Abstract
Extravillous trophoblasts (EVTs) play a central role in educating maternal leukocytes, endometrial stromal and endothelial cells to generate a receptive decidual microenvironment tailored to accept the semi-allogeneic fetus. HLA-G, a non-classical HLA class I molecule endowed with immune-regulatory functions, is primarily expressed on EVTs lining the placenta and on the naturally occurring tolerogenic dendritic cells, named DC-10, which are enriched in the human first trimester decidua. Decidual DC-10 are involved in HLA-G-mediated tolerance at the maternal–fetal interface. EVTs not only establish a tolerogenic microenvironment through the interaction with maternal innate and adaptive cells but also orchestrate placenta vascular and tissue remodeling, leading to a successful pregnancy. Here, we discuss the potential implications of the HLA-G-mediated cross-talk among the cells present at the maternal–fetal interface, and its role in maintaining a positive relationship between the mother and the fetus.
Keywords: HLA-G, trophoblasts, dendritic cells, IL-10, T regulatory cells, vascular remodeling
Introduction
The maternal–fetal interface is composed of fetal trophoblasts intermingled with maternal leukocytes, stromal, and endothelial cells that comprise the decidua. During implantation, trophoblasts, derived from the trophoectoderm surrounding the blastocyst, differentiate into the syncytiotrophoblasts that infiltrates the endometrium, and the cytotrophoblasts at the embryo side. The layer of syncytiotrophoblasts in contact with the decidua represents the extravillous trophoblasts (EVTs) (Figure 1). EVTs orchestrate bi-directional cross-talk between the mother and the fetus by providing structural and biochemical barriers, serving as an endocrine organ that support and regulate placental and fetal development and growth, and modulating maternal innate and adaptive immune responses (1).
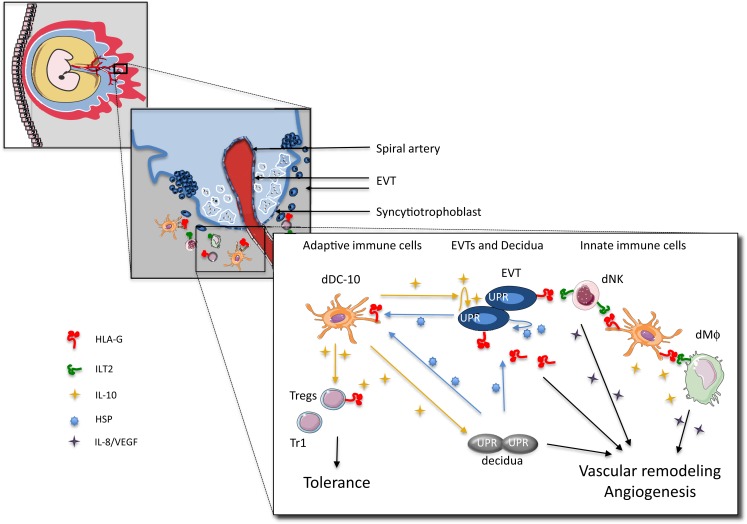
Figure 1.
Proposed model for cross-talk among embryo trophoblasts, decidual leukocytes, and stromal cells at the maternal–fetal interface in human first trimester pregnancy. EVTs express and secrete HLA-G, and release IL-10 (and TSLP), which instruct dAPCs to become tolerogenic DC (i.e., dDC-10 or TSLP-modulated dDC) secreting IL-10 and promoting the induction of a variety of Tregs (i.e., Tr1 cells, CD4+CD25+FOXP3+ Tregs, and CD4+HLA-G+ Tregs). Induced Tregs inhibit effector T cells, and, via IL-10 secretion, promote HLA-G expression on EVTs. EVTs via HLA-G directly promote dNK cell activation and the release of angiogenic factors. dDC-10 is HLA-G+ and can interact with either dNKs or dMΦ via ILT2, and promote their activation and pro-angiogenic effects. dDC-10 themselves secrete also pro-angiogenic factors supporting neo-vascularization. HSPs secreted by the maternal cells and trophoblasts contribute to the regulation of HLA-G expression on dAPCs and EVTs. Finally, IL-10 modulates the UPR pathway and regulates vascular uterine remodeling by HLA-G+ EVTs.
The evidence that, after embryo implantation, defective development and function of EVTs can lead to fetal loss and pregnancy-associated pathological conditions, including pre-eclampsia and intrauterine growth restriction (2–4), sustains the important role of EVTs in orchestrating the decidual modification for successful pregnancy. The expression of HLA-G, a non-classical HLA class I molecule, on EVTs contributes to trophoblast invasiveness, decidual cell differentiation, vascular remodeling, and maintenance of a local immunosuppressive state. A proper understanding of regulatory mechanisms that control EVTs interaction with the maternal niche is a critical issue in reproduction.
State of the Art
Hormonal regulation at the maternal–fetal interface
The endometrial microenvironment, constituted by luminal and glandular epithelial cells, stromal cells, fibroblasts, vascular smooth muscle cells, endothelial cells, leukocytes, endometrial stem cells, and dynamic leukocyte populations, undergoes cyclical changes regulated by sex hormones. In the absence of pregnancy, the endometrium is sloughed off at menstruation. In the post-menstrual proliferative phase, under estradiol stimulation, it undergoes rapid regeneration into a fertile soil capable to accept the embryo (5). During the secretory phase, the blood flow increases, the arteries branches, and the glands enlarge and start to secrete fluids rich in glycogen used by the embryo as an energy source in its early stages of growth. These processes are driven by the post-ovulatory rise of progesterone that inhibits the pro-proliferative effect of estradiol and, in mammals, induces a radical transformation of the endometrium (pre-decidualization) that heralds the limited period of endometrial receptivity, (“implantation window”) during which embryo attachment can take place (6). Pre-decidualization is primarily defined by the transformation of endometrial stromal cells into secretory epithelioid-like decidua cells and is characterized by massive influx of maternal innate immune cells and vascular remodeling (7).
