Naturally evolved double mutants in 5-enolpyruvylshikimate-3-phosphate synthase mimic engineered glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthase in crops and confer high-level glyphosate resistance.
Abstract
Glyphosate is the most important and widely used herbicide in world agriculture. Intensive glyphosate selection has resulted in the widespread evolution of glyphosate-resistant weed populations, threatening the sustainability of this valuable once-in-a-century agrochemical. Field-evolved glyphosate resistance due to known resistance mechanisms is generally low to modest. Here, working with a highly glyphosate-resistant Eleusine indica population, we identified a double amino acid substitution (T102I + P106S [TIPS]) in the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene in glyphosate-resistant individuals. This TIPS mutation recreates the biotechnology-engineered commercial first generation glyphosate-tolerant EPSPS in corn (Zea mays) and now in other crops. In E. indica, the naturally evolved TIPS mutants are highly (more than 180-fold) resistant to glyphosate compared with the wild type and more resistant (more than 32-fold) than the previously known P106S mutants. The E. indica TIPS EPSPS showed very high-level (2,647-fold) in vitro resistance to glyphosate relative to the wild type and is more resistant (600-fold) than the P106S variant. The evolution of the TIPS mutation in crop fields under glyphosate selection is likely a sequential event, with the P106S mutation being selected first and fixed, followed by the T102I mutation to create the highly resistant TIPS EPSPS. The sequential evolution of the TIPS mutation endowing high-level glyphosate resistance is an important mechanism by which plants adapt to intense herbicide selection and a dramatic example of evolution in action.
Modern herbicides make major contributions to global food production by easily removing weeds while maintaining sustainable soil conservation practices. However, persistent herbicide selection of huge weed numbers across vast areas has resulted in the widespread evolution of herbicide-resistant weed populations. Worldwide, there are currently more than 449 unique cases of herbicide resistance, with about 11 new cases reported annually, on average (Heap, 2015). Target site resistance due to target gene mutation is one of the major mechanisms enabling resistance evolution (Gressel, 2002; Powles and Yu, 2010).
The most important and globally used herbicide in crop fields is glyphosate (Duke and Powles, 2008). Glyphosate disrupts the shikimate pathway by specifically inhibiting 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS; Steinrücken and Amrhein, 1980). Glyphosate resistance was initially considered to be unlikely to evolve in nature based on the facts that intentional selection for glyphosate tolerance using whole plants and cell/tissue culture was unsuccessful, and laboratory-generated highly resistant EPSPS mutants displayed undesirable enzyme kinetics (Bradshaw et al., 1997; for review, see Pline-Srnic, 2006). This seemed to be true, as resistance was not found during the first 15 years of glyphosate use, primarily as a nonselective herbicide. However, unprecedented intensive glyphosate use for controlling large numbers of weeds over massive areas, especially after the introduction of glyphosate-resistant transgenic crops, imposed high selection pressure on weeds, resulting in the evolution of glyphosate resistance in populations of 32 weed species (Heap, 2015). Since the first identification of a resistance-endowing EPSPS point mutation, P106S, in a glyphosate-resistant Eleusine indica population (Baerson et al., 2002), several other resistance-endowing single-amino acid substitutions at P106 (P106T, P106A, and P106L) have been reported in glyphosate-resistant weeds (e.g. Ng et al., 2004; Yu et al., 2007; Kaundun et al., 2011; for review, see Sammons and Gaines, 2014). These single-codon EPSPS resistance mutations only endow low-level glyphosate resistance (2- to 3-fold the recommended rates). This is not surprising, because glyphosate is a competitive inhibitor of the second substrate, phosphoenolpyruvate (PEP; Boocock and Coggins, 1983), and is considered a transition state mimic of the catalyzed reaction course (Schönbrunn et al., 2001). Indeed, highly glyphosate-resistant EPSPS variants (e.g. mutants at G101 or T102) have greatly increased Km values (decreased affinity) for PEP when expressed in Escherichia coli (Eschenburg et al., 2002; Funke et al., 2009; for review, see Sammons and Gaines, 2014). In contrast, P106 substitutions confer weak glyphosate resistance but preserve adequate EPSPS functionality (Healy-Fried et al., 2007; for review, see Sammons and Gaines, 2014). Aside from P106 EPSPS gene mutations, there are other glyphosate resistance mechanisms, including EPSPS gene amplification and nontarget-site reduced glyphosate translocation/nontarget-site increased vacuole sequestration (Lorraine-Colwill et al., 2002; Gaines et al., 2010; Ge et al., 2010; for review, see Powles and Preston, 2006; Shaner, 2009; Powles and Yu, 2010; Sammons and Gaines, 2014). Generally, each of these mechanisms endows moderate-level (4- to 8-fold the recommended rates) glyphosate resistance.
Low-level glyphosate resistance due to the EPSPS P106 mutations was reported in Malaysian E. indica (Baerson et al., 2002; Ng et al., 2004). Recently, we reported a highly (more than 10-fold the recommended rates) glyphosate-resistant Malaysian E. indica population (Jalaludin et al., 2015). This paper investigates the high-level glyphosate resistance in this population, and is, to our knowledge, the first to reveal the sequential evolution of a double amino acid substitution in EPSPS.
