Abstract
Purpose
In the United States, 21 years is a critical age of legal and social transition, with changes in social programs such as public insurance coverage. Human immunodeficiency virus (HIV)–infected youth have lower adherence to care and medications and may be at risk of loss to follow-up (LTFU) at this benchmark age. We evaluated LTFU after the 22nd birthday for HIV-infected youth engaged in care. LTFU was defined as having no primary HIV visits in the year after the 22nd birthday.
Methods
All HIV-infected 21-year-olds engaged in care (2002–2011) at the HIV Research Network clinics were included. We assessed the proportion LTFU and used multivariable logistic regression to evaluate demographic and clinical characteristics associated with LTFU after the 22nd birthday. We compared LTFU at other age transitions during the adolescent/young adult years.
Results
Six hundred forty-seven 21-year-olds were engaged in care; 91 (19.8%) were LTFU in the year after turning 22 years. Receiving care at an adult versus pediatric HIV clinic (adjusted odds ratio [AOR], 2.91; 95% confidence interval [CI],1.42–5.93), having fewer than four primary HIV visits/year (AOR, 2.72; 95% CI, 1.67–4.42), and antiretroviral therapy prescription (AOR, .50; 95% CI, .41–.60) were independently associated with LTFU. LTFU was prevalent at each age transition, with factors associated with LTFU similar to that identified for 21-year-olds.
Conclusions
Although 19.8% of 21-year-olds at the HIV Research Network sites were LTFU after their 22nd birthday, significant proportions of youth of all ages were LTFU. Fewer than four primary HIV care visits/year, receiving care at adult clinics and not prescribed antiretroviral therapy, were associated with LTFU and may inform targeted interventions to reduce LTFU for these vulnerable patients.
Keywords: Adolescents, Youth, Young adults, Loss to follow-up, Attrition, Care, HIV Research Network
Loss to follow-up (LTFU) among patients with human immunodeficiency virus (HIV) infection has both individual and potential public health consequences. Patients who are at LTFU risk decline in their personal health because of unchecked HIV and may increase the transmission of HIV to others. The rate of LTFU among adult patients with HIV infection is high [1,2], and the rate of LTFU among HIV-infected youth may be even higher. A prior analysis of the HIV Research Network (HIVRN) cohort revealed a 10.8% LTFU for youth after they turned 18 years [3]. Farmer et al. [4] reported that 45% and 22% of nonperinatally HIV-infected (nPHIV) youth (aged 12–25 years) were retained 1 and 3 years after engaging in care, respectively.
With the advent of antiretroviral therapy (ART), most perinatally HIV-infected (PHIV) children are surviving beyond childhood [5,6]. PHIV patients, along with a growing number of nPHIV youth acquiring HIV infection through risk behaviors, comprise an emerging HIV-infected youth cohort [5,7,8]. Up to 90% of the HIV-infected children are covered by Medicaid [9], and significant proportions are in foster care [9,10]. Eighteen years is the age of majority in most states, and turning 19 years usually ends coverage on medical and social programs that cover children and adolescents (e.g., Medicaid, foster care). However, approximately 30 states have historically allowed extensions beyond the age of 18 years mostly through 21 years [11], at which point youth must transition out of these supportive programs and find alternative coverage or seek other means to develop social support systems. Thus, eligibility for these services continues through the age of 21 years, and the 22nd birthday is a critical benchmark age when youth must transition or “age out” of these programs [12,13].
Moreover, these programmatic changes are occurring at a time when youth, many with limited health and financial literacy [14], are expected to assume responsibility for their lives and to take more responsibility for their medical care (e.g., scheduling their appointments, refilling medications, applying, and renewing applications for social programs) [15]. Failing to assume responsibility, if not systematically and proactively evaluated and addressed, may result in decreased clinic attendance and potential LTFU [16,17]. The change in responsibility for one’s medical care as part of the overall transition to adulthood is not unique to HIV-infected youth but is also seen among youth living with other chronic diseases, including sickle cell disease, cystic fibrosis, congenital heart disease, and juvenile-onset diabetes mellitus [18,19]. For youth with these chronic illnesses, poor outcomes such as nonadherence and suboptimal engagement and retention in care have been identified. Minority race/ethnicity and lower socioeconomic status have been also associated with these outcomes [20]; notably, these risk factors are more prevalent among HIV-infected youth [8,21].
Special circumstances among youth living with HIV infection may raise the probability of LTFU. For PHIV youth, the steady, longstanding relationships with providers are often the only stable relationships in their lives and may promote continued engagement in care [22]. Disruption of this relationship may occur with transition, leading to greater potential for attrition from care. On the other hand, nPHIV youth have often not been engaged in any care relationship for long periods, making the sudden need for intense engagement with a care provider challenging.
In light of the legal and social changes that may occur to HIV-infected youth as they age, we sought to evaluate LTFU in the year after each birthday from the 18th through the 25th in a large multisite HIV cohort in the United States with a special focus on turning 22 years, when many youth lose eligibility for key social programs.
Methods
Study setting and participants
This was a retrospective cohort study of both PHIV and nPHIV youth who were enrolled in care at the HIVRN clinics between 2002 and 2011. The HIVRN is a consortium of high-volume U.S. clinic sites that provide primary and subspecialty care to HIV-infected patients of all ages [23]. Twenty-two sites contributed eligible patients to this analysis. Adolescents and young adults were followed at six pediatric and 16 adult regionally distributed sites (Northeast [9], Southern [5], West [5], and Midwest [3]).
