Abstract
Background
Little is known about population-based maternal, child, and system characteristics associated with high hospital resource use for children with orofacial clefts (OFC) in the US.
Methods
This was a statewide, population-based, retrospective observational study of children with OFC born between 1998 and 2006, identified by the Florida Birth Defects Registry whose records were linked with longitudinal hospital discharge records. We stratified the descriptive results by cleft type [cleft lip with cleft palate (CLP), cleft lip (CL) and cleft palate (CP)] and by isolated vs. non-isolated OFC (accompanied by other coded major birth defects). We used Poisson regression to analyze associations between selected characteristics and high hospital resource use (≥90th percentile of estimated hospitalized days and inpatient costs) for birth, post-birth, and total hospitalizations initiated before age two years.
Results
Our analysis included 2,129 children with OFC. Infants who were born low birth weight (<2500 grams) were significantly more likely to have high birth hospitalization costs for CLP [adjusted prevalence ratio (aPR): 1.6 (95% confidence interval (CI): 1.0–2.7)], CL [aPR: 3.0 (95% CI: 1.1–8.1)], and CP [aPR: 2.3 (95% CI: 1.3–4.0)]. Presence of multiple birth defects was significantly associated with a three- to eleven-fold and a three- to nine-fold increase in the prevalence of high costs and number of hospitalized days, respectively; at birth, post-birth before age two years and overall hospitalizations.
Conclusion
Children with CP had the greatest hospital resources use. Additionally, the presence of multiple birth defects contributed to greater inpatient days and costs for children with OFC.
Keywords: orofacial clefts, health services research, resource use, hospitalization, cost, cleft lip, cleft palate
INTRODUCTION
Orofacial clefts (OFC) include cleft lip with cleft palate (CLP), cleft lip (CL), and cleft palate (CP). In the United States, the estimated prevalence is 5.6 per 10,000 live births for cleft lip with cleft palate, 5.9 per 10,000 live births for cleft palate, and 3.1 per 10,000 live births for cleft lip (Mai et al., 2014). OFC are among the most common birth defects and are an important public health concern (Mai et al., 2014; Parker et al., 2010; Yazdy et al., 2007).
OFC can impair the development of speech, hearing, language, and psychomotor and cognitive skills, which can create physical and emotional stress for children and their families and can result in substantial medical costs. OFC are surgically correctable, often requiring multiple procedures. Although the timing of repair may vary by institutional protocol or by clinical circumstances, guidelines recommend that primary surgical closure of the cleft lip and cleft palate should occur within the first 12 and 18 months of life, respectively (American Cleft Palate-Craniofacial Association, 2009). Consequently, most hospitalizations and costs for infants with OFC are associated with surgical repairs (Abbott et al., 2011; Abbott and Meara, 2011). In addition, other costs are related to non-operative clinical services (e.g., speech, audiological, dental/orthodontic, and psychosocial services), special education, early intervention, loss of parental time from work, and travel.
Several recent studies have examined mean or median health service use and expenditures among children with OFC in the United States (Basseri et al., 2011; Boulet et al., 2009; Cassell et al., 2008; Centers for Disease Control and Prevention, 2007; Deleyiannis et al., 2013; Nguyen et al., 2013; Russo and Elixhauser, 2007; Weiss et al., 2009). Of these eight recent studies reporting health care costs or expenditures for children with different types of OFC in the United States, four used data from the Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP) (Basseri et al., 2011; Centers for Disease Control and Prevention, 2007; Nguyen et al., 2014; Russo and Elixhauser, 2007); one used MarketScan® Commercial Claims and Encounters databases (Boulet et al., 2009); one used data from one U.S. hospital (Deleyiannis et al., 2013); and two used statewide, population-based birth defects registry data (Cassell et al., 2008; Weiss et al., 2009). It is difficult to compare the findings of these studies due to the different methods employed and variation in the cleft phenotype classifications.
Although these studies provide valuable information on average medical costs for children with OFC, little is known about the associations of payer status and other factors with high hospital resource use for children with OFC. The purpose of this study was to examine factors associated with high hospital resource use (defined as ≥90th percentile of estimated hospitalized days and inpatient costs) for children aged 0–2 with OFC, stratified by cleft type and presence of other major birth defects, using a statewide, population-based birth defects registry.
METHODS
Study population
This study was a retrospective, observational study of children with OFC born between January 1, 1998, and December 31, 2006, identified by the Florida Birth Defects Registry (FBDR) whose records were linked with longitudinal hospital discharge data through December 31, 2008. The FBDR is a passive, statewide, population-based surveillance system that identifies infants with birth defects during the first year of life using multiple databases, including hospital discharge records from Florida’s Agency for Health Care Administration (AHCA) (Salemi et al., 2010; Salemi et al., 2011). The study population included children with an International Classification of Disease, 9th revision; Clinical Modification (ICD-9-CM) code in the FBDR for OFC (749.00–749.25) whose mothers were residents of Florida at the time of delivery and who had at least one inpatient discharge record on file during the study period, 1998–2008. Adopted children, prospective adoptees, and children whose mothers delivered out-of-state were excluded (Salemi et al., 2010; Salemi et al., 2011). In addition, children without an AHCA birth hospitalization were excluded from the study population in the present analysis.
Longitudinal data linkage
Historically, only hospital discharge records for the first year of life were available through the linkage between the FBDR and AHCA for children identified as having at least one FBDR-eligible ICD-9-CM code (Salemi et al., 2010; Salemi et al., 2011; Salemi et al., 2012). More recently, a subset of FBDR children with specific birth defects, including OFC, were linked to AHCA discharge records beyond the first year of life as part of a collaborative project between the University of North Carolina at Charlotte, University of South Florida, Florida Department of Health FBDR, and the Centers for Disease Control and Prevention’s (CDC) National Center on Birth Defects and Developmental Disabilities.
For this study, the data included live births from January 1, 1998, to December 31, 2006, to allow for two full years of hospital discharge data for the last birth cohort. A stepwise deterministic strategy was used to link birth certificate records to hospital inpatient, ambulatory, and emergency department database, using the child’s social security number (SSN), maternal SSN, child’s date of birth, and child’s sex (Salemi et al., 2013). The linkage was done in four stages: 1) linked infant birth and maternal delivery hospital inpatient records together to create a maternal-infant dyad; 2) linked maternal-infant dyads from stage 1 to infant birth certificate records; 3) linked infant birth hospital discharge inpatient records directly to birth certificate records for infants where maternal-infant dyads were not available; 4) combined valid links from stages 2 and 3 that created the base dataset for a given birth cohort. The linking stages were constructed hierarchically; exact matches had the highest confidence, and inexact matches had lower confidence. When a link was established during a given stage, the record was removed from the pool of available records to be linked during subsequent, lower-confidence stages. Linkages were conducted separately for singleton and multiple births due to the increased complexity of linking records for multiple births (Salemi et al., 2013).
Hospitalizations and costs
The number of hospitalized days and estimated hospital costs were analyzed based on hospitalizations initiated, but not necessarily completed, before age two or within the first two years of life. This time period was chosen because primary operative correction of cleft lip and/or cleft palate is typically completed by that age (American Cleft Palate-Craniofacial Association, 2009). Number of hospitalized days and hospital costs were assessed for birth, post-birth, and total hospitalizations before age two. Multiple admission records were merged in the event of hospital transfers (Colvin et al., 2009). Transfers were defined as inpatient admissions that occurred on the same day that the child was discharged from a previous hospitalization or admissions within one day of a previous discharge with an accompanying “transfer” code.
Year-specific statewide cost-to-charge ratios were used for conversion of inpatient charges to estimated costs. According to AHRQ’s State Inpatient Database, the average all-payer inpatient hospital cost-to-charge ratio in Florida ranged from 0.355 in 2001 (209 hospitals reporting) to 0.294 in 2008 (217 hospitals reporting) (Agency for Healthcare Research and Quality, 2013). The cost-to-charge ratio for 2001 (0.355) was used to convert inpatient charges to estimated costs for the years 1998–2001 because 2001 was the earliest year available. Hospital costs from different years were converted to 2012 equivalent costs in U.S. dollars using the Producer Price Index for hospital services (U.S. Bureau of Labor Statistics, 2013).
Case classification
Children with OFC were classified into three mutually exclusive groups based on the ICD-9-CM code used for their principal diagnosis. The three types of OFC included in this study were: “cleft of secondary hard and/or soft palate” (CP) for ICD-9-CM code 749.0; “cleft lip with/without cleft alveolus” (CL) for code 749.1; and “cleft lip with cleft secondary hard and/or soft palate” (CLP) for code 749.2. Cleft uvula (code 749.02) was excluded from consideration due to variability in ascertainment, diagnosis, and coding. If ICD-9-CM codes for both CL and CP were present, the child was classified as having CLP. When multiple birth defects codes were present, a clinician reviewed the ICD-9-CM codes and made case-specific decisions regarding classification (e.g., children with OFC and a code for “other anomalies of nose” [ICD-9-CM code 748.1] were classified as isolated OFC) (Rasmussen et al., 2003).