RESULTS
EPSPS Gene Sequencing Revealed a Double Amino Acid Substitution in EPSPS
To identify the basis of very high-level glyphosate resistance, a 301-bp EPSPS DNA fragment covering the highly conserved region (95LFLGNAGTAMRPL107) of the EPSPS gene was analyzed from 43 resistant plants. These resistant individuals were found to have the known weak resistance mutation at codon 106 (CCA to TCA), but importantly, also display a very rare mutation at codon 102 (ACT to ATT). Therefore, in this naturally evolved, highly glyphosate-resistant E. indica population, there are two resistance-endowing EPSPS amino acid substitutions, T102I and P106S. Cloning of the EPSPS complementary DNA (cDNA) fragment covering the 102 and 106 codons from resistant individuals revealed the two mutations were always present in the same EPSPS gene fragment, confirming the double amino acid substitution in a single EPSPS allele. This double amino acid substitution, T102I + P106S, is hereinafter referred to as the TIPS mutation. Based on the sequence information obtained, derived cleaved amplified polymorphic sequence (dCAPS) markers for the T102I and P106S mutations were developed (Supplemental Fig. S1). Analysis (by the dCAPS markers and sequencing) of 193 individuals (untreated) in the resistant population (Table I) revealed that 84% of the individuals are resistant mutants and 16% are the wild type. Only a very small percentage (1.6%) of the plants analyzed were homozygous mutants for the TIPS mutation (referred to as the RR genotype), about 30% were homozygous solely for the P106S mutation (rr), and nearly one-half were the resistant mutants of Rr. Importantly, neither the single T102I mutants, heterozygous P106S single mutants (r/wild type), nor heterozygous TIPS mutants (R/wild type) were found from the samples analyzed. Therefore, only three alleles (R, r, and the wild type) were found in the samples examined, and the frequency of the mutant TIPS allele (R) is only one-half of the P106S allele (r). To better understand the resistance allele frequencies, a more detailed analysis of the field population together with herbicide histories is needed. The full EPSPS cDNA sequences (1,338 bp) were compared among individuals of the wild type, P106S, and the TIPS mutant (GenBand accession no. KM078728). Except for the single-nucleotide polymorphisms at the 102 and 106 codons, there was only one single-nucleotide polymorphism that resulted in an amino acid change, a P381 in wild-type individuals, but an L381 in mutant individuals. However, blast results showed that this amino acid residue is not conserved in plant EPSPS, and the P381L mutation has been previously proven to be irrelevant to glyphosate resistance in E. indica (Baerson et al., 2002).
Table I. Genotype and allele frequencies determined for 193 E. indica individuals by the dCAPS method developed for the T102I and P106S mutations (see Supplemental Fig. S1).
| Genotypes | No. of Individuals Detected | Genotype Frequency | Alleles | Allele Frequency |
|---|---|---|---|---|
| % | % | |||
| RR | 3 | 1.6 | 102I-106S (R) | 26 |
| rr | 65 | 34 | T102-106S(r) | 58 |
| Wild type | 31 | 16 | T102-P106 (wild type) | 16 |
| Rr | 94 | 49 | ||
| R/wild type | 0 | 0 | ||
| r/wild type | 0 | 0 |
Plants Homozygous for the TIPS Mutation Displayed High-Level Glyphosate Resistance
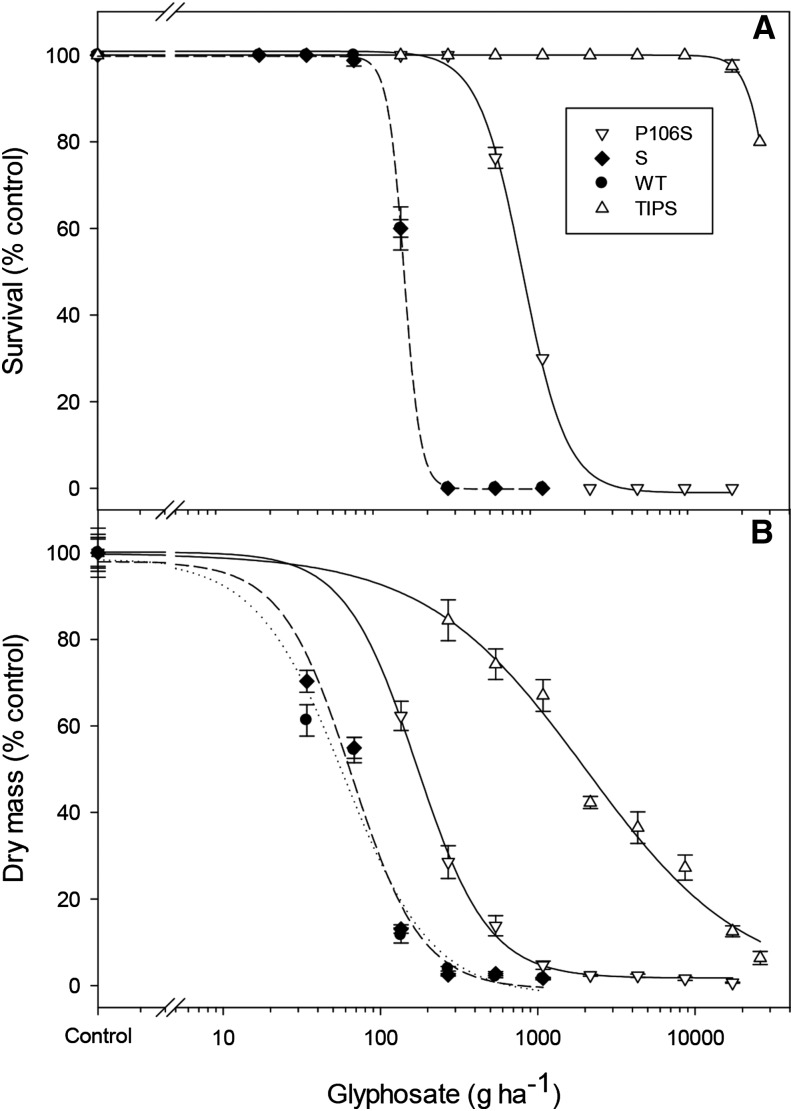
To characterize the glyphosate-resistant genotypes, from within the resistant population, we produced three purified subpopulations with individuals homozygous for the wild type, P106S, and TIPS EPSPS, respectively, and conducted detailed glyphosate dose response studies. To examine the possible involvement of any other glyphosate resistance mechanisms in these purified subpopulations, an herbicide-susceptible (S) E. indica population was also included as a further reference. The S and wild-type populations were found to be identically fully susceptible to glyphosate (Table II; Figs. 1, A and B and 2), indicating no major additional glyphosate resistance mechanisms present in the purified subpopulations. As expected, the P106S population is only moderately resistant to glyphosate, with 30% survival at the recommended field rate. Conversely, homozygous TIPS mutant plants were found to be highly glyphosate resistant, such that an LD50 could not be determined and therefore must be greater than the highest glyphosate rate used (25,900 g ha−1; Fig. 1A; Supplemental Fig. S2). Based on the glyphosate LD50 ratios (Table II), homozygous TIPS mutants are highly (more than 180-fold) resistant, whereas, as expected, homozygous P106S mutants are only moderately (5.6-fold) resistant. The homozygous TIPS plants, therefore, can tolerate more than 20-fold the recommended glyphosate rate of 1,080 g ha−1. Although the TIPS mutants survived high glyphosate doses, their growth was affected (Fig. 1B), resulting in a lower glyphosate GR50 ratio than the LD50 ratio (Table II).