There are no adolescent-only sites included in the network. All pediatric sites in the HIVRN care for youth up through their 25th birthday, with no expectation of transition to adult care before the 25th birthday. They are expected to have completed transfer before their 26th birthday. Adult sites care for individuals aged 18 years or more [24].
Sites annually abstract specified data elements from patients’ medical records; abstracted data are assembled into a uniform database, as previously described [25]. All patients are offered enrollment in the HIVRN, excluding one-time consultations and incarceratedindividuals;99%of the patients participated. The study was approved by the Johns Hopkins Institutional Review Board and institutional review boards at each participating institution.
For the primary analysis of LTFU after turning 22 years, patients were included if they were engaged in care at HIVRN clinics, were 21 years old between the years 2002 and 2011, and were followed at a site that contributed data through 2012, allowing assessment of follow-up for the year after the 22nd birthday for all eligible patients. Engaged in care was defined as having at least one CD4 value and one outpatient visit within the year of interest. To provide a comparison with other age transitions, we repeated the same analyses for each age transition from 17 turning 18 years to 24 turning 25 years, with appropriate changes to the eligibility criteria for each analysis.
Patients from all self-reported HIV acquisition risk categories were included. To group the patients who likely had more similar disease trajectories and experience with the medical system, we broadly classified them further as PHIV (including those infected via transfusion) or nPHIV (sexual acquisition, injection drug use, or mixed risk) [26]. The rationale for including youth infected with HIV via transfusion with PHIV youth included younger age at diagnosis, longer duration of infection and time at pediatric sites, limited numbers of transfusion-acquired youth (N = 16), and disease trajectory more similar to PHIV, than nPHIV youth [27].
Data collection and measures
The primary outcome of interest was LTFU, defined as no primary HIV outpatient provider visits during the 1 year (365 days) after the 22nd birthday. We queried sites about whether further information was available about the whereabouts of youth identified to be LTFU and if and where they were accessing medical care. Sites were asked to passively and retrospectively verify that youth identified as LTFU were actually lost versus transitioned to other care sites. To avoid undue burden to the sites, particularly the higher volume adult sites, we did not ask the sites to conduct any additional investigation beyond what was known to the site investigators/clinical team. The sites verified that only four patients noted as LTFU had actually transferred to other clinical sites and another three had died. These patients were excluded from the LTFU analysis.
Demographic and clinical data were collected from the clinical database. Race/ethnicity was based on self-reported data and classified as white, black, Hispanic, and other/unknown. The number of primary HIV outpatient provider visits during the year before the birthday of interest was categorized as fewer than four or four or more visits per year, on the basis of contemporary Department of Health and Human Services’ recommendations for quarterly clinical follow-up during the study period [28]. Visits were limited to visits with an HIV health care provider, excluding emergency department, administrative, laboratory testing, nurse-only, or psychiatry visits. Site of care was classified as adult or pediatric. Mental health and substance abuse data were incomplete and therefore not evaluated. Insurance was categorized as insured (private insurance, Medicaid, Medicare, and dual insurance coverage with Medicaid/Medicare) versus uninsured/Ryan White/other (e.g., county health program). ART was defined as concomitant prescription of three or more antiretroviral drugs from at least two classes, including acceptable alternate triple nucleoside/nucleotide–based regimens (i.e., zidovudine/lamivudine/abacavir) [29]. The first available CD4 T-cell count and the first reported HIV-1 RNA viral load (VL) test in the calendar year before the birthday of interest were used. For descriptive analyses, CD4 was categorized as <200,200–349, 350–499, and ≥500 cells/mm3 and VL was categorized as <2.6, 2.6–<4.0, 4.0–<4.70, 4.70–<5.0, ≥5.0 log10 HIV-1 RNA copies/mL.
We used logistic regression to assess demographic and clinical factors associated with LTFU (race/ethnicity, gender, insurance [insured vs. uninsured], number of HIV provider outpatient visits, log10 HIV-1 RNA and CD4 categories, ART prescription, site of care, and calendar year). Separate analyses were performed for each age transition. Data were analyzed using Stata 11.0 (Stata Corp., College Station, TX).