In the presence of the embryo, the human chorionic gonadotropin (hCG) sustains the full decidualization of the endometrium via stimulation of progesterone production. hCG is the most specific embryo-derived signal observed in humans and the hCG gene is transcribed as early as the two-cell stage (8, 9). Being released before embryo implantation, hCG also acts on endometrial cells in a paracrine way by inducing their differentiation characterized by secretion of prolactin, leukemia inhibitory factor (LIF), and IL-6 (10, 11). Furthermore, hCG promotes angiogenesis by increasing vessel sprouting of endothelial cells and secretion of vascular endothelial growth factor (VEGF) (12, 13). The immunomodulatory properties of hCG are multiple (13): it regulates decidual natural killer (dNK) cell proliferation, contributing to the remodeling of decidual spiral arterioles (14, 15); it induces CXCL8 production by monocytes (16); it influences tolerogenic dendritic cells (DCs) proliferation and differentiation (17); and it contributes to recruitment of T regulatory cells (Tregs) (18).
The pre-ovulatory peak of estrogen is important for proliferation of the uterine epithelium in preparation for implantation, while rising progesterone after ovulation is required for implantation of the embryo and decidual differentiation. Together with hCG, progesterone and estradiol are also essential for the programing of a local tolerogenic environment (19). Progesterone polarizes T-cell responses toward an anti-inflammatory phenotype, favoring T(helper)h2 while dampening Th1 and Th17 cells, and inducing Tregs via thymic stromal lymphopoietin (TSLP) (20–22). The increased concentration of progesterone at the maternal–fetal interface may play a role in regulating HLA-G gene expression (23). Progesterone induces up-regulation of HLA-G in primary cultures of first trimester cytotrophoblasts through the binding to an alternative progesterone response element in the HLA-G promoter (24).
Estradiol regulates the immune system by affecting T and B cells, and down regulating NK cell cytotoxicity (25). Interestingly, estradiol helps to regulate fetal tolerance during pregnancy by expanding Tregs and their suppressive function (26, 27).
Dendritic cells, by expressing specific receptors, are susceptible to stimulation with hCG, progesterone, and estradiol. Pregnancy hormones can either activate or reduce the stimulatory activity of monocyte-derived DCs. Consistent up-regulation of IL-10 production by human DCs has been observed upon stimulation with pregnancy hormones [as reviewed in Ref. (28)].
HLA-G-expressing trophoblast at the maternal-fetal interface
HLA-G has well-recognized immunomodulatory activities, is low polymorphic [reviewed in Ref. (29)], and has limited tissue distribution [reviewed in Ref. (30)]. HLA-G was the first HLA class I molecule identified on EVTs (31). EVTs, forming the placental interface with the maternal systemic circulation, do not express HLA class I, but as they differentiate to invade the decidua and contact maternal decidual leukocytes, they begin to express HLA-G (32). All EVTs, syncytiotrophoblasts (33), interstitial and endovascular trophoblasts, and placental bed giant cells are HLA-G positive [reviewed in Ref. (34)].
By alternative splicing of the primary transcript, four membrane-bound (HLA-G1 to -G4) and three soluble (HLA-G5 to -G7) isoforms can be generated [reviewed in Ref. (35)]. In addition, a soluble isoform, named shed HLA-G1, is released after proteolytic cleavage of the membrane-bound HLA-G1 by metalloproteinases (36, 37). Through the interaction with the inhibitory receptors immunoglobulin-like transcript (ILT)2 and ILT4, and the killer immunoglobulin-like receptor (KIR)2DL4, HLA-G regulates innate and adaptive immune responses and participates in promoting tolerance [reviewed in Ref. (38)].
During the last decade, it has become evident that the expression of HLA-G on EVTs is not primarily involved in protecting the fetus from the attack by maternal cells, but it plays an important role in tissue remodeling. HLA-G expressed or secreted by EVTs controls their decidual and endovascular invasion. EVTs can express membrane-bound or shed HLA-G1, and soluble HLA-G2, -G5, and -G6 (39–43) (Table 1). Studies in placental sections demonstrated that β2m-bound HLA-G is expressed by all EVTs, whereas more distal EVTs at the invasion front express the free heavy chain (FHC) HLA-G (40). It has been proposed that the selective expression of FHC–HLA-G, which is not recognized by ILT2 (44), may limit the inhibition of dNKs while allowing these cells to secrete factors required for successful pregnancy. In vitro studies showed that treatment of primary trophoblasts with HLA-G5 stimulates cell invasion and increases the production of metalloproteinases and urokinase, known to remodel the endometrial extracellular matrix (45, 46). Moreover, the interaction between HLA-G on EVTs and dNKs leads to CXCL8 and CXCL10 secretion that in turn, via stimulation of CXCR1 and CXCR3, promote EVTs invasiveness (14). Thereby, HLA-G-expressing EVTs regulate decidual invasion in both autocrine and paracrine manner.
