Abstract
The neuropeptide oxytocin may be an effective therapeutic strategy for the currently untreatable social and communication deficits associated with autism. Our recent paper reported that oxytocin mitigated autistic behavioral deficits through the restoration of activity in the ventromedial prefrontal cortex (vmPFC), as demonstrated with functional magnetic resonance imaging (fMRI) during a socio-communication task. However, it is unknown whether oxytocin exhibited effects at the neuronal level, which was outside of the specific task examined. In the same randomized, double-blind, placebo-controlled, within-subject cross-over clinical trial in which a single dose of intranasal oxytocin (24 IU) was administered to 40 men with high-functioning autism spectrum disorder (UMIN000002241/000004393), we measured N-acetylaspartate (NAA) levels, a marker for neuronal energy demand, in the vmPFC using 1H-magnetic resonance spectroscopy (1H-MRS). The differences in the NAA levels between the oxytocin and placebo sessions were associated with oxytocin-induced fMRI signal changes in the vmPFC. The oxytocin-induced increases in the fMRI signal could be predicted by the NAA differences between the oxytocin and placebo sessions (P=0.002), an effect that remained after controlling for variability in the time between the fMRI and 1H-MRS scans (P=0.006) and the order of administration of oxytocin and placebo (P=0.001). Furthermore, path analysis showed that the NAA differences in the vmPFC triggered increases in the task-dependent fMRI signals in the vmPFC, which consequently led to improvements in the socio-communication difficulties associated with autism. The present study suggests that the beneficial effects of oxytocin are not limited to the autistic behavior elicited by our psychological task, but may generalize to other autistic behavioral problems associated with the vmPFC.
Introduction
Cumulative evidence has demonstrated that oxytocin significantly affects social and affiliated behaviors.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Although oxytocin may also promote aggression and other antisocial behaviors in certain circumstances,1,11,12 previous studies have suggested the enhancing effect of oxytocin on social behavior in neuropsychiatric disorders, such as autism spectrum disorders (ASD).13, 14, 15, 16, 17, 18, 19
To evaluate the potential therapeutic effects of oxytocin on the social deficits associated with ASD, we conducted a double-blind, cross-over, randomized controlled trial by administering a single dose of oxytocin to 40 high-functioning men with ASD. We then performed both functional magnetic resonance imaging (fMRI) and 1H-magnetic resonance spectroscopy (1H-MRS). As the main outcome of the clinical trial, we recently reported that intranasal oxytocin increased the frequency of judgments based on non-verbal communication information by increasing originally diminished brain activity using fMRI.20 This finding is concordant with the results from prior behavioral studies that reported the beneficial effects of oxytocin on the social deficits associated with ASD.19,21, 22, 23, 24, 25 However, this significant increase in originally diminished brain activity was quantified as a change in the blood-oxygen-level-dependent signal during a socio-communication psychological task measured by fMRI. Thus, it remains unknown whether there are the effects of oxytocin on neurochemical that preceded the effects of oxytocin observed during fMRI.
To address this issue, we aimed to identify the neurochemical influence of oxytocin not directly related to any psychological task, assuming that such influence would trigger a task-specific fMRI signal change. Using 1H-MRS, we examined the influence of oxytocin on N-acetylaspartate (NAA) levels. Altered NAA levels measured with 1H-MRS are thought to be one of the most robust metabolite abnormalities underlying the neurochemical aspects of ASD pathophysiology.26,27 Together with its derivative, NAA synthesized in neurons acts on endothelial cells and modulates regional blood flow to supply energy to neurons.28 Thus, although the NAA levels are also influenced by the density of neurons in the region,29 the regional levels of NAA or differences in these levels reflect the regional demand of neuronal energy.30,31
A number of previous studies have demonstrated that the ventromedial prefrontal cortex (vmPFC)/anterior cingulate cortex (ACC) is involved in a variety of social cognitive components disturbed in individuals with ASD, such as empathy, emotion recognition and theory of mind.32, 33, 34 In fact, a line of previous fMRI and 1H-MRS studies demonstrated the dysfunction of this region in individuals with ASD.26,34, 35, 36, 37 Additionally, our recent systematic screening and meta-analysis showed that the vmPFC/ACC has received the most attention in 1H-MRS studies of ASD.26 In addition, previous studies have suggested that the vmPFC/ACC is an area greatly affected by oxytocin.15,24,38,39 Therefore, we examined the vmPFC/ACC40 in the present clinical trial because it is involved in the pathophysiology of disturbed social cognition in individuals with ASD and is most sensitive to oxytocin.
In this context, we hypothesized that the influence of oxytocin on NAA levels in the vmPFC/ACC underlies our recently reported fMRI signal changes in the same brain region and the associated changes in behavior. If there are task-independent oxytocin's influences on neurochemical that underlie task-dependent blood-oxygen-level-dependent signal changes, this would support the possibility of broad clinical use of oxytocin in individuals with ASD. To test this possibility, we measured the NAA levels in the same clinical trial in the vmPFC/ACC immediately after the fMRI measurement in both the oxytocin and placebo conditions after a 1-week interval (Figures 1 and 2). We examined the relationship between the influence of oxytocin on the NAA levels and the oxytocin-induced changes in the task-dependent fMRI signal. We then conducted a path analysis to clarify the relationships between the oxytocin and NAA levels, the fMRI signal change and the observed behavioral changes.
