Abstract
The GH/IGF1 response of somatotropinomas to somatostatin analogues (SSA) is associated with their pattern of somatostatin receptor (sst1–sst5) expression. Recently, we demonstrated that expression of a truncated sst5-variant (sst5TMD4) can influence the secretory response of somatotropinomas to SSA-therapy; however, its potential relationship with aggressive features (e.g. invasion/proliferation) is still unknown. Here, we show that sst5TMD4 is present in 50% of non-functioning pituitary-adenomas (NFPA) (n = 30) and 89% of somatotropinomas (n = 36), its expression levels being highest in somatotropinomas > > NFPAs > > > normal pituitaries (negligible expression; n = 8). In somatotropinomas, sst5TMD4 mRNA and protein levels correlated positively, and its expression was directly associated with tumor invasiveness (cavernous/sphenoid sinus), and inversely correlated with age and GH/IGF1 reduction after 3–6 months with octreotide-LAR therapy. GNAS+ somatotropinomas expressed lower sst5TMD4 levels. ROC analysis revealed sst5TMD4 expression as the only marker, within all sst-subtypes, capable to predict tumor invasiveness in somatotropinomas. sst5TMD4 overexpression increased cell viability in cultured somatotropinoma (n = 5). Hence, presence of sst5TMD4 associates with increased aggressive features and worse prognosis in somatotropinomas, thereby providing a potentially useful tool to refine somatotropinoma diagnosis, predict outcome of clinical response to SSA-therapy and develop new therapeutic targets.
Keywords: sst5TMD4, Acromegaly, Invasion, Proliferation
Introduction
Acromegaly results from increased release of GH and IGF1 caused by a GH-producing adenoma (somatotropinomas). Consequently, the main therapeutic goal in this disease is reduction of circulating GH and IGF1 levels and of tumor size, particularly in patients presenting macroadenomas (around 65% of the cases), wherein invasiveness becomes a crucial problem [1]. Somatostatin and its receptors (sst), especially sst2 (and to a lesser extent sst5), comprise the critical regulatory system for the negative control of hormonal secretion and tumor size in pituitary somatotropinomas [2]. Accordingly, medical treatment with synthetic somatostatin analogues (SSA [2]; synthetic versions of somatostatin presenting longer half-life and more stability) has been successfully used to treat GH secreting adenomas (i.e. to obtain biochemical control of the patients and/or achieve tumor shrinkage); however, a significant percentage of patients are biochemically resistant to SSA therapy and tumor shrinkage is also unsuccessful [3–6].
Lack of sst expression in tumor cells has been postulated as a logical key molecular determinant underlying SSA resistance. In line with this, we previously demonstrated that sst2 expression directly correlates with percent decrease of GH and IGF1 levels and tumor volume reduction in response to octreotide-LAR in a population of Brazilian acromegalic patients [7,8]. Yet, we also found, unexpectedly, a negative correlation between sst5 expression and percent decrease of GH and IGF1 after octreotide-LAR treatment [7,8]. Soon thereafter, our studies on independent populations of Spanish [9] and French [10] acromegalic patients revealed the existence of novel truncated variants of the sst5 receptor, which could be clinically relevant. Specifically, the truncated receptors, termed sst5TMD5 and sst5TMD4 based on the number of transmembrane domains (TMDs) [9], are extremely infrequent in normal tissues but sst5TMD5 is present in a subset of pituitary adenomas while sst5TMD4 is frequently present in pituitary tumors [9], where they seem to exhibit a subcellular localization. More interestingly, and in line with that mentioned above, the expression of sst5TMD4 was negatively linked to poorer clinical response in a series of patients with partial resistance to SSA-therapy [10]. On a different scenario, we have demonstrated that sst5TMD4 presence is associated with poor prognosis in breast cancer, and its expression in a germane cell model (MCF-7 cell line) increases malignancy features (proliferation, invasiveness and migration) [11]. Similarly, sst5TMD4 presence is associated with poor prognosis in thyroid cancer [12].
However, the possible influence of sst5TMD4 on aggressiveness features in somatotropinomas, beyond reduced SSA-response (i.e. invasion/proliferation abilities), has not been reported hither-to. In addition, the putative association between the levels of truncated sst5 variants, especially sst5TMD4, and the response to octreotide-LAR therapy was not originally explored in the well-characterized, and previously reported, population of Brazilian acromegalic patients [7,8,13], as the truncated receptors were not yet discovered at that time. Accordingly, this study has three main goals: 1) To evaluate and compare truncated sst5 variant levels in somatotropinomas, normal pituitaries and non-functioning pituitary adenomas (NFPA); 2) to assess potential correlations between truncated sst5 variant expression levels in somatotropinomas and response to SSA-therapy and/or presence of GNAS mutation (indicative of good response to SSA-therapy) [14]; and 3) to investigate the potential role of the truncated sst5 variants in conferring aggressiveness features to GH secreting adenomas (i.e. invasion/proliferation abilities) in vivo and in vitro.
Materials and methods
Patients and samples
For in vivo studies, we used a set of 74 pituitary samples (66 pituitary adenomas and 8 normal pituitaries; Tables 1–3), in which demographic/clinical data of patients, profile of sst1-5, and presence of GNAS oncogene in somatotropinomas were previously characterized [13,15]. Briefly, somatotropinoma (n = 36; median age: 38 years old; Table 1) and NFPA (n = 30; median age: 51 years old; Table 2) samples were obtained during transsphenoidal surgery of pituitary adenoma (PA) tumors. None of the patients belongs to pituitary adenoma kindred. In acromegalic patients, only 3 patients were treated with octreotide-LAR before surgery, and prevalence of GNAS oncogene was 10% [15]. In line with this last point, we have previously reviewed [16] that the prevalence of the GNAS oncogene in somatotropinomas is approximately 40%; however it has been shown that this prevalence dramatically varies between studies (ranging from 4.4 to 55%) considering previous series from several countries. The reason for this low prevalence is not known, but is most likely the result from randomness, since the recruitment of patients was not based in any pre-defined criteria that could bias the selection of non GNAS-mutated patients.
Table 1.