Results
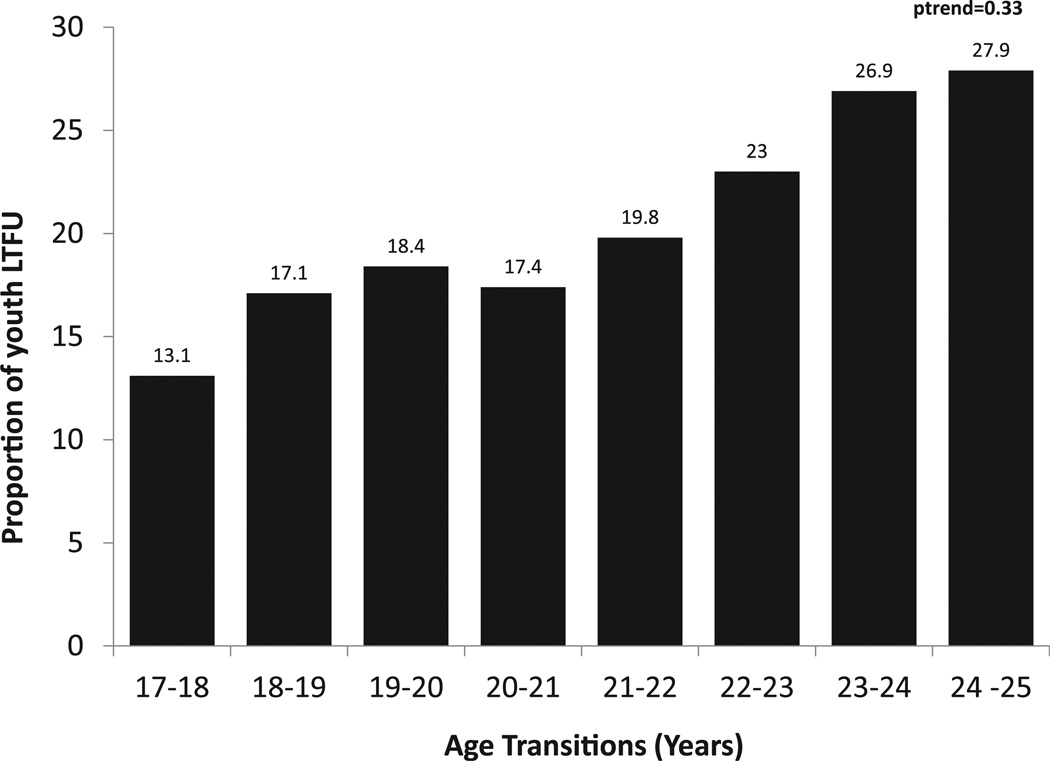
Between 2002 and 2011, 647 HIV-infected 21-year-olds were engaged in care for at least 1 year at HIVRN sites. The demographic breakdown included 62.4% male; 72.9% black and 13.9% Hispanic; 61.3% insured (Table 1). The median CD4 count was 452 cells/mm3 (range, 312–625), with 65.8% prescribed ART. Of the 21-year-olds who were engaged in care, 128 (19.8%) were LTFU in the year after their 22nd birthday. The proportions of patients turning various ages between 18 and 25 years of age who were LTFU are displayed in Figure 1. Although the proportion LTFU appeared to increase with age, this was not significant (p-trend = .33).
Table 1.
Demographic and clinical characteristics of 21-year-old human immunodeficiency virus (HIV)–infected youth engaged in care at HIV Research Network sites (N = 647)
| Characteristic | N (%) |
|---|---|
| Race | |
| White | 69 (10.7) |
| Black | 472 (72.9) |
| Hispanic | 90 (13.9) |
| Other/unknown | 16 (2.5) |
| Male gender | 404 (62.4) |
| HIV acquisition risk | |
| Men who have sex with men | 275 (42.5) |
| Perinatally HIV infected | 96 (14.8) |
| Blood transfusion | 16 (2.5) |
| Heterosexual | 232 (35.9) |
| Injection drug use | 9 (1.4) |
| Other/unknown | 14 (2.2) |
| Perinatal/blood HIV-infected youth | 112 (17.3) |
| Nonperinatal HIV-infected youth | 535 (82.7) |
| CD4 category (cells/mm3) | |
| <50 | 24 (3.7) |
| 50–200 | 58 (9.0) |
| >200–349 | 118 (18.2) |
| 350–499 | 181 (28.0) |
| ≥500 | 266 (41.1) |
| Median CD4 cells/mm3 (interquartile range) | 452 (312–625) |
| HIV viral load category (log10 copies/mL)b | |
| <2.6 | 219 (34.8) |
| 2.6–<4.0 | 219 (34.8) |
| 4.0–<4.70 | 110 (17.5) |
| 4.70–<5.0 | 31 (4.9) |
| ≥5.0 | 51 (8.1) |
| Antiretroviral therapy prescribed | 426 (65.8) |
| Site of HIV care | |
| Adult site | 359 (55.7) |
| Pediatric site | 286 (44.3) |
| Insureda | 395 (61.3) |
| Less than four outpatient visits in the year | 209 (32.3) |
| before the 22nd birthday |
Insured includes Medicare, Medicaid, dual (Medicare/Medicaid), private insurance, and other insurance, whereas uninsured included Ryan White and uninsured patients.
Seventeen patients with no available viral load measurements in year they were 21 years old.
Figure 1.
Proportion lost to follow-up (LTFU) in the subsequent year for human immunodeficiency virus (HIV)–infected youth in the HIV Research Network cohort.
Factors associated with higher risk of LTFU after the 22nd birthday in univariate analyses included higher VL, being in care at an adult clinic, fewer than four outpatient HIV provider visits, and not being prescribed ART in the year before the 22nd birthday (Table 2). There was no statistically significant difference in LTFU for PHIV youth compared with nPHIV youth. In multivariable analyses, having a VL greater than 5.0 log10 copies/mL versus undetectable VL (adjusted odds ratio [AOR], 1.95; 95% confidence interval [CI], 1.11–3.43), being in care at an adult HIV clinic (AOR, 2.91; 95% CI, 1.42–5.93), and having fewer than four outpatient HIV provider visits (AOR, 2.72; 95% CI, 1.67–4.42) remained independently associated with higher likelihood of LTFU (Table 2). Being prescribed ART was associated with a decreased likelihood of LTFU (AOR, .50; 95% CI, .41–.60). Other factors, specifically, HIV acquisition risk, insurance status, and CD4 category were not associated with LTFU (Table 2).
Table 2.
