Abstract
Objective
To compare analgesia provided by carprofen or tramadol in dogs after enucleation.
Design
Randomized, masked trial
Animals
Forty-three dogs
Procedures
Client-owned dogs admitted for routine enucleation were randomly assigned to receive either carprofen or tramadol orally 2 hours prior to surgery and 12 hours after the first dose. Dogs were scored for pain at baseline, and postoperatively at 0.25, 0.5, 1, 2, 4, 6, 8, 24, and 30 hours after extubation. Dogs received identical premedication and inhalation anesthesia regimens, including premedication with hydromorphone. If the total pain score was ≥9, if there was a score ≥ 3 in any one category, or if the visual analog scale score (VAS) was ≥35 combined with a palpation score of >0, rescue analgesia (hydromorphone) was administered and treatment failure was recorded. Characteristics between groups were compared with a Student’s t-test and Fisher’s exact test. The incidence of rescue was compared between groups using a log rank test. Pain scores and VAS scores between groups were compared using repeated measures ANOVA.
Results
There was no difference in age (p=0.493), gender (p=0.366) or baseline pain scores (p=0.288) between groups. Significantly more dogs receiving tramadol required rescue analgesia (6/21) compared to dogs receiving carprofen (1/22; p=0.035). Pain and VAS scores decreased linearly over time (p=0.038, p<0.001, respectively). There were no significant differences in pain (p=0.915) or VAS scores (p=0.372) between groups at any time point (dogs were excluded from analysis after rescue).
Conclusions and Clinical Relevance
This study suggests that carprofen, with opioid premedication, provides more effective post-operative analgesia than tramadol in dogs undergoing enucleation.
Enucleation is one of the more common ophthalmic surgeries performed in both general and specialty veterinary practices. This procedure is often performed due to an intractably painful eye, secondary to glaucoma, corneal rupture, and other causes. The surgical procedure itself is also painful for dogs, considering that the adnexa, globe and orbit are richly innervated with sensory nerves.1 Animals with post-operative ocular pain may self-traumatize, which can lead to undesirable postoperative complications such as dehiscence and/or secondary infection. Few studies have addressed the management of postoperative pain in ocular surgery; however, local anesthetic infiltration of the retrobulbar space has been shown to provide excellent analgesia after enucleation in dogs.2 This published technique3, however, requires some technical skill and may not be an analgesic method that many veterinarians would feel competent using without prior training.
Among analgesic drugs, non-steroidal anti-inflammatory drugs (NSAIDs) remain the most popular for oral administration for dogs. There are, however, several oral analgesic medications with different mechanisms of action that are gaining popularity for the treatment of post-surgical pain in dogs, but studies of their actual efficacy are limited. Among those medications, tramadol is the most intriguing, as its metabolites have mechanisms of action that suggest it is a multimodal analgesic that targets many points along the pain processing pathway. Tramadol has been available in oral and injectable formulations outside the USA for many years. It is available in the oral formulation in the USA and has quickly gained popularity as an analgesic for dogs with both acute and chronic pain. Tramadol is an isomeric drug, of which the (+) enantiomer is a weak mu opioid agonist with analgesic potency about 1/10th that of morphine.4,5 In addition, the (+) enantiomer acts within the spinal cord dorsal horn to inhibit serotonin reuptake, thereby providing analgesia in much the same way that the SSRI drugs do.5 The (-) enantiomer of tramadol inhibits norepinephrine reuptake in the spinal cord dorsal horn, thus providing yet another mechanism for analgesia.6 Early research indicated when given orally to dogs, at doses of 4 mg/kg, tramadol achieves therapeutic plasma levels within 5 minutes and persists in plasma at measurable concentrations for between 5 – 10 hours.7 More recent research showed that at a dose of 10mg/kg given orally to dogs, many of the metabolites thought to be important for opioid-mediated analgesia achieved very low plasma concentrations, suggesting that the reported analgesic effects may be independent of opioid activity.8 Despite tramadol’s interesting mechanisms, little is published regarding its analgesic efficacy in dogs when given orally for either post-surgical pain or chronic pain. Due to its ease of use and safety profile, many practitioners use tramadol commonly despite limited and conflicting data as to its efficacy.
In comparison to tramadol, there are many published reports of carprofen’s efficacy for moderate post-surgical pain in dogs. All of these studies compare carprofen to analgesic drugs that are generally considered to be of moderate effectiveness.9-12 Carprofen provides analgesia by reducing prostaglandin synthesis in injured tissues by virtue of cyclooxygenase II inhibition.13 Because this drug may also inhibit cyclooxygenase I, there is the potential for carprofen to cause renal and hepatic toxicity, GI ulceration or upset, and inhibit platelet adhesion. Because of these side effects, and because of its relatively moderate analgesic effects, carprofen should not be used in animals with renal or hepatic disease or as the single analgesic in those undergoing major surgical procedures.