Demographic, laboratory, qPCR data (sst5TMD5 and sst5TMD4 mRNA copy number levels) and immunohistochemistry analysis (IHC) of sst5TMD4 in individual somatotropinomas. mRNA copy number have been corrected by a normalization factor (NF) derived from the expression of three control genes (glyceraldehyde-3-phosphate dehydrogenase, GAPDH; hypoxanthine ribosyltransferase, HPRT1; and beta-actin, ACTB).
| No. | S | A | Basal GH (µg/L) |
Basal IGF1 (% ULRV) |
Tumor volume (cm3) |
mRNA copy number/NF | sst5TMD4 IHC | ||
|---|---|---|---|---|---|---|---|---|---|
| sst5TMD5 | sst5TMD4 | Intensity | % cells | ||||||
| 1 | F | 24 | 139.0 | 151.4 | 4.0 | 30 | 262 | ++ | 37.5 |
| 2 | F | 26 | 5.7 | 110.6 | NA | 18 | 347 | ++ | 25.0 |
| 3 | F | 31 | 3.2 | 103.0 | NA | 0 | 715 | + | 3.0 |
| 4† | F | 36 | 133 | 181 | 4.7 | 0 | 71 | − | 0.0 |
| 5 | F | 40 | 6.8 | 221.9 | 0.2 | 0 | 86 | NA | NA |
| 6 | F | 41 | 33.6 | 239 | 15.5 | 12 | 136 | + | 8.1 |
| 7† | F | 42 | 10.3 | 171.2 | 10.9 | 0 | 103 | NA | NA |
| 8 | F | 45 | 4.2 | 144.8 | 0.1 | 0 | 1413 | NA | NA |
| 9 | F | 48 | 2.3 | 197.3 | NA | 0 | 0 | NA | NA |
| 10 | F | 51 | 2.5 | 276.3 | NA | 0 | 93 | ++ | 49.0 |
| 11 | F | 31 | NA | NA | NA | 0 | 773 | + | 3.1 |
| 12 | F | 32 | NA | NA | NA | 0 | 103 | − | 0 |
| 13 | F | 32 | NA | NA | NA | NA | NA | NA | NA |
| 14 | F | 37 | NA | NA | NA | NA | NA | NA | NA |
| 15 | F | 39 | NA | NA | NA | 0 | 214 | ++ | 61.9 |
| 16 | F | 40 | 2.1 | 616.0 | NA | 0 | 312 | NA | NA |
| 17 | F | 41 | NA | NA | NA | 0 | 392 | +++ | 89.3 |
| 18 | F | 42 | NA | NA | NA | 0 | 0 | − | 0.0 |
| 19 | F | 46 | NA | NA | NA | 0 | 36 | NA | NA |
| 20 | F | 52 | 3.71 | NA | NA | 0 | 241 | − | 0.0 |
| 21 | F | 59 | 0.5 | 148.0 | NA | 0 | 261 | − | 0.0 |
| 22 | M | 28 | 5.7 | 190.9 | NA | 0 | 242 | ++ | 25.0 |
| 23 | M | 31 | 197 | 238 | NA | 0 | 147 | NA | NA |
| 24 | M | 31 | 46 | 177 | 11.6 | 0 | 497 | − | 0.0 |
| 25^ | M | 33 | 17 | 377 | 24.5 | 0 | 1167 | NA | NA |
| 26 | M | 34 | 21.3 | 209.5 | 2.1 | 10 | 312 | ++ | 54.0 |
| 27^ | M | 38 | 112.0 | 137.0 | 3.3 | 0 | 494 | + | 5.0 |
| 28† | M | 50 | 7.3 | 277.5 | NA | 0 | 0 | NA | NA |
| 29 | M | 58 | 2.8 | 193.0 | NA | 0 | 89 | NA | NA |
| 30 | M | 62 | 36.0 | 300.4 | 5.2 | 0 | 182 | − | 0.0 |
| 31 | M | 18 | NA | NA | NA | 0 | 201 | + | 68.7 |
| 32 | M | 26 | NA | NA | NA | 0 | 84 | − | 0.0 |
| 33† | M | 28 | NA | NA | NA | 0 | 81 | − | 0.0 |
| 34 | M | 30 | 1.5 | 207.9 | NA | 0 | 632 | +++ | 90.0 |
| 35 | M | 33 | NA | NA | NA | 0 | 347 | NA | NA |
| 36 | M | 38 | NA | NA | NA | NA | NA | NA | NA |
| 37 | M | 45 | NA | NA | NA | 3 | 500 | NA | NA |
| 38^ | M | 63 | NA | NA | NA | 0 | 0 | NA | NA |
| 39# | M | 46 | 32.7 | 479 | NA | 0 | 101 | NA | NA |
| Median | − | 38 | 7.1 | 197.3 | 4.7 | 0 | 208 | − | − |
NA: not available sample; 0: zero copies. In IHC analysis: −, +, ++, +++ stand for not expressed and low, medium, and high intensities of the signal expression; % cell: percentage of positive cells. S: sex; A: age at diagnosis;
: microadenoma;
: patients that used octreotide-LAR before surgery;
: patients presenting GNAS mutation;
GH levels in µg/L; IGF1 levels: ULRV, upper limit of the reference values; tumor volume in cm3. It should be noted that expression levels of sst system in sample nos. 13, 14 and 36 were not included in this and the previous study [13] due to the limited available amount of cDNA; however, samples are arranged similarly to the previous study in order to keep the order of the samples in both reports. Basal GH and IGF1 levels and tumor volume data were previously reported [8].
Table 3.
Demographic and qPCR data: somatostatin receptor subtype sst5TMD5 and sst5TMD4 mRNA levels in individual normal pituitaries estimated mRNA copy number corrected by a normalization factor (NF) derived from the expression of three control genes (GAPDH, HPRT1 and ACTB).
| No. | S | A | sst5TMD5/NF | sst5TMD4/NF |
|---|---|---|---|---|
| 1 | F | 30 | 2 | 2 |
| 2 | F | 40 | 0 | 34 |
| 3 | F | 40 | 0 | 1 |
| 4 | M | 34 | 0 | 12 |
| 5 | M | 40 | 3 | 0 |
| 6 | M | 40 | 6 | 1 |
| 7 | M | 42 | 0 | 1 |
| 8 | M | 50 | 0 | 1 |
| Median | 40 | 0 | 1 |
Table 2.
Demographic and qPCR data somatostatin receptor subtype sst5TMD5 and sst5TMD4 mRNA levels in individual non-functioning pituitary adenomas estimated mRNA copy number corrected by a normalization factor (NF) derived from the expression of three control genes (GAPDH, HPRT1 and ACTB).
| No. | S | A | sst5TMD5/NF | sst5TMD4/NF |
|---|---|---|---|---|
| 1 | F | 18 | 0 | 5 |
| 2 | F | 25 | 0 | 0 |
| 3 | F | 43 | 0 | 27 |
| 4 | F | 47 | 0 | 111 |
| 5 | F | 49 | 0 | 0 |
| 6 | F | 50 | 0 | 0 |
| 7 | F | 52 | 0 | 6 |
| 8 | F | 57 | 0 | 0 |
| 9 | F | 61 | 0 | 0 |
| 10 | F | 68 | 0 | 0 |
| 11 | F | 72 | 0 | 11 |
| 12 | F | 74 | 0 | 61 |
| 13 | F | 80 | 0 | 100 |
| 14 | F | 81 | 138 | 0 |
| 15 | F | 84 | 0 | 73 |
| 16 | M | 31 | 0 | 28 |
| 17 | M | 32 | 0 | 20 |
| 18 | M | 32 | 0 | 25 |
| 20 | M | 39 | 0 | 13 |
| 21 | M | 40 | 0 | 69 |
| 22 | M | 44 | 0 | 0 |
| 23 | M | 46 | 0 | 20 |
| 24 | M | 48 | 0 | 0 |
| 25 | M | 48 | 38 | 216 |
| 26 | M | 51 | 0 | 0 |
| 27 | M | 53 | 0 | 0 |
| 28 | M | 53 | 0 | 37 |
| 29 | M | 56 | 0 | 72 |
| 30 | M | 60 | 0 | 0 |
| Median | 51 | 0 | 9 |
As previously reported, normal pituitaries (n = 8; median age 40 years old; Table 3) were obtained during autopsy, after accidental death [13,15]. Tumors were classified as invasive or noninvasive according to radiological criteria as previously reported [17]. As previously published [8], patients with acromegaly were classified as pharmacologically controlled when achieving the criteria of normal IGF1 levels for age, as well as GH <2.5 µg/L (indeed, all patients also achieved the more stringent criteria of 1 µg/L). As previously published [8], basal GH and IGF levels and tumor volume data of the acromegalic patients are indicated in Table 1 in order to improve global insight of the data.