Although recent recommendations propose classifying OFC as syndromic/non-syndromic rather than isolated/non-isolated, in a passive, ICD-9-CM code-based surveillance system, the isolated/non-isolated OFC classification is more practical and realistic (Watkins et al., 2014). Isolated OFC was defined as having no other ICD-9-CM code for any major birth defect but minor defects could be present. Non-isolated OFC was defined as the presence of any other ICD-9-CM code for a major, unrelated birth defect in addition to OFC. Additionally, presence of an ICD-9-CM code for a single gene or chromosomal syndrome would lead to a non-isolated classification (Rasmussen et al., 2003).
Variable construction and statistical analysis
Variables of interest included the following and are explained below: (1) selected maternal and child demographics; (2) Adequacy of Prenatal Care Utilization Index (APNCU) (Kotelchuck, 1994); (3) principal expected healthcare payer at the birth hospitalization; and (4) birth-hospital nursery level (I, II, or III [highest]) (American Academy of Pediatrics Committee on Fetus and Newborn, 2012).
Maternal demographics included age, race/ethnicity, nativity, parity, and education. Child demographics of interest included sex, preterm birth, birth weight, death, plurality, and presence of other major FBDR-eligible birth defects.
The APCNU Index is a measure of the adequacy of both initiation of and the receipt of prenatal care services; adequacy is classified as “inadequate,” “intermediate,” and “adequate/adequate plus”.
The child’s principal expected healthcare payer was defined as the payer recorded on the birth hospitalization discharge record. This was used in place of a composite measure of payer type based on multiple hospitalizations to avoid the reverse causation bias that can result if high resource use leads to subsequent change in payer status. Payers were categorized as private (i.e., employer-based insurance, including Tricare), public (i.e., Medicaid, Medicare, and other state and local government insurance in Florida, such as KidCare) or no insurance or self-pay/uninsured, which was defined as no insurance or less than 30% coverage.
Hospital nursery level was recorded as the highest possible level in the facility (i.e., a hospital with Level II and III beds was classified as Level III).
All variables were stratified by isolated and non-isolated CLP, CL, and CP. Pearson chi-square statistics were used to compare demographic characteristics of mothers and children with non-isolated and isolated OFC. Hospitalized days and estimated costs were presented as mean (with standard deviation), median (with interquartile range), and 90th percentile estimates for birth, post-birth, and total hospitalizations before age two years. Not all children had hospital discharge records available for both the birth and post-birth periods. Analyses for post-birth hospitalizations were restricted to children with a birth hospitalization who had additional admissions following the birth hospitalization; all children were included in the total hospitalization analyses. Wilcoxon rank-sum tests were used to detect differences in hospital resource utilization by phenotypic class and by isolated vs. non-isolated status.
High resource use was defined as the number of hospitalized days and estimated inpatient costs ≥90th percentile and was assessed by multivariable analysis. Given the large sample size, the design of our study, and the fact that our outcomes were not rare, Poisson regression models were constructed to produce adjusted prevalence ratios (aPR) and 95% confidence intervals (CI) for each cleft type for each period of hospitalization (birth, post-birth, and total) before age two years (Langlois et al., 2013; Zou, 2004). The results for each analysis were reported only for cells with at least five observations for both hospitalized days and cost within the relevant time period; infants who were high utilizers in one time period were not necessarily high utilizers in another period. We investigated statistical interactions for multiple combinations of variables to assess effect modification (e.g, prenatal care and multiple birth defects) and did not find evidence of any interaction; however, we had limited power to assess these interactions. The variables of interest were selected a priori, as described above.
All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). This study was approved by the Institutional Review Boards at the University of North Carolina at Charlotte, Florida Department of Health (FDOH), and CDC.
RESULTS
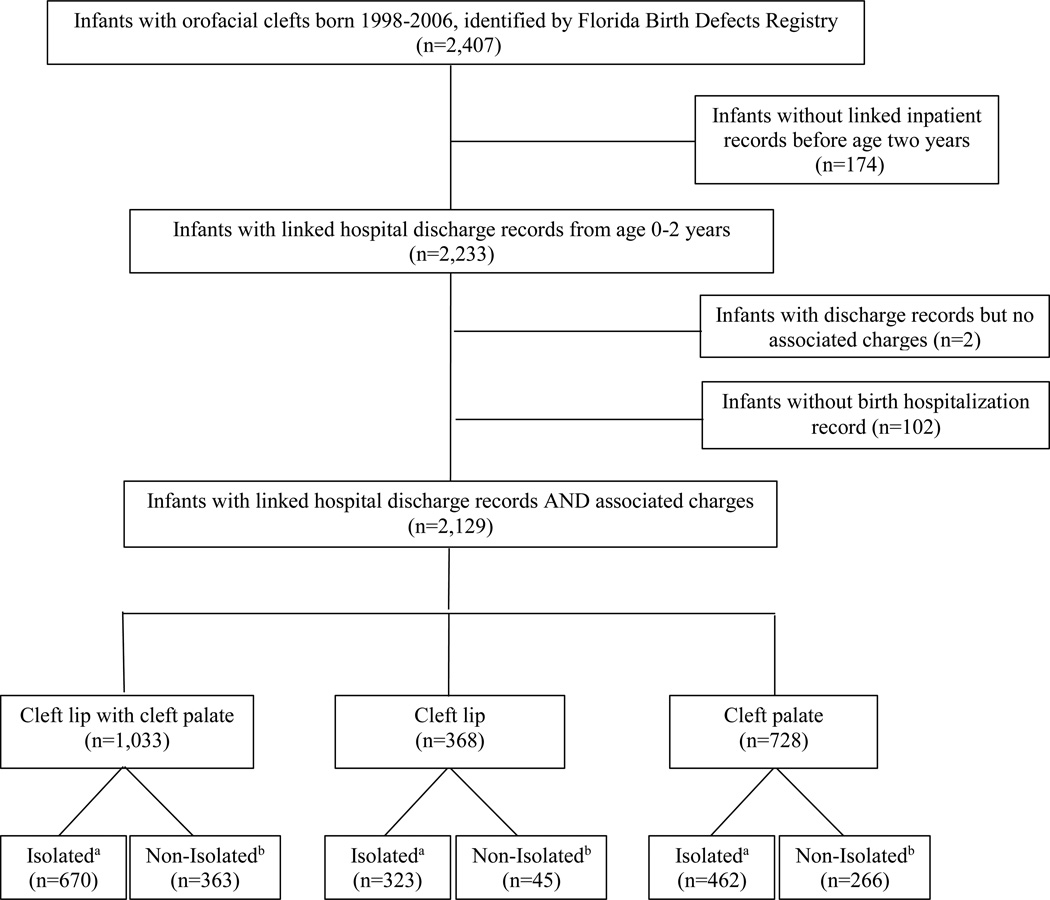
Of the 2,407 children with OFC born from 1998 through 2006 and identified by the FBDR, 174 children were excluded due to lack of linked hospital discharge records or the presence of hospital discharge records without associated charges. Children excluded were more likely to be born to Hispanic mothers and foreign-born mothers and were more likely to be twins or higher order multiples than children included in the analysis. Children without a birth hospitalization record (n=102) and those with discharge records who did not have associated charges (n=2) were also excluded from this analysis. The number of children included in the study sample was 2,129, with a phenotypic distribution of 48.5% CLP (n=1,033), 17.3% CL (n=368), and 34.2% CP (n=728). Isolated OFC was found in 68.3% (n=1,455) of children (Figure 1).
Figure 1.
Inclusion flowchart for children with orofacial clefts, born 1998–2006, in the Florida Birth Defects Registry and linked with hospital discharge data up toage two years
The distribution of maternal age was significantly different when comparing isolated to non-isolated CLP (p=0.02), with a larger percentage of children with isolated CLP born to mothers less than 25 years old (Table 1). This difference was not observed for children with CL or CP. Children with non-isolated CLP and CP were more likely to be born to foreign born mothers (p=0.0002 and p=0.009, respectively), and children with all subtypes of non-isolated OFC were more likely to be born in facilities with a higher nursery care level (CLP: p=<0.0001, CL: p=0.03, and CP: p=0.0009, respectively) than their counterparts with isolated OFC (Table 1). More than 70% of mothers reported having received adequate/adequate plus prenatal care for all subtypes of OFC. In addition, compared to children with isolated OFC, significantly higher rates of preterm birth (< 37 weeks’ gestation) and low birth weight (<2,500 grams) were observed for children with all subtypes of non-isolated OFC. Additionally, higher rates of death at birth hospitalization and before age two were observed in children with non-isolated CLP compared with isolated CLP as well as higher rates of death before age two in children with non-isolated CP or CLP compared with isolated CP or CLP (Table 1).
Table 1.