Table II. Parameter estimates of the nonlinear regression analysis (the logistic 3-parameter model) of herbicide rates causing 50% plant mortality (LD50) or growth reduction (GR50) for glyphosate-susceptible (S), EPSPS wild-type, and homozygous P106S and TIPS EPSPS E. indica mutants.
se is in parentheses.
| Genotype | a | b | X0 g ha−1 | P Value for X0 | Ratio to S of X0 |
|---|---|---|---|---|---|
| LD50 | |||||
| S | 99.7 (0.22) | 8.71 (1.61) | 142 (1.3) | <0.0001 | 1 |
| Wild type | 99.7 (0.22) | 8.71 (1.61) | 142 (1.3) | <0.0001 | 1 |
| P106S | 101 (1.78) | 3.14 (0.78) | 798 (29) | <0.0001 | 5.6 |
| TIPS | >25,900 | >182 | |||
| GR50 | |||||
| S | 98.3 (8.42) | 1.65 (0.43) | 65 (8.0) | 0.0013 | 1 |
| Wild type | 97.8 (6.12) | 1.98 (0.37) | 57 (10.8) | 0.0063 | 0.88 |
| P106S | 100 (1.96) | 1.76 (0.12) | 173 (7.3) | <0.0001 | 2.67 |
| TIPS | 99.8 (3.87) | 0.85 (0.08) | 2,023 (299) | 0.0005 | 31.1 |
Figure 1.
Glyphosate dose response: mortality (A) and dry mass (B) of susceptible (S), EPSPS wild type (WT), and homozygous P106S and TIPS mutant E. indica.
Figure 2.
Glyphosate dose response of susceptible (S), EPSPS wild type (WT), homozygous P106S, and homozygous TIPS mutant E. indica.
TIPS Encodes a Highly Glyphosate-Resistant EPSPS
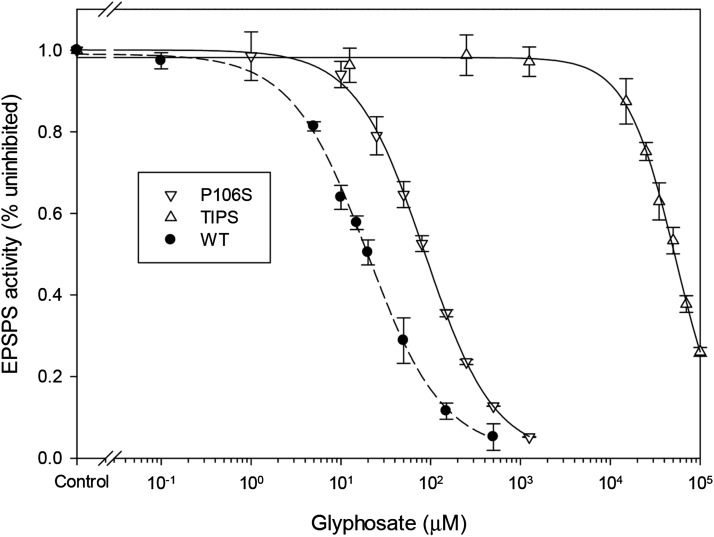
To further characterize the TIPS mutation at the EPSPS level, the wild-type, P106S, and TIPS E. indica 5-enolpyruvylshikimate-3-phosphate synthase (EiEPSPS) were expressed in E. coli, and the activity and herbicide dose causing 50% in vitro inhibition (IC50) of the His-tagged recombinant EiEPSPS variants were determined (Table III). As expected, based on glyphosate IC50 ratios, the E. coli-expressed P106S variant is moderately (4.3-fold) resistant to glyphosate, whereas the TIPS variant is highly (2,647-fold) resistant (Fig. 3; Table III). These results confirm that TIPS EiEPSPS is essentially insensitive to glyphosate, with an IC50 value of 54 mM, and is therefore responsible for very high-level glyphosate resistance as observed at the whole-plant level (Fig. 1). Notably, while incurring no significant changes in the Km (PEP), the E. coli-expressed TIPS variant displayed an EPSPS-specific activity greatly (16-fold) lower than the wild type (Table III), indicating a resistance cost at the enzyme level due to reduced catalytic efficiency.
Table III. Glyphosate IC50 and EPSPS activity, Km (PEP) of E. coli-expressed EiEPSPS variants.
se from the nonlinear regression analysis is in parentheses. The parameter estimates for IC50 can be found in Supplemental Table S2. The χ2 for goodness of fit of the kinetic data (Vmax and Km) is 0.8 (α = 0.05).
Figure 3.
Glyphosate dose response of E. coli-expressed E. indica EPSPS variants.
DISCUSSION
In the biotechnology search for glyphosate-tolerant crops, various EPSPS double mutations have been generated using site-directed mutagenesis and expressed in E. coli and plants (e.g. Spencer et al., 2000; Howe et al., 2002; Lebrun et al., 2003; Kahrizi et al., 2007; Alibhai et al., 2010). The P106 and then later the TIPS mutation were first found empirically in a mutational screen in Salmonella sp. (Comai et al., 1983; Stalker et al., 1985). The TIPS mutation was engineered into tobacco (Nicotiana tabacum; Arnaud et al., 1998) and field tested for glyphosate tolerance (Lebrun et al., 2003). The TIPS EPSPS was then used to produce the first generation commercially successful glyphosate-tolerant transgenic corn (GA21; Spencer et al., 2000). Here, we demonstrate that this TIPS mutation has now evolved in nature.