For in vitro studies, somatotropinoma specimens were obtained during transsphenoidal surgery (n = 7), placed in sterile cold media and dispersed into single cells for culture by enzymatic and mechanical disruption following the methods previously reported [18].
This study was in accordance with the ethical standards of the Helsinki Declaration of the World Medical Association, and it was approved by the Ethic Committees of the Hospital Universitario Clementino Fraga Filho (Brazil) and of the University of Cordoba (Spain). Informed consent was obtained from each patient or relative, in case of autopsy, before study entry.
RNA isolation, reverse transcription, and analysis of truncated sst5 variant gene expression by quantitative real-time PCR (qPCR)
Details of RNA extraction, quantification, reverse-transcription (RT), application of qPCR and primer sequences used to measure the expression levels of human transcripts included in this study have been previously reported elsewhere by our group [9,19]. Briefly, samples were processed for recovery of total RNA, its concentration quantified, and 1 µg was reverse transcribed. cDNAs were amplified by qPCR and run against synthetic standards for each transcript of interest to estimate mRNA copy number. As previously described, to control for variations in the amount of RNA used in the RT reaction and the efficiency of the RT reaction, the expression level (copy-number) of the transcripts of interest was adjusted by the normalization factor (NF) within the sample calculated from the expression levels of three control genes [glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta-actin (ACTB) and hypoxanthine ribosyltransferase (HPRT1)] using the GeNorm3.3 application [20].
Immunohistochemical analysis (IHC) of sst5TMD4 in somatotropinomas
IHC was performed using the Ultra-Sensitive ABC Peroxidase Staining Kits and the Metal Enhanced DAB Substrate Kit (Thermo Scientific, Rockford, IL, USA) as previously described but with some modifications [9]. Specifically, the sections were deparaffinized and boiled for 20 min in 10mMcitric acid (pH 6.0) to unmask the epitopes and were treated with 3% hydrogen peroxide for 10 min to inhibit the endogenous peroxidase activity. Then, sections were treated with blocking solution (PBS + 1% BSA + 0.1% Triton-X) for 30 min and the specific sst5TMD4 primary rabbit antibody [9] was subsequently added (it should be mentioned that a specific antibody for sst5TMD5 is not available and that is the reason why IHC was not performed for sst5TMD5). After an overnight incubation with the primary antibody at 4 °C, the sections were washed and incubated with an anti-rabbit horseradish peroxidase-conjugated antibody (Cell Signaling, Danvers, MA, USA) for 60 min, the ABC complex for 30 min and 3,3′-diaminobenzidine tetrahydrochloride for 10 min. The sections were counterstained with hematoxylin. Negative controls were obtained by omitting the primary antibodies or preadsorbing the sst5TMD4-specific antibody with molar excess of the immunizing peptide. Cell number counting and signal intensity estimation was performed independently by 3 different researchers in a blinded manner and a minimum of 1000 cells were analyzed.
Pituitary adenoma cell culture, cloning of truncated sst5 receptors (sst5TMD4 and sst5TMD5) into pCDNA3.1+ expression vector, transfection of cultured somatotropinoma cells and measurement of in vitro cell viability
To explore the functional consequences of the presence of truncated sst5 variants on cell viability, we overexpressed sst5TMD4 and sst5TMD5 in human primary somatotropinoma cell cultures. Details of the cloning of truncated sst5 variants (sst5TMD4 and sst5TMD5) and transfection methods into cultured cells have been previously reported elsewhere by our group [9,19]. Briefly, 7 human somatotropinoma obtained after transsphenoidal surgery were dispersed into single cells by enzymatic and mechanical disruption and cultured onto tissue culture plates in a serum containing medium, as previously indicated [18]. Then, dispersed GH secreting adenoma cells were plated in a 6-well plate using DMEM supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic, and transfected with 1 µg of sst5TMD4 (n = 5) or sst5TMD5 (n = 3) plasmid using Lipofectamine 2000 (Life Technologies, Barcelona, Spain). Somatotropinoma cells transiently transfected with empty pCDNA3.1+ (mock transfected) were used as negative control. Efficacy of the transfection was assessed by qPCR. After 24 h, transfected cells were detached and plated (10,000 cells/well) in a 96-well plate, and cell viability rate was assessed using the alamar-Blue reagent (Biosource International; Camarillo, CA) in the FlexStation-3 system (Molecular Devices; Madrid, Spain), as previously reported [18]. Unfortunately, commonly used models of tumor pituitary cell lines (i.e. GH3, GH4 or GC cells) could not be employed in these assays, as they derive from rodent pituitary tumor cells that endogenously express truncated sst5 variant orthologues [21], which would confound and preclude an adequate analysis of the functional consequences of the presence of human sst5 variants in these cell lines.
Statistical analysis and receiver operating characteristic (ROC) curve of sst5TMD4, sst2 and sst5
All statistical analyses were performed by SPSS 19.0 (Chicago, IL) and GraphPad Prism 6.0 (La Jolla, CA). sst5TMD4 expression among normal pituitaries, somatotropinomas and NFPA samples was analyzed using Kruskal–Wallis test (p < 0.001) followed by a manual analysis with Mann–Whitney for each pair of samples using Bonferroni’s correction (p was considered statistically significant if <0.017). Correlations between variables were assessed using Spearman’s correlation test. As previously reported [18] to normalize values within each different in vitro experiments, the values obtained in the in vitro cell viability experiments were compared with mock-transfected controls (set at 100%). p < 0.05 was considered significant. When p-values ranged between <0.1 and >0.05, a trend for significance was indicated where appropriate.
As previously reported [12], ROC was performed for evaluation of diagnostic test sensibility and specificity. Specifically, in this study ROC was used as a tool to measure how well the expression of sst5TMD4, sst2 and sst5 could distinguish between the diagnostic groups [patients presenting extension into sinus (total invasion and invasion into cavernous or sphenoid sinus)]. Statistical analysis of ROC curves was performed by calculating the Area under the Curve (AUC) of each receptor and comparing them with the AUC of the reference line using Student’s t-test.