Selected demographics of Florida-born children with orofacial clefts in the Florida Birth Defects Registry and linked with longitudinal hospital discharge data before age two years, 1998–2006
| Total (n=2,129) |
Cleft lip with cleft palate (n=1,033) |
Cleft lip (n=368) |
Cleft palate (n=728) |
||||
|---|---|---|---|---|---|---|---|
| Isolateda (n=670) |
Non-Isolatedb (n=363) |
Isolateda (n=323) |
Non-Isolatedb (n=45) |
Isolateda (n=462) |
Non-Isolatedb (n=266) |
||
| Maternal Characteristics | |||||||
| Mother’s age, years | |||||||
| <20 | 228 (10.7) | 94 (14.0) | 42 (11.6) | 21 (6.5) | NR | 46 (10.0) | 24 (9.0) |
| 20–24 | 545 (25.6) | 183 (27.3) | 89 (24.5) | 70 (21.7) | 11 (24.4) | 123 (26.6) | 69 (25.9) |
| 25–29 | 538 (25.3) | 175 (26.1) | 90 (24.8) | 88 (27.2) | 12 (26.7) | 107 (23.2) | 66 (24.8) |
| 30–34 | 511 (24.0) | 143 (21.3) | 86 (23.7) | 96 (29.7) | 11 (24.4) | 115 (24.9) | 60 (22.6) |
| ≥ 35 | 307 (14.4) | 75 (11.2) | 56 (15.4) | 48 (14.9) | 10 (22.2) | 71 (15.4) | 47 (17.7) |
| P=0.02 | P=0.37 | P=0.40 | |||||
| Mother’s race / ethnicity | |||||||
| White, non-Hispanic | 1,308 (61.4) | 447 (66.7) | 187 (51.5) | 214 (66.3) | 23 (51.1) | 287 (62.1) | 150 (56.4) |
| Black, non-Hispanic | 335 (15.7) | 88 (13.1) | 70 (19.3) | 41 (12.7) | 10 (22.2) | 76 (16.5) | 50 (18.8) |
| Hispanic | 417 (19.6) | 118 (17.6) | 91 (25.1) | 54 (16.7) | 10 (22.2) | 86 (18.6) | 58 (21.8) |
| Asian / Pacific Islander and American Indian / Alaskan | 54 (2.5) | 14 (2.1) | 12 (3.3) | 12 (3.7) | NR | 8 (1.7) | 7 (2.6) |
| P=<0.0001 | P=0.13 | P=0.22 | |||||
| Mother's nativity, foreign-born | 411 (19.3) | 107 (16.0) | 91 (25.1) | 60 (18.6) | 9 (20.0) | 79 (17.1) | 65 (24.4) |
| P=0.0002 | P=0.82 | P=0.009 | |||||
| Mother’s education | |||||||
| Less than high school graduate | 438 (20.6) | 140(20.9) | 88 (24.2) | 49 (15.2) | 9 (20.0) | 92 (19.9) | 60 (22.6) |
| High school graduate or equivalent | 722 (33.9) | 231 (34.5) | 127 (35.0) | 100 (31.0) | 16 (35.6) | 158 (34.2) | 90 (33.8) |
| At least some college or university | 953 (44.8) | 294 (43.9) | 146 (40.2) | 171 (52.9) | 19 (42.2) | 210 (45.5) | 113 (42.5) |
| P=0.18 | P=0.69 | P=0.92 | |||||
| Mother’s marital status, married | 1,292 (60.7) | 394 (58.8) | 220 (60.6) | 227 (70.3) | 22 (48.9) | 272 (58.9) | 157 (59.0) |
| P=0.57 | P=0.004 | P=0.97 | |||||
| Parity, ≥ 1 previous live birth | 1,257 (59.2) | 396 (59.2) | 210 (58.2) | 191 (59.1) | 32 (71.1) | 271 (58.8) | 157 (59.0) |
| P=0.75 | P=0.12 | P=0.95 | |||||
| System Characteristics | |||||||
| Adequacy of Prenatal Care Utilization Indexc | |||||||
| Inadequate | 214 (10.0) | 67 (10.0) | 42 (11.6) | 23 (7.1) | NR | 46 (10.0) | 33 (12.4) |
| Intermediate | 188 (8.8) | 58 (8.7) | 32 (8.8) | 28 (8.7) | 6 (13.3) | 42 (9.1) | 22 (8.3) |
| Adequate/adequate plus | 1,601 (75.2) | 506 (75.5) | 271 (74.7) | 249 (77.1) | 33 (73.3) | 352 (76.2) | 190 (71.4) |
| P=0.37 | P=0.68 | P=0.86 | |||||
| Principal payer at birth hospitalizationd | |||||||
| Private | 1,054 (49.5) | 313 (46.7) | 161 (44.3) | 191 (59.1) | 25 (55.6) | 234 (50.7) | 130 (48.9) |
| Public | 989 (46.5) | 332 (49.6) | 185 (51.0) | 116 (35.9) | 18 (40.0) | 209 (45.2) | 129 (48.5) |
| Self/underinsured/charity | 86 (4.0) | 25 (3.7) | 17 (4.7) | 16 (5.0) | NR | 19 (4.1) | 7 (2.6) |
| P=0.37 | P=0.74 | P=0.95 | |||||
| Birth hospital nursery care level | |||||||
| I | 559 (26.3) | 206 (30.8) | 64 (17.6) | 92 (28.5) | 8 (17.8) | 135 (29.2) | 54 (20.3) |
| II | 580 (27.2) | 187 (27.9) | 81 (22.3) | 103 (31.9) | 11 (24.4) | 132 (28.6) | 66 (24.8) |
| III | 983 (46.2) | 275 (41.0) | 216 (59.5) | 128 (39.6) | 26 (57.8) | 194 (42.0) | 144 (54.1) |
| P=<0.0001 | P=0.03 | P=0.0009 | |||||
| Child Characteristics | |||||||
| Sex, female | 968 (45.5) | 245 (36.6) | 161 (44.4) | 123 (38.1) | 18 (40.0) | 267 (57.8) | 154 (57.9) |
| P=0.01 | P=0.80 | P=0.98 | |||||
| Preterm birth (< 37 weeks) | 375 (17.7) | 93 (13.9) | 89 (24.7) | 43 (13.4) | 11 (24.5) | 64 (13.9) | 75 (28.2) |
| P=<0.0001 | P=0.001 | P=<0.0001 | |||||
| Low birth weight (<2,500 grams) | 373 (17.5) | 71 (10.6) | 113 (31.1) | 27 (8.4) | 13 (28.9) | 66 (14.3) | 83 (31.2) |
| P=<0.0001 | P=<0.0001 | P=<0.0001 | |||||
| Death before age 2 years | 197 (9.2) | 15 (2.2) | 92 (25.3) | NR | 12 (26.7) | 14 (3.0) | 62 (23.3) |
| P=<0.0001 | NR | P=<0.0001 | |||||
| Death during birth hospitalization | 38 (1.8) | 8 (1.2) | 15 (4.1) | NR | NR | NR | 10 (3.8) |
| P=<0.0001 | NR | NR | |||||
| Plurality, singleton | 2,090 (98.2) | 658 (98.2) | 357 (98.4) | 316 (97.8) | 42 (93.3) | 455 (98.5) | 262 (98.5) |
| P=0.69 | P=0.16 | P=0.83 | |||||
NOTE: Orofacial clefts sub-types are mutually exclusive
NR: not reported due to cell counts of < 5.
Results presented as N (%). Percentages may not sum to 100% due to missing values; Significant differences using Pearson chi-square in the distribution of characteristics for children with isolated versus non-isolated orofacial clefts were indicated in bold (p<0.05).
Isolated = no International Classification of Disease, 9th revision; Clinical Modification (ICD-9-CM) code for any other major birth defect present in the Florida Birth Defects Registry; could include minor birth defects or other birth defects related to orofacial clefts.
Non-isolated= ICD-9-CM codes for other major birth defects present in the Florida Birth Defects Registry, including syndromes.
Adequacy of Prenatal Care Utilization (APNCU) Index is a measure of the adequacy of both initiation of and the receipt of prenatal care services; adequacy is classified as “adequate,” “intermediate,” and “adequate/adequate plus” (Kotelchuck M. 1994. The adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. American Journal of Public Health 84(9):1486–1489).
Private insurance included employer-based insurance (including military coverage, Civilian Health and Medical Program of the Uniformed Services (CHAMPUS) and Tricare). Public insurance included Medicare, Medicaid, and other state and local government insurance in Florida, such as the state's Children’s Health Insurance Program (CHIP), KidCare. Self or under-insured was defined as no insurance or less than 30% coverage.
Congenital heart defects (ICD-9-CM: 745.00–747.90) were the most prevalent grouping of other major defects among children with non-isolated CLP (63.4%) and CL (57.8%), and the second most prevalent grouping of defects for children with non-isolated CP (59.0%). Musculoskeletal defects (ICD-9-CM: 754.00–756.90) were the most prevalent grouping of defects in children with non-isolated CP (61.3%) and the second most prevalent type of defects in children with non-isolated CLP (37.2%) and CL (35.6%) (Table 2).
Table 2.
Distribution of other major birth defects for Florida-born children with non-isolated orofacial clefts, 1998–2006
| Cleft lip with cleft palate (n=363) |
Cleft lip (n=45) |
Cleft palate (n=266) |
|
|---|---|---|---|
| Accompanying defecta | N (%) | N (%) | N (%) |
| Congenital heart defects | 230 (63.4) | 26 (57.8) | 157 (59.0) |
| Musculoskeletal defects | 135 (37.2) | 16 (35.6) | 163 (61.3) |
| Chromosomal abnormalities | 87 (24.0) | 6 (13.3) | 59 (22.2) |
| Genitourinary defects | 69 (19.0) | 6 (13.3) | 60 (22.6) |
| Congenital lung abnormalities | 54 (14.9) | 5 (11.1) | 33 (12.4) |
| Eye/Ear defects | 48 (13.2) | 14 (31.1) | 28 (10.5) |
| Gastrointestinal defects | 47 (13.0) | NR | 48 (18.1) |
| Central nervous system defects | 11 (3.0) | NR | 8 (3.0) |
Notes: Orofacial clefts sub-types are mutually exclusive; Non-isolated= International Classification of Disease, 9th revision; Clinical Modification (ICD-9-CM) codes for other major birth defects present in the Florida Birth Defects Registry, including syndromes.