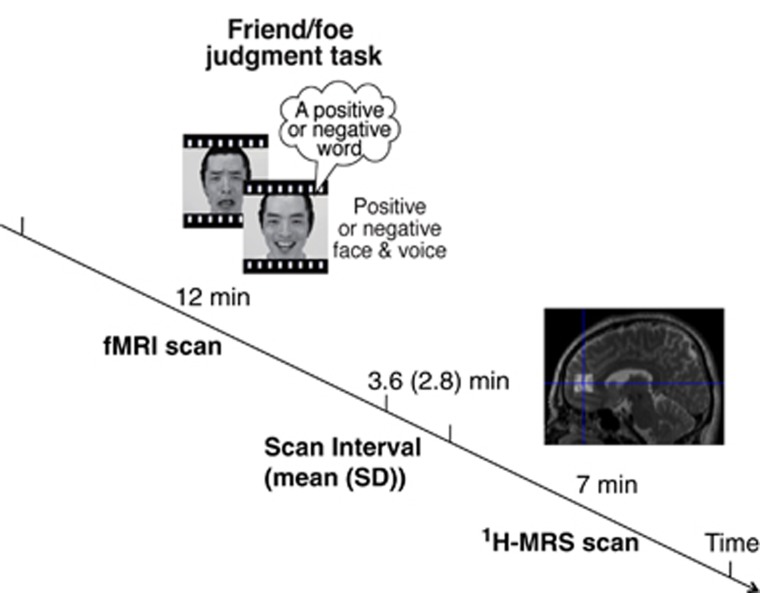
Figure 1.
Experimental design. Relationship between the timing of the 12-min functional magnetic resonance imaging (fMRI) scan and the 7-min magnetic resonance spectroscopy (MRS) scan. The interval between the end of the fMRI scan and the start of the MRS scan was 3.6±2.8 (mean±s.d.) min. During the fMRI scan, the participants were asked to make a ‘friend or foe' judgment of the actor in each movie based on the socio-communication content in which the verbal and non-verbal information conflicted.
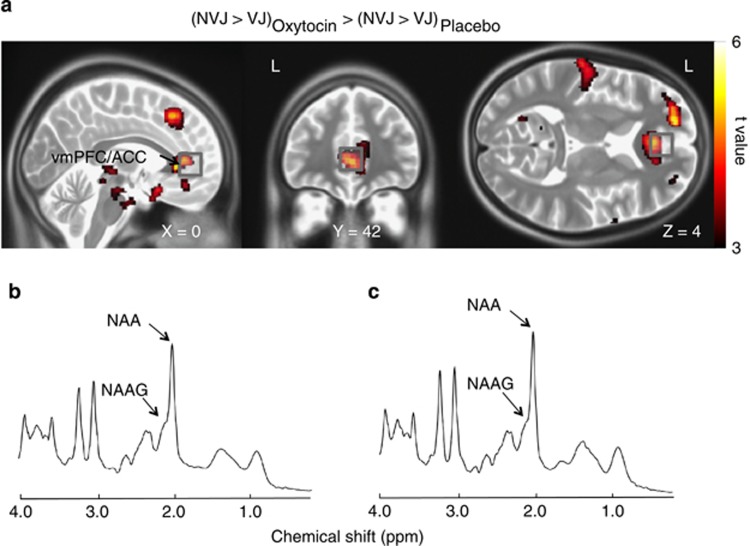
Figure 2.
Anatomical details between the 1H-magnetic resonance spectroscopy (1H-MRS) volume-of-interest (VOI) and functional magnetic resonance imaging (fMRI) signal change. (a) Brain regions that showed a significant effect of oxytocin on the fMRI signal associated with socio-communication behavior (non-verbal communication information-based judgment (NVJ)-specific activity>verbal communication information-based judgment (VJ)) (that is, the ventromedial prefrontal/anterior cingulate cortices (vmPFC/ACC) and the dorsomedial prefrontal cortex (dmPFC), P<0.001, uncorrected for the purpose of presentation) are overlaid on orthogonal slices. Gray squares represent the 1H-MRS VOIs (20 × 20 × 20 mm). Representative spectrum of (b) oxytocin and (c) placebo sessions in a study participant as fit by the LCModel.