Although it is reasonable to assume that enucleation is a painful procedure, evaluation of this pain in canine patients can be challenging.14-16 Veterinarians must utilize clinical impressions of pain, expectations of potential pain and careful observation to assess true levels of discomfort. Single observer, subjective observation and interpretation of specific pain-related behaviors17,18 is the best way to evaluate pain in animals despite the potential for observer bias.15,19,20 The pain scoring system utilized in the present study was validated in a previous study published by our group.2
The objective of the study reported here was to compare tramadol with another commonly administered oral analgesic, the non-steroidal anti-inflammatory drug (NSAID) carprofen, for analgesic efficacy in dogs following enucleation surgery. Because of its multiple proposed mechanisms of action, we hypothesized that tramadol would be a superior analgesic compared with carprofen in dogs undergoing this type of surgery.
Material and Methods
Animals
This study was approved by the University of Wisconsin-Madison School of Veterinary Medicine, and conformed to the guidelines of the Association for Research in Vision and Ophthalmology regarding animal use in research. After obtaining informed consent, client-owned dogs were admitted to the UW Veterinary Care for enucleation surgery as indicated by ophthalmic examination. Exclusion criteria were the presence of any source of pain other than the affected eye, significantly elevated hepatic or renal enzymes, inability to remain in the hospital for 30 hours of pain scoring, or administration of analgesics less than 24 hours prior to inclusion in the study.
Procedures
All dogs had complete ophthalmic examinations, including slit lamp biomicroscopy, indirect ophthalmoscopy and applanation tonometry, and complete physical examinations. Pre-operative diagnostic evaluation included PCV/TP and a chemistry panel. Other diagnostic tests, such as chest radiographs and abdominal ultrasound were performed as indicated by signalment and historical/clinical findings.
Dogs were randomly assigned to receive either 2.2 mg/kg of carprofena PO or 5mg/kg of tramadol hydrochlorideb PO. Block randomization was done to account for the long duration of the study (2.5 years) by using a commercially available randomization software program (Randomization Plan Generator, available at www.randomization.com. Accessed on 8/1/2010). Medication was prepared by hospital pharmacy staff, dispensed in a treat designed with a pouch for pill administrationc, and administered to the dogs by a student not involved in pain scoring. Study drugs were given 2 hours prior to anesthetic premedication and again 12 hours later.
Prior to anesthesia, dogs were premedicated with hydromorphoned (0.2mg/kg IM) and midazolame (0.2mg/kg IM). After placement of a venous catheter, anesthesia was induced with propofolf (2 – 6 mg/kg) to effect or ketamine hydrochlorideg (5 mg/kg)/diazepamh (0.25 mg/kg) to effect, then maintained with isofluranei in oxygen. Standard balanced electrolyte fluids (10ml/kg/hr) during anesthesia and prophylactic antibiotic adminstration (cefazolin sodiumj 22mg/kg IV at induction and 6 hours later SC) were provided. Transpalpebral enucleation was performed by one of three veterinary ophthalmology residents under the supervision of a board-certified veterinary ophthalmologist, with or without placement of an intraorbital prosthesis depending on owner preference. The time between study drug administration and extubation (time 0) was calculated for each group.
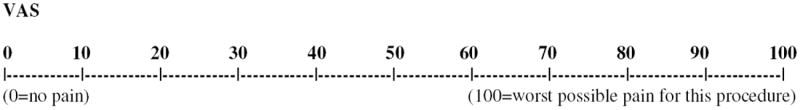
Dogs were evaluated and scored for pain prior to receiving test article (baseline), and postoperatively at 0.25, 0.5, 1, 2, 4, 6, 8, 24, and 30 hours after extubation by one of two trained observers masked to treatment groups, using a pain scoring system modified from previously published studies (Appendix A).2,21 This pain scale included 5 behavioral categories that were assessed outside the cage and then in the cage with the animal, and included vocalization, posture, activity, mental status and response to palpation of the surgical site. The minimum possible score was 0; the maximum possible score was 20 with 3 or 4 being the highest possible score for each category. Additionally, the observer assigned a visual analog score from 0 (no pain) to 100 (worst possible pain for this procedure). The two observers scored several dogs together prior to the study to ensure comparable scoring techniques, however, the majority of the scoring on the study was done by one author (CD). If the total pain score was ≥9, if there was a score ≥3 in any one category, or if the visual analog score (VAS) was ≥35 combined with a palpation score of > 0, rescue analgesia (hydromorphone 0.1-0.2mg/kg IM or IV) was administered, treatment failure was recorded, and the patient re-assessed to ensure any pain was below our minimum threshold. After treatment failure, the dog’s pain scores were excluded from further analysis, although the dogs continued to be scored to ensure comfort. If emergence delirium or dysphoria was considered a factor in causing an increased pain score, particularly in the areas of vocalization and posture combined with a palpation score of 0, 0.01mg/kg acepromazine maleatek was given IV and the patient re-scored. If the pain score decreased to below the minimum threshold, no rescue medication was administered and the post-acepromazine scores were used for analysis.