In target site resistance evolution for acetolactate synthase- and acetyl coenzyme A carboxylase-inhibiting herbicides, highly resistant yet fit individuals with single target site mutations are common (Vila-Aiub et al., 2009), as these herbicides have large binding sites in and adjacent to the enzyme catalytic site, resulting in contacts with amino acids that are nonessential for structure or function (Sammons et al., 2007; Powles and Yu, 2010). The most dramatic example is the multiple (more than 10) different amino acid substitutions at P197 in acetolactate synthase (Tranel and Wright, 2002; Tranel et al., 2015). The transition state inhibitor designation for glyphosate comes from the observation that PEP contacts 17 amino acids responsible for catalysis, which necessarily prevents any substitutions of these essential amino acids (Schönbrunn et al., 2001). The P106S EPSPS provides relatively low glyphosate resistance (Arnaud et al., 1998), whereas the T102I EPSPS alone endows high-level resistance, but with drastically decreased affinity for the second substrate, PEP (Kishore et al., 1992; Funke et al., 2009). The concomitant mutations at the 106 and 102 codons are merely adjacent to the active site and together make very small fractional Ångstrom modifications structurally to the EPSPS active site, therefore selectively impacting glyphosate binding more than PEP (Funke et al., 2009). Hence, the TIPS mutation endows high-level glyphosate resistance with acceptable affinity for PEP.
Multiple mutations of a single-pesticide target site gene are known in adaptive evolution of fungicide or insecticide resistance (Brunner et al., 2008; Karasov et al., 2010). Accumulation of multiple mutations in a single allele in insects and fungi can be achieved via intragenic recombination between preexisting resistant alleles in natural populations, in response to increased selective pressure (Mutero et al., 1994; Brunner et al., 2008). However, this is unlikely to occur in E. indica, as the single-codon mutation T102I was not detected in the resistant population, and is therefore unlikely to preexist in the population. Indeed, the very poor fitness of the kinetics of the T102I mutant enzyme (Alibhai et al., 2010) suggests this mutation would be unfit and even lethal when obtained alone. This lack of fitness of the T102I mutation explains why this single mutation has not been observed in nature.
The notion that compensatory mutations may require a particular evolution trajectory to prevent lethal mutants is discussed by Weinreich et al. (2006), where a series of five amino acid point mutations providing 100,000-fold resistance (compared with susceptible counterparts) to beta-lactamase in a matrix of combinations were studied to reveal a defined successful evolutionary pathway. Here, for the glyphosate-resistant E. indica, our data suggest that the TIPS evolved sequentially under intense glyphosate selection. First, the weak P106S mutation was selected, enriched, and reached homozygosity, and then was followed by the T102I mutation to create the highly resistant TIPS EPSPS. This TIPS EPSPS enables plants to survive high glyphosate rates. Indeed, many glyphosate-resistant E. indica populations in Malaysia and other countries have been found to possess mutations at P106 (Baerson et al., 2002; Ng et al., 2003, 2004; Kaundun et al., 2008), and in Malaysia, glyphosate was used frequently (every month) and continually (5–10 years) at increased glyphosate rates (0.72–1.92 kg ha−1) to control E. indica (Ng et al., 2004). Therefore, evolution of at least the TIPS mutation can be expected from other glyphosate-resistant E. indica populations in Malaysia and other countries.
Will there be other EPSPS double mutations in nature? In addition to the TIPS mutant, various EPSPS double mutants at 102 and 106 were intentionally produced and the kinetics of E. coli-expressed EPSPS variants studied. Compared with the wild-type and T102I mutant alone, double mutants such as T102I + P106A, T102I + P106T, or T102L + P106A also show favorable kinetics comparable with or even better than TIPS (Alibhai et al., 2010; for review, see Sammons and Gaines, 2014). As various amino acid substitutions at P106 have been identified (e.g. P106A, P106S, P106T, or P106L) in glyphosate-resistant weed species (for review, see Sammons and Gaines, 2014), the evolution and selection for other EPSPS double variants is also possible where glyphosate selection is intense.
Does the decreased catalytic efficiency of TIPS EPSPS result in a whole-plant resistance cost due to possible impact on the shikimate pathway? The very low percentage (1.6%) of resistant individuals homozygous for the TIPS EPSPS (RR) as compared with the higher percentage (49%) of resistant individuals of the Rr genotype (Table I) may suggest: (1) the additional T102I mutation is a recent event, and given that E. indica is a self-pollinated species, homozygosity at 102 can be increased in a few generations; and/or (2) a significant resistance cost is associated with homozygous TIPS mutants when glyphosate selection is relaxed. The latter correlates with the measured low catalytic turnover of the E. coli-expressed TIPS EiEPSPS (Vmax in Table III). This decreased catalytic efficiency then translates to the significantly reduced plant growth (Fig. 4, above-ground dry weight per plant of the wild type [4.14 ± 0.24 g], P106S [4.06 ± 0.34 g], and TIPS [1.29 ± 0.05 g; n = 20–25]) and fecundity we have observed for the homozygous TIPS plants (data not shown). Consequently, RR TIPS mutants are outperformed over time by Rr TIPS mutants, which may suffer less or little fitness cost and therefore proliferate in the population. Due to predominant self pollination in E. indica, a very low level of outcrossing (if any) between wild-type and homozygous P106S (rr) mutants may produce a small number of heterozygous P106S (r/wild-type) mutants. However, as expected, these individuals are unable to survive the field or higher glyphosate rates, and hence are selected against. This would explain why the r/wild-type mutants were not detected in the resistant population. We have fitness studies underway with the wild-type, P106S (rr), and TIPS (RR, Rr) EPSPS mutants. However, if this fitness cost from decreased catalytic efficiency is offset, for instance, by gene duplication of the TIPS gene as required for commercial crops (CaJacob et al., 2004), then evolution of Roundup Ready-like E. indica may be expected in nature, especially in species exhibiting EPSPS gene amplification (for review, see Sammons and Gaines, 2014), where the tandem repeat nature of the duplication (Jugulam et al., 2014) may facilitate incorporation of the necessary point mutations, and gene duplication is free of fitness cost (Vila-Aiub et al., 2014).
Figure 4.
Reduced growth of the homozygous EPSPS TIPS mutants, as compared with the wild-type (WT) and homozygous P106S mutants. E. indica plants are 5 weeks after transplanting.
