant Pathol 125: 445–458 [Google Scholar]
- Audenaert K, Vanheule A, Höfte M, Haesaert G (2013) Deoxynivalenol: a major player in the multifaceted response of Fusarium to its environment. Toxins (Basel) 6: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16: 561–569 [DOI] [PubMed] [Google Scholar]
- Beccari G, Covarelli L, Nicholson P (2011) Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol 60: 671–684 [Google Scholar]
- Becher R, Weihmann F, Deising HB, Wirsel SGR (2011) Development of a novel multiplex DNA microarray for Fusarium graminearum and analysis of azole fungicide responses. BMC Genomics 12: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16: 497–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini L, Caporale C, Testa M, Proietti S, Caruso C (2009) Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett 583: 2865–2871 [DOI] [PubMed] [Google Scholar]
- Bi C, Chen F, Jackson L, Gill BS, Li W (2011) Expression of lignin biosynthetic genes in wheat during development and upon infection by fungal pathogens. Plant Mol Biol Rep 29: 149–161 [Google Scholar]
- Birkett MA, Bruce TJA, Pickett JA (2010) Repellent activity of Nepeta grandiflora and Nepeta clarkei (Lamiaceae) against the cereal aphid, Sitobion avenae (Homoptera: Aphididae). Phytochem Lett 3: 139–142 [Google Scholar]
- Boenisch MJ, Schäfer W (2011) Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottalico A, Perrone G (2002) Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J Plant Pathol 108: 611–624 [Google Scholar]
- Christensen SA, Nemchenko A, Park YS, Borrego E, Huang PC, Schmelz EA, Kunze S, Feussner I, Yalpani N, Meeley R, et al. (2014) The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. Mol Plant Microbe Interact 27: 1263–1276 [DOI] [PubMed] [Google Scholar]
- Conrath U. (2009) Priming of induced plant defense responses. InVanLoon LC, ed, Plant Innate Immunity, Vol 51 Elsevier, Amsterdam, The Netherlands, pp 361–395 [Google Scholar]
- Conrath U. (2011) Molecular aspects of defence priming. Trends Plant Sci 16: 524–531 [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, et al. (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19: 1062–1071 [DOI] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214: 537–545 [DOI] [PubMed] [Google Scholar]
- Desmond OJ, Edgar CI, Manners JM, Maclean DJ, Schenk PM, Kazan K (2005) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol 67: 171–179 [Google Scholar]
- Desmond OJ, Manners JM, Stephens AE, Maclean DJ, Schenk PM, Gardiner DM, Munn AL, Kazan K (2008) The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol Plant Pathol 9: 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleesschauwer D, Gheysen G, Höfte M (2013) Hormone defense networking in rice: tales from a different world. Trends Plant Sci 18: 555–565 [DOI] [PubMed] [Google Scholar]
- Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, Xue S, Jia H, Ma Z (2011) Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 6: e19008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25: 417–440 [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101: 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Contreras CF, Dalvi C, Li T, Engelberth M (2013) Early transcriptome analyses of Z-3-hexenol-treated Zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. PLoS ONE 8: e77465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Seidl-Adams I, Schultz JC, Tumlinson JH (2007) Insect elicitors and exposure to green leafy volatiles differentially upregulate major octadecanoids and transcripts of 12-oxo phytodienoic acid reductases in Zea mays. Mol Plant Microbe Interact 20: 707–716 [DOI] [PubMed] [Google Scholar]
- Feng B, Dong Z, Xu Z, An X, Qin H, Wu N, Wang D, Wang T (2010) Molecular analysis of lipoxygenase (LOX) genes in common wheat and phylogenetic investigation of LOX proteins from model and crop plants. J Cereal Sci 52: 387–394 [Google Scholar]