Results
Expression of truncated somatostatin receptor 5 variants (sst5TMD4 and sst5TMD5) in somatotropinomas, NFPA and normal pituitary
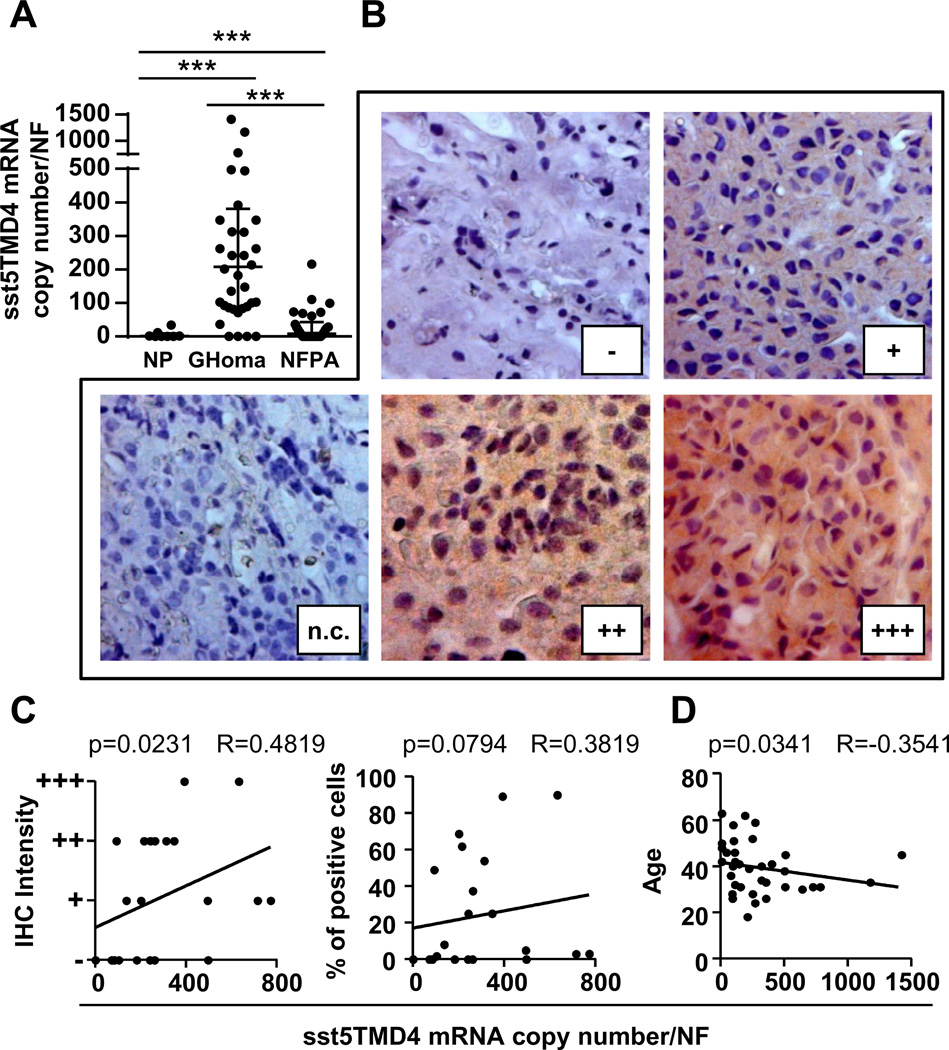
Our results indicate that truncated sst5 variants are differentially expressed in human pituitary adenomas and that they are not relevantly expressed in normal pituitaries. Specifically, the sst5TMD4 variant is expressed in a high proportion of somatotropinomas (89%; 32/36; median: 208 mRNA copies; Table 1), in 50% of the NFPA (15/30; median: 9 mRNA copies; Table 2) and in 2 of the 8 normal pituitaries analyzed (median: 1 mRNA copy; Table 3). Interestingly, we also found that the sst5TMD4 expression levels were significantly higher in somatotropinomas than in NFPA (Fig. 1A; somatotropinomas > > > NFPA > > > normal pituitaries). However, the sst5TMD5 variant was only detected in a small subset of the samples analyzed [11% of somatotropinomas (n = 4/36), 7% of NFPAs (n = 2/30) and, 0% of normal pituitaries; Tables 1–3], and sst5TMD5 expression levels were significantly lower as compared with truncated sst5TMD4 in somatotropinomas (comparing the 4 samples coexpressing sst5TMD4/sst5TMD5; Table 1). For this reason (i.e. only 4 samples available expressing sst5TMD5), we could not perform correlations between its expression and the clinical data available.
Fig. 1.
(A) Median sst5TMD4 mRNA levels in normal pituitaries (NP; n = 8), somatotropinomas (GHomas; n = 36) and non-functioning pituitary adenomas (NFPA; n = 30). (B) Representative IHC images of sst5TMD4 in somatotropinomas. (C) Positive correlation between IHC intensity and percentage of positive cells with sst5TMD4 mRNA levels. (D) Negative correlation between the age of acromegalic patients at diagnosis and sst5TMD4 levels. (A–D) mRNA copy number corrected by a normalization factor (NF) derived from the expression of three control genes (HPRT1, ACTB, and GAPDH).
IHC of sst5TMD4 in 22 paraffin-embedded somatotropinoma available samples (Table 1 and Fig. 1B) revealed a positive correlation between mRNA levels and IHC data (both with signal intensity and percent of stained cells; Fig. 1C). Unfortunately, formalin fixed paraffin-embedded samples from NFPA patients were not available, and therefore, we could not perform IHC analysis of sst5TMD4 in these samples.