Factors associated with loss to follow-up after the 22nd birthday
| Univariate (OR) |
Multivariable (AOR)a |
|
|---|---|---|
| Male gender | .77 (.48–1.24) | .95 (.63–1.43) |
| Race/ethnicity | ||
| White | 1.0 (Ref) | 1.0 (Ref) |
| Black | .54 (.28–1.05) | .76 (.32–1.80) |
| Hispanic | 1.19 (.52–2.71) | .87 (.46–1.62) |
| Human immunodeficiency virus (HIV) acquisition risk | ||
| Not perinatally HIV-infected/no blood transfusion | 1.0 (Ref) | 1.0 (Ref) |
| Perinatally HIV-infected/blood transfusion | .68 (.36–1.29) | 1.13 (.65–1.96) |
| CD4 category (cells/mm3) | ||
| <50 | 1.0 (Ref) | 1.0 (Ref) |
| 50–199 | .70 (.15–3.23) | .70 (.10–4.48) |
| 200–349 | .97 (.47–2.01) | .85 (.27–2.65) |
| 350–499 | 1.04 (.43–2.53) | .64 (.16–2.47) |
| >500 | .90 (.36–2.28) | .73 (.23–2.34) |
| HIV viral load (VL) category (log10 copies/mL) | ||
| <2.6 | 1.0 (Ref) | 1.0 (Ref) |
| 2.6–<4.0 | 2.31 (1.53–3.50) | 1.58 (.98–2.55) |
| 4.0–<4.70 | 1.96 (1.21–3.19) | 1.43 (.88–2.32) |
| 4.70–<5.0 | 2.58 (1.03–6.48) | 1.86 (.67–5.15) |
| ≥5.0 | 2.54 (1.38–4.66) | 1.95 (1.11–3.43) |
| Site of HIV care | ||
| Pediatric site | 1.0 (Ref) | 1.0 (Ref) |
| Adult site | 3.91 (1.52–10.0) | 2.91 (1.42–5.93) |
| Insurance statusb | ||
| Uninsured | 1.0 (Ref) | 1.0 (Ref) |
| Insured | 1.26 (.65–2.44) | 1.60 (.92–2.78) |
| Number of HIV provider outpatient visits in the year before their | ||
| 22nd birthday | ||
| ≥4 | 1.0 (Ref) | 1.0 (Ref) |
| <4 | 4.06 (2.13–7.75) | 2.72 (1.67–4.42) |
| Antiretroviral therapy (ART) prescribedb | ||
| No | 1.0 (Ref) | 1.0 (Ref) |
| Yes | .38 (.30–.48) | .50 (.41–.60) |
Table entries are odds ratios (OR) or adjusted odds ratios (AOR) and 95% confidence intervals. Bolded values are statistically significant at the level of p < .05.
Multivariable logistic regression model including gender, race/ethnicity, clinic utilization, insurance status, HIV acquisition risk, ART prescribed, CD4 and VL category, adult vs. pediatric treatment site, and calendar year. Table entries are odds ratios (OR) or adjusted odds ratios (AOR) and 95% confidence intervals. Bolded values are statistically significant at the level of p < .05.
Insured includes Medicare, Medicaid, dual (Medicare/Medicaid), private insurance, and other insurance, while uninsured included Ryan White and uninsured patients.
In analyses of other age transitions (Table 3) infrequent clinic attendance was significantly associated with LTFU for most ages. Receiving care at an adult HIV clinic site was associated with LTFU only for transitions from 20 to 21, 21 to 22, and 22 to 23 years of age. Having ART prescribed was associated with lower likelihood of LTFU for all but two age transitions (Tables 3).
Table 3.
Factors independently associated with loss to follow-up at the various age transitionsa
| 17–18 Years; N = 312 |
18–19 Years; N = 378 |
19–20 Years; N = 422 |
20–21 Years; N = 529 |
21–22 Years; N = 647 |
22–23 Years; N = 718 |
23–24 Years; N = 795 |
24–25 Years; N = 841 |
|
|---|---|---|---|---|---|---|---|---|
| Male gender | .84 (.50–1.41) | .58 (.31–1.10) | 1.01 (.62–1.67) | 1.05 (.64–1.73) | .95 (.63–1.43) | 1.20 (.71–2.00) | .94 (.69–1.28) | .86 (.49–1.51) |
| Race/ethnicity | ||||||||
| White | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Black | .38 (.13–1.09) | .41 (.11–1.43) | 1.04 (.35–3.05) | .74 (.37–1.46) | .76 (.32–1.80) | .51 (.27–.98) | .073 (.43–1.26) | .80 (.47–1.36) |
| Hispanic | 2.62 (.57–12.2) | 1.68 (.27–10.3) | .29 (.06–1.29) | .33 (.12–.92) | .87 (.46–1.62) | .54 (.24–1.23) | .86 (.48–1.53) | .57 (.29–1.12) |
| Human immunodeficiency virus (HIV) acquisition risk | ||||||||
| Not perinatally HIV-infected/no blood transfusion | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Perinatally HIV-infected/blood transfusion | .37 (.10–1.27) | 1.27 (.55–2.90) | .67 (.33–1.44) | 1.08 (.60–1.97) | 1.13 (.65–1.96) | .86 (.48–1.54) | .82 (.45–1.50) | .42 (.11–1.58) |
| CD4 category (cells/mm3) | ||||||||
| <50 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 50–199 | .37 (.003–47.5) | 2.58 (.59–11.3) | .39 (.14–1.05) | .48 (.14–1.66) | .70 (.10–4.48) | 2.16 (.56–8.39) | 1.47 (.96–2.25) | .86 (.36–2.08) |