Sample size calculation
Sample size estimation was calculated via comparison of the proportion of patients in each study category expected to demonstrate effective pain control. Estimates of such proportions were figured conservatively, based on previous work in dogs undergoing lens extraction,20 extrapolation from studies evaluating the efficacy of analgesic regimens for a variety of surgical procedures22,23 and successful strategies for pain control in our experience with enucleation for painful conditions in dogs. We predict that pain will be effectively managed, meaning rescue analgesia was not required, in <50% of patients receiving carprofen and ≥85% of patients receiving tramadol. The significance level was set at 0.05 and the power of the test was set at 80%, allowing calculation of the number of subjects required per group as n=20.24
Statistical analysis
Select characteristics between groups were compared with a Student’s t-test (age, baseline pain scores, time between study drug administration and extubation), Fisher’s exact test (gender), and Wilcoxon rank sum test (baseline VAS scores). The incidence of rescue analgesia was compared between groups using a log rank test, and pain scores and log VAS scores between groups were compared using repeated measures ANOVA. Significance was inferred at P < 0.05.
Results
Forty-four dogs were enrolled in the study. One dog in the tramadol group was excluded from analysis due to behavior which prevented accurate pain scoring, resulting in 22 dogs in the carprofen (C) group and 21 dogs in the tramadol (T) group. There was no difference in mean age, gender, baseline pain scores or median baseline VAS scores between groups (Table 1). Reasons for enucleation and breed distribution were similar between groups (Table 1). Twenty-one of 22 in the carprofen group had painful ocular conditions prior to surgery, while 19/21 dogs in the tramadol group had painful ocular conditions prior to surgery. Of the three dogs with non-painful ocular conditions requiring enucleation, only one of these dogs, in the tramadol group, required rescue analgesia.
Table 1.
Distribution of the age, sex, reason for enucleation, and baseline pain scores for dogs enrolled in a study comparing analgesia provided by carprofen or tramadol after enucleation. Age and baseline pain scores were compared with a Student’s t test and birth gender was compared with a Fisher’s exact test
| Variable | Carprofen (n=22) | Tramadol (n=21) | P value |
|---|---|---|---|
|
| |||
| Mean (range) age (y) | 7.1(1-13) | 6.4 (2-12) | 0.493 |
|
| |||
| Birth gender | 13 F, 9 M | 9 F, 12 M | 0.366 |
|
| |||
| Mean (SD)* baseline pain score | 2.0 (1.5) | 1.6 (1.1) | 0.288 |
|
| |||
| Median (range) baseline VAS score | 27.5 (0-50) | 20.0 (0-60) | 0.492 |
|
| |||
| Ocular disease (No. of dogs) | Glaucoma (15) | Glaucoma (18) | n/a |
| Blind, ulcerated (2) | Blind, ulcerated (1) | ||
| KCS non-responder (1) | Intraocular tumor (1, non-painful) | ||
| Rupture (1) | Phthsical (1, non-painful) | ||
| Proptosis (1) | |||
| Phthsical (1, non-painful) | |||
|
| |||
| Breeds (No. of dogs, n=1 if not notated) | Bassett (4), Mixed (5), Springer, Golden retriever, Cockapoo, Miniature Pinscher, Labrador retriever, American Bulldog, Bichon Frise, Cocker spaniel, Scottish terrier, Cavalier King Charles Spaniel, Greater Swiss Mountain dog, Pekingese | Mixed (5), Labrador retriever (3), Cocker spaniel (2), Siberian Husky (2), Parson Jack Russell (2), Pit bull (2), German shepherd, Boston terrier, Bassett, Miniature Poodle, Golden retriever | n/a |
Maximum score of 20
Three dogs in the carprofen group and 4 dogs in the tramadol group received ketamine to to induce anesthesia. One f these dogs in the tramadol group required rescue analgesia. All remaining dogs in the study received propofol to induce anesthesia.
The time of study drug administration to extubation (time 0) was determined for each group. The average time from study drug administration for the carprofen group was 3 hr 52 minutes (range 3 hr to 5 hr 10 minutes) and the average time for the tramadol group was 3 hr 52 minutes (range 2 hr 50 min to 5 hr 10 min; p=0.74)
Significantly more dogs receiving tramadol required rescue analgesia (6/21) compared to dogs receiving carprofen (1/22; p=0.035, Fig 1). There were no significant differences in pain scores (Fig 2) or VAS scores (Fig 3) between groups at any time point (dogs were excluded from analysis after rescue). On average (95% CI), over all time points, tramadol dogs had a pain score that was 0.03 (-0.60, 0.66) points higher than carprofen dogs (p=0.915), but tramadol dogs had a VAS score that was, on average, -0.16 (-0.45, 0.25) units lower than carprofen dogs (p=0.372). The single dog rescued in the carprofen group was rescued at 30 minutes after extubation. Of the dogs rescued in the tramadol group, 1 was rescued at extubation, 1 at 15 minutes post extubation, and 3 at 6 hours, and 1 at 8 hours All but one dog was rescued due to a score of 3 on palpation (will not allow general surgical area to be touched), typically with a VAS score of >35; the remaining dog (in the tramadol group) was rescued with a score of 2 on palpation (increased whining or painful expression when surgical area touched), a score of 3 on activity (rolling and thrashing) and a VAS score of >35.
Figure 1.