Association between sst5TMD4 expression and age of acromegalic patients, the in vivo response of acromegalic patients to octreotide-LAR and GNAS mutation status
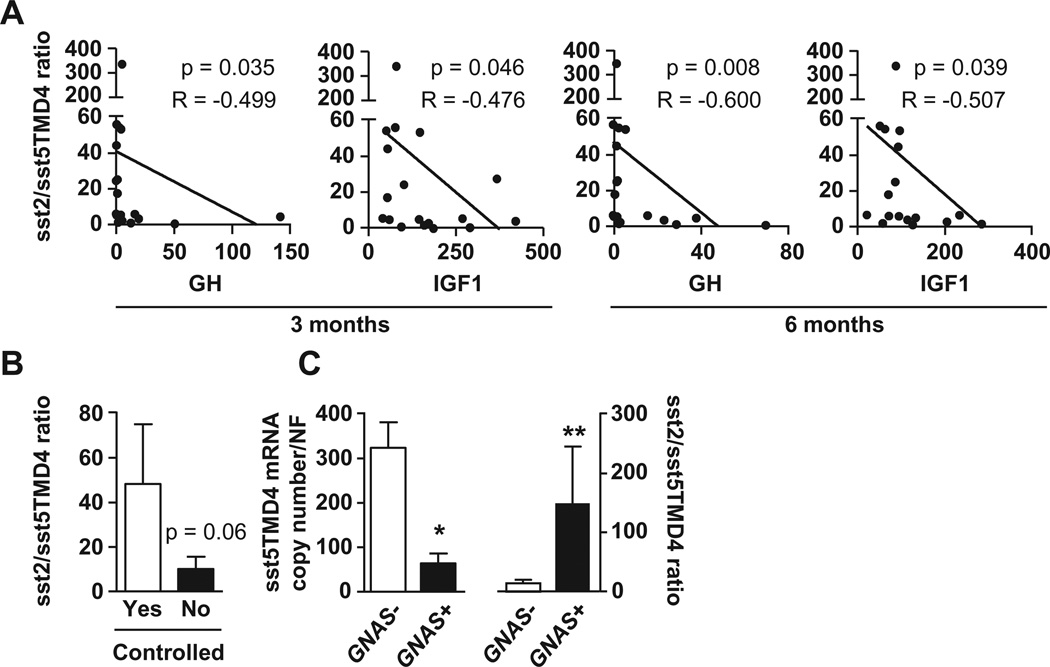
We found that sst5TMD4 expression levels showed a clear inverse correlation with age of patients with somatotropinomas (Fig. 1D). It should be noted that this correlation with age was exclusive of sst5TMD4, and it was not previously observed with other receptors (sst1–sst5) in this population of patient subtypes [7,8,13]. Interestingly, no correlations were found between the pharmacological response to octreotide-LAR therapy and individual sst5TMD4 expression levels; however, we found a marked inverse correlation between sst2/sst5TMD4 ratio and percent decrease of GH and IGF1 levels after 3 and 6 months postsurgical octreotide-LAR treatment [Fig. 2A; no significant correlations for sst5/sst5TMD4 ratio (data not-shown)]. Additionally, a higher sst2/sst5TMD4 ratio was observed among patients achieving hormonal control (GH and IGF1 levels after octreotide-LAR therapy) compared with uncontrolled patients (Fig. 2B; p = 0.06). Furthermore, our data indicate that sst5TMD4 expression was significantly lower in patients positive for GNAS mutation than in patients with no mutation, while a higher sst2/sst5TMD4 ratio was observed in patients with GNAS positive tumors (Fig. 2C).
Fig. 2.
(A) Negative correlations between sst2/sst5TMD4 ratio and GH and IGF1 levels after 3 and 6 months postsurgical treatment with octreotide-LAR in acromegalic patients. (B) sst2/sst5TMD4 ratio in pharmacologically controlled and uncontrolled patients with GH secreting adenomas (GHomas). (C) sst5TMD4 expression and sst2/sst5TMD4 ratio in patients with GNAS negative and positive tumors.
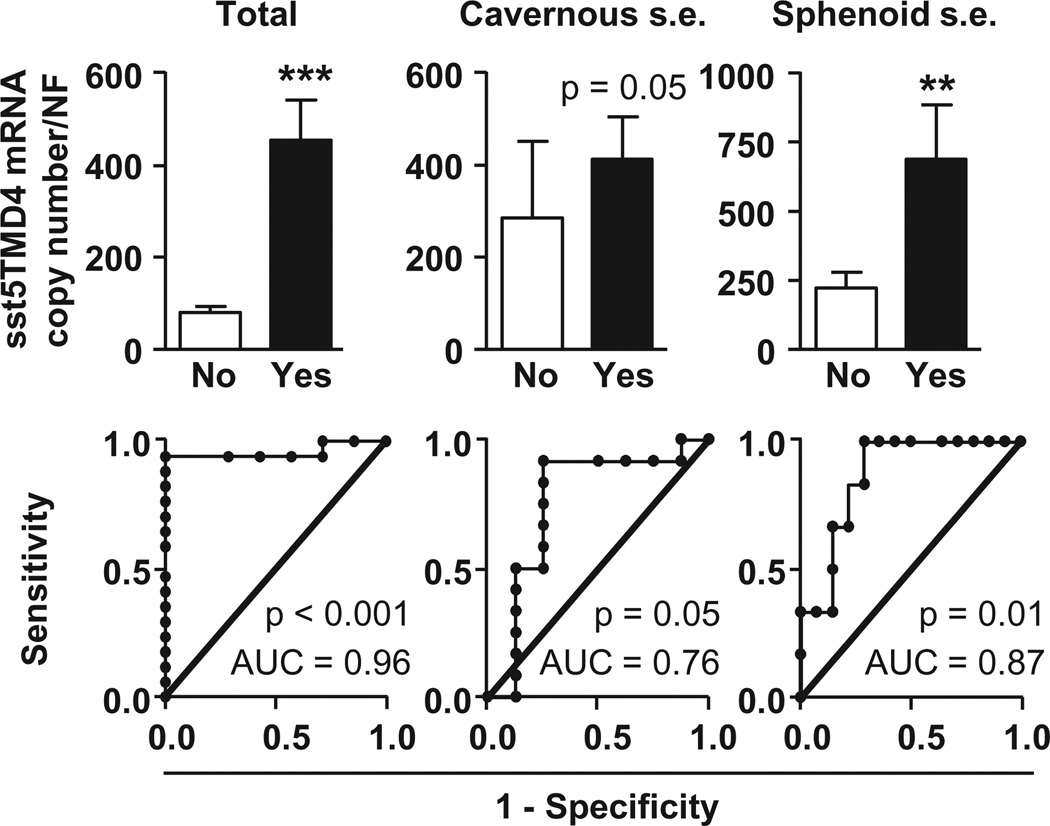
Association between sst5TMD4 expression and invasion of intracranial structures
We found that expression levels of sst5TMD4, but not sst2 or sst5, were significantly higher in somatotropinomas presenting extension into sinus [total (cavernous + sphenoid) and individual sinus extension; Fig. 3, top-panels and Supplemental Fig. S1A and B)]. In fact, ROC analysis of sst2, sst5 and sst5TMD4 showed that only the expression of sst5TMD4 discriminates between the two diagnostic groups of invasiveness [patients presenting total and individual (cavernous or sphenoid sinus) extension into sinus; Fig. 3, bottom-panels; the closer the ROC curve is to the upper left corner of the graphic (i.e., higher sensitivity and specificity), the higher the overall accuracy of the marker used]; conversely, sst2 and sst5 expressions showed a poor ability to distinguish between the two somatotropinoma-populations (ROC curves similar to the reference line; Supplemental Fig. S1A and B). It should be mentioned that similar clinical data from NFPA patients (invasiveness) were not available and therefore, we could not determine the potential association between sst5TMD4 expression and increased aggressive features in these patients.
Fig. 3.
sst5TMD4 expression levels in invasive somatotropinomas [total (cavernous + sphenoid) sinus extension; left, top-panel], as well as in tumors with cavernous (middle, top-panel) or sphenoid (right, top-panel) sinus extension; Receiver operating characteristics (ROC) curve to determine the accuracy of sst5TMD4 receptor as diagnostic test to discriminate between invasive and non-invasive somatotropinomas (bottom-panels).
