Abstract
Using the 9L experimental brain tumor model, we studied long-term tumor regression and immunologic consequences of tumor killing in a model of in vivo gene transfer of the herpes simplex virus 1 thymidine kinase (HSV-TK) gene and ganciclovir (GCV) treatments. Fibroblasts modified to produce retroviral vectors carrying the HSV-TK gene were implanted into established 9L brain tumors in Fischer 344 rats to carry out gene transfer. Animals were then treated with parenteral GCV. Significant tumor regression was seen following GCV treatments in short-term experiments (17 days) as quantified by measurements of tumor volume. In long-term studies, 7 of 32 (22%) treated animals survived 90 days. Histologic examination of the brains of the successfully treated animals demonstrated residual tumor cells and inflammatory cells consisting predominantly of macrophages/microglia and T cells in the hemisphere with the residual tumor cyst. Rats surviving 90 days rejected repeat tumor injections into the contralateral brain and flank, whereas identical tumor injections in naive animals resulted in both brain and flank tumors. The presence of significant anti-tumor immunity following HSV-TK and GCV treatments suggests that the immune system plays a critical role in the sustained tumor regressions associated with these treatments. These findings show that while HSV-TK and GCV treatments can result in long-term tumor regressions in this model, the success of these treatments could be improved by better understanding the role played by the host's immune systems.
Full text
PDF
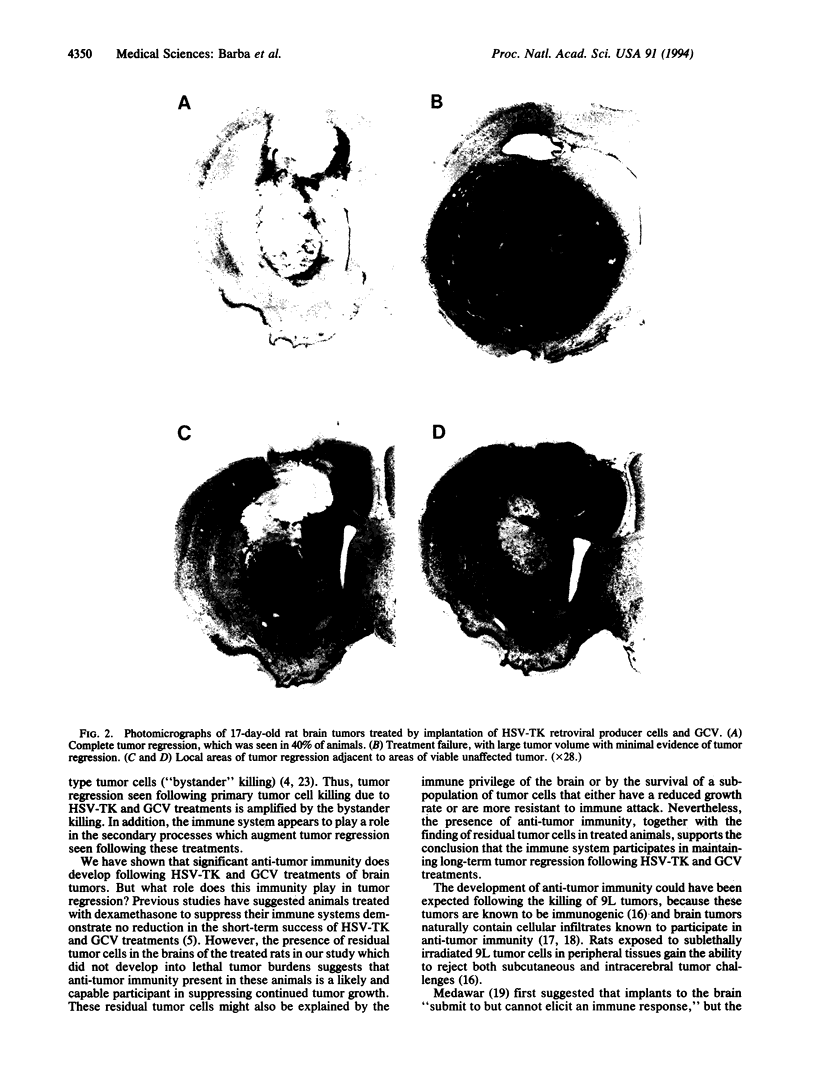
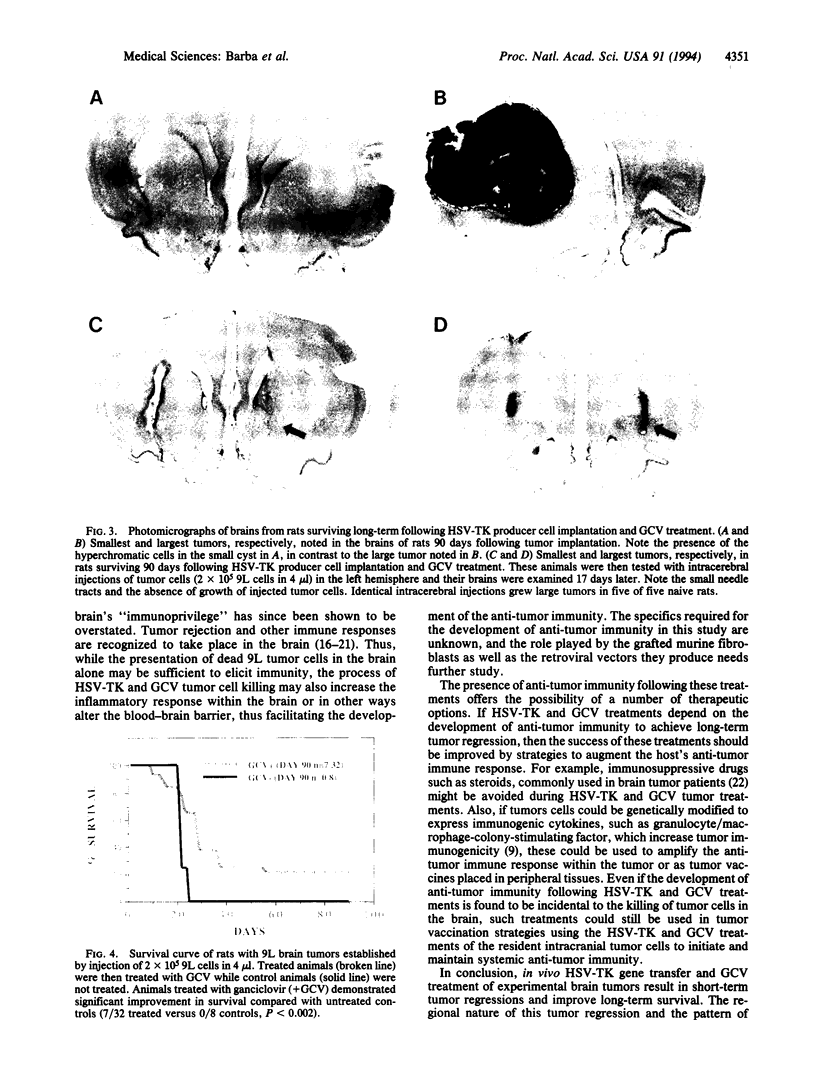

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barba D., Hardin J., Ray J., Gage F. H. Thymidine kinase-mediated killing of rat brain tumors. J Neurosurg. 1993 Nov;79(5):729–735. doi: 10.3171/jns.1993.79.5.0729. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzeddine Z. D., Martuza R. L., Platika D., Short M. P., Malick A., Choi B., Breakefield X. O. Selective killing of glioma cells in culture and in vivo by retrovirus transfer of the herpes simplex virus thymidine kinase gene. New Biol. 1991 Jun;3(6):608–614. [PubMed] [Google Scholar]