Therefore, the evolutionary recipe to high-level glyphosate resistance in weedy plant species under glyphosate selection may have these primary components: (1) overexpression of EPSPS, as already reported in four weed species with gene duplication; (2) P106S (or T/A) EPSPS, as documented in six weed species (for review, see Sammons and Gaines, 2014); (3) acquiring the second EPSPS T102I mutation, as described here for the first time; and (4) combining with other glyphosate resistance-endowing mechanisms that would have an additive impact on the resistance magnitude, as demonstrated in glyphosate-resistant Lolium rigidum (Yu et al., 2007; Ge et al., 2012; Nandula et al., 2013).
In summary, this research is, to our knowledge, the first to report the evolution of an EPSPS double mutation (TIPS) conferring very high-level glyphosate resistance in crop fields. The TIPS mutation mimics the biotechnology-derived glyphosate-tolerant EPSPS, demonstrating that laboratory and field selection methods are linked. This is a dramatic manifestation of the power of evolution in action and how nature responds and adapts to manipulated environment stresses. This is also a very clear example that herbicide sustainability demands much greater diversity in weed control tactics than reliance on a single herbicide.
MATERIALS AND METHODS
Plant Material
The glyphosate-resistant Eleusine indica population was originally collected from several patches within a palm oil nursery in Jerantut, Malaysia (Jalaludin et al., 2010), and the resistant and susceptible populations used in the current study were further characterized by Jalaludin et al. (2015). Seeds were germinated on 0.6% (w/v) agar for 4 to 7 d, and germinating seedlings were transplanted into plastic pots (12–20 per pot) filled with potting mix (50% [v/v] moss peat and 50% [v/v] river sand) and grown in a greenhouse during the summer growing season (December–March) with an average day/night temperature of 30°C/24°C and a 13-h photoperiod under natural sunlight. When seedlings reached the three- to four-leaf stage, they were treated with various rates of commercial glyphosate using a cabinet sprayer with a spray volume of 112 L ha−1 at a pressure of 200 kPa and a speed of 1 m s−1. Each treatment contained at least three to four replicate pots. Visual assessment for mortality was made 3 to 4 weeks after treatment. Plants were recorded as alive if they were actively growing and tillering after herbicide treatment and as dead if there was little new growth and no new tiller formation.
EPSPS Sequencing and cDNA Cloning
Genomic DNA was extracted from the leaf tissue of resistant and susceptible plants and total RNA isolated using the Plant RNeasy Mini Kit (Qiagen). Genomic DNA contamination was removed using the TURBO-DNA free kit (Ambion). For EPSPS DNA partial sequencing, a pair of published primers (Ng et al., 2003) was used to amplify a highly conserved region (95LFLGNAGTAMRPL107; refer to plant EPSPS numbering system) in which point mutations conferring glyphosate resistance in plants and bacteria have been found (Sammons and Gaines, 2014). The forward primer EleuEPSPS-F (5′-GCGGTAGTTGTTGGCTGTGGTG-3′) and the reverse primer EleuEPSPS-R (5′-TCAATCCGACAACCAAGTCGC-3′; Ng et al., 2003) amplify a 301-bp DNA (includes 99-bp intron) fragment covering the potential mutation sites. Using the same primer pairs, a 202-bp (without intron) cDNA fragment was amplified from pregenotyped plants and cloned into the pGEM-T vector (Promega) and transformed into Escherichia coli. White colonies with putative inserts were used as templates for PCR reamplification and sequencing of the 202-bp fragment. The PCR was conducted in a 25-µL volume that consisted of 1 to 2 µL of genomic DNA or cDNA, 0.5 µM each primer, and 12.5 µL of 2× GoTaq Green Master Mix (Promega). The PCR was run with the following profile: 94°C for 4 min; 40 cycles of 94°C for 30 s, 57°C (annealing temperature) for 30 s, and 72°C for 30 to 50 s; followed by a final extension step of 7 min at 72°C. For EPSPS full-cDNA sequencing, a 1,338-bp cDNA was amplified with Phusion High-Fidelity DNA Polymerase (New England Biolabs, Inc.) using the primer pair (EiRT1 and EiLFT1; Baerson et al., 2002).
The PCR was conducted in a 20-µL volume that consisted of 1 µL (80 ng) of cDNA, 0.5 µM each primer, and 4 µL of 5× Phusion HF buffer, 200 µM deoxyribonucleotide triphosphate, and 0.2 µL of Phusion DNA Polymerase. The PCR was run with the following profile: 98°C for 30 s; 40 cycles of 98°C for 10 s, 72°C for 50 s; followed by a final extension step of 10 min at 72°C. The PCR product was purified from agarose gel as described earlier and sequenced with the primers (EiRT1 and EiLFT1) and an internal forward primer (5′-CTCTTCTTGGGGAATGCTGGA-3′; Kaundun et al., 2008).
The PCR product was purified from agarose gel with Wizard SV Gel and PCR Clean-up System (Promega) and sequenced by commercial services (LotteryWest, State Biomedical Facility Genomics). All sequence chromatograms were visually checked for quality and consistency before sequences were assembled and aligned.