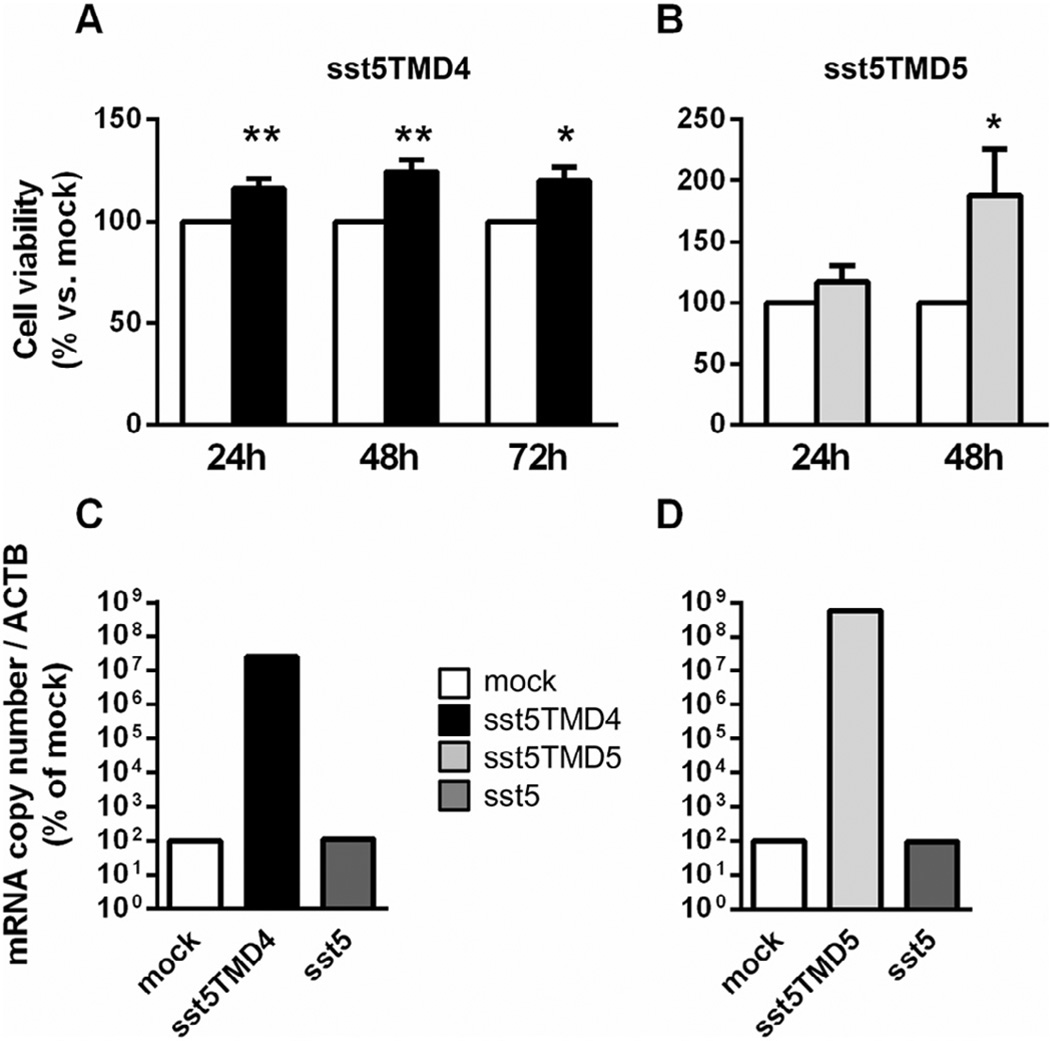
Overexpression of truncated somatostatin receptor 5 variants increased cell viability abilities of somatotropinomas
We found that overexpression of truncated sst5 variants was directly associated with increased cell viability in cultured somatotropinoma cells compared to controls (mock-transfected somatotropinoma cells). Specifically, somatotropinoma cells transfected with sst5TMD4 significantly increased cell viability 24, 48 and 72 h after transfection (Fig. 4A), whereas cell viability was only significantly increased in sst5TMD5-transfected cells 48 h after transfection (Fig. 4B). Validation of transfection was performed using qPCR as shown in Fig. 4C and D which showed that only sst5TMD4 or sst5TMD5, but not sst5, was overexpressed in these experiments.
Fig. 4.
Cell viability (24-, 48- and/or 72-h) in primary culture of somatotropinoma transfected with sst5TMD4 (A, n = 5; black columns) and sst5TMD5 (B, n = 3; gray-columns) as compared with mock-transfected control cells (white-columns). Representative validation by qPCR sst5TMD4 (C, black bar) and sst5TMD5 (D, light gray bar) demonstrating an increase in sst5TMD4, but not in sst5 (C and D, dark gray bar), mRNA levels. Data are expressed as percent of mock, set at 100%.
Discussion
Departing from a clinically and molecularly well characterized series of pituitary adenomas (for demographic/clinical data and sst1–5 expression profile in these samples see ref. 13), in this report we demonstrate that truncated sst5 variants are expressed differentially in pituitary adenomas [e.g. sst5TMD4 in 89% of somatotropinomas vs. 50% of NFPAs (median: 208 vs. 9 mRNA copies)], and that they are not relevantly expressed in normal pituitaries [irrelevant median expression (1 copy) based on method detection limit for ssts] [7,9], which is consistent with our previous reports using smaller cohorts of samples (n = 6, 22 and 1, respectively) [9]. Altogether, these data indicate that truncated sst5 variants, especially sst5TMD4 (which is expressed in a high proportion of pituitary adenomas), could play a relevant pathophysiological role in pituitary adenomas, particularly in GH secreting adenomas, where its expression levels are notably higher than sst5TMD5 (comparing the 4 samples coexpressing sst5TMD4/sst5TMD5; Table 1), and also higher than in NFPAs (Fig. 1A). Additionally, we have found a positive correlation between sst5TMD4 mRNA and protein (IHC data) expression levels in somatotropinomas, suggesting that this mRNA is appropriately translated to build sst5TMD4 protein, and that sst5TMD4 expression levels may serve as a reliable indicator to perform further analyses.
Interestingly, sst5TMD4 expression showed a clear inverse correlation with age of patients with GHomas, which was not observed previously with other sst-subtypes [7,8,13]. Since previous results indicate age of acromegalic patients as a good clinical predictor of biochemical activity and aggressive features in acromegaly [3,22], our finding showing an inverse correlation between sst5TMD4 expression and age suggests that expression of sst5TMD4 could be associated, perhaps causally, with these clinical parameters in somatotropinomas. In fact, in line with this, we previously reported that sst5TMD4 expression is associated with poorer secretory response in a population of French acromegalic patients partially resistant to SSA therapy [10]. However, no studies have analyzed hitherto the relationship between sst5TMD4 expression, aggressive features and pharmacological response to octreotide-therapy in acromegaly. Hence, we subsequently studied whether sst5TMD4 expression is associated with aggressiveness in GH secreting adenomas in vivo (i.e., reduced pharmacological response to octreotide-LAR and invasive features, in the Brazilian acromegalic population) and in vitro (i.e., proliferative capacities in cultured somatotropinoma cells expressing high sst5TMD4 levels).