- Finiti I, de la O Leyva M, Vicedo B, Gómez-Pastor R, López-Cruz J, García-Agustín P, Real MD, González-Bosch C (2014) Hexanoic acid protects tomato plants against Botrytis cinerea by priming defence responses and reducing oxidative stress. Mol Plant Pathol 15: 550–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Henika PR, Mandrell RE (2002) Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot 65: 1545–1560 [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM (2008) Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol 180: 722–734 [DOI] [PubMed] [Google Scholar]
- Gao CS, Kou XJ, Li HP, Zhang JB, Saad ASI, Liao YC (2013) Inverse effects of Arabidopsis NPR1 gene on fusarium seedling blight and fusarium head blight in transgenic wheat. Plant Pathol 62: 383–392 [Google Scholar]
- Gatehouse JA. (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156: 145–169 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Goswami RS, Kistler HC (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5: 515–525 [DOI] [PubMed] [Google Scholar]
- Heil M. (2008) Indirect defence via tritrophic interactions. New Phytol 178: 41–61 [DOI] [PubMed] [Google Scholar]
- Heil M. (2014) Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytol 204: 297–306 [Google Scholar]
- Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9: 813–817 [DOI] [PubMed] [Google Scholar]
- Heil M, Ton J (2008) Long-distance signalling in plant defence. Trends Plant Sci 13: 264–272 [DOI] [PubMed] [Google Scholar]
- Imathiu SM, Ray RV, Back M, Hare MC, Edwards SG (2009) Fusarium langsethiae pathogenicity and aggressiveness towards oats and wheat in wounded and unwounded in vitro detached leaf assays. Eur J Plant Pathol 124: 117–126 [Google Scholar]
- Ito K, Tanaka T, Hatta R, Yamamoto M, Akimitsu K, Tsuge T (2004) Dissection of the host range of the fungal plant pathogen Alternaria alternata by modification of secondary metabolism. Mol Microbiol 52: 399–411 [DOI] [PubMed] [Google Scholar]
- Jansen C, von Wettstein D, Schäfer W, Kogel KH, Felk A, Maier FJ (2005) Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc Natl Acad Sci USA 102: 16892–16897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E, Van Langenhove H, Simpraga M, Steppe K, Amelynck C, Schoon N, Muller JF, Dewulf J (2010) Variation in biogenic volatile organic compound emission pattern of Fagus sylvatica L. due to aphid infection. Atmos Environ 44: 227–234 [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324: 89–91 [DOI] [PubMed] [Google Scholar]
- Karban R, Shiojiri K, Huntzinger M, McCall AC (2006) Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87: 922–930 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT (2006) Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148: 280–292 [DOI] [PubMed] [Google Scholar]
- Koga H, Dohi K, Nakayachi O, Mori M (2004) A novel inoculation method of Magnaporthe grisea for cytological observation of the infection process using intact leaf sheaths of rice plants. Physiol Mol Plant Pathol 64: 67–72 [Google Scholar]
- Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94: 619–628 [Google Scholar]
- Kravchuk Z, Vicedo B, Flors V, Camañes G, González-Bosch C, García-Agustín P (2011) Priming for JA-dependent defenses using hexanoic acid is an effective mechanism to protect Arabidopsis against B. cinerea. J Plant Physiol 168: 359–366 [DOI] [PubMed] [Google Scholar]
- Lancashire PD, Bleiholder H, Vandenboom T, Langeluddeke P, Stauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119: 561–601 [Google Scholar]
- Li G, Yen Y (2008) Jasmonate and ethylene signaling pathway may mediate Fusarium head blight resistance in wheat. Crop Sci 48: 1888–1896 [Google Scholar]
- Liu X, Meng J, Starkey S, Smith CM (2011) Wheat gene expression is differentially affected by a virulent Russian wheat aphid biotype. J Chem Ecol 37: 472–482 [DOI] [PubMed] [Google Scholar]
- López MA, Bannenberg G, Castresana C (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11: 420–427 [DOI] [PubMed] [Google Scholar]
- Makandar R, Nalam VJ, Lee H, Trick HN, Dong Y, Shah J (2012) Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol Plant Microbe Interact 25: 431–439 [DOI] [PubMed] [Google Scholar]