- Freeman S. M., Abboud C. N., Whartenby K. A., Packman C. H., Koeplin D. S., Moolten F. L., Abraham G. N. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993 Nov 1;53(21):5274–5283. [PubMed] [Google Scholar]
- Gage F. H., Kawaja M. D., Fisher L. J. Genetically modified cells: applications for intracerebral grafting. Trends Neurosci. 1991 Aug;14(8):328–333. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bagger P., Bendtsen T. F., Evans S. M., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988 Oct;96(10):857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., de Tribolet N., von Fliedner V. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg. 1988 Mar;68(3):438–448. doi: 10.3171/jns.1988.68.3.0438. [DOI] [PubMed] [Google Scholar]
- Moolten F. L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986 Oct;46(10):5276–5281. [PubMed] [Google Scholar]
- Moolten F. L., Wells J. M. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990 Feb 21;82(4):297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Ram Z., Culver K. W., Walbridge S., Blaese R. M., Oldfield E. H. In situ retroviral-mediated gene transfer for the treatment of brain tumors in rats. Cancer Res. 1993 Jan 1;53(1):83–88. [PubMed] [Google Scholar]
- Short M. P., Choi B. C., Lee J. K., Malick A., Breakefield X. O., Martuza R. L. Gene delivery to glioma cells in rat brain by grafting of a retrovirus packaging cell line. J Neurosci Res. 1990 Nov;27(3):427–439. doi: 10.1002/jnr.490270322. [DOI] [PubMed] [Google Scholar]
- Sloan D. J., Wood M. J., Charlton H. M. The immune response to intracerebral neural grafts. Trends Neurosci. 1991 Aug;14(8):341–346. doi: 10.1016/0166-2236(91)90159-r. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Tzeng J. J., Barth R. F., Orosz C. G., James S. M. Phenotype and functional activity of tumor-infiltrating lymphocytes isolated from immunogenic and nonimmunogenic rat brain tumors. Cancer Res. 1991 May 1;51(9):2373–2378. [PubMed] [Google Scholar]