| 200–349 | .95 (.02–40.2) | 2.26 (.54–9.47) | .40 (.19–.83) | .62 (.19–1.97) | .85 (.27–2.65) | 2.86 (.81–10.1) | 1.05 (.58–1.93) | .82 (.35–1.88) |
| 350–499 | .48 (.01–17.0) | 2.49 (.43–14.4) | .25 (.10–.67) | .23 (.05–1.17) | .64 (.16–2.47) | 2.03 (.55–7.44) | .60 (.36–1.02) | .43 (.17–1.08) |
| >500 | .56 (.01–26.9) | 3.61 (1.00–13.1) | .21 (.10–.46) | .41 (.05–3.08) | .73 (.23–2.34) | 1.39 (.40–4.84) | .80 (.51–1.25) | .43 (.21–.88) |
| Viral load (VL) category (log10 copies/mL) | ||||||||
| <2.6 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 2.6–<4.0 | .44 (.13–1.44) | 1.32 (.62–2.78) | 2.10 (.89–5.00) | .58 (.34–1.01) | 1.58 (.98–2.55) | .89 (.52–1.53) | 1.23 (.69–2.19) | .91 (.53–1.54) |
| 4.0–<4.70 | .09 (.02–.44) | 4.81 (1.65–14.00) | 3.87 (1.38–10.9) | .64 (.28–1.48) | 1.43 (.88–2.32) | .88 (.50–1.52) | 1.43 (.75–2.72) | 1.40 (.73–2.69) |
| 4.70–<5.0 | 1.23 (.004–357.2) | 3.28 (1.13–9.51) | 2.12 (.75–5.98) | 1.06 (.66–1.71) | 1.86 (.67–5.15) | .59 (.27–1.29) | 1.23 (.43–3.52) | 1.23 (.55–2.77) |
| ≥5.0 | .17 (.01–3.29) | 3.11 (.66–14.63) | 2.15 (.70–6.63) | .87 (.30–2.54) | 1.95 (1.11–3.43) | .69 (.41–1.15) | 1.42 (.60–3.29) | 1.02 (.51–2.05) |
| Site of HIV care | ||||||||
| Pediatric site | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Adult site | 4.34 (.70–26.9) | .95 (.30–3.05) | 2.33 (.92–5.93) | 2.99 (1.68–5.33) | 2.91 (1.42–5.93) | 2.45 (1.13–5.33) | .98 (.60–1.60) | .66 (.42–1.03) |
| Insurance status | ||||||||
| Uninsured | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Insured | .44 (.13–1.54) | .74 (.36–1.55) | 1.24 (.33–4.59) | .96 (.65–1.42) | 1.60 (.92–2.78) | 1.03 (.71–1.47) | .85 (.55–1.33) | 1.04 (.72–1.50) |
| Number of HIV provider outpatient visits in the year prior | ||||||||
| ≥4 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| <4 | 2.44 (.82–7.29) | 2.04 (.74–5.61) | 5.51 (1.94–15.7) | 3.23 (1.83–5.72) | 2.72 (1.67–4.42) | 3.02 (1.89–4.82) | 2.80 (1.77–4.44) | 3.59 (2.19–5.87) |
| Antiretroviral therapy (ART) prescribeda,b | ||||||||
| No | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Yes | .12 (.06–.24) | .70 (.36–1.36) | .90 (.38–2.13) | .46 (.26–.80) | .50 (.41–.60) | .47 (.29–.77) | .85 (.53–1.39) | .57 (.35–.95) |
Output is adjusted odds ratio (AOR). Bolded values are statistically significant at the level of p < .05.
Multivariable logistic regression model including gender, race/ethnicity, clinic utilization, insurance status, HIV acquisition risk, ART prescribed, CD4 and VL category adult vs. pediatric treatment site, and calendar year.
Insured includes Medicare, Medicaid, dual (Medicare/Medicaid), private insurance, and other insurance, while uninsured included Ryan White and uninsured patients.
Discussion
Recently, there has been significant discussion of the continuum of HIV care and the striking decline in the numbers of persons living with HIV who reach each successive stage of the continuum right from awareness of infection, linkage to care, engagement, to retention in care. Youth are at high risk for this attrition [30–32]. Herein, we report factors associated with the dynamics of LTFU for youth, which may be important in explaining a component of the continuum of care for youth.
Among the 647 21-year-old youth who were engaged in care at HIVRN sites, one in five (20%) were LTFU in the year after their 21st birthday. This rate is higher than the 10% we previously reported among youth who turned 18 years [3]. The LTFU rate for those turning 22 years was not especially high, contrary to our hypothesis. In fact, LTFU rates trended (not statistically significant) to be higher among those turning 23, 24, or 25 years. This suggests ongoing risk for LTFU as youth enter late adolescence and adulthood. Key factors associated with LTFU include attending fewer than four HIV primary provider visits, not being prescribed ART, and being followed at an adult HIV clinical site (vs. pediatric site) in the prior year.