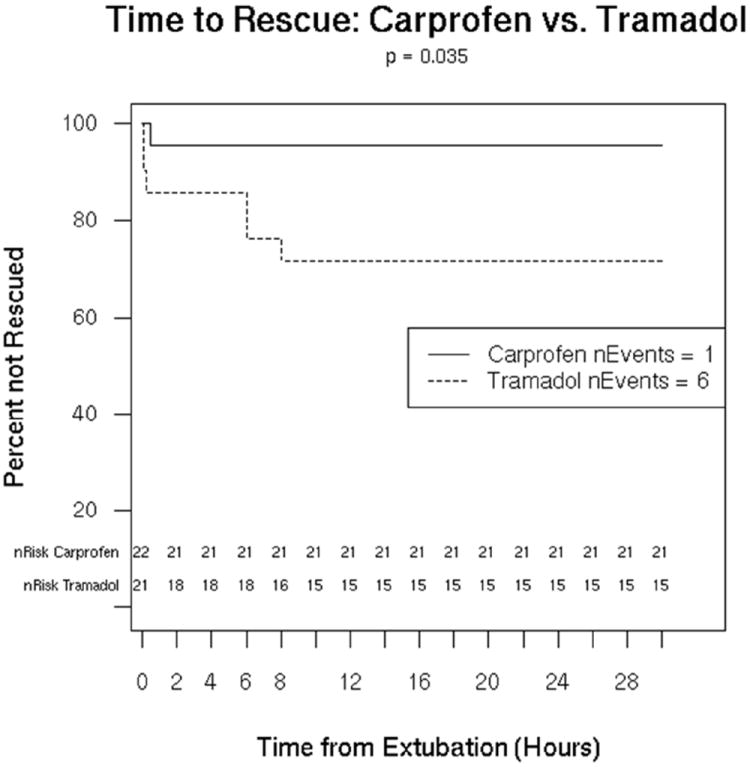
Survival curve of successful treatment (ie, no rescue analgesia needed) by treatment groups. The number of dogs with treatment failure was significantly greater in dogs receiving tramadol (p=0.035) than in dogs receiving carprofen.
Figure 2.
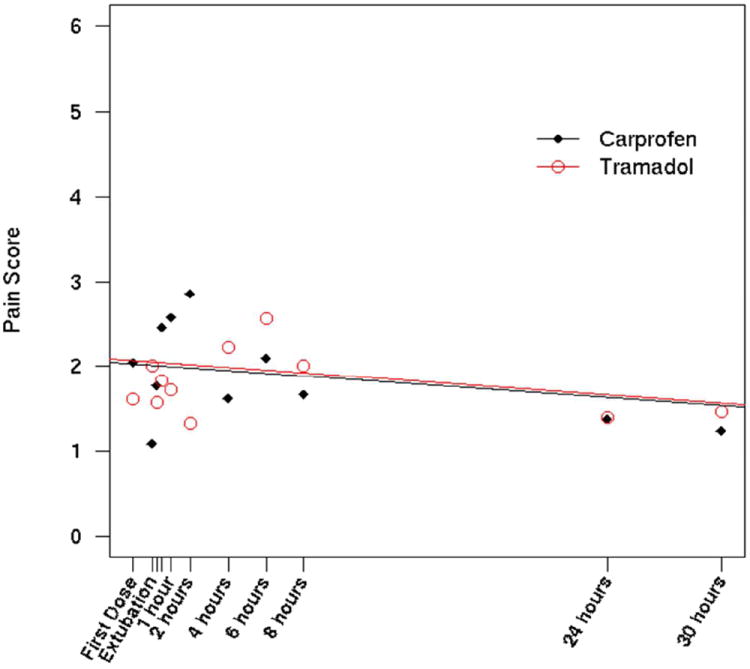
Comparison of pain scores over time between dogs receiving tramadol and dogs receiving carprofen. There was no difference between groups at any time point.
Fig 3.
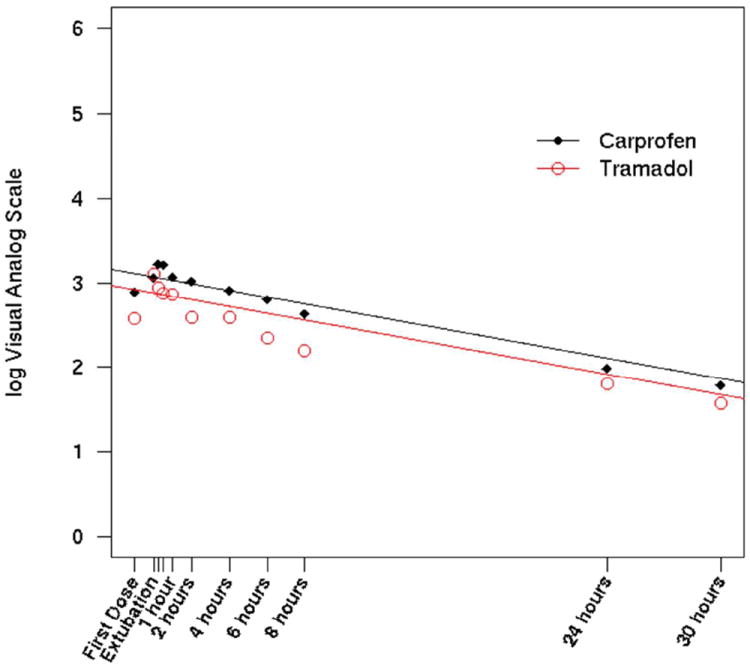
Comparison of VAS over time between dogs receiving tramadol and dogs receiving carprofen. There was no difference between groups at any time point. Log scores of VAS were used to normalize the data.