NR: not reported due to cell counts of < 5.
Birth defect groups are not mutually exclusive- ICD-9-CM codes used: congenital heart defects (745.00–747.90), musculoskeletal defects (754.00–756.90), chromosomal abnormalities (758.00–758.90), genitourinary (752.00 – 753.90), congenital lung abnormalities (748.00-478.90), eye/ear defects (743.00–744.90) gastrointestinal (750.00–751.90), central nervous system (740.00 –742.90).
Birth hospitalizations represented 35.0% of the total 6,087 hospitalizations during the study period and, overall, accounted for 64.5% of all hospitalized days and 57.3% of hospitalization costs (data not shown). Mean and median number of hospitalized days and inpatient costs during the birth hospitalization were higher among children with non-isolated OFC than those with isolated OFC for all subtypes and were higher for children with isolated CLP and CP than children with isolated CL. The same trends were observed for post-birth hospitalizations and total hospitalizations before age two years (Table 3).
Table 3.
Inpatient resource use for Florida-born children with orofacial clefts before age two years by hospitalization period, 1998–2006
| Total | Cleft lip with cleft palate | Cleft lip | Cleft palate | ||||
|---|---|---|---|---|---|---|---|
| Isolateda | Non-Isolatedb | Isolateda | Non-Isolatedb | Isolateda | Non-Isolatedb | ||
| Birth hospitalization | |||||||
| Number of children with admissions during birth hospitalizationc | 2,129 | 670 | 363 | 323 | 45 | 462 | 266 |
| Mean (SD) hospitalized daysc,d | 12.2 (24.8) | 6.4 (15.3)^ | 20.3 (28.7)* | 3.8 (8.1) | 14.7 (17.6)* | 8.5 (19.4)^ | 31.7 (41.9)* |
| Median (IQR) hospitalized days | 3 (2–8) | 3 (2–4)^ | 8 (3–24)* | 2 (2–3) | 5 (2–23)* | 2 (2–4)^ | 15 (4–42)* |
| 90th Percentile hospitalized days | 33 | 10 | 54 | 5 | 41 | 15 | 99 |
| Mean (SD) inpatient coste,d ($) | 18,740 (57,210) | 7,480 (29,981)^ | 32,760 (58,459)* | 2,872 (12,681) | 28,607 (48,778)* | 10,057 (34,450)^ | 60,654 (117,821)* |
| Median (IQR) inpatient cost ($) | 1,454 (674–10,299) | 1,106 (641–4,201)^ | 10,984 (2,762–32,229)* | 699 (486–1,129) | 11,921 (1,631–38,428)* | 901 (588–2,977)^ | 17,199 (4,728–60,248)* |
| 90th Percentile inpatient cost ($) | 42,801 | 10,239 | 96,911 | 4,543 | 81,319 | 15,769 | 173,729 |
| Post-birth hospitalizations initiated before age two years | |||||||
| Number of children with admissions initiated during period | 1,258 | 419 | 258 | 120 | 20 | 255 | 186 |
| Median (IQR) Inpatient admissions (n) | 1 (1–3) | 2 (1–2) | 2 (1–4) | 1 (1–1) | 1 (1–3) | 1 (1–2) | 2 (1–4) |
| Mean (SD) hospitalized days | 11.4 (25.7) | 5.2 (8.8)^ | 21.7 (37.0)* | 3.2 (8.9) | 13.9 (28.7)* | 8.0 (19.7)^ | 21.1 (36.7)* |
| Median (IQR) hospitalized days | 3 (2–8) | 3 (1–5)^ | 7 (2–25)* | 2 (1–3) | 3 (1–8)* | 2 (2–6)^ | 7 (2–24)* |
| 90th Percentile hospitalized days | 28 | 10 | 64 | 6 | 50 | 15 | 64 |
| Mean (SD) inpatient cost ($) | 23,616 (60,061) | 10,612 (14,591)^ | 41,471 (73,470)* | 6,604 (17,727) | 40,432 (79,325)* | 13,892 (29,887)^ | 50,643 (112,006)* |
| Median (IQR) inpatient cost ($) | 7,445 (4,364–15,743) | 7,265 (4,499–12,339)^ | 15,459 (6,138–42,502)* | 4,236 (3,240–6,237) | 8,696 (4,671–39,183)* | 5,545 (4,034–8,372)^ | 12,802 (5,907–40,902)* |
| 90th Percentile inpatient cost ($) | 49,041 | 17,547 | 99,194 | 9,171 | 122,323 | 25,540 | 139,331 |
| Total hospitalizations initiated before age two years | |||||||
| Number of children with admissions initiated | 2,129 | 670 | 363 | 323 | 45 | 462 | 266 |
| Median (IQR) Inpatient admissions (n) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 2 (1–4) |
| Mean (SD) hospitalized days | 18.9 (37.3) | 9.6 (17.8)^ | 35.8 (49.7)* | 4.9 (10.1) | 20.9 (29.6)* | 12.9 (27.2)^ | 46.5 (61.0)* |
| Median (IQR) hospitalized days | 5 (3–15) | 5 (3–9)^ | 14 (6–45)* | 3 (2–4) | 9 (3–28)* | 4 (2–8)^ | 25 (7–59)* |
| 90th Percentile hospitalized days | 51 | 16 | 93 | 8 | 63 | 33 | 124 |
| Mean (SD) inpatient cost ($) | 32,695 (86,007) | 14,116 (34,546)^ | 62,235 (98,165)* | 5,326 (17,061) | 46,577 (76,980)* | 17,724 (45,604)^ | 96,066 (175,594)* |
| Median (IQR) inpatient cost ($) | 7,639 (1,619–20,952) | 7,011 (1,622–13,871)^ | 23,105 (9,766–68,177)* | 1,292 (568–5,476) | 16,305 (2,583–51,112)* | 4,836 (906–10,5157)^ | 35,611 (13,488–91,957)* |
| 90th Percentile inpatient cost ($) | 74,447 | 23,306 | 173,024 | 9,007 | 127,478 | 43,110 | 248,059 |
Note: Orofacial clefts sub-types are mutually exclusive
SD: standard error; IQR: interquartile range
Indicates p<0.05, comparison group = isolated cleft lip (Wilcoxon rank-sum test)
Indicates p<0.05, comparison group = isolated orofacial clefts (Wilcoxon rank-sum test)
Isolated = no International Classification of Disease, 9th revision; Clinical Modification (ICD-9-CM) code for any other major birth defect present in the Florida Birth Defects Registry; could include minor birth defects or other birth defects related to clefts.
Non-isolated= ICD-9-CM codes for other major birth defects present in the Florida Birth Defects Registry, including syndromes.
Hospitalized days refers to the number of days that a child spent in the hospital for all admissions initiated within the specified period.
Hospitalizations were assessed as continuous episodes of hospital care, regardless of whether a transfer took place. Multiple admission records were merged into one if a child was admitted to a hospital on the same day as a discharge from a previous admission, or if the child was admitted to a hospital on the day after a previous discharge with an accompanying “transfer” code.
Presented as 2012 values. Estimated costs calculated as total charges adjusted using state-wide year-specific cost-to-charge ratios. Inpatient charges included all hospital facility charges (excluded professional fees): Pharmacy, medical and surgical supply, laboratory, radiology and other imaging, cardiology, operating room, anesthesia, recovery room, emergency room (if an inpatient admission originated in the emergency room), treatment or observation room (if a visit resulted in an inpatient admission) charges.
During the birth hospitalization, none of the maternal characteristics were found to be statistically significantly associated with high hospital resource use (Table 4). Among other characteristics examined, birth hospital nursery care level was associated with high resource use for children with CLP. Children born at hospitals with level II nurseries were less likely to have had high resource use than those born at hospitals with level III nurseries.
Table 4.