Materials and methods
Participants and diagnoses
We used 1H-MRS, an outcome measure of the clinical trial (UMIN000002241/000004393), to assess the relationship between the influence of oxytocin on the neurochemicals measured by 1H-MRS and the oxytocin-induced changes in fMRI signals, which was recently reported as a main outcome of the clinical trial.20 Therefore, the descriptions of the study participants, how the sample size was determined and the diagnostic protocol are detailed in our previous paper (see the eMethods section ‘Participants' and ‘Diagnosis') (Supplementary Figure 1).20 Briefly, of the 323 individuals with probable ASD who visited The University of Tokyo Hospital or Showa University Karasuyama Hospital between 1 November 2009 and 30 April 2011, 40 high-functioning ASD men participated because of their firm diagnosis, age (⩾20 years), full-scale intelligence quotient (>80) and written consent. Of the 33 participants included in the main analysis in the recent fMRI report,20 two participants were also excluded from the main analysis here because of the low quality 1H-MRS spectra (see the eMethods section ‘Participants').
Randomized and masked drug administration
The method used for randomizing intranasal administration has been described elsewhere.20 Briefly, this study adopted a randomized, placebo-controlled, double-blind, within-subjects cross-over experimental design. The individuals with ASD received a single dose of oxytocin (24 IU; Syntocinon-Spray, Novartis, Basel, Switzerland)41, 42, 43 or placebo at a 1-week interval in a pseudo-random order 40 min before each MR scanning session (see the eMethods section ‘Drug administration').
Structural MRI acquisition, 1H-MRS acquisition and spectrum quantification
The scanning protocols for the structural MRI and 1H-MRS acquisition, post-processing procedures and quantification of the 1H-MRS spectra were the same as in our previous studies40,44 (see the eMethods section ‘Structural MRI acquisition'). Briefly, MRI data were obtained using a 3-T scanner (GE Signa HDxt, Waukesha, WI, USA). A stimulated echo acquisition model was applied to obtain the proton MR spectrum from a volume-of-interest (VOI) at the vmPFC. All spectra were quantified with LCModel (version 6.1-4F, Stephen Provencher, Oakville, ON, Canada), and the quality of each spectrum was assessed for inclusion in the analysis (see the eMethods sections ‘1H-MRS acquisition', ‘Spectrum quantification' and ‘Spectrum quality'). Because we had an a priori hypothesis for the influence of oxytocin on NAA levels, we also conducted an exploratory analysis of NAA and NAA plus N-acetylaspartylglutamate levels. For the exploratory analyses, we also measured other major metabolites, including creatine, glutamine plus glutamine, myo-inositol and choline-containing compounds.
Tissue segmentation within the VOI
Three-dimensional fast spoiled gradient-echo (3D-FSPGR) images were used to calculate the volumes of different tissue types (that is, the gray matter volume, the white matter volume and the cerebrospinal fluid) using the segmentation tool in SPM8 (www.fil.ion.ucl.ac.uk/spm). The method used for tissue segmentation was the same as in our previous studies (see the eMethods section ‘Tissue segmentation within VOI').40,44
fMRI task/stimuli, scanning and analysis
The fMRI task, stimuli, scanning45,46 and analysis20 have been reported in our recent publications. Details are provided in the Supplementary Information (see the eMethods section ‘fMRI task and stimuli' and ‘fMRI scanning').
Extraction of fMRI signals from the exact location for the 1H-MRS VOI and mPFC regions
Because it is important to examine metabolite/fMRI signal relationships in the same anatomical area, the average fMRI signal change was calculated for the 1H-MRS VOI, cubic with 20 × 20 × 20 mm (center of the VOI: x=0, y=42, z=4, the Montreal Neurological Institute coordinates). The effect of oxytocin on the fMRI signal change from the 1H-MRS VOI was examined by comparing the average fMRI signal between the oxytocin and placebo sessions using a paired t-test. Further, the potential associations between the oxytocin-induced fMRI signal change in the VOI and the change in socio-communication behavior were also investigated using Pearson's correlation analysis (that is, judgment of others' hostility primarily based on non-verbal communicative cues, such as facial expression and voice prosody, rather than the verbal content of speech). For exploratory purposes, we also extracted the average fMRI signal change from the two brain regions indicated by recently published fMRI data from the present clinical trial that exhibited significant effects from oxytocin administration, and these changes were associated with the socio-communication behavior described elsewhere (see the eMethods section ‘fMRI task and stimuli').20 The first area was the vmPFC/ACC (x=2, y=34, z=8), which partially overlapped with the 1H-MRS VOI, and the second area was the dorsomedial prefrontal cortex (x=0, y=30, z=52), which did not overlap with the VOI.
Statistical analysis
All statistical analyses were conducted using the Statistical Package for Social Science (SPSS) Version 21.0 and Amos Version 21.0 (SPSS Inc., Chicago, IL, USA). To assess the potential differences in the tissue composition within the VOIs, the spectrum quality and the metabolite levels between oxytocin and placebo sessions, we conducted paired t-tests.
Regression analyses between 1H-MRS and fMRI signals
Single linear regression analyses were performed to ascertain the effects of metabolite level differences between the oxytocin and placebo sessions and the extracted fMRI signal change in the VOI and two mPFC regions. Because we had an a priori hypothesis that differences in NAA levels between oxytocin and placebo sessions underlie the fMRI signal changes extracted from the 1H-MRS VOI in the vmPFC, the statistical threshold was set at P<0.05 for the VOI and at P<0.025 (=0.05/2, brain regions) for the other mPFC regions.