Three dogs were given acepromazine, two in the tramadol group and one in the carprofen group. All three dogs were given acepromazine for a vocalization score of 3 (continuous vocalization), and posture scores of 2 (abnormal posture and/or uncomfortable, continuous position change). Two dogs had a score of 0 on palpation (normal, allows palpation of surgical site), however, the third dog was thrashing too much to be easily or accurately palpated. All dogs had decreased vocalization scores and posture scores, with palpation scores of zero, after administration of acepromazine, so they were not administered rescue analgesia and their post-acepromazine scores at that time point were used for analysis.
Discussion
Results from the current study suggest that, with a mu agonist opioid premedication, carprofen provides more effective analgesia than tramadol after enucleation. In this study, significantly more dogs in the tramadol group required rescue analgesia, despite identical premedication and similar distribution of breeds and reasons for enucleation. Pain scores and VAS scores were not different between groups at any time point. Dogs were excluded from analysis after rescue analgesia, thus dogs in both groups that continued to be pain scored could be expected to have similar levels of pain. The majority of dogs in this study that required rescue did so due to a high score on palpation of the surgical site rather than due to an overall high pain score, which also contributed to similar pain and VAS scores between groups across time.
One possible confounding factor is the administration of ketamine to induce anesthesia in a small number of dogs in each group, given that ketamine has been reported to have analgesic effects in dogs undergoing ovariohysterectomy.25 Because the number of dogs receiving ketamine were similar between groups, and because one dog which received ketamine required rescue analgesia, the use of ketamine as an induction agent seems unlikely to have had an effect on our results. Interestingly, one report showed pre-operative ketamine was no better than saline 20 minutes post extubation.25
Efficacy of analgesic treatment in a particular situation cannot be proven until a placebo-controlled, randomized, masked clinical trial is performed. We recently published such a trial that assessed pain in dogs after enucleation and the pain scoring system used in that trial strongly identified dogs that were treated with the placebo medication, indicating that our pain scoring system was sensitive to moderate or severe pain.2 Because results from this published study by our group include data from a placebo-controlled population that demonstrate the pain scoring system is sensitive to post-operative pain, this study utilized a positive control (carprofen) and a test drug (tramadol) with the same randomized, masked trial design using a slightly modified pain score system as we previously described. While every pain scoring method or scale has its strengths and weaknesses, the pain scoring method used here, a modified Melbourne Pain scale,21,26 has been used in two prior ocular studies in dogs,2,27 both of which had placebo groups. In both of those studies, this pain scale was effective at identifying pain in placebo animals. Given our previous experience with this scale, we did not include a placebo group in the current study to confirm the sensitivity of our scale. Using the criteria of a score of ≥ 3, the highest or second highest score possible in each category, as our definition for treatment failure, proved to be the most sensitive way to assess for pain, and a painful response to palpation was the most common reason for treatment failure. The sensitivity of the palpation category in detecting pain was a surprising finding. We suspect this may be due in part to relatively low levels of pain post enucleation, resulting in lower scores in the other categories that represent a greater overall pain response.
Published studies since the start of the current study on the analgesic efficacy of tramadol in dogs, while limited, have reported mixed results. One study compared oral tramadol, carprofen, ABT-116 (a TRPV1 antagonist) and a placebo for pain due to hip osteoarthritis as assessed by an owner questionnaire, activity monitoring and gait analysis.28 Dogs showed no difference in activity as measured by an accelerometer between carprofen, tramadol and ABT-116, however, fewer rescue medications were required in dogs receiving carprofen. In this same study, however, owners reported via questionnaire increased activity and decreased pain with both tramadol and carprofen as compared to placebo.28 An uncontrolled open label study in dogs with severe chronic neoplastic pain who failed NSAID therapy reported improved quality of life scores with oral tramadol treatment.29 In this study, however, owner reported “placebo effect” could have confounded the results, as a placebo effect can account for up to 40% of the perceived improvements in quality of life,30 so these results should be interpreted cautiously. Davila and colleagues compared oral tramadol to firoxocib and tramadol in combination with firocoxib after cranial cruciate repair, and found that tramadol alone resulted in higher pain scores and a higher rate of rescue analgesia compared to firoxocib and firoxocib/tramadol.31 This study suggests NSAIDs are more potent analgesics than tramadol alone in some situations.
In the current study, not every dog receiving tramadol failed treatment, suggesting that for some dogs tramadol can provide effective analgesia provided an opioid is included in the analgesic protocol and the pain is similar to what was expected in the current study. Several studies have examined the pharmacokinetics of oral tramadol in dogs, but these studies were limited to 2 studies in Beagles,7,32 and one with Greyhounds.8 These studies measured different metabolites with different methods, so comparison is somewhat difficult, and all three studies revealed a large variation between subjects. These three studies were also not representative of the variety of dog breeds in the general population. There is genetic variation among humans in the metabolism of tramadol, in that some are poor metabolizers of tramadol, while others can be extensive or even ultra-metabolizers of tramadol.33 These differences in metabolism are important clinically, demonstrated by either a lack of, or poor, analgesia with tramadol in humans who are poor metabolizers.33 Differences in metabolism between individual dogs or even breeds of dogs could explain some of the failures of tramadol analgesia in this study. Further work with a wider variety of dog breeds, determination of how tramadol is metabolized in dogs, as well as which metabolites are active, will be important in our understanding of tramadol’s efficacy.