Factors associated with high (90th %) inpatient resource use* for Florida-born children with orofacial clefts, 1998–2006
| Cleft lip with cleft palatea | Cleft lipa | Cleft palatea | ||||
|---|---|---|---|---|---|---|
| Hospitalized daysb,c | Costc,d | Hospitalized daysb,c | Costc,d | Hospitalized daysb,c | Costc,d | |
| Adjusted Prevalence Ratios (95% CI) | Adjusted Prevalence Ratios (95% CI) | Adjusted Prevalence Ratios (95% CI) | ||||
| Birth hospitalizations | ||||||
| Ne=105, 928 | Ne=104, 929 | Ne=39, 329 | Ne=36, 332 | Ne=73, 655 | Ne=72, 656 | |
| Maternal Characteristics | ||||||
| Mother’s age, years | ||||||
| <20 | 0.78 (0.34–1.77) | 0.73 (0.31–1.69) | --g | --g | 0.72 (0.28–1.85) | 0.57 (0.20–1.60) |
| 20–24 | 0.78 (0.46–1.37) | 0.71 (0.40–1.25) | 0.83 (0.30–2.33) | 0.42 (0.14–1.32) | 1.00 (0.52–1.91) | 0.90 (0.46–1.77) |
| 25–29 | Referent | Referent | Referent | Referent | Referent | Referent |
| 30–34 | 0.94 (0.54–1.63) | 0.98 (0.56–1.69) | 1.06 (0.40–2.82) | 0.90 (0.35–2.34) | 0.84 (0.41–1.73) | 0.94 (0.46–1.88) |
| ≥ 35 | 0.72 (0.36–1.44) | 0.97 (0.52–1.82) | 1.03 (0.33–3.19) | 0.86 (0.27–2.20) | 0.76 (0.34–1.70) | 0.97 (0.45–2.07) |
| Mother’s race / ethnicity | ||||||
| White, non-Hispanic | Referent | Referent | Referent | Referent | Referent | Referent |
| Black, non-Hispanic | 1.24 (0.74–2.08) | 1.16 (0.68–2.01) | 1.02 (0.38–2.73) | 0.77 (0.27–2.20) | 0.91 (0.48–1.73) | 0.92 (0.47–1.81) |
| Hispanic | 1.15 (0.58–2.27) | 1.61 (0.87–2.96) | 1.06 (0.36–3.15) | 0.94 (0.29–3.04) | 1.25 (0.59–2.64) | 1.83 (0.89–3.75) |
| Mother's nativity, foreign-born | 0.84 (0.45–1.59) | 0.81 (0.44–1.48) | 1.09 (0.35–3.35) | 0.60 (0.16–2.20) | 0.96 (0.45–2.04) | 0.91 (0.45–1.85) |
| Mother’s education | ||||||
| Less than high school graduate | 1.02 (0.57–1.82) | 0.93 (0.52–1.68) | 1.86 (0.64–5.42) | 2.08 (0.65–6.69) | 0.92 (0.43–1.98) | 0.79 (0.35–1.75) |
| High school graduate or equivalent | 1.11 (0.68–1.83) | 0.95 (0.58–1.55) | 1.06 (0.44–2.55) | 1.10 (0.43–2.84) | 1.39 (0.78–2.48) | 1.32 (0.74–2.34) |
| At least some college or university | Referent | Referent | Referent | Referent | Referent | Referent |
| Mother’s marital status, not married | 0.90 (0.72–1.14) | 0.92 (0.73–1.16) | 0.97 (0.60–1.57) | 1.02 (0.63–1.67) | 0.91 (0.67–1.24) | 1.05 (0.77–1.43) |
| Parity, ≥ 1 previous live birth | 0.76 (0.48–1.18) | 0.75 (0.48–1.18) | 0.65 (0.29–1.48) | 0.79 (0.31–2.03) | 0.77 (0.46–1.27) | 0.79 (0.35–1.75) |
| System Characteristics | ||||||
| Adequacy of Prenatal Care Utilization Index | ||||||
| Inadequate | 0.67 (0.33–1.33) | 0.74 (0.37–1.47) | --g | --g | 0.86 (0.38–1.94) | 1.33 (0.63–2.82) |
| Intermediate | 1.05 (0.49–2.27) | 0.64 (0.25–1.63) | --g | --g | 1.17 (0.49–2.81) | 1.10 (0.45–2.67) |
| Adequate/adequate plus | Referent | Referent | Referent | Referent | Referent | Referent |
| Principal payer at birth hospitalizationf | ||||||
| Private | Referent | Referent | Referent | Referent | Referent | Referent |
| Public | 1.56 (0.93–2.62) | 1.63 (0.98–2.71) | 1.63 (0.65–4.08) | 1.59 (0.63–3.97) | 1.66 (0.87–3.17) | 1.21 (0.64–2.31) |
| Self/underinsured/charity | 1.67 (0.62–4.55) | 1.72 (0.64–4.63) | --g | --g | --g | --g |
| Birth hospital nursery care level | ||||||
| I | 0.62 (0.35–1.11) | 0.65 (0.37–1.16) | 0.71 (0.27–1.88) | --g | 0.85 (0.44–1.64) | 0.73 (0.36–1.47) |
| II | 0.51 (0.28–0.94) | 0.54 (0.30–0.97) | 0.68 (0.26–1.82) | 0.61 (0.23–1.62) | 0.71 (0.37–1.38) | 0.77 (0.41–1.45) |
| III | Referent | Referent | Referent | Referent | Referent | Referent |
| Child Characteristics | ||||||
| Sex, female | 1.39 (1.13–1.70) | 1.32 (1.09–1.62) | 1.13 (0.79–1.62) | 1.19 (0.82–1.74) | 0.80 (0.62–1.02) | 0.86 (0.67–1.10) |
| Preterm birth (< 37 weeks) | 1.93 (1.19–3.14) | 2.11 (1.30–3.43) | 2.19 (0.90–5.36) | 0.78 (0.28–2.15) | 1.60 (0.92–2.77) | 1.58 (0.90–2.77) |
| Low birth weight (<2,500 grams) | 2.67 (1.63–4.34) | 1.63 (1.00–2.66) | 1.96 (0.76–5.03) | 3.01 (1.11–8.14) | 2.68 (1.50–4.80) | 2.25 (1.26–4.02) |
| Non-isolated birth defects | 3.47 (2.18–5.52) | 4.01 (2.51–6.40) | 5.93 (2.84–12.40) | 11.02 (4.86–24.97) | 3.34 (1.95–5.72) | 4.86 (2.71–8.74) |
| Post-birth hospitalizations initiated before age two years | ||||||
| Ne=69, 964 | Ne=68, 965 | Ne=17, 351 | Ne=14, 354 | Ne=46, 682 | Ne=44, 684 | |
| Maternal Characteristics | ||||||
| Mother’s age, years | ||||||
| <20 | 1.07 (0.38–3.02) | 0.91 (0.33–2.52) | --g | --g | 0.78 (0.23–2.70) | 0.79 (0.23–2.75) |
| 20–24 | 1.62 (0.81–3.22) | 1.23 (0.64–2.40) | --g | --g | 1.18 (0.52–2.68) | 1.01 (0.43–2.38) |
| 25–29 | Referent | Referent | Referent | Referent | Referent | Referent |
| 30–34 | 0.89 (0.40–1.97) | 0.77 (0.36–1.67) | --g | --g | 0.67 (0.26–1.73) | 0.63 (0.23–1.74) |
| ≥ 35 | 1.44 (0.66–3.15) | 0.93 (0.42–2.09) | --g | --g | --g | 0.81 (0.28–2.38) |
| Mother’s race / ethnicity | ||||||
| White, non-Hispanic | Referent | Referent | Referent | Referent | Referent | Referent |
| Black, non-Hispanic | 1.38 (0.70–2.71) | 1.88 (0.95–3.70) | --g | --g | 1.23 (0.51–2.95) | 1.11 (0.45–2.71) |
| Hispanic | 1.07 (0.51–2.25) | 0.99 (0.45–2.17) | --g | --g | 1.59 (0.65–3.90) | 1.42 (0.57–3.55) |
| Mother's nativity, foreign-born | 0.97 (0.46–2.04) | 0.92 (0.44–1.93) | --g | --g | 0.89 (0.36–2.25) | 1.11 (0.44–2.80) |
| Mother’s education | ||||||
| Less than high school graduate | 0.96 (0.47–1.96) | 1.02 (0.48–2.17) | --g | --g | 1.13 (0.47–2.72) | 0.94 (0.38–2.32) |
| High school graduate or equivalent | 0.93 (0.51–1.70) | 1.22 (0.66–2.23) | 2.80 (0.84–9.34) | 1.85 (0.51–6.67) | 1.06 (0.50–2.27) | 1.01 (0.46–2.21) |
| At least some college or university | Referent | Referent | Referent | Referent | Referent | Referent |
| Mother’s marital status, not married | 0.82 (0.61–1.10) | 0.78 (0.58–1.06) | 2.31 (1.15–4.66) | 1.41 (0.62–3.21) | 0.73 (0.50–1.06) | 0.89 (0.61–1.31) |
| Parity, ≥ 1 previous live birth | 1.05 (0.61–1.85) | 1.01 (0.57–1.79) | 1.58 (0.41–6.09) | 1.12 (0.29–4.33) | 0.98 (0.51–1.89) | 1.14 (0.57–2.25) |
| System Characteristics | ||||||
| Adequacy of Prenatal Care Utilization Index | ||||||
| Inadequate | 1.02 (0.49–2.15) | 0.68 (0.30–1.54) | --g | --g | 0.82 (0.31–2.19) | 0.69 (0.25–1.87) |
| Intermediate | 1.32 (0.59–2.92) | 0.96 (0.40–2.33) | --g | --g | --g | --g |
| Adequate/adequate plus | Referent | Referent | Referent | Referent | Referent | Referent |
| Principal payer at birth hospitalizationf | ||||||
| Private | Referent | Referent | Referent | Referent | Referent | Referent |