The effects of NAA level differences between the two pharmacological sessions on the behavioral changes during the fMRI task, which was significantly associated with the fMRI signal change in the 1H-MRS VOI (see Results), were also examined using a single linear regression analysis by regressing out the fMRI signal change in the VOI. The statistical threshold was set at P<0.05.
The statistical analysis of the associations between the other metabolite level differences with the fMRI signal is described in the Supplementary Information (see the eMethods section ‘Relationship between the influence of oxytocin on other metabolite levels and oxytocin-induced fMRI signal change').
Assessing the effects of potential confounding factors on the relationship between 1H-MRS and fMRI signals
The time interval between the fMRI and 1H-MRS scans may have influenced the relationship between oxytocin–placebo differences in NAA levels and fMRI signal changes. Thus, we subtracted the interval between the fMRI and 1H-MRS scans within each session and calculated the difference in the intervals between the two different pharmacological sessions. This variable was used to control for the effect of differences in the timing schedule in the trials.
The effects of the order of drug administration were assessed by conducting two regression analyses in the two independent groups: the group in which oxytocin was administered first and the other group in which placebo was administered first. Then, the difference in the regression coefficient between the two groups was assessed using Fisher's r to z transformation. To test whether the NAA–fMRI signal association remained after controlling for the effects of drug administration order, a single linear regression analysis of the oxytocin–placebo differences in NAA levels and fMRI signal changes was conducted in which the oxytocin/placebo administration order was a covariate (see the eMethods section ‘Explore whether both the oxytocin administered first group and the placebo administered first groups preserve significant NAA/fMRI signal relation').
In addition, we examined whether the relationship between NAA levels and fMRI signal changes was preserved after including participants who were excluded from the main analysis because of psychotropic medications or unusual behavior during the fMRI task (see the eMethods section ‘Exploring whether the result is preserved after expanding participants').
Path analysis
A path analysis was conducted to elucidate the multiple relationships between the influences of oxytocin on the NAA levels, the oxytocin-induced changes in fMRI signals and the effects of oxytocin on socio-communication behavior, with a standard maximum likelihood estimation. Pathways between these three variables were proposed and expressed quantitatively as path coefficients. The models constructed can be evaluated using multiple measures. The measures used were the goodness-of-fit index (GFI), a measure of the overall model fit, the adjusted GFI, which is the GFI adjusted for the degrees of freedom used to evaluate the overall model fit, and the Akaike information criteria. If the GFI and adjusted GFI values are greater than 0.90, the model is considered to have a good fit. The Akaike information criteria is a measure that compares more than two models with a good fit; a smaller value indicates a good fit. The fitness of residuals was assessed using the root mean square error of approximation (RMSEA), which is based on the non-centrality parameter, and specifically considers the approximation error. For the RMSEA, values<0.08 indicate a good fit. In general, when the model obtains more than two good scores, including the RMSEA, the model is considered to have a good fit.
Results
Relationship between the influence of oxytocin on NAA levels and oxytocin-induced fMRI signal changes
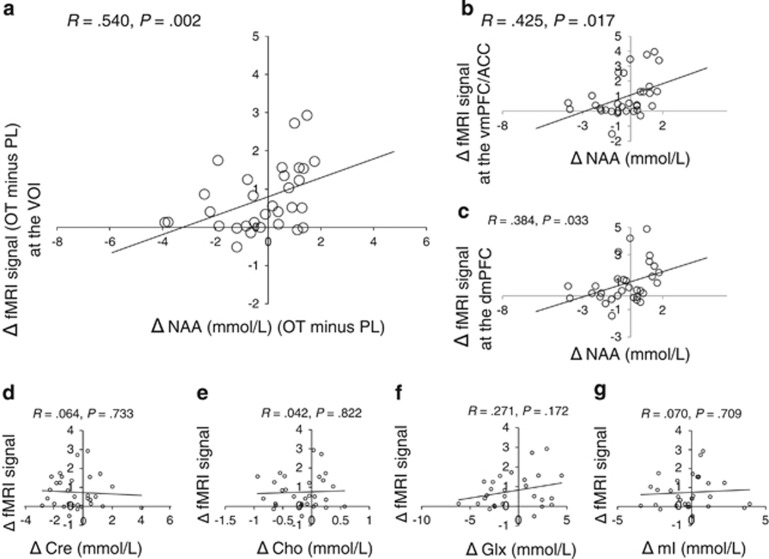
A paired t-test did not detect a significant difference in the spectral quality or tissue composition between the oxytocin and placebo sessions (P>0.088) (see the eResults section ‘Difference in spectral quality and tissue composition between two sessions'). Although significant differences in the NAA levels between the oxytocin and placebo conditions were not detected (t30=1.315, P=0.198; Supplementary Table 2), a linear regression analysis demonstrated a significant relationship between the influence of oxytocin on NAA levels and the oxytocin-induced changes in the fMRI signal from the vmPFC VOI (R=0.540, P=0.002, n=31; Figure 3a). It should be noted that the NAA–fMRI signal relationship was also observed with NAA+N-acetylaspartylglutamate, an alternative measure of NAA and its derivatives, instead of NAA alone (R=0.439, P=0.013, n=31; Supplementary Figure 2).