dCAPS Marker Development and Genotyping
Based on the EPSPS sequence information obtained from the susceptible (20 plants) and resistant samples (at least 80), we developed dCAPS markers for detecting mutation(s) at 102 and 106 using the Web-based dCAPS finder version 2.0 software. The A-to-T mismatch was introduced in the forward primer RsaIF (5′-TGCAGCTCTTCTTGGGGAATGCTGGTA-3′) two nucleotides upstream of the 102 codon (i.e. N+2 position) to create a restriction site for RsaI (GT˄AC) in the wild-type sequence. Any nucleotide mutations resulting in substitution of the T102 would abolish the restriction site. Therefore, the primer pair RsaIF and EleuEPSPS-R was used to amplify a 234-bp fragment that was then digested with RsaI. The wild-type sequence would generate a single digested 208-bp band, whereas the mutant sequence at 102 would generate an undigested 234-bp band (Supplemental Fig. S1. A heterozygous sequence at 102 would produce both the 208- and 234-bp bands (Supplemental Fig. S1). Similarly, a G-to-C mismatch was introduced in the forward primer Sau96IF (5′-CTCTTCTTGGGGAATGCTGGAACTGCAATGGGA-3′) at the N+3 position of the 106 codon to create a restriction site for Sau96I (G˄GNCC) in the wild-type sequence. Any mutations resulting in substitution of the P106 would abolish the restriction site. Therefore, the primer pair Sau96IF and EleuEPSPS-R was used to amplify a 233-bp fragment that was then digested with Sau96I. The wild-type sequence would produce a single digested 202-bp band, whereas the mutant sequence at 106 would produce an undigested 233-bp band (Supplemental Fig. S1). A heterozygous sequence at 106 would produce both the 202- and 233-bp bands (Supplemental Fig. S1; no heterozygous sequences at 106 were detected). PCR conditions were similar to those described earlier except that the annealing temperature was 62°C. Restriction digestions were carried out according to the manufacturer’s recommendations (New England Biolabs), and digestion patterns were viewed on 2% (w/v) agarose gels (electrophoresis at 90–100 V for 50–80 min) stained with ethidium bromide. The accuracy of the two markers was confirmed by comparing sequencing and marker analysis results of over 40 samples.
It is noticed that the two dCAPS markers were designed to only detect mutant and wild-type sequences at the 102 and 106 codons without knowing the nature of the specific mutation. We confirmed that the resistant population only possessed the TIPS mutations, so the two dCAPS markers can be used for genotyping in the population. If used in other uncharacterized E. indica populations, the specific mutations have to be determined by sequencing.
Generation of Purified Subpopulations
Plants (seven to 12) that were confirmed by sequencing and marker analysis to be homozygous for the wild type, P106S, or TIPS mutation were bulk selfed in isolation to produce seeds to enable respective subpopulations. Progeny plants (10–12) from each of these purified subpopulations were randomly marker analyzed to confirm their genotype and homozygosity prior to use for subsequent glyphosate dose response.
E. coli Transformation
Total RNA was isolated from the E. indica (hereafter known as Ei) P106S EPSPS mutant. cDNA was synthesized, and the mature P106S EiEPSPS cassette was PCR amplified using the primer pair 1 and 2 (Supplemental Table S1; Baerson et al., 2002). The PCR product was inserted into the pCR Blunt II TOPO vector and verified by sequencing. The P106S EiEPSPS was converted to the wild type (primer pair 3 and 4, Supplemental Table S1) and the double mutant TIPS EiEPSPS (primer pair 5 and 6, Supplemental Table S1) using the Phusion site-directed mutagenesis kit (Thermo Scientific). After sequencing verification, these three genes were PCR amplified using the primer pair 7 and 8, and the PCR products were digested by NdeI and then inserted into the NdeI site of pET19-b vector to form an N-terminal His-tag fusion to facilitate the downstream purification of the enzymes.
EPSPS Purification and Activity Assay
The BL21 (DE3) cells (Invitrogen) harboring the EiEPSPS constructs in pET-19b vector were cultured in the MagicMedia E. coli Expression Medium (Invitrogen) according to the dual temperature protocol provided by the media supplier. Soluble proteins were extracted from frozen cells using the B-per bacterium extraction reagent supplied with DNase I, lysozyme, and Halt Protease inhibitor cocktail (Thermo Scientific). After centrifugation at 21,000g for 5 min, the supernatant fraction was used to purify His-tagged EPSPS with HisPur Ni-NTA resin (Thermo Scientific). The binding buffer was made of 20 mm Tris (pH 8.0), 500 mm NaCl, 10 mm imidazole, and 0.03% (v/v) Triton X-100; washing buffer contained 20 mm Tris (pH 8.0), 500 mm NaCl, 50 mm imidazole, and 0.03% Triton X-100; and elution buffer contained 20 mm Tris (pH 8.0), 500 mm NaCl, and 500 mm imidazole. The eluted enzyme was kept in storage buffer (10 mm MOPS, 10% [v/v] glycerin, 0.5 mM EDTA, 2.5 mm beta-mercaptoethanol) after buffer exchange using Amicon Ultra 0.5-mL centrifugal filters (MWCO 30 kD) three times for 5 min at 14,000g. The protein content was quantified using the Pierce 660 nm Protein Assay kit (Thermo Scientific), and purification results were analyzed by SDS-PAGE electrophoresis.
Activity of purified EPSPS variants was measured by the coupled assay that measures continuous release of inorganic phosphate using the EnzChek Phosphate Assay Kit (E-6646, Invitrogen; Gaines et al., 2010). For IC50 measurement of EiEPSPS variants, various concentrations of glyphosate were used (wild type, 0, 0.1, 5, 10, 20, 50, 150, and 500 μm; P106S, 0, 1, 10, 25, 50, 80, 150, 500, and 1,250 μm; and TIPS, 0, 12.5, 1,250, 15,000, 25,000, 35,000, 50,000, 70,000, and 100,000 μm), holding shikimate 3-phosphate constant at 0.1 mm. To measure the Km (PEP) of the EiEPSPS variants, shikimate 3-phosphate concentration was fixed at 0.1 mm, and various amounts of PEP (2.5, 5, 10, 15, 20, 25, 40, 60, and 80 μm) were used.
Statistics
The LD50, GR50, or IC50 was estimated by nonlinear regression using the three-parameter logistic curve model y = a/[1 + (X/X0)b], where a is the maximum plant response close to untreated controls, X0 is the dose giving 50% response, and b is the slope around X0. The estimates were obtained using the Sigmaplot software (version 12.3, Systat Software, Inc.), and the test (α = 0.05) was used to test significance of the regression parameters. The Km (PEP) and Vmax were calculated by nonlinear fit of the data to the Michaelis-Menten equation, ν = VS/(Km + S), where S is the concentration of the substrate pyruvate, ν is the reaction velocity at any PEP concentration, and V is the maximal reaction velocity. The kinetic values were obtained using GraFit (version 7.0.3, Erithacus Software Ltd.), and χ2 (α = 0.05) was used to test goodness of fit. Glyphosate dose response experiments were repeated at least twice with similar results, so all data were pooled and evaluated for a composite line fit. Each EPSPS kinetic assay contained three technical replicates, and three independent enzyme extracts were used for each assay set.