- Pańka D, Piesik D, Jeske M, Baturo-Cieśniewska A (2013) Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J Plant Physiol 170: 1010–1019 [DOI] [PubMed] [Google Scholar]
- Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DW, Jenkinson P, McLeod L (1995) Fusarium ear blight (scab) in small-grain cereals: a review. Plant Pathol 44: 207–238 [Google Scholar]
- Pastor V, Luna E, Mauch-Mani B, Ton J, Flors V (2013) Primed plants do not forget. Environ Exp Bot 94: 46–56 [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Piesik D, Pańka D, Delaney KJ, Skoczek A, Lamparski R, Weaver DK (2011) Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.). J Plant Physiol 168: 878–886 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28: 489–521 [DOI] [PubMed] [Google Scholar]
- Prasad V, Upadhyay RS (2010) Alternaria alternata f.sp lycopersici and its toxin trigger production of H2O2 and ethylene in tomato. J Plant Pathol 92: 103–108 [Google Scholar]
- Proctor RH, Desjardins AE, McCormick SP, Plattner RD, Alexander NJ, Brown DW (2002) Genetic analysis of the role of trichothecene and fumonisin mycotoxins in the virulence of Fusarium. Eur J Plant Pathol 108: 691–698 [Google Scholar]
- Purahong W, Alkadri D, Nipoti P, Pisi A, Lemmens M, Prodi A (2012) Validation of a modified Petri-dish test to quantify aggressiveness of Fusarium graminearum in durum wheat. Eur J Plant Pathol 132: 381–391 [Google Scholar]
- Qi PF, Johnston A, Balcerzak M, Rocheleau H, Harris LJ, Long XY, Wei YM, Zheng YL, Ouellet T (2012) Effect of salicylic acid on Fusarium graminearum, the major causal agent of fusarium head blight in wheat. Fungal Biol 116: 413–426 [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JDG (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49: 317–343 [DOI] [PubMed] [Google Scholar]
- Rotter BA, Prelusky DB, Pestka JJ (1996) Toxicology of deoxynivalenol (vomitoxin). J Toxicol Environ Health 48: 1–34 [DOI] [PubMed] [Google Scholar]
- Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC (2013a) Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int J Mol Sci 14: 17781–17811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala A, Mirabella R, Mugo C, Matsui K, Haring MA, Schuurink RC (2013b) E-2-hexenal promotes susceptibility to Pseudomonas syringae by activating jasmonic acid pathways in Arabidopsis. Front Plant Sci 4: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Liu X, Wang LJ, Liu X, Chen MS, Starkey S, Bai J (2010) Aphid feeding activates expression of a transcriptome of oxylipin-based defense signals in wheat involved in resistance to herbivory. J Chem Ecol 36: 260–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17: 260–270 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM (2002) Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131: 227–235 [DOI] [PubMed] [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Röse USR, Schnitzler JP (2006) Practical approaches to plant volatile analysis. Plant J 45: 540–560 [DOI] [PubMed] [Google Scholar]
- Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49: 16–26 [DOI] [PubMed] [Google Scholar]
- Vanheule A, Audenaert K, De Boevre M, Landschoot S, Bekaert B, Munaut F, Eeckhout M, Höfte M, De Saeger S, Haesaert G (2014) The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. Int J Food Microbiol 181: 28–36 [DOI] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103: 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meulebroek L, Bussche JV, Steppe K, Vanhaecke L (2012) Ultra-high performance liquid chromatography coupled to high resolution Orbitrap mass spectrometry for metabolomic profiling of the endogenous phytohormonal status of the tomato plant. J Chromatogr A 1260: 67–80 [DOI] [PubMed] [Google Scholar]
- Vicedo B, Flors V, de la O Leyva M, Finiti I, Kravchuk Z, Real MD, García-Agustín P, González-Bosch C (2009) Hexanoic acid-induced resistance against Botrytis cinerea in tomato plants. Mol Plant Microbe Interact 22: 1455–1465 [DOI] [PubMed] [Google Scholar]
- Walter S, Nicholson P, Doohan FM (2010) Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol 185: 54–66 [DOI] [PubMed] [Google Scholar]