Figure 3.
Relationships between the oxytocin-induced differences in 1H-magnetic resonance spectroscopy (1H-MRS) measures and the changes in functional magnetic resonance imaging (fMRI) signal. Scatterplots show the relationships between the oxytocin-induced N-acetylaspartate (NAA) differences (NAA levels at oxytocin (OT) sessions minus NAA levels at placebo (PL) sessions) and the task-evoked fMRI signal changes in (a) the volume-of-interest (VOI), (b) the ventromedial prefrontal cortex (vmPFC)/anterior cingulate cortex (ACC) and (c) the dorsomedial prefrontal cortex (dmPFC). No significant relationship was detected between the changes in the fMRI signal and the differences in (d) creatine (Cre), (e) choline-containing compounds (Cho), (f) glutamine and glutamate (Glx) or (g) myo-Inositol (mI) levels.
A significant NAA–fMRI signal relationship was also observed in the vmPFC/ACC region (R=0.425, P=0.017, n=31; Figure 3b), but was not preserved in the dorsomedial prefrontal cortex after correction for multiple comparisons (R=0.384, P=0.033, n=31; Figure 3c). Importantly, although the location of the 1H-MRS VOI was not identical to that of the activation peak in the vmPFC/ACC that was detected in our recently published fMRI data from the present clinical trial,20 we also detected the same effects of oxytocin on the fMRI signal in the 1H-MRS VOI (that is, significantly increased fMRI signal, t30=4.875, P<0.001 and a significant positive correlation with increased use of judgments based on non-verbal communicative cues, R=0.853, P<0.001, n=31; Supplementary Figure 3).
The association between the fMRI signals and NAA levels remained significant in an additional regression analysis that controlled for the difference in the fMRI-1H-MRS scan interval between the oxytocin and placebo sessions (R=0.550, P=0.006, n=31), which indicated that this relationship does not depend on the time interval between the fMRI and 1H-MRS scans.
Furthermore, no significant difference in the regression coefficient between the group in which oxytocin was administered first and the group in which placebo was administered first was observed (R=0.237 and R=0.737, respectively, t=1.325, P=0.196), which indicated that there was no significant carry-over effect. In addition, the association between the fMRI signals and NAA levels also remained in the regression analysis that controlled for differences in the administration order (R=0.618, P=0.001, n=31), which indicated that the relationship does not rely on the order of drug administration. These findings suggest that possible neuronal changes during the 1-week interval are not necessary to observe the association between the NAA levels and the fMRI signal.
Furthermore, additional analyses demonstrated that the relationship between NAA and fMRI signal was preserved even when the subjects who were on medication or exhibited unusual behavior during the fMRI tasks were included (P<0.014) (see the eResults section ‘Relationship between oxytocin's influence on the other metabolite levels and the oxytocin-induced fMRI signal change'). In addition, associations between other metabolite levels and fMRI signal were not observed, including creatine (Figure 3d), choline-containing compounds (Figure 3e), glutamine plus glutamine (Figure 3f) and myo-inositol (Figure 3g) (P>0.172).
Statistical confirmation of the hypothesized model
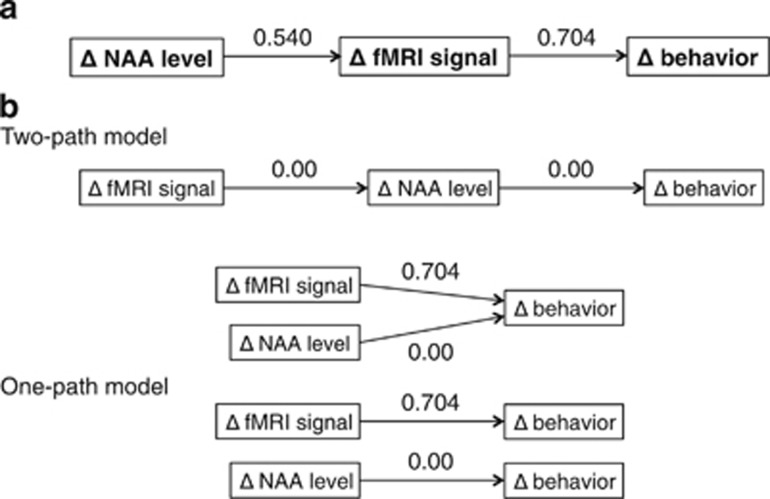
Although a correlation analysis demonstrated that the influence of oxytocin on NAA levels was significantly related to an increased frequency of judgments based on non-verbal information (R=0.454, P=0.010, n=31; Supplementary Figure 3), no significant relationship between these parameters was observed when the fMRI signal change was regressed out (R=0.124, P=0.515), which suggests that the influence of oxytocin on NAA levels is not directly linked to oxytocin-induced increases in the frequency of judgments based on non-verbal information. Thus, we hypothesized that differences in NAA levels underlie the fMRI changes and associated behavioral changes. A path analysis demonstrated that the hypothesized model had satisfactory indices of the GFI (GFI=0.990, adjusted GFI=0.940 and a RMSEA<0.001; Table 1). Furthermore, among the possible models that are consistent with the notion that the oxytocin-induced neural changes (that is, metabolite level and/or fMRI signal) underlie behavioral change,15 the hypothesized model had the smallest value of Akaike information criteria=10.462. These results suggest that the hypothesized model is the best fit model (Table 1; Figure 4).