| Public | 1.60 (0.84–3.03) | 1.50 (0.79–2.87) | --g | --g | 2.38 (1.07–5.29) | 3.32 (1.41–7.81) |
| Self/underinsured/charity | --g | --g | --g | --g | --g | --g |
| Child Characteristics | ||||||
| Sex, female | 1.60 (1.24–2.07) | 1.59 (1.23–2.06) | 1.28 (0.76–2.17) | 1.20 (0.67–2.16) | 0.71 (0.52–0.96) | 0.74 (0.54–1.02) |
| Preterm birth (23–36 weeks) | 0.88 (0.44–1.74) | 0.79 (0.40–1.58) | --g | --g | 0.66 (0.29–1.48) | 0.35 (0.14–0.86) |
| Low birth weight (<2,500 grams) | 1.03 (0.54–1.97) | 1.04 (0.58–1.97) | --g | --g | 1.65 (0.77–3.57) | 1.94 (0.89–4.24) |
| Death before age 2 years | 1.35 (0.75–2.43) | 1.70 (0.96–3.01) | --g | --g | 2.05 (0.97–4.32) | 2.17 (1.01–4.65) |
| Non-isolated birth defects | 9.43 (4.66–19.06) | 9.70 (4.65–20.30) | 4.88 (1.62–14.68) | 9.20 (2.86–29.55) | 2.83 (1.44–5.56) | 3.30 (1.62–6.74) |
| Total hospitalizations initiated before age two years | ||||||
| Ne=105, 928 | Ne=104, 929 | Ne=39, 329 | Ne=37, 331 | Ne=73, 655 | Ne=72, 656 | |
| Maternal Characteristics | ||||||
| Mother’s age, years | ||||||
| <20 | 0.50 (0.21–1.19) | 0.69 (0.30–1.57) | --g | --g | 0.52 (0.19–1.47) | 0.63 (0.23–1.74) |
| 20–24 | 0.70 (0.40–1.22) | 0.85 (0.49–1.49) | 0.66 (0.24–1.80) | 0.46 (0.16–1.36) | 0.99 (0.52–1.88) | 0.86 (0.44–1.68) |
| 25–29 | Referent | Referent | Referent | Referent | Referent | Referent |
| 30–34 | 0.81 (0.46–1.43) | 0.81 (0.44–1.47) | 1.00 (0.38–2.61) | 1.00 (0.39–2.56) | 0.63 (0.30–1.32) | 0.66 (0.33–1.36) |
| ≥ 35 | 0.94 (0.50–1.76) | 0.98 (0.52–1.85) | 1.32 (0.46–3.84) | 1.11 (0.37–3.30) | 0.50 (0.21–1.18) | 0.54 (0.23–1.23) |
| Mother’s race / ethnicity | ||||||
| White, non-Hispanic | Referent | Referent | Referent | Referent | Referent | Referent |
| Black, non-Hispanic | 1.33 (0.79–2.25) | 1.73 (1.01–2.97) | 0.84 (0.31–2.28) | 0.68 (0.22–2.07) | 0.91 (0.47–1.74) | 0.96 (0.49–1.88) |
| Hispanic | 0.99 (0.51–1.93) | 1.40 (0.74–2.64) | 0.61 (0.19–1.99) | 0.64 (0.19–2.22) | 0.95 (0.43–2.09) | 1.48 (0.70–3.12) |
| Mother's nativity, foreign-born | 1.05 (0.57–1.96) | 0.93 (0.51–1.69) | 0.98 (0.30–3.25) | 0.85 (0.23–3.07) | 1.22 (0.57–2.62) | 1.14 (0.55–2.37) |
| Mother’s education | ||||||
| Less than high school graduate | 1.17 (0.66–2.09) | 1.20 (0.67–2.16) | 2.48 (0.87–7.10) | 2.84 (0.95–8.48) | 0.97 (0.47–2.03) | 0.66 (0.30–1.47) |
| High school graduate or equivalent | 1.09 (0.66–1.80) | 1.13 (0.68–1.86) | 1.38 (0.59–3.25) | 1.32 (0.54–3.20) | 1.22 (0.68–2.18) | 1.27 (0.72–2.25) |
| At least some college or university | Referent | Referent | Referent | Referent | Referent | Referent |
| Mother’s marital status, not married | 0.86 (0.68–1.09) | 0.80 (0.63–1.02) | 1.21 (0.73–1.99) | 0.93 (0.55–1.57) | 0.91 (0.68–1.23) | 0.91 (0.67–1.24) |
| Parity, ≥ 1 previous live birth | 0.66 (0.42–1.03) | 0.71 (0.45–1.12) | 0.53 (0.23–1.22) | 0.52 (0.22–1.25) | 0.90 (0.54–1.50) | 0.91 (0.55–1.51) |
| System Characteristics | ||||||
| Adequacy of Prenatal Care Utilization Index | ||||||
| Inadequate | 0.59 (0.30–1.19) | 0.63 (0.32–1.24) | 0.93 (0.24–3.55) | 0.58 (0.11–2.87) | 0.78 (0.34–1.76) | 0.98 (0.45–2.14) |
| Intermediate | 0.72 (0.32–1.61) | 0.93 (0.45–1.91) | --g | --g | 1.21 (0.52–2.80) | 0.97 (0.40–2.38) |
| Adequate/adequate plus | Referent | Referent | Referent | Referent | Referent | Referent |
| Principal payer at birth hospitalizationf | ||||||
| Private | Referent | Referent | Referent | Referent | Referent | Referent |
| Public | 1.91 (1.14–3.21) | 1.71 (1.02–2.87) | 1.07 (0.40–2.85) | 1.37 (0.51–3.68) | 1.95 (1.02–3.71) | 1.67 (0.89–3.16) |
| Self/underinsured/charity | --g | --g | --g | --g | --g | --g |
| Child Characteristics | ||||||
| Sex, female | 1.48 (1.20–1.82) | 1.44 (1.18–1.77) | 1.27 (0.89–1.80) | 1.28 (0.88–1.87) | 0.80 (0.63–1.03) | 0.92 (0.72–1.18) |
| Preterm birth (< 37 weeks) | 1.59 (0.99–2.56) | 1.28 (0.78–2.10) | 1.43 (0.56–3.69) | 1.26 (0.49–3.22) | 1.41 (0.80–2.48) | 1.29 (0.74–2.27) |
| Low birth weight (<2,500 grams) | 2.06 (1.25–3.38) | 1.49 (0.90–2.44) | 2.77 (0.99–7.71) | 3.47 (1.26–9.58) | 1.81 (1.00–3.30) | 2.52 (1.40–4.56) |
| Death before age 2 years | 1.41 (0.89–2.24) | 1.78 (1.13–2.80) | 0.44 (0.11–1.72) | 0.80 (0.23–2.66) | 2.08 (1.18–3.66) | 1.80 (1.03–3.16) |
| Non-isolated birth defects | 4.75 (2.85–7.92) | 6.59 (3.74–11.61) | 6.45 (3.10–13.42) | 9.64 (4.43–20.94) | 3.18 (1.82–5.54) | 4.34 (2.36–7.96) |
Notes: Orofacial clefts sub-types are mutually exclusive; Bolded results identify significant findings at p<0.05. Non-isolated= International Classification of Disease, 9th revision; Clinical Modification (ICD-9-CM) codes for other major birth defects present in the Florida Birth Defects Registry, including syndromes and minor birth defects.
CI=Confidence interval
High inpatient resource use defined as resource use ≥ 90th percentile for hospitalized days or estimated inpatient costs.
Adjusted models controlled for all characteristics listed in the table.
Hospitalized days referred to the number of days that an infant spent in the hospital for all admissions initiated within the specified period.
Hospitalizations were assessed as continuous episodes of hospital care, regardless of whether a transfer took place. Multiple admission records were merged into one if a child was admitted to a hospital on the same day as a discharge from a previous admission, or if the child was admitted to a hospital on the day after a previous discharge with an accompanying “transfer” code.
Estimated costs calculated as total charges adjusted using state-wide year-specific cost-to-charge ratios and standardized as 2012 values. Inpatient charges included all hospital facility charges (excluded professional fees): pharmacy, medical and surgical supply, laboratory, radiology and other imaging, cardiology, operating room, anesthesia, recovery room, emergency room (if an inpatient admission originated in the emergency room), treatment or observation room (if a visit resulted in an inpatient admission) charges.
Number of children classified as high hospital resource users, all others
Private insurance included employer-based insurance (including military coverage, Civilian Health and Medical Program of the Uniformed Services (CHAMPUS) and Tricare). Public insurance included Medicare, Medicaid, and other state and local government insurance in Florida, such as KidCare. Self or under-insured was defined as no insurance or or less than 30% coverage.
Estimates were not calculated due to cell counts <5 for comparisons of 10th vs. 90th percentiles for both number of hospitalized days and cost.