Table 1.
Expression pattern of HLA-G-related molecules on cells at the maternal–fetal interface.
| Cell types | HLA-G isoforms (reference) | HLA-G receptors (reference) |
|||
|---|---|---|---|---|---|
| ILT2 | ILT4 | KIR2DL4 | |||
| EVTs | HLA-G1 (39, 40) | Neg (47) | Neg (47) | n.t. | |
| shed HLA-G1 (40, 42) | |||||
| HLA-G2 (42) | |||||
| HLA-G5 (41) | |||||
| HLA-G6 (43) | |||||
| Syncytiotrophoblasts | HLA-G5 (33) | Neg (47) | Neg (47) | n.t. | |
| Endothelial cells | Maternal endothelium | n.t. | Neg (47) | Neg (47) | n.t. |
| Fetal vessels | n.t. | Neg (47) | n.t. | n.t. | |
| Endometrial stromal cells | n.t. | Pos (47) | Neg (47) | n.t. | |
| dNK | Total CD56+ | Neg (48) | Posowlow (49) | Neg (49) | Pos (49–51) |
| CD4+ | Total CD4+ | n.t. | Pos (52) | n.t. | Pos (52) |
| CD4+HLA-G+ | HLA-G1 (53, 54) | n.t. | n.t. | n.t. | |
| CD8+ | Total CD8+ | n.t. | n.t. | n.t. | n.t. |
| CD8+HLA-G+ | HLA-G1 (53) | n.t. | n.t. | n.t. | |
| Macrophages | CD14+CD163+ | Neg (55) | Pos (50, 56) | Pos (50, 56) | n.t. |
| DCs | DC-SIGN+ | HLA-G1 (57) | n.t. | Pos (57) | n.t. |
| DC-10 | HLA-G1 (53) | Pos (53) | Pos (53) | n.t. | |
The indicated markers have been tested on cells at the maternal–fetal interface and demonstrated to be expressed (Pos) or not (Neg).
The indicated markers have not been tested yet (n.t.).
The presence of soluble HLA-G in embryo culture supernatants positively associates with embryo implantation (58–60). The interaction of HLA-G with ILT2 on endometrial stromal cells (47) might contribute to the remodeling of uterine vascularization, and EVT migration and invasion (61, 62). Moreover, the interaction between EVTs and resident dNKs that express both ILT2, although at low levels, and KIR2DL4 (49, 50) guarantees the correct arterial remodeling (Table 1). In contrast to peripheral NK, dNKs are poorly cytotoxic and secrete, in addition to IFN-γ, the pro-angiogenic factors VEGF, placental growth factor (PLGF), angiopoietin 1 and 2, and transforming growth factor (TGF)-β1 (14, 63–66). These molecules promote the uterine vascular changes necessary for maximizing maternal blood flow through the placenta. Moreover, the perivascular localization of dNKs in a microenvironment enriched in EVT-derived soluble HLA-G enables the formation of uterine spiral arteries (67). In vitro studies show that the interaction between HLA-G5 and shed HLA-G1, with KIR2DL4 in the early endosome of activated NKs promotes phenotypical and physiological changes leading to cellular senescence, which sustains the secretion of pro-angiogenic mediators (49, 51). Exposure of macrophages (MΦ) isolated from the first trimester decidua to HLA-G-expressing cell lines induces secretion of IL-6, CXCL8, and TNF-α that activate dNK-mediated vascular remodeling (50). Hence, the cross-talk between HLA-G-expressing/secreting EVTs and decidual innate cells coordinate the tissue remodeling necessary for a successful pregnancy.
It cannot be overlooked that EVTs-derived HLA-G also induces tolerogenic immune responses leading to semi-allogeneic fetus acceptance. In addition to dNKs, MΦ, DCs, effector and regulatory T cells, and B cells infiltrate the decidua (52, 68, 69), which are likely to be important determinants in tolerance induction. dMΦ are characterized by low levels of CD86 coupled with the expression of the immunomodulatory molecule indoleamine 2,3-dioxigenase (IDO) (70), and by IL-10 production (50, 71, 72). Gene expression profiling demonstrated that dMΦ from the first trimester of pregnancy express genes functionally related to immunomodulation and tissue remodeling (73). In vitro studies showed that exposure of U937 cells to HLA-G5 or HLA-G6 modulates IL-10 and TGF-β secretion (74). Based on these data, and on the fact that dMΦ express ILT2 and ILT4 (50, 56) (Table 1), it was postulated that, in the presence of dNK-derived IFN-γ, dMΦ in contact with HLA-G+EVTs and exposed to soluble HLA-G are induced to secrete IL-10 and TGF-β, which limit T-cell responses and promotes tolerance (74).
Plasmacytoid (BDCA-2+) and myeloid (BDCA-1+ and BDCA-3+) DCs have been also identified at the maternal–fetal interface (53, 75, 76). In early human pregnancy, DC-SIGN+ dDCs, characterized by low expression of CD86 and DEC-205, were described (77). DC-SIGN+ dDCs might be involved in re-programing the local immune response since they are associated with GM-CSF- and IL-10-secreting large granular lymphocytes that inhibit their maturation, and possibly favor tolerogenic responses (78). It has been shown that a population resembling DC-SIGN+ dDCs that express ILT4 can be differentiated in vitro (57, 76), suggesting that these cells can be also modulated by HLA-G+ decidual resident cells (Table 1). Our group identified a peculiar subset of tolerogenic DCs at the maternal–fetal interface in the first trimester of pregnancy. These DCs, termed DC-10, express HLA-G and ILT4 and secrete IL-10, thus are potentially involved in promoting tolerance (53) (Table 1). Future investigation is warranted to define whether dDC-10 and DC-SIGN+ dDCs are distinct populations of tolerogenic APCs, or cells at different stages of differentiation.