The E. indica TIPS cDNA sequence information can be found in GenBank with an accession number KM078728.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. dCAPS markers for T102I and P106S.
Supplemental Figure S2. Survival of TIPS plants at very high glyphosate rates.
Supplemental Table S1. Primers used in cloning the EiEPSPS.
Supplemental Table S2. Parameter estimation of nonlinear regression of herbicide IC50.
Supplementary Material
Acknowledgments
We thank Dr. Martin Vila-Aiub, Mike Ashworth, and anonymous reviewers for comments on the article.
Glossary
- EPSPS
5-enolpyruvylshikimate-3-phosphate synthase
- PEP
phosphoenolpyruvate
- cDNA
complementary DNA
- TIPS
T102I and P106S
- dCAPS
derived cleaved amplified polymorphic sequences
- S
susceptible
- LD50
50% plant mortality
- GR50
50% growth reduction
- EiEPSPS
Eleusine indica 5-enolpyruvylshikimate-3-phosphate synthase
- IC50
herbicide dose causing 50% in vitro inhibition
Footnotes
This work was supported by the Grains Research and Development Corporation of Australia and the Australian Research Council.
References
- Arnaud L, Sailland A, Lebrun M, Pallett K, Ravanel P, Nurit F, Tissut M (1998) Physiological behavior of two tobacco lines expressing EPSP synthase resistant to glyphosate. Pestic Biochem Physiol 62: 27–39 [Google Scholar]
- Alibhai MF, CaJacob C, Feng PCC, Heck GR, Qi Y, Flasinski S, Stallings W, inventors. August 5, 2010. Glyphosate resistant Class I 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS). Monsanto Technology LLC: US 7,723,575 B2. pp. 1-49
- Baerson SR, Rodriguez DJ, Tran M, Feng Y, Biest NA, Dill GM (2002) Glyphosate-resistant goosegrass: identification of a mutation in the target enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Plant Physiol 129: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock MR, Coggins JR (1983) Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett 154: 127–133 [DOI] [PubMed] [Google Scholar]
- Bradshaw LD, Padgette SR, Kimball SL, Wells BH (1997) Perspectives on glyphosate resistance. Weed Technol 11: 189–198 [Google Scholar]
- Brunner PC, Stefanato FL, McDonald BA (2008) Evolution of the CYP51 gene in Mycosphaerella graminicola: evidence for intragenic recombination and selective replacement. Mol Plant Pathol 9: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaJacob CA Feng PCC, Heck GR, Alibhai MF, Sammons RD, Padgette SR (2004). Engineering resistance to herbicides. In Christou P, Klee H, eds, Handbook of Plant Biotechnology. John Wiley & Sons Ltd., Hoboken, NJ, pp. 353–372 [Google Scholar]
- Comai L, Sen LC, Stalker DM (1983) An altered aroA gene product confers resistance to the herbicide glyphosate. Science 221: 370–371 [DOI] [PubMed] [Google Scholar]
- Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64: 319–325 [DOI] [PubMed] [Google Scholar]
- Eschenburg S, Healy ML, Priestman MA, Lushington GH, Schönbrunn E (2002) How the mutation glycine96 to alanine confers glyphosate insensitivity to 5-enolpyruvyl shikimate-3-phosphate synthase from Escherichia coli. Planta 216: 129–135 [DOI] [PubMed] [Google Scholar]
- Funke T, Yang Y, Han H, Healy-Fried M, Olesen S, Becker A, Schönbrunn E (2009) Structural basis of glyphosate resistance resulting from the double mutation Thr97 -> Ile and Pro101 -> Ser in 5-enolpyruvylshikimate-3-phosphate synthase from Escherichia coli. J Biol Chem 284: 9854–9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines TA, Zhang W, Wang D, Bukun B, Chisholm ST, Shaner DL, Nissen SJ, Patzoldt WL, Tranel PJ, Culpepper AS, et al. (2010) Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc Natl Acad Sci USA 107: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, d’Avignon DA, Ackerman JJH, Collavo A, Sattin M, Ostrander EL, Hall EL, Sammons RD, Preston C (2012) Vacuolar glyphosate-sequestration correlates with glyphosate resistance in ryegrass (Lolium spp.) from Australia, South America, and Europe: a 31P NMR investigation. J Agric Food Chem 60: 1243–1250 [DOI] [PubMed] [Google Scholar]
- Ge X, d’Avignon DA, Ackerman JJH, Sammons RD (2010) Rapid vacuolar sequestration: the horseweed glyphosate resistance mechanism. Pest Manag Sci 66: 345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressel J. (2002). Molecular Biology of Weed Control. Taylor and Francis Ltd., London, pp 504 [Google Scholar]
- Healy-Fried ML, Funke T, Priestman MA, Han H, Schönburnn E (2007) Structural basis of glyphosate tolerance resulting from mutations of Pro101 in Escherichia coli 5-enolpyruvylshikimate-3-phosphate synthase. J Biol Chem 282: 32949–32955 [DOI] [PubMed] [Google Scholar]
- Heap I (2015) International Survey of Herbicide Resistant Weeds. Online. www.weedscience.com (January 25, 2015)
- Howe AR, Gasser CS, Brown SM, Padgette SR, Hart J, Parker GB, Fromm ME, Armstrong CL (2002) Glyphosate as a selective agent for the production of fertile transgenic maize (Zea mays L.) plants. Mol Breed 10: 153–164 [Google Scholar]
- Jalaludin A, Ngim J, Bakar BHJ Alias Z (2010) Preliminary findings of potentially resistant goosegrass (Eleusine indica) to glufosinate-ammonium in Malaysia. Weed Biol Manage 10: 256–260 [Google Scholar]
- Jalaludin A, Yu Q, Powles SB (2015) Multiple resistance across glufosinate, glyphosate, paraquat and ACCase-inhibiting herbicides in an Eleusine indica population. Weed Res 55: 82–89 [Google Scholar]