Several child characteristics were associated with resource use during the birth hospitalization. Females with CLP were more likely than males to have had a high number of hospitalized days and inpatient costs (aPR: 1.4; 95% CI: 1.1–1.7 and aPR: 1.3; 95% CI: 1.1–1.6, respectively). Being born preterm was also significantly associated with high number of hospitalized days and inpatient costs for children with CLP (aPR: 1.9; 95% CI: 1.2–3.1 and aPR: 2.1; 95% CI: 1.3–3.4, respectively). History of low birth weight was significantly associated with a higher number of hospitalized days for children with CLP (aPR 2.7; 95% CI: 1.6–4.3), higher inpatient costs for children with CL (aPR 3.0; 95% CI: 1.1–8.1), and hospitalized days and higher inpatient costs for children with CP (aPR 2.7; 95% CI: 1.5–4.8; aPR 2.3; 95% CI: 1.3–4.0, respectively). The only consistent association with high resource use during both birth and post-birth hospitalizations stratified by OFC subtype was the presence of another major birth defect. Children with all subtypes of OFC who also had another major birth defect were 3.3–5.9 times more likely to have had a high number of hospitalized days and 4.0–11.0 times more likely to have had high inpatient costs during the birth hospitalization (Table 4).
Because fewer children had post-birth hospitalizations, the post-birth analyses had less power to detect associations, particularly for children with CL (Table 4). Among children with CP, those with public insurance were 2.4 times more likely to have had a high number of hospitalized days and 3.3 times more likely to have had high inpatient costs than their counterparts with private insurance. Also, among children with CP, being born low birth weight was associated with higher likelihoods of high hospitalized days and high inpatient costs.
Similar to the birth hospitalization results, females with CLP were more likely to have had a high number of hospitalized days and inpatient costs than males with CLP during the post-birth period. Among children with CLP, the presence of other major birth defects was an even stronger predictor of high post-birth resource use than for birth hospitalizations. Children with non-isolated OFC were 2.8–9.4 and 3.3–9.7 times more likely to have had a high number of hospitalized days and inpatient costs, respectively (Table 4).
The results for the analysis of total hospitalizations from birth to age two were similar to those that have been previously described for birth and post-birth hospitalizations (Table 4). Females were more likely than males to have high resource use among children with CLP and were less likely to have a high number of hospitalized days among children with CP. Being born low birth weight was a significant predictor of a high number of total hospitalized days for children with CLP and CP and high inpatient costs for children with CL and CP. Children with non-isolated OFC were more likely to have a high number of total hospitalized days and inpatient costs for all OFC subtypes. When examining factors associated with total hospitalizations initiated before age two years, public insurance at the birth hospitalization was a predictor of higher number of hospitalized days and costs for children with CLP and higher number of hospitalized days for children with CP compared to those with private insurance.
DISCUSSION
This study is the largest population-based study of hospital use in children with orofacial clefts in the United States and among the few to examine maternal, child, and system characteristics associated with high resource use in these children. This report describes high hospital resource use for Florida-born children with OFC, stratified by OFC subtype. About 68% of children had isolated OFC, which is similar to distributions found in several other studies (Cassell et al., 2008; Watkins et al., 2014; Weiss et al., 2009). The number of hospitalized days and estimated inpatient costs were significantly greater for children with non-isolated OFC compared to isolated OFC for all OFC subtypes, which was to be expected and similar to other previous findings (Boulet et al., 2009; Cassell et al., 2008; Weiss et al., 2009).
This large population-based study can be compared with several studies that examined hospital use and costs for children with OFC by cleft subtypes (Basseri et al., 2011; Boulet et al., 2009; Cassell et al., 2008; Centers for Disease Control and Prevention, 2007; Deleyiannis et al., 2013; Nguyen et al., 2013; Russo and Elixhauser, 2007; Weiss et al., 2009). Our results were similar to recent studies in that the presence of other major birth defects, in addition to OFC, was associated with both higher hospitalization costs (Boulet et al., 2009; Cassell et al., 2008; Weiss et al., 2009) and hospitalized days (Weiss et al., 2009). Our study is most comparable to that of Weiss et al. (2009) in terms of study design and analysis. Although we observed the highest mean costs and hospitalized days for children with isolated and non-isolated CP during birth and post-birth hospitalizations, Weiss et al. (2009) observed a similar association in children with isolated CP at birth hospitalization only. Our findings support those from a more recent study in which researchers concluded that compared to children with the other two OFC subtypes, the highest hospital charges and payments were reported for children with isolated CP (Deleyiannis et al., 2013). However, that study only used one hospital for its study sample and included a small study sample (Deleyiannis et al., 2013). In addition, of the two studies that used birth defects registry data and examined healthcare use in the first year of life, one concluded that children with CP incurred the highest cost (Boulet et al., 2009), whereas the other found that CLP was associated with the highest total cost followed closely by CP (Cassell et al., 2008). The differences observed in these two studies may be due to ascertainment definitions, coding differences, payer type examined (private vs. public) and study time frame.
Although our study utilized data from a single state, our results are similar to studies that utilized national databases, such as the AHRQ HCUP Kids’ Inpatient Database and the Nationwide Inpatient Sample (Centers for Disease Control and Prevention, 2007; Russo and Elixhauser, 2007). Russo and Elixhauser (2007) also observed a higher number of mean hospitalized days and costs for children with CP compared to those with cleft lip with or without cleft palate and similar hospitalization costs for both groups; however, a major limitation of that study was the lack of information on the presence or absence of other major birth defects as well as lack of inclusion of three distinct groups of OFC. A study of hospitalized days and charges in children with selected birth defects in the United States also found that neonates with CP had a higher number of hospitalized days and costs compared to children with cleft lip with or without cleft palate (Centers for Disease Control and Prevention, 2007); that study did not distinguish between cleft lip and cleft lip with palate and did not stratify by the presence of other major birth defects.
Our study was subject to several limitations, including the use of a passive birth defects surveillance system, the FBDR, in which ICD-9-CM codes were used to identify and classify children with OFC and other birth defects instead of verbatim or clinically-verified diagnoses. We were not able to capture post-birth hospitalizations that occurred out-of-state or at a Florida hospital or clinic that does not report to AHCA. While approximately 290 Florida hospitals report data to the AHCA, not all hospitals are required to report to AHCA, such as military hospitals (Florida Agency for Health Care Administration, 2013). Therefore, post-birth and total hospitalization costs may be an underestimate of the total resource use of the study cohort. Additionally, we did not have a comparison group of unaffected children, so we were unable to make comparisons between the hospital resource use of Florida-born children with OFC and that of unaffected children. However, the focus of this analysis was an internal comparison of costs for children with OFC by subtype and isolated versus non-isolated OFC. Furthermore, we did not have information on the prenatal experience, including any prenatal diagnosis of OFC or other major birth defects. However, only 3% to 33% of OFC are diagnosed prenatally (varies by subtype) (Johnson et al., 2009; Robbins et al., 2010); lack of prenatal diagnosis is potentially a limitation because mothers of fetuses with a prenatal diagnosis of a birth defect may be referred to, or elect to, deliver in a hospital with higher level of neonatal care, thus accruing higher hospitalized days and costs. Also, we observed a higher proportion of Hispanic and foreign-born mothers among children with non-isolated OFC compared to children with isolated OFC. Because our study only included live-born children, this difference could reflect differential access to prenatal diagnostics or rates of elective termination between Hispanic and non-Hispanic white women, as Hispanic women may be less likely to terminate a pregnancy due to birth defects (Jones et al., 2002).
Another limitation of our study was the use of a statewide cost-to-charge ratio to convert inpatient charges to costs, which may be problematic due to variability in cost mark-up between hospitals and departments within a hospital (Rogowski et al., 1999). Also, 2001 cost-to-charge ratios were used to impute the missing cost-to-charge ratios for 1998–2000 because these ratios were unavailable for years prior to 2001. Thus, costs for hospitalizations during 1998–2000 are a slight underestimate given that cost-to-charge ratios have decreased over time. Another limitation is that hospital discharge data only include facility fees and thus professional fees are excluded. The implication is that hospitalization costs per child could be understated by about one-fifth in the present analysis, based on an analysis of claims data from California (Rogowski et al., 1998).
An additional limitation was that some birth hospitalization costs may have been applied to the mothers’ hospital discharge records. Because we did not have access to maternal delivery records, we could not account for any costs on the maternal delivery record related to the child. Also, some of our results must be interpreted with caution due to small numbers and wide confidence intervals. Lastly, our study only reflects information from one state and may not be generalizable to other states and the U.S. population.
Strengths of our study included the use of data from the FBDR, which included multiple sources of birth defects ascertainment (Salemi et al., 2010; Salemi et al., 2011; Salemi et al., 2012). Our study used a combination of statewide, population-based registry data linked to longitudinal hospital data. While not actively ascertained, the FBDR’s overall completeness of ascertainment of birth defects, including OFC, is high, about 88%, although ascertainment varies by specific birth defect (Salemi et al., 2011; Salemi et al., 2012). Although our study only included one state, Florida was the fourth most populous state in 2010, ranked fourth in annual number of live births in the United States and has one of the highest rates of in- and out-migration (Martin et al., 2012; Perry, 2003; Schachter, 2004). Additionally, in 2010, Florida ranked third in annual live births to Hispanic women and first in annual live births to African-American women (Martin et al., 2012). We also were able to convert charges to current dollar costs. Finally, we were able to analyze the associations between high resource use and a wide variety of maternal, child, and other characteristics by OFC subtype, which was another strength of the study.