It is not surprising that Tregs are present in the decidua during pregnancy. An increased frequency of CD4+FOXP3+ Tregs in the peripheral blood of pregnant women has been shown (79) and the accrual of these cells has been described in human decidua with controversial results (53, 76, 80, 81). Recent evidence indicated that CD4+FOXP3+ Tregs might be generated in situ (57). A population of CD4+ T cells expressing HLA-G, termed CD4+HLA-G+ T cells, representing up to 20% of the decidua-infiltrating CD4+ cells, have been recently reported (53, 54) (Table 1).
Open Issues
Trophoblast-maternal APCs cross-talk: role of HLA-G-mediated signals
For the acceptance of the semi-allogeneic fetus, a crucial role is played by the trophoblasts themselves. In addition to express/secrete HLA-G, EVTs release immune-modulatory mediators (i.e., IL-10 and TSLP), which are involved in promoting a pro-tolerogenic microenvironment. Our group characterized the tolerogenic DC-10 that are present in vivo and are inducible in vitro in the presence of IL-10. DC-10 are mature myeloid cells that spontaneously secrete IL-10 in the absence of IL-12, and express HLA-G, ILT2, ILT3, and ILT4. Importantly, DC-10 promote the induction of adaptive T regulatory type 1 (Tr1) cells via the IL-10-induced HLA-G/ILT4 pathway (82). Later, we demonstrated that DC-10 accumulate in human decidua during the first trimester of pregnancy (53). Based on this observation, we postulate that dDC-10 may represent the naturally occurring HLA-G-expressing DCs involved in re-programing the immune response toward tolerance. The recent observation that the frequency of dDC-10 in women with spontaneous abortion is lower compared to that observed in pregnant women sustains this hypothesis (our unpublished data). One of the important questions regarding dDC-10 is whether they are recruited in decidua during pregnancy or are induced in situ. Recently, it was demonstrated that the secretion of TSLP by EVTs induces CD11c+ dDCs to express co-stimulatory molecules and HLA-DR and to secrete IL-10 and TGF-β (83). TSLP-instructed DCs via TFG-β secretion induce CD4+CD25+FOXP3+ Tregs that inhibit effector T cells, and promote HLA-G expression on EVTs (83). Thus, the decidual microenvironment, enriched in TSLP and IL-10, produced by both EVTs and immune cells, sustains the expression of HLA-G on EVTs. In this scenario, the cross-talk between HLA-G-expressing EVTs and decidual myeloid cells might favor the generation of a set of tolerogenic DCs, including dDC-10 and TSLP-modulated CD11c+ dDCs, which co-operate in promoting tolerance via the generation of different subsets of Tregs: Tr1, CD4+CD25+FOXP3+, or CD4+HLA-G+ cells. As discussed above, EVT-derived HLA-G directs dMΦ toward a tolerogenic path, which contributes to the inhibition of effector T cells and to the induction of Tregs. The hypothesis that decidual tolerogenic APCs drive the differentiation of Tregs is supported by the higher frequency of peripherally induced Tregs (defined as Helios− iTreg) compared to the thymic-derived Tregs in decidua (57). Our group recently demonstrated that co-expression of CD49b and LAG-3 identified Tr1 cells in vivo (84); thus, the use of these biomarkers in conjunction with the expression of FOXP3, Helios, and HLA-G will better define Treg cell composition at the maternal–fetal interface and define their relationship and relative contribution in tolerance induction.
Tolerogenic DCs can also contribute to sustain the pro-angiogenic milieu in the decidua. dDC-10 through the HLA-G can interact with dNKs or dMΦ via ILT2 and promote their activation and the release of the angiogenic factors. Moreover, dDC-10 themselves secrete IL-8 and VEGF (our unpublished data), supporting their pro-angiogenic functions. Since dMΦ, dDC-10, and TSLP-modulated CD11c+ dDCs are characterized by the ability to secrete IL-10, they can also support the up-regulation of HLA-G on EVTs and on other decidual infiltrating cells (85), hence facilitating the establishment of an appropriate vascular bed at the maternal–fetal interface.
Trophoblast-decidua cross-talk: role of HLA-G-mediated signals
The pre-decidualization program entails the production of a plethora of transcription factors, cell cycle regulators, cytokines, and the activation of diverse signaling pathways (86). Full decidualization is then achieved upon embryo arrival. In view of the increased requirements for protein secretion during embryo implantation, cytoplasmic and endoplasmic reticulum (ER) stress responses are activated at the maternal–fetal interface. Cytoplasmic stress responses are characterized by the rapid stress-induced synthesis of heat shock proteins (HSPs) that allow cells to restore protein homeostasis and to be protected against molecular damage (87). Stress-induced HSPs are not only essential for regulating the state of intracellular folding, assembly, and translocation of proteins but are also potent modulators of the immune responses. Moreover, HSPs are necessary for placental development. Targeted deletion of HSP90 results in embryonic lethality (88). In primary decidualizing, endometrial stromal cells treated with embryo supernatants, genome wide expression profiling revealed that HSP70 was strongly increased (89).