Table 1. Results of the path analysis.
| Model | RMSEA | GFI | AGFI | AIC |
|---|---|---|---|---|
| Two paths model | ||||
| NAA→fMRI signal→behaviora | <0.001 | 0.990 | 0.940 | 10.462 |
| fMRI signal→NAA→behavior | 0.660 | 0.800 | −0.198 | 24.054 |
| NAA and fMRI signal→behavior | 0.558 | 0.837 | 0.024 | 20.333 |
| One path model | ||||
| NAA→behavior | 0.383 | 0.832 | 0.497 | 18.794 |
| fMRI signal→behavior | 0.611 | 0.729 | 0.188 | 32.387 |
Abbreviations: AGFI, adjusted goodness-of-fit index; AIC, Akaike information criterion; fMRI, functional magnetic resonance imaging; GFI, goodness-of-fit index; NAA, N-acetylaspartate; RMSEA, root mean square error of approximation.
Optimal model.
Figure 4.
Statistical confirmation of the hypothesized model. (a) A hypothesized model of the relationship between NAA level differences, fMRI signal changes and behavioral changes. Path coefficients (that is, standardized partial regression coefficients) are written above each arrow. (b) The model shown is more likely compared with all other possible models that are based on the hypothesis that oxytocin-induced behavioral changes are mediated by neural changes.
Discussion
The crucial finding in the present study is that the influence of oxytocin on NAA levels in the vmPFC/ACC, which is not confined to a specific psychological task, underlies the oxytocin-induced increase of originally diminished task-specific fMRI signal in the same brain region. Although a single dose of intranasal oxytocin administration did not significantly alter the NAA levels, the present findings suggest an important role for the vmPFC/ACC in the neural mechanisms of oxytocin-induced behavioral improvements in the socio-communication deficits associated with ASD. Importantly, the current findings cannot be directly extended to female subjects with ASD, ASD subjects with intellectual disabilities or typically developed individuals.
The fMRI signal change induced by oxytocin was related to differences in the NAA levels, but not with other metabolite changes. Regional NAA levels are determined by both static structural factors (for example, regional neuronal density29) and dynamic functional factors (for example, synthesis of NAA in each neuron that responds to neuronal energy demand).30,38 In the current study, we obtained the NAA levels in two pharmacological conditions with a 1-week interval in the same brain region in the same individual, suggesting that these changes are less likely due to structural changes. The sub-analyses in the current study also indicated no significant difference in the association between NAA levels and fMRI signal between the two groups that differed in the order of oxytocin administration. Although a 1-week interval may cause slight structural changes, the current results indicate that the significance of the relationship between the fMRI signal change and the differences in NAA levels remained regardless of the 1-week interval. It is possible for oxytocin-associated structural changes to occur within the interval between oxytocin administration and the 1H-MRS scan (mean±s.d.: 87.5±10.3 min), but it may be more likely that the difference in the NAA levels between the different pharmacological conditions reflects a difference in dynamic function rather than static structural factors. Previous studies also support this notion, suggesting that repeated evaluations of the NAA levels within a short interval (that is, 3–4 min) in the same brain region of the same individual may change because of dynamic changes in neuronal energy demand.30 Previous studies have also reported potential rapid changes in NAA levels (for example, within 10 min).30 Prior studies have also reported that a single intranasal administration of oxytocin induced an increase in plasma oxytocin levels within 30–150 min.47 We measured the NAA levels ~90 min after the intranasal administration of oxytocin/placebo. Thus, it can be assumed that there was sufficient time for changes in the NAA levels to occur between the oxytocin administration and the 1H-MRS scan, and that there may be an effect of oxytocin during the 1H-MRS scan. Taken together, the current finding suggests that intranasal oxytocin may influence the vmPFC/ACC neuronal energy demand that modulates the differences in NAA levels underlying the fMRI signal changes.
In the present study, we aimed to scan the participants during the period when oxytocin has the greatest influence in the brain. To achieve this purpose, we have traded off the temporal specificity against spatial specificity. Thus, we cannot conclude that the association of NAA levels with fMRI signal is regionally specific to the vmPFC/ACC by the current single-voxel 1H-MRS without a control VOI. However, the current study findings are consistent with the hypothesis that oxytocin may affect social interaction through the modulation of vmPFC/ACC function as a key site of a prefrontal-limbic circuit15,20,39,48,49 and reports a new result on the effect of oxytocin at the neurochemical level.