Finally, our findings can inform planning for hospital resources, treatment, and management of OFC. Furthermore, this information may be helpful for guiding clinicians, researchers, and public health officials concerned with treatment, management, and service planning for children with OFC, including counseling of an OFC prenatal diagnosis. Our study is important and fits with studies of other health outcomes for this population because it may be likely that the higher users of hospital care during infancy will have higher service use throughout their childhood and lifetime.
CONCLUSIONS
We observed longer hospitalizations and higher inpatients costs during the birth hospitalization and hospitalizations for the first two years of life for children with non-isolated OFC compared to children with isolated OFC, with the greatest resource use for children with CP. Children with CP had the greatest hospital resource use and the presence of other major birth defects was the most important predictor of high resource use, although factors, such as preterm birth and low birth weight, may be predictors of high resource use for some subsets of children. This study highlights the use of linking existing birth defects surveillance data with hospital discharge data to evaluate the impact of birth defects on the healthcare system.
ACKNOWLEDGEMENTS
The authors thank the March of Dimes Foundation for providing funding for various aspects of this project (Grant #: 5-FY09-533). The authors also thank the entire staff of the FBDR within the FDOH, the Children's Medical Services Program, and the Florida AHCA. Without these agencies, these data could not have been obtained. We also thank Jason Salemi, PhD, MPH, with the University of South Florida and Marie Bailey, MA, with FDOH for consultations on data linkages and variables. We also thank Adrienne Henderson, MPH, and Gloria Barker with AHCA, Florida Center for Health Information and Policy Analysis, and Karen Freeman, MPH, MS, with FDOH for consultations on hospital discharge data and hospitals.
Funding Source: Research Grant #5-FY09-533 from the March of Dimes Foundation supported various aspects of this project.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Abbott MM, Alkire BC, Meara JG. The value proposition: using a cost improvement map to improve value for patients with nonsyndromic, isolated cleft palate. Plast Reconstr Surg. 2011;127(4):1650–1658. doi: 10.1097/PRS.0b013e318208d25e. [DOI] [PubMed] [Google Scholar]
- Abbott MM, Meara JG. A microcosting approach for isolated, unilateral cleft lip care in the first year of life. Plast Reconstr Surg. 2011;127(1):333–339. doi: 10.1097/PRS.0b013e3181f95af3. [DOI] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP) [Accessed June 26, 2013];Cost-to-charge ratio files: 2010 Central Distributor State Inpatient Database. 2013 Available at: http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp.
- American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics. 2012;130(3):587–597. doi: 10.1542/peds.2012-1999. [DOI] [PubMed] [Google Scholar]
- American Cleft Palate-Craniofacial Association. Parameters for evaluation and treatment of patients with cleft lip/palate or other craniofacial anomalies. [Accessed May 19, 2014];2009 Available at: http://www.acpa-cpf.org/uploads/site/Parameters_Rev_2009.pdf. [Google Scholar]
- Basseri B, Kianmahd BD, Roostaeian J, et al. Current national incidence, trends, and health care resource utilization of cleft lip-cleft palate. Plast Reconstr Surg. 2011;127(3):1255–1262. doi: 10.1097/PRS.0b013e3182043af6. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Grosse SD, Honein MA, et al. Children with orofacial clefts: health-care use and costs among a privately insured population. Public Health Rep. 2009;124(3):447–453. doi: 10.1177/003335490912400315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell CH, Meyer R, Daniels J. Health care expenditures among Medicaid enrolled children with and without orofacial clefts in North Carolina, 1995–2002. Birth Defects Res A Clin Mol Teratol. 2008;82(11):785–794. doi: 10.1002/bdra.20522. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(2):25–29. [PubMed] [Google Scholar]
- Colvin L, Bower C. A retrospective population-based study of childhood hospital admissions with record linkage to a birth defects registry. BMC Pediatrics. 2009;9:32. doi: 10.1186/1471-2431-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleyiannis FW, TeBockhorst S, Castro D. The financial impact of multidisciplinary cleft care: an analysis of hospital revenue to advance program development. Plast Reconstr Surg. 2013;131(3):615–622. doi: 10.1097/PRS.0b013e31827c6ffb. [DOI] [PubMed] [Google Scholar]
- Florida Agency for Health Care Administration. Florida Agency for Health Care Administration, Disclaimer. [Accesed July 29, 2014];2013 Available at: http://www.floridahealthfinder.gov/CompareCare/Disclaimer.aspx.
- Johnson C, Honein M, Hobbs C, et al. Prenatal diagnosis of orofacial clefts, National Birth Defects Prevention Study, 1998–2004. Prenat Diagn. 2009;29(9):833–839. doi: 10.1002/pd.2293. [DOI] [PubMed] [Google Scholar]
- Jones RK, Darroch JE, Henshaw SK. Patterns in the socioeconomic characteristics of women obtaining abortions in 2000–2001. Perspect Sex Reprod Health. 2002;34(5):226–235. [PubMed] [Google Scholar]
- Kotelchuck M. The adequacy of Prenatal Care Utilization Index: its US distribution and association with low birthweight. Am J Public Health. 1994;84(9):1486–1489. doi: 10.2105/ajph.84.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois PH, Canfield MA, Swartz MD. Poisson versus logistic regression in a descriptive epidemiologic analysis of data from a birth defects registry. Birth Defects Res A Clin Mold Teratol. 2013;97(10):702–707. doi: 10.1002/bdra.23167. [DOI] [PubMed] [Google Scholar]
- Mai CT, Cassell CH, Meyer RE, et al. for the National Birth Defects Prevention Network. Birth defects data from population-based birth defects surveillance programs in the United States, 2007–2011: highlighting orofacial clefts. Birth Defects Res A clin Mol Teratol. 2014 doi: 10.1002/bdra.23329. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep. 2012;61(1):1–72. [PubMed] [Google Scholar]
- Nguyen C, Hernandez-Boussard T, Davies SM, et al. Cleft palate surgery: an evaluation of length of stay, complications, and costs by hospital type. Cleft Palate Craniofac J. 2014;51(4):412–419. doi: 10.1597/12-150. [DOI] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, et al. for the National Birth Defects Prevention Network. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Perry MJ. State-to-state migration flows: 1995–2000. [Accesed May 30, 2014];U.S. Census Bureau. 2003 Available at: http://www.census.gov/prod/2003pubs/censr-8.pdf. [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- Robbins J, Damiano P, Druschel C, et al. Prenatal diagnosis of orofacial clefts: association with maternal satisfaction, team care, and treatment outcomes. Cleft Palate Craniofac J. 2010;47(5):476–481. doi: 10.1597/08-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski J. Cost-effectiveness of care for very low birth weight infants. Pediatrics. 1998;102(1 Pt 1):35–43. doi: 10.1542/peds.102.1.35. [DOI] [PubMed] [Google Scholar]
- Rogowski J. Measuring the cost of neonatal and perinatal care. Pediatrics. 1999;103(1 Suppl E):329–335. [PubMed] [Google Scholar]
- Russo CA, Elixhauser A. Hospitalizations for birth defects, 2004. Rockville: U.S. Agency for Healthcare Research and Quality; 2007. pp. 1–9. Report number 24. [PubMed] [Google Scholar]
- Salemi JL, Hauser KW, Tanner JP, et al. Developing a database management system to support birth defects surveillance in Florida. J Registry Manag. 2010;37(1):10–15. [PubMed] [Google Scholar]
- Salemi JL, Tanner JP, Bailey M, et al. Creation and evaluation of a multi-layered maternal and child health database for comparative effectiveness research. J Registry Manag. 2013;40(1):14–28. [PubMed] [Google Scholar]
- Salemi JL, Tanner JP, Block S, et al. The relative contribution of data sources to a birth defects registry utilizing passive multisource ascertainment methods: does a smaller birth defects case ascertainment net lead to overall or disproportionate loss? J Registry Manag. 2011;38(1):30–38. [PubMed] [Google Scholar]
- Salemi JL, Tanner JP, Kennedy S, et al. A comparison of two surveillance strategies for selected birth defects in Florida. Public Health Rep. 2012;127(4):391–400. doi: 10.1177/003335491212700407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter JP. Geographical Mobiltiy: 2002–2003. [Accesed May 30, 2014];U.S. Census Bureau. 2004 Available at: http://www.census.gov/prod/2004pubs/p20-549.pdf.
- U.S. Bureau of Labor Statistics. Producer Price Index industry data: hospitals. [Accesed April 30, 2013];Series PCU622. 2013 Available at: http://www.bls.gov/cpi.
- Watkins S, Meyer R, Strauss R, et al. Classification, epidemiology, and genetics of orofacial clefts. Clin Plast Surg. 2014;41(2):149–163. doi: 10.1016/j.cps.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Weiss J, Kotelchuck M, Grosse SD, et al. Hospital use and associated costs of children aged zero-to-two years with craniofacial malformations in Massachusetts. Birth Defects Res A Clin Mol Teratol. 2009;85(11):925–934. doi: 10.1002/bdra.20635. [DOI] [PubMed] [Google Scholar]
- Yazdy MM, Honein MA, Rasmussen SA, et al. Priorities for future public health research in orofacial clefts. Cleft Palate Craniofac J. 2007;44(4):351–357. doi: 10.1597/06-233.1. [DOI] [PubMed] [Google Scholar]
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]