It is possible that our results may have differed if we had used a higher dose or more frequent dosing interval of tramadol. Dosing recommendations for oral tramadol are wide, and range from 2 – 10 mg/kg at a frequency of twice to three times daily.34 Higher doses of tramadol can be associated with sedation in dogs, and there are some anecdotal reports of serotonin syndrome in dogs receiving high or frequent doses of tramadol (Karol Mathews, personal communication). For these reasons we elected to use a mid-range dose of tramadol in this study, with a dosing interval that was equal to that of carprofen at the 2.2 mg/kg oral dose. Three of the dogs in the tramadol group were rescued at extubation or within 15 minutes of extubation, which was well within 8 hours of the initial tramadol administration. The remaining three dogs were rescued between 9-12 hours post initial tramadol administration, so increasing the frequency of tramadol may have altered our results, although administering an oral medication in the post operative period can be difficult.
Many studies have reported modest efficacy of carprofen for analgesia in dogs. Most of those studies, have compared carprofen to analgesia with opioid drugs with recognized modest efficacy such as buprenorphine and pethidine. In studies examining analgesia after ovariohysterectomy in dogs, Shih and colleagues reported that carprofen was as effective as the partial agonist opioid buprenorphine11, Dzikiti showed that a low dose of morphine was equivalent to carprofen,10 and Slingsby found that carprofen was superior to pethidine.12 In a study by Malek, in which dogs with osteoarthritis were treated with either an experimental TRPV1 receptor antagonist, carprofen or tramadol, fewer rescue analgesics were required in dogs treated with carprofen and owner-reported activity was increased with treatment of either tramadol or carprofen.28 For more severe pain, such as occurs after orthopedic surgery, carprofen alone was inadequate for analgesia.9
Interestingly, in the current study, all dogs that required rescue analgesia did so within 8 hours of extubation. All dogs were pain scored for the duration of the study, and no other dogs required rescue analgesia, even at 24 hours post operatively, prior to the administration of any other analgesic medications. All dogs had, however, received either carprofen or tramadol 12 hours after their first dose, or approximately 8 hours from the time of surgery. Similarly, in the study by Myrna et al,2 most patients needed rescue analgesia in the first few hours after surgery. These results suggest that pain after enucleation in dogs is most intense within the first 6 hours after recovery, and that pain may subside within 24 hours of surgery. This is important information for the planning of analgesic therapy, suggesting analgesia should be relatively aggressive in the immediate post-operative period, while an NSAID alone may be sufficient by 24 hours after surgery.
In conclusion, our results indicate that carprofen, combined with an opioid such as hydromorphone, provides excellent analgesia for enucleation in dogs and that tramadol, with an opioid such as hydromorphone, produces more variable and sometimes poor analgesia for this type of surgery. More studies are warranted to study the efficacy of tramadol in a wide population of dogs, under a variety of painful conditions, and at a wide range of doses before the usefulness of this drug as an oral analgesic can be fully confirmed.
Acknowledgments
Funded by a grant from the UW Companion Animal Fund and NIH NCATS grant UL1TR000427. The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Table 2.
| Category and descriptor | Score |
|---|---|
| From outside the cage: | |
| Vocalization (choose only one)* | |
| Not vocalizing | 0 |
| Slight vocalization but dysphoric | 1 |
| Intermittent vocalization | 2 |
| Continuous vocalization | 3 |
| Posture | |
| a) Guarding or protecting affected area | 2 |
| b) Position (choose only one) | |
| Lateral recumbency | 0 |
| Sternal recumbency | 1 |
| Sitting, standing, or comfortable | 1 |
| Standing with head hanging | 2 |
| Moving | 1 |
| Abnormal posture and/or uncomfortable, continuous position change | 2 |
| Activity (choose one) | |
| At rest | |
| Sleeping | 0 |
| Semi-conscious | 0 |
| Awake | 1 |
| Eating | 0 |
| Restless (pacing continuously; getting up and down) | 2 |
| Rolling and thrashing | 3 |
| From inside the cage: | |
| Mental status (choose only one)** Baseline minus current score = overall score | |
| too sedate to evaluate or dysphoric | 0 |
| Submissive | 1 |
| Uninterested in people (unusual for this dog) | 2 |
| Overtly friendly | 3 |
| Wary or Aggressive | 4 |
| Response to palpation (choose only one)*** | |
| Normal, allows palpation of amputation site | 0 |
| Allows but then moves away, tenses or looks when surgical area touched | 1 |
| Increased whining or painful expression when surgical area touched | 2 |
| Will not allow general surgical area to be touched | 3 |
| Vocalization (choose only one)* | |
| Not vocalizing | 0 |
| Vocalizing but responds to quiet voice and/or stroking | 1 |
| Vocalizing when touched | 2 |
| Intermittent vocalization | 2 |
| Continuous vocalization | 3 |
The minimum possible score is 0; the maximum possible score is 20.