No correlations were found between the pharmacological response to octreotide-LAR therapy and individual sst5TMD4 expression levels. This observation was not surprising despite its apparent discrepancy with our previous report where sst5TMD4 expression was related to lower GH levels in a subset of acromegalic patients [10], as our present study was performed in a larger, unselected cohort of patients, whereas the former set of patients was specifically analyzed because they had been previously found to be partially resistant to SSA therapy in vivo and/or in vitro [10]. Moreover, it is worth noting that, as our group and others have previously demonstrated, pharmacological SSA-therapy response in acromegaly might be more dependent on the relative expression levels between the critical sst-subtypes involved in negative control of hormonal secretion (i.e. low sst2/sst5 ratio is present in resistant somatotropinomas to SSA-therapy) than on individual expression levels of a single sst-subtype [7,23]. Accordingly, we next sought to determine the potential associations between sst2/sst5TMD4 or sst5/sst5TMD4 ratios and pharmacological response to octreotide-LAR. Interestingly, we found a marked inverse correlation between sst2/sst5TMD4 ratio and percent decrease of GH and IGF-I levels after 3 and 6 months postsurgical octreotide-LAR treatment suggesting that patients with high sst2 levels and low sst5TMD4 levels respond better to octreotide-LAR. In keeping with this notion is the higher sst2/sst5TMD4 ratio observed among patients achieving hormonal control (GH and IFG-I levels after octreotide-LAR therapy) compared with uncontrolled patients. Furthermore, when results from previous reports suggesting that presence of the GNAS mutation can be considered as a clinical indicator of a good response to SSA-therapy in acromegalic patients [14,22], and our previous data showing that patients with GNAS positive tumors expressed high levels of sst2 [15], are viewed in light of our present results showing that sst5TMD4 expression is significantly lower in patients positive for the GNAS mutation than in patients with no mutation (and additionally, a higher sst2/sst5TMD4 ratio is observed in patients with GNAS positive tumors), it is reasonable to suggest the existence of an overall negative association between sst5TMD4 presence and pharmacological response to SSA-therapy in acromegaly.
Results of this study, especially the connection between sst2/sst5TMD4 with clinical response in acromegalic patients, might be patho-physiologically relevant because: 1) in this and previous studies [9,10,24], sst5TMD4 and sst2 are often coexpressed in pituitary adenomas; 2) sst-subtypes are known to form homo/heterodimers, which modify functional, pharmacological, and signaling properties of individual sst in response to their natural and synthetic ligands [25]; 3) a similar association and functional connection also occurs between sst2 and truncated sst5 variants [11]; and, 4) in coexpression experiments, using FRET technology, we observed that sst2 preferentially localizes at the plasma membrane in the absence of truncated sst5TMD4 but when sst5TMD4 colocalizes with sst2 in the same cells, they interact and sst2 is partly retained in intracellular compartments, thus reducing/disrupting the normal inhibitory functioning of sst2, as sst5TMD4 would act as a dominant-negative modulator for sst2-mediating signaling [11].
Most importantly, we found that expression levels of sst5TMD4 were significantly higher in somatotropinomas presenting extension into the sinus (total and individual sinus extension). Of note, these differences in expression levels between patients with and without invasion were specific for sst5TMD4, at least in this patient population, and was not found for sst2 or sst5, suggesting that the relative sst5TMD4 expression levels might represent a potential unique molecular signature contributing to invasion abilities of somatotropinomas. In support of this, the ROC analysis of sst2, sst5 and sst5TMD4 demonstrated that only expression of sst5TMD4 discriminates between the two diagnostic groups of invasiveness [patients presenting extension into the sinus (total invasion and invasion into cavernous or sphenoid sinus)]. Importantly, in support of the invasion/proliferation abilities of truncated sst5 variants in somatotropinomas, our in vitro results revealed, for the first time, that overexpression of sst5TMD4 (and also of truncated sst5TMD5) enhanced cell viability of cultured somatotropinoma in a modest but consistent and significant manner in comparison with mock-transfected somatotropinoma cells.
In conclusion, our data demonstrate that presence of truncated sst5 variants, especially sst5TMD4, is associated with increased aggressiveness in GH secreting adenomas, thereby suggesting that sst5TMD4 could contribute to worsen somatotropinoma prognosis and may provide an attractive target for therapeutic research. Accordingly, it seems plausible that analysis of sst5TMD4 expression, particularly in relation to sst2, could represent in the future a genuine, valuable diagnostic and/or prognostic tool to help in predicting aggressive properties of somatotropinomas (proliferative and invasive capacities), and outcome of clinical response to SSA-therapy in these patients.
Supplementary Material
Acknowledgments
Funding
This work has been funded by the following grants: BIO-0139, CTS-1406, PI-639-2012, (Junta de Andalucía) BFU2013-43282 (Ministerio de Economía y Competitividad, Gobierno de España), PI13-00651 (Proyectos de Investigación en Salud FIS, funded by Instituto de Salud Carlos III), CIBERobn and Ayuda Merck Serono 2013 (to RML and JPC); fellowship P09-CTS-5051 (to AIC), “Sara Borrell” program CD11/00276 (to MDG); Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Merit Award and the National Institutes of Health: R21AG031465 and R01DK088133 (to RDK). CIBER is an initiative of Instituto de Salud Carlos III, Ministerio de Sanidad, Servicios Sociales e Igualdad, Spain.
M.D. Culler is an employee of IPSEN. R.M. Luque and J.P. Castano have received grants and lecture fees from Ipsen and Novartis. M.R. Gadelha has received research grants from Novartis and Pfizer, served as a principle investigator for clinical trials and speaker for Novartis and Ipsen. E. Venegas-Moreno and A. Soto-Moreno received grants and lecture fees from Ipsen, Novartis and Pfizer.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2015.01.037.
Footnotes
Authors’ contributions
Conception and design: RML, RDK, MDC, MRG, JPC; Development of methodology: RML, AI-C, LVN, GFT, DH-A, LK, MDG; Acquisition of clinical data: LVN, GFT, LK, EV-M, AM-C, MAG, AS-M, MRG; Analysis and interpretation of data: RML, AI-C, DH-A, MDG, JPC; Writing of the manuscript: RML, AI-C, MDG, MRG, JPC; Review and/or revision of the manuscript: RML, AI-C, LVN, GFT, DH-A, LK, EV-M, AM-C, MAG, AS-M, RDK, MDC, MDG, MRG, JPC; Study supervision: RML, MRG and JPC.
Conflict of interest
We wish to draw attention to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work.
The rest of the authors have nothing to disclose.