The definition of LTFU is study dependent, limiting comparisons among studies [33]. Rates of LTFU among adults have varied from 20% to 35% [1,2,34]. Recently, an analysis of HIVRN data revealed LTFU, defined as no subsequent visits in the 12 months since the last documented HIV provider visit, of 34.9% over a 2- to 8-year period [1]. This study included youth in the broader 18- to 30-year-old age group and did not look specifically at LTFU in younger adults. The high rates of LTFU among youth at all age transitions are worrisome.
Attending fewer than four HIV provider visits in the prior year was highly associated with LTFU in the subsequent year. The current Department of Health and Human Services’ guidelines for adult and adolescent patients suggest that two annual patient visits (including laboratory evaluation) may be sufficient for virologically suppressed patients on stable regimens [29]. Our data suggest that caution may be needed when considering implementing a reduced follow-up schedule for youth. Additionally, monitoring missed visits and using outreach programs/workers to reengage youth may be useful. Furthermore, practices such as long-term prescriptions without built-in appointments might be conservatively used, primarily for those patients who clearly demonstrate significant maturity and unwavering established engagement in care.
Being prescribed ART was strongly associated with a decreased likelihood of being LTFU. This finding is in line with other studies that have shown improved retention with ART initiation [35,36]. In a prior study of youth, attending four or more visits in the calendar year was highly associated with ART initiation. ART initiation and visit attendance are likely interrelated, as individuals who attend visits are likely demonstrating some commitment to their care, which increases the likelihood that ART will be prescribed. It is also likely that individuals who are on therapy feel as if they are attending the doctors for a reason and therefore more likely to attend appointments and stay in care [37]. The exact direction of the relationship is not clear.
Adult and pediatric HIV clinics differed in LTFU rates for youth. Our group previously reported that nPHIV youth followed at adult clinical sites were more likely to discontinue ART once initiated [38], and Ryscavage et al. [39] reported lower rates of virologic suppression for youth being seen at adult HIV clinics. It is conceivable that HIV-infected youth at adult clinics may not have the capacity to navigate their health care that is expected and may not be comfortable, leading to differential engagement in care at pediatric/adolescent versus adult HIV clinics. The structure of many adult clinics may include features that may not be considered youth friendly, such as higher patient censuses and patient-to-provider ratios, noncentralized care, and clinic hours that are unfavorable to schedules of younger patients [40]. Both PHIV- and nPHIV-infected youth may have challenges navigating the health care system. The PHIV youth’s prior interactions may have been directed by their pediatric caregivers, whereas nPHIV youth may have had limited interactions with the health care system. Both groups, however, are increasingly expected to take greater responsibility for their health care with age. Given the increasing age of PHIV youth and the increasing incidence of nPHIV youth, there will be a surge of aging youth transitioning to adult sites. The high rates of LTFU reported for adults [1,33,34] raise concern that adult sites may not be equipped to manage the additional challenges that youth transitioning into adult care bring, potentially increasing the LTFU rates for youth. In fact, we did observe an increase in LTFU with increasing age, although this was not statistically significant.
There are several important limitations to this study. Overall, sample sizes for youth less than the age of 21 years and of youth with transfusion-acquired infection were relatively small; therefore, our findings must be interpreted with caution, as we may be under-powered to detect characteristics associated with LTFU. Furthermore, the assumption that patients were LTFU if they did not have a documented outpatient visit at their respective HIVRN clinic may be wrong. Although we did check with clinic staff, it is possible that they were unaware that youth may have continued to engage in care at a clinical site outside the HIVRN because of geographic relocation, changes in insurance providers, or other factors. Third, our HIVRN demographics closely resemble that of the U.S. population; however, our sample is not nationally representative. Finally, although HIVRN does collect death data if that information is available, it is possible that some patients died and the sites were not aware of it. Data were not available to ascertain which patients transitioned out of foster care during the observation period. Mental health, substance abuse, socioeconomic status, and other psychosocial characteristics, which may be important factors affecting LTFU, could not be examined. We defined LTFU as having no outpatient visits in the year after the birthday. This definition differs slightly from “not being retained,” which is broader and would consider individuals not to be retained if they did not have at least one medical visit in each 4-month period of the year of study. A patient could be nonretained without being LTFU (e.g., only 1–3 visits in the year). Those without any outpatient visit in the calendar year after their birthday would be considered both nonretained and LTFU. The analysis was designed to look at the issue of LTFU and not specifically at transition from pediatric to adult providers; data on transition were not systematically collected. Finally, the analysis occurred before the introduction of the Affordable Care Act, and it will be important to assess the potential impact of LTFU in the era of Medicaid expansion under Affordable Care Act.
Although nearly one in five youth followed at HIVRN sites were LTFU after turning 22 years, the LTFU rates were also high in other age groups, and the rate after the unique transitional 22nd birthday was not exceptionally different than that at other ages. Overall, youth were at a high risk of falling out of care. Youth represent the age group with the highest incidence of HIV in the United States. Therefore, LTFU among youth, with resultant decreased access to and utilization of ART, viremia, and increased risk of transmission, is of significant concern and requires more research to identify root causes and interventions to improve outcomes. Interventions focused on supporting linkage and retention to care may enhance long-term engagement, and thereby improve ART use, virologic suppression, immune recovery, and ultimately overall health, while also reducing risk of transmission. Additional studies comparing structural and clinic characteristics of adult and pediatric HIV clinical sites may inform targeted efforts to better retain youth in care.
IMPLICATIONS AND CONTRIBUTION.