Because we obtained metabolite levels after the fMRI scan, task-specific neural activation may have affected NAA levels. Thus, both the NAA levels and fMRI signal change may reflect neural activation due to the specific psychological task. There are two arguments against this potential confound. First, NAA levels have previously been suggested to decrease during stimulation and begin to recover to baseline levels soon after the stimulation ends (that is, 5–6 min).30 In the current study, there was a 3.6-min interval between the end of the fMRI scan and the start of the 1H-MRS scan, which may be an adequate time to return to baseline NAA levels during the 7-min 1H-MRS scan. Thus, it is expected that there was little residual influence of the stimulation during fMRI on the NAA levels. In addition, the path analysis suggested that our hypothesized model statistically conforms more to three variables, including the NAA level differences, the fMRI signal changes and the behavioral changes, compared with the model in question, which assumes that the NAA differences were induced by fMRI changes. Although a limited number of previous studies have investigated the relationship between metabolite levels measured by MRS and the fMRI signal,50, 51, 52 taken together, the current findings suggest that the influence of oxytocin on NAA levels may underlie the fMRI signal and behavioral changes.
Although no significant difference was observed in the metabolite levels between the oxytocin and placebo sessions, the global trend was that all metabolites except choline-containing compounds were higher in the placebo session compared with the oxytocin session, which suggests that oxytocin induced global physiological changes in the brain. Multiple regression analysis showed a significant relationship between the differences in all major metabolites (NAA, creatine, myo-inositol, choline-containing compounds and glutamine plus glutamine) and fMRI signal changes; however, this association disappeared when NAA was excluded from the analysis (see eMethods and eResults). These results suggest that differences in NAA levels have an essential role in predicting fMRI signal change. However, because in vivo NAA is associated with water transportation, it is possible that oxytocin administration induced global physiological changes, such as global changes in brain water content. Future studies that address whether oxytocin induces global physiological effects in the brain should be performed.
Other factors may explain the association between the NAA/fMRI signal change other than oxytocin, such as motion artifact, system drift over time and global changes in the participants. Among these factors, we confirmed that there was no significant effect of motion artifacts during fMRI on the association between the NAA/fMRI signal changes. Magnetic field inhomogeneity in our scanner was monitored with daily basic quality control. In addition, there was no significant influence of system drift over time on the association between the NAA/fMRI signal change (see eMethods and eResults). In terms of global changes in the participants, we scanned all participants under approximately the same conditions in the same scanner at nearly the same time; further, all pairs of sessions were performed at a 1-week interval. We confirmed that there was no exacerbation of mental health problems during this clinical trial in any of the participants by a trained psychiatrist (HY) before each MRI scan during the sessions, which suggests that there were no global changes in the participants at the clinical level.
The absence of a significant effect of oxytocin on NAA levels prevents us from reporting the usability of 1H-MRS as a functional paradigm to detect the effect of oxytocin on the brain. A potential explanation for the absence of group level effects of oxytocin on NAA levels is that heterogeneity in the pathophysiology of ASD among participants may result in heterogeneous responses to oxytocin administration. For example, the single nucleotide polymorphism rs2254298A in the oxytocin receptor gene (OXTR), a risk allele for ASD in the Asian population,53 is associated with small gray matter volume in the vmPFC/ACC.49 Variance in the CD38 gene, which codes for a protein involved in oxytocin secretion processes,6 influences oxytocin plasma levels, social behavior and the response to externally administered oxytocin.54 In sum, it is assumed that individuals with ASD who have risk alleles in the OXTR and those in the CD38 genes exhibit different responses to oxytocin, and heterogeneity in the potential etiology may result in the absence of group level effects of oxytocin. However, to address this speculation and explore other possibilities, future studies that examine the relationship between genetic polymorphisms and the response to externally administered oxytocin should be performed.
The current study demonstrated that the influence of oxytocin on NAA levels in the vmPFC/ACC does not depend on specific psychological tasks, but instead underlies the oxytocin-induced increase of originally diminished task-specific fMRI signals in the vmPFC/ACC in adult males with ASD. These findings suggest the possibility that a single dose of intranasal oxytocin induces a general (that is, task independent) change in the regional neuronal energy demand in the vmPFC/ACC. Because this brain region is putatively involved in a variety of cognitive deficits observed in individuals with ASD,34, 35, 36 the results of the present study indicate that the potential benefits of oxytocin are not limited to specific autistic behavior elicited in a psychological task, but may be expanded to a relatively broad range of autistic behavioral problems involved in vmPFC/ACC dysfunction in adult males with high-functioning ASD.