The range of functions attributed to HSPs has expanded to encompass functions outside the cell (90). Extracellular HSPs may be able to play a role as danger signals (91). In this context, HSPs may interact with pattern recognition receptors, and activate pro-inflammatory signaling and transcription. Specifically, extracellular HSP60 was shown to allow communication between immune cells and other cells in the body (92), and HSP70 can be released from cells after acute stress in different cells, including cultured rat embryo cells (93), and peripheral blood mononuclear cells (94). Notably, HSPs can activate NKs and Tregs (95, 96). Evidence for regulation of HLA-G by HSPs is still scanty. HLA-G transcription was found to be induced upon heat shock in tumor cell lines, by heat shock transcription factor 1 (HSF1) binding to a heat shock element (HSE) present in HLA-G but not in other HLA class I genes (97). Moreover, mice mutant for Hsf1 have a thin spongiotrophoblast layer and die in utero (98). Further investigation is warranted to define if maternal/fetal-derived HSPs might contribute to the regulation of HLA-G expression on dDC-10 and EVTs.
Protein folding in the ER is essential to ensure normal cell function. Disruption of ER homeostasis causes accumulation of misfolded proteins in the ER, a condition referred to as ER stress. ER stress activates the unfolded protein response (UPR) to restore protein homeostasis within the ER. However, if ER stress is persistent and excessive, the ER homeostasis cannot be re-established and the UPR will induce apoptosis. Intriguingly, IL-10 is emerging as a novel modulator of the ER stress (99). Intestinal epithelial cells isolated from IL-10−/− mice exhibit increased expression levels of BiP, a prototypic marker for ER stress, suggestive of an increased ER stress in the absence of IL-10. Further observations revealed that IL-10 attenuates tunicamycin-induced ER stress through suppression of BiP (100). These studies consistently suggest a novel role for IL-10 in modulating ER stress (101). Under ER stress, which occurs during normal development of labyrinthine trophoblasts in the mouse placenta, transcriptional regulation of VEGF is mediated by the three master regulators of the UPR: IRE1a, PERK, and ATF6 (102). The modulation of the UPR pathway by IL-10, produced by dMΦ, dDC-10, and TSLP-modulated CD11c+ dDCs, might represent an additional mechanism to regulate vascular uterine remodeling and placentation.
Perspectives
The existence of mechanisms by which fetal and maternal cells simultaneously attract and modulate each other is intriguing. Upon blastocyst implantation into the uterine wall, trophoblasts differentiate into EVTs that possess the ability to coordinate the cross-talk at the interface via the expression of HLA-G. Accumulating evidence indicate that EVTs play a key role in orchestrating a number of molecular and cellular decidual modifications by (i) regulating cell-migration in the decidua, (ii) supporting the induction of the pro-angiogenic decidual microenvironment necessary for effective vascular remodeling, (iii) inhibiting effector innate and adaptive immune responses, and (iv) promoting a tolerogenic loop in which resident cells are instructed to become tolerogenic. These functions are regulated through the finely tuned specific interactions of HLA-G-expressing EVTs with maternal innate immune cells, adaptive immune cells, and non-immune cells (Figure 1). The interplay among these cells supports the development of an appropriate maternal–fetal niche. Pregnancy hormones are essential to fully support the niche, although their role in regulating HLA-G expression has not been investigated yet (29).
We suggest that the integration and exchange between fetal and maternal blood vessels at the interface is likely to be contributed by multiple mechanisms, including trophoblast interaction with dNKs and resident/recruited APCs, as well as by the IL-10-driven tolerance and regulation of the UPR pathway in decidual and trophoblast cells.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Telethon Italy grant number TGT11E02, by the Italian Ministry of Health, RF-2011-02346774, by the European grant for European cooperation in science and technology (Action to Focus and Accelerate Cell-based Tolerance-inducing Therapies), and by the Fondazione Giorgio Pardi, Milan, Italy.