- Jugulam M, Niehues K, Godar AS, Koo DH, Danilova T, Friebe B, Sehgal S, Varanasi VK, Wiersma A, Westra P. , et al. (2014) Tandem amplification of a chromosomal segment harboring 5-enolpyruvylshikimate-3-phosphate synthase locus confers glyphosate resistance in Kochia scoparia. Plant Physiol 166: 1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrizi D, Salmanian AH, Afshari A, Moieni A, Mousavi A (2007) Simultaneous substitution of Gly96 to Ala and Ala183 to Thr in 5-enolpyruvylshikimate-3-phosphate synthase gene of E. coli (k12) and transformation of rapeseed (Brassica napus L.) in order to make tolerance to glyphosate. Plant Cell Rep 26: 95–104 [DOI] [PubMed] [Google Scholar]
- Karasov T, Messer PW, Petrov DA (2010) Evidence that adaptation in Drosophila is not limited by mutation at single sites. PLoS Genet 6: e1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaundun SS, Dale RP, Zelaya IA, Dinelli G, Marotti I, McIndoe E, Cairns A (2011) A novel P106L mutation in EPSPS and an unknown mechanism(s) act additively to confer resistance to glyphosate in a South African Lolium rigidum population. J Agric Food Chem 59: 3227–3233 [DOI] [PubMed] [Google Scholar]
- Kaundun SS, Zelaya IA, Dale RP, Lycett AJ, Carter P, Sharples KR, McIndoe E (2008) Importance of the P106S target-site mutation in conferring resistance to glyphosate in a goosegrass (Eleusine indica) population from the Philippines. Weed Sci 56: 637–646 [Google Scholar]
- Kishore GM, Padgette SR, Fraley RT (1992) History of herbicide-tolerant crops, methods of development and current state of the art - emphasis on glyphosate tolerance. Weed Technol 6: 626–634 [Google Scholar]
- Lebrun M, Sailland A, Freyssinet G and Degryse E, inventors. May 20, 2013. Mutated 5-enolpyruvylshikimate-3-phosphate synthase, gene coding for said protein and transformed plants containing said gene. Bayer CropScience S.A.: US 6,566,587. pp. 1-17
- Lorraine-Colwill DF, Powles SB, Hawkes TR, Hollinshead PH, Warner SAJ, Preston C (2002) Investigations into the mechanism of glyphosate resistance in Lolium rigidum. Pestic Biochem Physiol 74: 62–72 [Google Scholar]
- Mutero A, Pralavorio M, Bride JM, Fournier D (1994) Resistance-associated point mutations in insecticide-insensitive acetylcholinesterase. Proc Natl Acad Sci USA 91: 5922–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandula VK, Ray JD, Ribeiro DN, Pan Z, Reddy KN (2013) Glyphosate resistance in tall waterhemp (Amaranthus tuberculatus) from Mississippi is due to both altered target-site and nontarget-site mechanisms. Weed Sci 61: 374–383 [Google Scholar]
- Ng C, Wickneswary R, Salmijah S, Teng YT, Ismail BS (2004) Glyphosate resistance in Eleusine indica (L.) Gaertn. from different origins and polymerase chain reaction amplification of specific alleles. Aust J Agric Res 55: 407–414 [Google Scholar]
- Ng CH, Wickneswari R, Salmijah S, Teng YT, Ismail BS (2003) Gene polymorphisms in glyphosate-resistant and -susceptible biotypes of Eleusine indica from Malaysia. Weed Res 43: 108–115 [Google Scholar]
- Pline-Srnic W. (2006) Physiology mechanisms of glyphosate resistance. Weed Technol 20: 290–300 [Google Scholar]
- Powles SB. (2008) Evolved glyphosate-resistant weeds around the world: lessons to be learnt. Pest Manag Sci 64: 360–365 [DOI] [PubMed] [Google Scholar]
- Powles SB, Preston C (2006) Evolved glyphosate resistance in plants: Biochemical and genetic basis of resistance. Weed Technol 20: 282–289 [Google Scholar]
- Powles SB, Yu Q (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61: 317–347 [DOI] [PubMed] [Google Scholar]
- Sammons RD, Gaines TA (2014) Glyphosate resistance: state of knowledge. Pest Manag Sci 70: 1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons RD, Heering DC, DiNicola N, Glick H, Elmore GA (2007) Sustainability and stewardship of glyphosate and glyphosate-resistant crops. Weed Technol 21: 347–354 [Google Scholar]
- Schönbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JNS, Kabsch W (2001) Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc Natl Acad Sci USA 98: 1376–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner DL. (2009) Role of translocation as a mechanism of resistance to glyphosate. Weed Sci 57: 118–123 [Google Scholar]
- Spencer M, Mumm R, Gwyn J inventors. March 21, 2000. Glyphosate resistant maize lines. Dekalb Genetics Corporation: US 6,040,497. pp. 1-59 [Google Scholar]
- Stalker DM, Hiatt WR, Comai L (1985) A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J Biol Chem 260: 4724–4728 [PubMed] [Google Scholar]
- Steinrücken HC, Amrhein N (1980) The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun 94: 1207–1212 [DOI] [PubMed] [Google Scholar]
- Tranel PJ, Wright TR (2002) Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci 50: 700–712 [Google Scholar]
- Tranel PJ, Wright TR, Heap IM (2015) Mutations in herbicide-resistant weeds to ALS inhibitors. http://www.weedscience.com January 27, 2015)
- Vila-Aiub MM, Goh SS, Gaines TA, Han H, Busi R, Yu Q, Powles SB (2014) No fitness cost of glyphosate resistance endowed by massive EPSPS gene amplification in Amaranthus palmeri. Planta 239: 793–801 [DOI] [PubMed] [Google Scholar]
- Vila-Aiub MM, Neve P, Powles SB (2009) Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol 184: 751–767 [DOI] [PubMed] [Google Scholar]
- Weinreich DM, Delaney NF, Depristo MA, Hartl DL (2006) Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312: 111–114 [DOI] [PubMed] [Google Scholar]
- Yu Q, Cairns A, Powles S (2007) Glyphosate, paraquat and ACCase multiple herbicide resistance evolved in a Lolium rigidum biotype. Planta 225: 499–513 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.