Young people living with human immunodeficiency virus are at increased risk of loss to follow-up (LTFU). Identifying factors associated with LTFU, such as being followed at adult sites, attending fewer visits, and not initiating antiretroviral therapy, may inform strategies to minimize LTFU in this high-risk population.
Acknowledgments
HIV Research Network Participating Sites and Principal Investigators: Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.); Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.); Community Health Network, Rochester, New York (Roberto Corales, D.O.); Drexel University, Philadelphia, Pennsylvania (Jeffrey Jacobson, M.D., Sara Allen, C.R.N.P.); Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.); Montefiore Medical Group, Bronx, New York (Robert Beil, M.D.); Montefiore Medical Center, Bronx, New York (Lawrence Hanau, M.D.); Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.); Parkland Health and Hospital System, Dallas, Texas (Ank Nijhawan, M.D., Muhammad Akbar, M.D.); St. Jude Children’s Research Hospital, Memphis, Tennessee (Aditya Gaur, M.D.); St. Luke’s Roosevelt Hospital Center, New York, New York (Victoria Sharp, M.D., Stephen Arpadi, M.D.); Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.); University of California, San Diego, California (W. Christopher Mathews, M.D.); Wayne State University, Detroit, Michigan (Jonathan Cohn, M.D.).
Data Coordinating Centers: Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A.).
Funding Sources
The HIV Research Network is supported by the Agency for Healthcare Research and Quality (290-11-00007c) and the Johns Hopkins Center for AIDS Research (P30 AI094189).
Conflicts of Interest: We confirm all co-authors contributed significantly to this work and have seen and agree with the contents of the article. K.A.G has received research funding from Tibotec and served as a scientific consultant to Tibotec and Bristol Meyers Squibb. A.L.A is supported by the National Institutes of Allergy and Infectious Diseases (1K23AI084549-01A1), Lana Lee is supported by the National Institutes of Child Health and Development (T32HD052459) and the Health Resources Services Administration/Maternal Child Health (5T71MC08054), B.R.Y is supported by the National Institutes of Health/National Institutes of Mental Health (K23-MH097647), and K.N.A is supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (K01-AI093197). Fred Hellinger, Ph.D., John Fleishman, Ph.D., and Irene Fraser, Ph.D., were supported by the Agency for Healthcare Research and Quality, Rockville, Maryland; and Robert. Mills, Ph.D., and Faye Malitz, M.S., were supported by the Health Resources and Services Administration, Rockville, Maryland.
Footnotes
Disclaimer: The views expressed in this article are those of the authors. No official endorsement by Department of Health and Human Services, the National Institutes of Health, or the Agency for Healthcare Research and Quality is intended or should be inferred.
Preliminary findings of this study were presented at the 2012 International AIDS Society and 2013 Society of Adolescent Health and Medicine Annual Meetings.
References
- 1.Fleishman JA, Yehia BR, Moore RD, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249–259. doi: 10.1097/QAI.0b013e318258c696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samet JH, Freedberg KA, Savetsky JB, et al. Discontinuation from HIV medical care: Squandering treatment opportunities. J Health Care Poor Underserved. 2003;14:244–255. doi: 10.1353/hpu.2010.0798. [DOI] [PubMed] [Google Scholar]
- 3.Agwu AL, Althoff K, Rutstein RK, et al. for the HIV Research Network. Factors associated with falling out of care for older adolescents in the HIV Research Network, in 19th International AIDS Conference; 2012; Washington, DC. [Google Scholar]
- 4.Farmer C, Yehia BR, Fleishman JA, et al. A.A.f.t.H.R. Network. Factors associated with retention among non-perinatally HIV-infected youth in the HIV Research Network. J Pediatr Infect Dis Soc. 2014 doi: 10.1093/jpids/piu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: Children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 6.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18579. doi: 10.7448/IAS.16.1.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agwu A, Ellen J. Rising rates of HIV infection among young US men who have sex with men. Pediatr Infect Dis J. 2009;28:633–634. doi: 10.1097/INF.0b013e3181afcd22. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Diagnosis of HIV infection and AIDS in the United States and dependent areas. HIV Surveillance Report. 2011:21. [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. Fact Sheet: Medicaid and the Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS) Epidemic in the United States. [Accessed July 30, 2014];Health Care Financing Review. 2000 22:117–122. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/HealthCareFinancingReview/downloads/00fallpg117.pdf. [Google Scholar]
- 10.U.S. Departement of Health and Human Services. Assistant Secretary for Planning and Evaluation. A Report on Infants and Children with HIV Infection in Foster Care. [Accessed June 1, 2014];1989 Nov; Available at: http://aspe.hhs.gov/daltcp/reports/hivinfec.pdf.