References
- 1.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol (2006) 6(8):584–94. 10.1038/nri1897 [DOI] [PubMed] [Google Scholar]
- 2.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet (2001) 2(7):538–48. 10.1038/35080570 [DOI] [PubMed] [Google Scholar]
- 3.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science (2005) 308(5728):1592–4. 10.1126/science.1111726 [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer PL, Pearton DJ. Trophoblast development. Reproduction (2012) 143(3):231–46. 10.1530/REP-11-0374 [DOI] [PubMed] [Google Scholar]
- 5.Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev (2006) 27(1):17–46 10.1210/er.2004-0021 [DOI] [PubMed] [Google Scholar]
- 6.Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol (1992) 6(2):351–71 10.1016/S0950-3552(05)80092-6 [DOI] [PubMed] [Google Scholar]
- 7.Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol (2009) 200(6):e1–6. 10.1016/j.ajog.2008.11.037 [DOI] [PubMed] [Google Scholar]
- 8.Fishel SB, Edwards RG, Evans CJ. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro. Science (1984) 223(4638):816–8. 10.1126/science.6546453 [DOI] [PubMed] [Google Scholar]
- 9.Jurisicova A, Antenos M, Kapasi K, Meriano J, Casper RF. Variability in the expression of trophectodermal markers beta-human chorionic gonadotrophin, human leukocyte antigen-G and pregnancy specific beta-1 glycoprotein by the human blastocyst. Hum Reprod (1999) 14(7):1852–8. 10.1093/humrep/14.7.1852 [DOI] [PubMed] [Google Scholar]
- 10.Han SW, Lei ZM, Rao CV. Treatment of human endometrial stromal cells with chorionic gonadotropin promotes their morphological and functional differentiation into decidua. Mol Cell Endocrinol (1999) 147(1–2):7–16. 10.1016/S0303-7207(98)00240-8 [DOI] [PubMed] [Google Scholar]
- 11.Perrier d’Hauterive S, Charlet-Renard C, Berndt S, Dubois M, Munaut C, Goffin F, et al. Human chorionic gonadotropin and growth factors at the embryonic-endometrial interface control leukemia inhibitory factor (LIF) and interleukin 6 (IL-6) secretion by human endometrial epithelium. Hum Reprod (2004) 19(11):2633–43. 10.1093/humrep/deh450 [DOI] [PubMed] [Google Scholar]
- 12.Berndt S, Perrier d’Hauterive S, Blacher S, Pequeux C, Lorquet S, Munaut C, et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J (2006) 20(14):2630–2. 10.1096/fj.06-5885fje [DOI] [PubMed] [Google Scholar]
- 13.Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d’Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol (2010) 85(1):93–8. 10.1016/j.jri.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med (2006) 12(9):1065–74. 10.1038/nm1452 [DOI] [PubMed] [Google Scholar]
- 15.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest (2014) 124(5):1872–9. 10.1172/JCI68107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosaka K, Fujiwara H, Tatsumi K, Yoshioka S, Sato Y, Egawa H, et al. Human chorionic gonadotropin (HCG) activates monocytes to produce interleukin-8 via a different pathway from luteinizing hormone/HCG receptor system. J Clin Endocrinol Metab (2002) 87(11):5199–208. 10.1210/jc.2002-020341 [DOI] [PubMed] [Google Scholar]
- 17.Wan H, Versnel MA, Leijten LM, van Helden-Meeuwsen CG, Fekkes D, Leenen PJ, et al. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J Leukoc Biol (2008) 83(4):894–901. 10.1189/jlb.0407258 [DOI] [PubMed] [Google Scholar]
- 18.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol (2009) 182(9):5488–97. 10.4049/jimmunol.0803177 [DOI] [PubMed] [Google Scholar]
- 19.Siiteri PK, Febres F, Clemens LE, Chang RJ, Gondos B, Stites D. Progesterone and maintenance of pregnancy: is progesterone nature’s immunosuppressant? Ann N Y Acad Sci (1977) 286:384–97 10.1111/j.1749-6632.1977.tb29431.x [DOI] [PubMed] [Google Scholar]
- 20.Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J Reprod Immunol (2009) 83(1–2):60–4. 10.1016/j.jri.2009.06.262 [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Dong B, Wang H, Zeng Z, Liu W, Chen N, et al. Progesterone suppresses Th17 cell responses, and enhances the development of regulatory T cells, through thymic stromal lymphopoietin-dependent mechanisms in experimental gonococcal genital tract infection. Microbes Infect (2013) 15(12):796–805. 10.1016/j.micinf.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, et al. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology (2010) 151(11):5477–88. 10.1210/en.2010-0426 [DOI] [PubMed] [Google Scholar]
- 23.Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol (2013) 97(1):43–50. 10.1016/j.jri.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 24.Yie SM, Li LH, Li GM, Xiao R, Librach CL. Progesterone enhances HLA-G gene expression in JEG-3 choriocarcinoma cells and human cytotrophoblasts in vitro. Hum Reprod (2006) 21(1):46–51. 10.1093/humrep/dei305 [DOI] [PubMed] [Google Scholar]
- 25.Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol (2013) 13(4):576–81. 10.1016/j.coph.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 26.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol (2004) 173(4):2227–30. 10.4049/jimmunol.173.4.2227 [DOI] [PubMed] [Google Scholar]
- 27.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol (2005) 170(1–2):85–92. 10.1016/j.jneuroim.2005.08.023 [DOI] [PubMed] [Google Scholar]
- 28.Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol (2014) 5:196. 10.3389/fimmu.2014.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castelli EC, Veiga-Castelli LC, Yaghi L, Moreau P, Donadi EA. Transcriptional and posttranscriptional regulations of the HLA-G gene. J Immunol Res (2014) 2014:734068. 10.1155/2014/734068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curigliano G, Criscitiello C, Gelao L, Goldhirsch A. Molecular pathways: human leukocyte antigen G (HLA-G). Clin Cancer Res (2013) 19(20):5564–71. 10.1158/1078-0432.CCR-12-3697 [DOI] [PubMed] [Google Scholar]