Acknowledgments
HY had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of data analysis. We thank Drs Hideyuki Inoue, Yasumasa Nippashi, Motomu Suga, Yoshitsugu Yanagihara and Miho Kuroda for their support of this study. A part of this study is a result of the ‘Development of biomarker candidates for social behavior' project under the Strategic Research Program for Brain Sciences by the MEXT. We acknowledge the support provided by KAKENHI (22689034 to HY) and a grant from the Japan Society for the Promotion of Science Research Foundation for Young Scientists (222882 to TW).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Guastella A, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick K, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol. 2011;32:426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders. Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Kim H, Sherman D, Sasaki J, Xu J, Chu T, Ryu C, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc Natl Acad Sci USA. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, et al. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci USA. 2014;111:1987–1992. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Barth ME, Johnson SL, Gotlib IH, Johnson SC. Oxytocin receptor (oxtr) polymorphisms and attachment in human infants. Front Psychol. 2011;2:200. doi: 10.3389/fpsyg.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci USA. 2011;108:19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Scheele D, Kendrick KM, Becker B, Schafer L, Schwalba K, et al. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA. 2012;109:18144–18149. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Neuroscience. The promise and perils of oxytocin. Science. 2013;339:267–269. doi: 10.1126/science.339.6117.267. [DOI] [PubMed] [Google Scholar]
- Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJMH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Van IMH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Yee JR, Hurlemann R, Rilling JK, Chen FS, Meyer-Lindenberg A, et al. Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction. J Neurosci. 2012;32:14109–14117. doi: 10.1523/JNEUROSCI.3327-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, Olivier B. Intranasal administration of oxytocin: Behavioral and clinical effects, a review. Neurosci Biobehav Rev. 2013;37:1445–1465. doi: 10.1016/j.neubiorev.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23:123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry. 2014;71:166–175. doi: 10.1001/jamapsychiatry.2013.3181. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74:164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry. 2012;2:e69. doi: 10.1038/tp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, Critchley HD, Schmitz N, McAlonan G, Van Amelsvoort T, Robertson D, et al. Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry. 2002;59:885–891. doi: 10.1001/archpsyc.59.10.885. [DOI] [PubMed] [Google Scholar]
- Baslow MH. The vertebrate brain, evidence of its modular organization and operating system: insights into the brain's basic units of structure, function, and operation and how they influence neuronal signaling and behavior. Front Behav Neurosci. 2011;5:5. doi: 10.3389/fnbeh.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager T, Hansen A, Laursen H. Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:780–788. doi: 10.1097/00004647-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Hrabe J, Guilfoyle DN. Dynamic relationship between neurostimulation and N-acetylaspartate metabolism in the human visual cortex: evidence that NAA functions as a molecular water pump during visual stimulation. J Mol Neurosci. 2007;32:235–245. doi: 10.1007/s12031-007-0049-9. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci. 2012;7:727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Soc Neurosci. 2011;6:22–30. doi: 10.1080/17470911003683185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, et al. Neural signatures of autism. Proc Natl Acad Sci USA. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Geda E, Sacco K, D'Agata F, Duca S, Geminiani G, et al. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J Neurol Neurosurg Psychiatry. 2011;82:1304–1313. doi: 10.1136/jnnp.2010.239111. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology. 2013;38:962–974. doi: 10.1016/j.psyneuen.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Abe O, Yahata N, Kuwabara H, Natsubori T, Iwashiro N, et al. Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Transl Psychiatry. 2012;2:e178. doi: 10.1038/tp.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y, et al. Reduced frontal glutamate+glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia Schizophr Bulladvance online publication, 10 September 2013;PMID: 24023251 (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Watanabe T, Yahata N, Abe O, Kuwabara H, Inoue H, Takano Y, et al. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PLoS One. 2012;7:e39561. doi: 10.1371/journal.pone.0039561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yahata N, Kawakubo Y, Inoue H, Takano Y, Iwashiro N, et al. Network structure underlying resolution of conflicting non-verbal and verbal social information. Soc Cogn Affect Neurosci. 2014;9:767–775. doi: 10.1093/scan/nst046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Grunder G, et al. Oxytocin plasma concentrations after single intranasal oxytocin administration - a study in healthy men. Neuropeptides. 2012;46:211–215. doi: 10.1016/j.npep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Suga M, Yahata N, Inoue H, Tochigi M, Abe O, et al. Reply to: neurogenetic effects of OXTR rs2254298 in the extended limbic system of healthy caucasian adults. Biol Psychiatry. 2011;70:e41–e42. doi: 10.1016/j.biopsych.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Hutcheson NL, Reid MA, White DM, Kraguljac NV, Avsar KB, Bolding MS, et al. Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res. 2012;140:136–142. doi: 10.1016/j.schres.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N, Schweinsburg B, Cohen D, Müller R, Courchesne E. N-acetyl aspartate in autism spectrum disorders: regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97. doi: 10.1016/j.brainres.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Yamasue H. Function and structure in social brain regions can link oxytocin-receptor genes with autistic social behavior. Brain Dev. 2013;35:111–118. doi: 10.1016/j.braindev.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Sauer C, Montag C, Worner C, Kirsch P, Reuter M. Effects of a common variant in the CD38 gene on social processing in an oxytocin challenge study: possible links to autism. Neuropsychopharmacology. 2012;37:1474–1482. doi: 10.1038/npp.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.