- 11.Pergamit MR, McDaniel M, Chen V, et al. Washington, D.C: U.S. Department of Health and Human Services; [Accessed June 1, 2014]. Assistant Secretary for Planning and Evaluation Report: Providing Medicaid to Youth Formerly in Foster Care under the Chafee Option: Informing Implementation of the Affordable Care Act. November 2012. Availatle at: http://158.74.49.3/hsp/13/ChafeeMedicaidReport/rpt2.cfm. [Google Scholar]
- 12.Peters CM, Dworsky A, Courtney ME, et al. Chapin Hall Issue Brief. Chicago: Chapin Hall at the University of Chicago, Chicago IL; 2009. [Accessed June 1, 2014]. Extending Foster Care to Age 21: Weighing the Costs to Government against the Benefits to Youth. Available at: www.chapinhall.org. [Google Scholar]
- 13.Permanency Planning for Children Department, N.C.o.J.a.F.C.J., The Foster Care Independence Act of 1999 and the John F. Chafee Foster Care and Independence Program. 2002 [Google Scholar]
- 14.Murphy DA, Lam P, Naar-King S, et al. Health literacy and antiretroviral adherence among HIV-infected adolescents. Patient Educ Couns. 2010;79:25–29. doi: 10.1016/j.pec.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly T. Transition from care: Status and outcomes of youth who age out of foster care. Child Welfare. 2003;82:727–746. [PubMed] [Google Scholar]
- 16.Dietz E, Clum GA, Chung S, et al. Adherence to scheduled appointments among HIV-infected female youth in five U.S. cities. J Adolesc Health. 2010;46:278–283. doi: 10.1016/j.jadohealth.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudy BJ, Murphy DA, Harris DR, et al. Patient-related risks for non-adherence to antiretroviral therapy among HIV-infected youth in the United States: A study of prevalence and interactions. AIDS Patient Care STDS. 2009;23:185–194. doi: 10.1089/apc.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle MP, Farukhi Z, Nosky ML. Strategies for improving transition to adult cystic fibrosis care, based on patient and parent views. Pediatr Pulmonol. 2001;32:428–436. doi: 10.1002/ppul.1154. [DOI] [PubMed] [Google Scholar]
- 19.Betz CL. Adolescents in transition of adult care: Why the concern? Nurs Clin North Am. 2004;39:681–713. doi: 10.1016/j.cnur.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Lotstein DS, Kuo AA, Strickland B, Tait F. The transition to adult health care for youth with special health care needs: Do racial and ethnic disparities exist? Pediatrics. 2010;126(Suppl 3):S129–S136. doi: 10.1542/peds.2010-1466F. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Pediatric HIV Surveillance (through 2010) 2012. [Accessed June 1, 2014]; Available at http://www.cdc.gov/hiv/library/slideSets/.
- 22.New York State Department of Health AIDS Institute. Transitioning HIV Infected Adolescents into Adult Care. New York, NY: 2011. [Accessed May 30, 2014]. Available at http://www.hivguidelines.org/clinical-guidelines/adolescents/transitioning-hiv-infected-adolescents-into-adult-care/. [Google Scholar]
- 23.HIV Research Network (HIVRN) [Accessed August 15, 2013]; Available at: https://cds.johnshopkins.edu/hivrn/index.cfm. [Google Scholar]
- 24.Yehia BR, Gebo KA, Hicks PB, et al. Structures of care in the clinics of the HIV Research Network. AIDS Patient Care STDS. 2008;22:1007–1013. doi: 10.1089/apc.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebo KA, Moore RD, Fleishman JA. The HIV Research Network: A unique opportunity for real time clinical utilization analysis in HIV. Hopkins HIV Rep. 2003;15:5–6. [PubMed] [Google Scholar]
- 26.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in pediatric HIV infection. [Accessed June 30, 2014]; Available at: http://aidsinfo.nih.gov/guidelines.
- 27.Nielsen K, McSherry G, Petru A, et al. A descriptive survey of pediatric human immunodeficiency virus-infected long-term survivors. Pediatrics. 1997;99:E4. doi: 10.1542/peds.99.4.e4. [DOI] [PubMed] [Google Scholar]
- 28.Archive of Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed May 30, 2014]; Available at: http://www.hivatis.org/Guidelines/ArchivedGuidelines.aspx. [Google Scholar]
- 29.Department of Health and Human Services. Panel of antiretroviral guidelines for, adults and adolescents, Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; [June 30, 2014]. Available at: http://aidsinfo.nih.gov/guidelines. [Google Scholar]
- 30.Lamb MR, Fayorsey R, Nuwagaba-Birbonwoha H, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS. 2014;28:559–568. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) Fact Sheet: HIV in the United States: The stages of care. 2012 [Google Scholar]
- 32.Park MJ, et al. The health status of young adults in the United States. J Adolesc Health. 2006;39:305–317. doi: 10.1016/j.jadohealth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Yehia BR, et al. Comparing different measures of retention in outpatient HIV care. AIDS. 2012;26:1131–1139. doi: 10.1097/QAD.0b013e3283528afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(Suppl 1):43–47. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helleberg M, Engsig FN, Kronborg G, et al. Retention in a public healthcare system with free access to treatment: A Danish nationwide HIV cohort study. AIDS. 2012;26:741–748. doi: 10.1097/QAD.0b013e32834fa15e. [DOI] [PubMed] [Google Scholar]
- 37.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: A systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agwu AL, Siberry GK, Ellen J, et al. Predictors of highly active antiretroviral therapy utilization for behaviorally HIV-1-infected youth: Impact of adult versus pediatric clinical care site. J Adolesc Health. 2012;50:471–477. doi: 10.1016/j.jadohealth.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryscavage P, Anderson EJ, Sutton SH, et al. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58:193–197. doi: 10.1097/QAI.0b013e31822d7564. [DOI] [PubMed] [Google Scholar]
- 40.Yehia BR, Agwu AL, Schranz A, et al. Conformity of pediatric/adolescent HIV clinics to the patient-centered medical home care model. AIDS Patient Care STDS. 2013;27:272–279. doi: 10.1089/apc.2013.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]