- 31.Ellis SA, Sargent IL, Redman CW, McMichael AJ. Evidence for a novel HLA antigen found on human extravillous trophoblast and a choriocarcinoma cell line. Immunology (1986) 59(4):595–601. [PMC free article] [PubMed] [Google Scholar]
- 32.Loke YW, King A, Burrows TD. Decidua in human implantation. Hum Reprod (1995) 10(Suppl 2):14–21 10.1093/humrep/10.suppl_2.14 [DOI] [PubMed] [Google Scholar]
- 33.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol (2003) 171(3):1376–84. 10.4049/jimmunol.171.3.1376 [DOI] [PubMed] [Google Scholar]
- 34.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol (2008) 29(7):313–21. 10.1016/j.it.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 35.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol (2008) 29(3):125–32. 10.1016/j.it.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 36.Dong Y, Lieskovska J, Kedrin D, Porcelli S, Mandelboim O, Bushkin Y. Soluble nonclassical HLA generated by the metalloproteinase pathway. Hum Immunol (2003) 64(8):802–10. 10.1016/S0198-8859(03)00093-4 [DOI] [PubMed] [Google Scholar]
- 37.Rizzo R, Trentini A, Bortolotti D, Manfrinato MC, Rotola A, Castellazzi M, et al. Matrix metalloproteinase-2 (MMP-2) generates soluble HLA-G1 by cell surface proteolytic shedding. Mol Cell Biochem (2013) 381(1–2):243–55. 10.1007/s11010-013-1708-5 [DOI] [PubMed] [Google Scholar]
- 38.Amodio G, Sales de Albuquerque R, Gregori S. New insights into HLA-G mediated tolerance. Tissue Antigens (2014) 84(3):255–63. 10.1111/tan.12427 [DOI] [PubMed] [Google Scholar]
- 39.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science (1990) 248(4952):220–3. 10.1126/science.2326636 [DOI] [PubMed] [Google Scholar]
- 40.Gonen-Gross T, Goldman-Wohl D, Huppertz B, Lankry D, Greenfield C, Natanson-Yaron S, et al. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLoS One (2010) 5(1):e8941. 10.1371/journal.pone.0008941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales PJ, Pace JL, Platt JS, Langat DK, Hunt JS. Synthesis of beta(2)-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology (2007) 122(2):179–88. 10.1111/j.1365-2567.2007.02623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, et al. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol (2003) 171(11):6215–24. 10.4049/jimmunol.171.11.6215 [DOI] [PubMed] [Google Scholar]
- 43.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J (2005) 19(7):681–93. 10.1096/fj.04-2078rev [DOI] [PubMed] [Google Scholar]
- 44.Gonen-Gross T, Achdout H, Arnon TI, Gazit R, Stern N, Horejsi V, et al. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and beta 2-microglobulin-free HLA-G molecules. J Immunol (2005) 175(8):4866–74. 10.4049/jimmunol.175.8.4866 [DOI] [PubMed] [Google Scholar]
- 45.Bai SX, Wang YL, Qin L, Xiao ZJ, Herva R, Piao YS. Dynamic expression of matrix metalloproteinases (MMP-2, -9 and -14) and the tissue inhibitors of MMPs (TIMP-1, -2 and -3) at the implantation site during tubal pregnancy. Reproduction (2005) 129(1):103–13. 10.1530/rep.1.00283 [DOI] [PubMed] [Google Scholar]
- 46.Guo Y, Lee CL, So KH, Gao J, Yeung WS, Yao Y, et al. Soluble human leukocyte antigen-g5 activates extracellular signal-regulated protein kinase signaling and stimulates trophoblast invasion. PLoS One (2013) 8(10):e76023. 10.1371/journal.pone.0076023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntire RH, Sifers T, Platt JS, Ganacias KG, Langat DK, Hunt JS. Novel HLA-G-binding leukocyte immunoglobulin-like receptor (LILR) expression patterns in human placentas and umbilical cords. Placenta (2008) 29(7):631–8. 10.1016/j.placenta.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A (1997) 94(21):11520–5. 10.1073/pnas.94.21.11520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol (2006) 4(1):e9. 10.1371/journal.pbio.0040009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li CHB, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci U S A (2009) 106(14):5767–72. 10.1073/pnas.0901173106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajagopalan S, Long EO. Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodelling. Proc Natl Acad Sci U S A (2012) 109(50):20596–601. 10.1073/pnas.1208248109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lombardelli L, Aguerre-Girr M, Logiodice F, Kullolli O, Casart Y, Polgar B, et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4(+) T cells and macrophages. J Immunol (2013) 191(7):3651–62. 10.4049/jimmunol.1300567 [DOI] [PubMed] [Google Scholar]
- 53.Amodio G, Mugione A, Sanchez AM, Vigano P, Candiani M, Somigliana E, et al. HLA-G expressing DC-10 and CD4(+) T cells accumulate in human decidua during pregnancy. Hum Immunol (2013) 74(4):406–11. 10.1016/j.humimm.2012.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu P, Santner-Nanan B, Joung S, Peek MJ, Nanan R. Expansion of CD4(+) HLA-G(+) T cell in human pregnancy is impaired in pre-eclampsia. Am J Reprod Immunol (2014) 71(3):217–28. 10.1111/aji.12195 [DOI] [PubMed] [Google Scholar]
- 55.Sedlmayr P, Morales P, Trummer S, Wascher K, Azzola D, Blaschitz A, et al. Absence of HLA-G expression in macrophages of human decidua. Am J Reprod Immunol (2002) 48(2):96–102. 10.1034/j.1600-0897.2002.01116.x [DOI] [PubMed] [Google Scholar]
- 56.Petroff MG, Sedlmayr P, Azzola D, Hunt JS. Decidual macrophages are potentially susceptible to inhibition by class Ia and class Ib HLA molecules. J Reprod Immunol (2002) 56(1–2):3–17. 10.1016/S0165-0378(02)00024-4 [DOI] [PubMed] [Google Scholar]