Does not include alert barking.
For this category, score recorded is the score obtained after surgery minus the score obtained before surgery.
Palpate around the general surgical area starting at the dorsal end and working toward incision site. If the dog scores a 0 for palpation, total pain score may be attributable to dysphoria. Administer acepromazine at 0.01 mg/kg IV before rescue analgesia.
Rescue analgesia: If total pain score is ≥ 9 or VAS is ≥ 35 and palpation score is > 0.
Footnotes
Rimadyl, Pfizer Animal Health, New York, NY.
Tramadol hydrochloride, Caraco Pharmaceutical Laboratories, LTD, Detroit, MI
Pill Pocket, The Nutro Company, Franklin, TN
Hydromorphone HCl, Westward, Eatontown, NJ
Midazolam, Caraco Pharmaceutical Laboratories, LTD, Detroit, MI
Propoflo, Abbott Laboratories, North Chicago, IL
Ketamine HCl, Fort Dodge Animal Health, Fort Dodge, IA
Diazepam, Hospira Inc, Lake Forest, IL
Isoflurane, Piramal Healthcare Limited, Digwal Village, Anhra Pradesh, India
Cefazolin sodium, WG Critical Care, LLC, Paranus, NJ
Acepromazine maleate, Boehringer Ingelheim Vetmedica Inc, St. Joseph, MO
References
- 1.Murphy C, Pollack R. The eye. In: Evans H, editor. Miller’s Anatomy of the Dog. 3. Philadelphia: W.B. Saunders; 1993. pp. 1009–1057. [Google Scholar]
- 2.Myrna KE, Bentley E, Smith LJ. Effectiveness of injection of local anesthetic into the retrobulbar space for postoperative analgesia following eye enucleation in dogs. J Am Vet Med Assoc. 2010;237:174–177. doi: 10.2460/javma.237.2.174. [DOI] [PubMed] [Google Scholar]
- 3.Accola P, Bentley E, Smith L, et al. Development of a retrobulbar injection technique for ocular surgery and analgesia in dogs. J Am Vet Med Assoc. 2006;229:220–225. doi: 10.2460/javma.229.2.220. [DOI] [PubMed] [Google Scholar]
- 4.Ide S, Minami M, Ishihara K, et al. Mu opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacology. 2006;51:651–658. doi: 10.1016/j.neuropharm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Ogata J, Minami K, Uezono Y, et al. The inhibitory effects of tramadol on 5-hydroxytryptamine type 2C receptors expressed in Xenopus oocytes. Anesth Analg. 2004;98:1401–1406. doi: 10.1213/01.ane.0000108963.77623.a4. table of contents. [DOI] [PubMed] [Google Scholar]
- 6.Sagata K, Minami K, Yanagihara N, et al. Tramadol inhibits norepinephrine transporter function at desipramine-binding sites in cultured bovine adrenal medullary cells. Anesth Analg. 2002;94:901–906. doi: 10.1097/00000539-200204000-00024. table of contents. [DOI] [PubMed] [Google Scholar]
- 7.Giorgi M, Del Carlo S, Saccomanni G, et al. Pharmacokinetic and urine profile of tramadol and its major metabolites following oral immediate release capsules administration in dogs. Vet Res Commun. 2009;33:875–885. doi: 10.1007/s11259-009-9236-1. [DOI] [PubMed] [Google Scholar]
- 8.Kukanich B, Papich MG. Pharmacokinetics and antinociceptive effects of oral tramadol hydrochloride administration in Greyhounds. Am J Vet Res. 2011;72:256–262. doi: 10.2460/ajvr.72.2.256. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann H, Nolte I, Kramer S. Comparison of analgesic efficacy of preoperative or postoperative carprofen with or without preincisional mepivicaine epidural anesthesia in canine pelvic or femoral fracture repair. Vet Surg. 2007;36:623–632. doi: 10.1111/j.1532-950X.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 10.Dzikiti T, Joubert K, Venter L, et al. Comparison of morphine and carprofen administered alone or combination for analgesia in dogs undergoing ovariohysterectomy. J S Afr Vet Assoc. 2006;77:120–126. doi: 10.4102/jsava.v77i3.358. [DOI] [PubMed] [Google Scholar]
- 11.Shih A, Robertson S, Isaza N, et al. Comparison between analgesic effects of burpenorphine, carprofen, and buprenorphine for canine ovariohysterectomy. Vet Anaesth Analg. 2008;35:69–79. doi: 10.1111/j.1467-2995.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 12.Slingsby L, Waterman-Peason A. Analgesic Effects in dogs of carprofen and pethidine together compared with the effects of either drug alone. Vet Rec. 2001;148:441–444. doi: 10.1136/vr.148.14.441. [DOI] [PubMed] [Google Scholar]
- 13.Kay-Mugford P, Benn S, LaMarre J, et al. In vitro effects of nonsteroidal anti-inflammatory drugs on cyclooxygenase activitiy in dogs. Am J Vet Res. 2000;61:802–210. doi: 10.2460/ajvr.2000.61.802. [DOI] [PubMed] [Google Scholar]