References
- 1.Melmed S. Acromegaly pathogenesis and treatment. J. Clin. Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front. Neuroendocrinol. 2013;34:228–252. doi: 10.1016/j.yfrne.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr. Rev. 2011;32:247–271. doi: 10.1210/er.2010-0002. [DOI] [PubMed] [Google Scholar]
- 4.Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J. Mol. Endocrinol. 2014;52:R223–R240. doi: 10.1530/JME-14-0011. [DOI] [PubMed] [Google Scholar]
- 5.Giustina A, Mazziotti G, Maffezzoni F, Amoroso V, Berruti A. Investigational drugs targeting somatostatin receptors for treatment of acromegaly and neuroendocrine tumors. Expert Opin. Investig. Drugs. 2014;23:1619–1635. doi: 10.1517/13543784.2014.942728. [DOI] [PubMed] [Google Scholar]
- 6.Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, et al. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat. Rev. Endocrinol. 2014;10:243–248. doi: 10.1038/nrendo.2014.21. [DOI] [PubMed] [Google Scholar]
- 7.Taboada GF, Luque RM, Bastos W, Guimaraes RF, Marcondes JB, Chimelli LM, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1–5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur. J. Endocrinol. 2007;156:65–74. doi: 10.1530/eje.1.02313. [DOI] [PubMed] [Google Scholar]
- 8.Taboada GF, Luque RM, Neto LV, Machado Ede O, Sbaffi BC, Domingues RC, et al. Quantitative analysis of somatostatin receptor subtypes (1–5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur. J. Endocrinol. 2008;158:295–303. doi: 10.1530/EJE-07-0562. [DOI] [PubMed] [Google Scholar]
- 9.Duran-Prado M, Gahete MD, Martinez-Fuentes AJ, Luque RM, Quintero A, Webb SM, et al. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J. Clin. Endocrinol. Metab. 2009;94:2634–2643. doi: 10.1210/jc.2008-2564. [DOI] [PubMed] [Google Scholar]
- 10.Duran-Prado M, Saveanu A, Luque RM, Gahete MD, Gracia-Navarro F, Jaquet P, et al. A potential inhibitory role for the new truncated variant of somatostatin receptor 5, sst5TMD4, in pituitary adenomas poorly responsive to somatostatin analogs. J. Clin. Endocrinol. Metab. 2010;95:2497–2502. doi: 10.1210/jc.2009-2247. [DOI] [PubMed] [Google Scholar]
- 11.Duran-Prado M, Gahete MD, Hergueta-Redondo M, Martinez-Fuentes AJ, Cordoba-Chacon J, Palacios J, et al. The new truncated somatostatin receptor variant sst5TMD4 is associated to poor prognosis in breast cancer and increases malignancy in MCF-7 cells. Oncogene. 2012;31:2049–2061. doi: 10.1038/onc.2011.389. [DOI] [PubMed] [Google Scholar]
- 12.Puig-Domingo M, Luque RM, Reverter JL, Lopez-Sanchez LM, Gahete MD, Culler MD, et al. The truncated isoform of somatostatin receptor5 (sst5TMD4) is associated with poorly differentiated thyroid cancer. PLoS ONE. 2014;9:e85527. doi: 10.1371/journal.pone.0085527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neto LV, Machado Ede O, Luque RM, Taboada GF, Marcondes JB, Chimelli LM, et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J. Clin. Endocrinol. Metab. 2009;94:1931–1937. doi: 10.1210/jc.2008-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlier A, Gunz G, Zamora AJ, Morange-Ramos I, Figarella-Branger D, Dufour H, et al. Prognostic and therapeutic consequences of Gs alpha mutations in somatotroph adenomas. J. Clin. Endocrinol. Metab. 1998;83:1604–1610. doi: 10.1210/jcem.83.5.4797. [DOI] [PubMed] [Google Scholar]
- 15.Taboada GF, Neto LV, Luque RM, Cordoba-Chacon J, de Oliveira Machado E, de Carvalho DP, et al. Impact of gsp oncogene on the mRNA content for somatostatin and dopamine receptors in human somatotropinomas. Neuroendocrinology. 2011;93:40–47. doi: 10.1159/000322040. [DOI] [PubMed] [Google Scholar]
- 16.Taboada GF, Tabet AL, Naves LA, de Carvalho DP, Gadelha MR. Prevalence of gsp oncogene in somatotropinomas and clinically non-functioning pituitary adenomas: our experience. Pituitary. 2009;12:165–169. doi: 10.1007/s11102-008-0136-0. [DOI] [PubMed] [Google Scholar]
- 17.Kasuki Jomori de Pinho L, Vieira Neto L, Armondi Wildemberg LE, Gasparetto EL, Marcondes J, de Almeida Nunes B, et al. Low aryl hydrocarbon receptor-interacting protein expression is a better marker of invasiveness in somatotropinomas than Ki-67 and p53. Neuroendocrinology. 2011;94:39–48. doi: 10.1159/000322787. [DOI] [PubMed] [Google Scholar]
- 18.Luque RM, Ibanez-Costa A, Lopez-Sanchez LM, Jimenez-Reina L, Venegas-Moreno E, Galvez MA, et al. A cellular and molecular basis for the selective desmopressin-induced ACTH release in Cushing disease patients: key role of AVPR1b receptor and potential therapeutic implications. J. Clin. Endocrinol. Metab. 2013;98:4160–4169. doi: 10.1210/jc.2013-1992. [DOI] [PubMed] [Google Scholar]
- 19.Gahete MD, Cordoba-Chacon J, Hergueta-Redondo M, Martinez-Fuentes AJ, Kineman RD, Moreno-Bueno G, et al. A novel human ghrelin variant (In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: potential pathophysiological relevance. PLoS ONE. 2011;6:e23302. doi: 10.1371/journal.pone.0023302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordoba-Chacon J, Gahete MD, Duran-Prado M, Pozo-Salas AI, Malagon MM, Gracia-Navarro F, et al. Identification and characterization of new functional truncated variants of somatostatin receptor subtype 5 in rodents. Cell. Mol. Life Sci. 2010;67:1147–1163. doi: 10.1007/s00018-009-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadelha MR, Kasuki L, Korbonits M. Novel pathway for somatostatin analogs in patients with acromegaly. Trends Endocrinol. Metab. 2013;24:238–246. doi: 10.1016/j.tem.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Saveanu A, Gunz G, Dufour H, Caron P, Fina F, Ouafik L, et al. Bim-23244, a somatostatin receptor subtype 2- and 5-selective analog with enhanced efficacy in suppressing growth hormone (GH) from octreotide-resistant human GH-secreting adenomas. J. Clin. Endocrinol. Metab. 2001;86:140–145. doi: 10.1210/jcem.86.1.7099. [DOI] [PubMed] [Google Scholar]
- 24.Gatto F, Feelders R, van der Pas R, Kros JM, Dogan F, van Koetsveld PM, et al. beta-Arrestin 1 and 2 and G protein-coupled receptor kinase 2 expression in pituitary adenomas: role in the regulation of response to somatostatin analogue treatment in patients with acromegaly. Endocrinology. 2013;154:4715–4725. doi: 10.1210/en.2013-1672. [DOI] [PubMed] [Google Scholar]
- 25.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.