- 14.Conzemius MG, Hill CM, Sammarco JL, et al. Correlation between subjective and objective measures used to determine severity of postoperative pain in dogs. J Am Vet Med Assoc. 1997;210:1619–1622. [PubMed] [Google Scholar]
- 15.Holton LL, Scott EM, Nolan AM, et al. Relationship between physiological factors and clinical pain in dogs scored using a numerical rating scale. J Small Anim Pract. 1998;39:469–474. doi: 10.1111/j.1748-5827.1998.tb03681.x. [DOI] [PubMed] [Google Scholar]
- 16.Holton LL, Scott EM, Nolan AM, et al. Comparison of three methods used for assessment of pain in dogs. J Am Vet Med Assoc. 1998;212:61–66. [PubMed] [Google Scholar]
- 17.Hansen B. Through a glass darkly: using behavior to assess pain. Seminars in Veterinary Medicine and Surgery (Small Animal) 1997;12:61–74. doi: 10.1016/s1096-2867(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 18.Sackman J. Pain and its management. Vet Clin North Am. 1997;27:1487–1504. doi: 10.1016/s0195-5616(97)50135-5. [DOI] [PubMed] [Google Scholar]
- 19.Capner CA, Lascelles BD, Waterman-Pearson AE. Current British veterinary attitudes to perioperative analgesia for dogs. Vet Rec. 1999;145:95–99. doi: 10.1136/vr.145.4.95. [DOI] [PubMed] [Google Scholar]
- 20.Smith L, Shih A, Bentley E, et al. Systemic lidocaine infusion an analgesic for intraocular surgery in dogs: a pilot study. J Vet Anes Analg. 2004;31:53–63. doi: 10.1111/j.1467-2995.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 21.Firth AM, Haldane SL. Development of a scale to evaluate postoperative pain in dogs. J Am Vet Med Assoc. 1999;214:651–659. [PubMed] [Google Scholar]
- 22.Lascelles BD, Cripps P, Jones A, et al. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg. 1998;27:568–582. doi: 10.1111/j.1532-950x.1998.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 23.Sammarco JL, Conzemius MG, Perkowski SZ, et al. Postoperative analgesia for stifle surgery: a comparison of intra-articular bupivacaine, morphine, or saline. Vet Surg. 1996;25:59–69. doi: 10.1111/j.1532-950x.1996.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 24.Friedman L, Furberg C, DeMets D. Fundamentals of clinical trials. 3. New York: Springer-Verlag; 1999. Sample Size; pp. 94–129. [Google Scholar]
- 25.Slingsby L, Waterman-Pearson AE. The post-operative analgesic effects of ketamine after canine ovariohysterectomy-a comparison between pre- or post-operative administration. Research in Veterinary Sci. 2000;69:147–152. doi: 10.1053/rvsc.2000.0406. [DOI] [PubMed] [Google Scholar]
- 26.Hansen B. Assessment of pain in dogs: Veterinary clinical studies. ILAR. 2003;44:197–205. doi: 10.1093/ilar.44.3.197. [DOI] [PubMed] [Google Scholar]
- 27.Clark JS, Bentley E, Smith LJ. Evaluation of topical nalbuphine or oral tramadol as analgesics for corneal pain in dogs: a pilot study. Vet Ophthalmol. 2011;14:358–364. doi: 10.1111/j.1463-5224.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 28.Malek S, Sample SJ, Schwartz Z, et al. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet Res. 2012;8:185. doi: 10.1186/1746-6148-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flor PB, Yazbek KV, Ida KK, et al. Tramadol plus metamizole combined or not with anti-inflammatory drugs is clinically effective for moderate to severe chronic pain treatment in cancer patients. Vet Anaesth Analg. 2013;40:316–327. doi: 10.1111/vaa.12023. [DOI] [PubMed] [Google Scholar]
- 30.Conzemius MG, Evans R. Caregiver placebo effect for dogs with lameness for osteoarthritis. J Am Vet Med Assoc. 2012;241:1314–1319. doi: 10.2460/javma.241.10.1314. [DOI] [PubMed] [Google Scholar]
- 31.Davila D, Keeshen TP, Evans RB, et al. Comparison of the analgesic efficacy of perioperative firocoxib and tramadol administration in dogs undergoing tibial plateau leveling osteotomy. J Am Vet Med Assoc. 2013;243:225–231. doi: 10.2460/javma.243.2.225. [DOI] [PubMed] [Google Scholar]
- 32.Kukanich B, Papich M. Pharmocokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Therap. 2004;27:239–246. doi: 10.1111/j.1365-2885.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirchheiner J, Keulen JT, Bauer S, et al. Effects of the CYP2D6 gene duplication on the pharmacokinetics and pharmacodynamics of tramadol. J Clin Psychopharmacol. 2008;28:78–83. doi: 10.1097/JCP.0b013e318160f827. [DOI] [PubMed] [Google Scholar]
- 34.Gaynor J, Muir W. Drugs used for the treatment of paind and pain-related anxiety in dogs and cats. In: Gaynor J, Muir W, editors. Handbook of Veterinary Pain Management. 1. St. Louis: Mosby; 2002. [Google Scholar]