- 57.Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol (2012) 181(6):2149–60. 10.1016/j.ajpath.2012.08.032 [DOI] [PubMed] [Google Scholar]
- 58.Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, et al. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol (2002) 32(2):311–5. [DOI] [PubMed] [Google Scholar]
- 59.Noci I, Fuzzi B, Rizzo R, Melchiorri L, Criscuoli L, Dabizzi S, et al. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum Reprod (2005) 20(1):138–46. 10.1093/humrep/deh572 [DOI] [PubMed] [Google Scholar]
- 60.Rebmann V, Switala M, Eue I, Grosse-Wilde H. Soluble HLA-G is an independent factor for the prediction of pregnancy outcome after ART: a German multi-centre study. Hum Reprod (2010) 25(7):1691–8. 10.1093/humrep/deq120 [DOI] [PubMed] [Google Scholar]
- 61.Das P, Ezashi T, Schulz LC, Westfall SD, Livingston KA, Roberts RM. Effects of fgf2 and oxygen in the bmp4-driven differentiation of trophoblast from human embryonic stem cells. Stem Cell Res (2007) 1(1):61–74. 10.1016/j.scr.2007.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizzo R, Vercammen M, van de Velde H, Horn PA, Rebmann V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol Life Sci (2011) 68(3):341–52. 10.1007/s00018-010-0578-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med (2003) 198(8):1201–12. 10.1084/jem.20030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopcow HDAD, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A (2005) 102(43):15563–8. 10.1073/pnas.0507835102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lash GE, Robson SC, Bulmer JN. Review: functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta (2010) 31(Suppl):S87–92. 10.1016/j.placenta.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 66.Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol (2013) 190(8):3939–48. 10.4049/jimmunol.1202582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol (2005) 42(4):511–21. 10.1016/j.molimm.2004.07.035 [DOI] [PubMed] [Google Scholar]
- 68.Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol (2003) 162(3):887–96. 10.1016/S0002-9440(10)63884-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest (2008) 118(12):3954–65. 10.1172/JCI36682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol (2003) 131(3):498–505. 10.1046/j.1365-2249.2003.02092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McIntire RH, Ganacias KG, Hunt JS. Programming of human monocytes by the uteroplacental environment. Reprod Sci (2008) 15(5):437–47. 10.1177/1933719107314065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunt JS, Petroff MG. IFPA senior award lecture: reproductive immunology in perspective – reprogramming at the maternal-fetal interface. Placenta (2013) 34(Suppl):S52–5. 10.1016/j.placenta.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One (2008) 3(4):e2078. 10.1371/journal.pone.0002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McIntire RH, Morales PJ, Petroff MG, Colonna M, Hunt JS. Recombinant HLA-G5 and -G6 drive U937 myelomonocytic cell production of TGF-beta1. J Leukoc Biol (2004) 76(6):1220–8. 10.1189/jlb.0604337 [DOI] [PubMed] [Google Scholar]
- 75.Ban YL, Kong BH, Qu X, Yang QF, Ma YY. BDCA-1+, BDCA-2+ and BDCA-3+ dendritic cells in early human pregnancy decidua. Clin Exp Immunol (2008) 151(3):399–406. 10.1111/j.1365-2249.2007.03576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu P, Nanan RK. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front Immunol (2014) 5:125. 10.3389/fimmu.2014.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod (2004) 70(4):1018–23. 10.1095/biolreprod.103.022640 [DOI] [PubMed] [Google Scholar]
- 78.Laskarin G, Kammerer U, Rukavina D, Thomson AW, Fernandez N, Blois SM. Antigen-presenting cells and materno-fetal tolerance: an emerging role for dendritic cells. Am J Reprod Immunol (2007) 58(3):255–67. 10.1111/j.1600-0897.2007.00511.x [DOI] [PubMed] [Google Scholar]
- 79.Schumacher A, Zenclussen AC. Regulatory T cells: regulators of life. Am J Reprod Immunol (2014) 72(2):158–70 10.1111/aji.12238 [DOI] [PubMed] [Google Scholar]
- 80.Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod (2010) 82(4):698–705. 10.1095/biolreprod.109.081208 [DOI] [PubMed] [Google Scholar]
- 81.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology (2004) 112(1):38–43. 10.1111/j.1365-2567.2004.01869.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood (2010) 116(6):935–44. 10.1182/blood-2009-07-234872 [DOI] [PubMed] [Google Scholar]
- 83.Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol (2014) 192(4):1502–11 10.4049/jimmunol.1203425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med (2013) 19(6):739–46. 10.1038/nm.3179 [DOI] [PubMed] [Google Scholar]
- 85.Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, et al. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol (1999) 11(5):803–11. 10.1093/intimm/11.5.803 [DOI] [PubMed] [Google Scholar]
- 86.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med (2012) 18(12):1754–67. 10.1038/nm.3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol (1998) 16(9):833–8. 10.1038/nbt0998-833 [DOI] [PubMed] [Google Scholar]
- 88.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development (2000) 127(1):1–11. [DOI] [PubMed] [Google Scholar]
- 89.Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, et al. Uterine selection of human embryos at implantation. Sci Rep (2014) 4:3894. 10.1038/srep03894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett (2007) 581(19):3689–94. 10.1016/j.febslet.2007.04.044 [DOI] [PubMed] [Google Scholar]
- 91.Matzinger P. The danger model: a renewed sense of self. Science (2002) 296(5566):301–5. 10.1126/science.1071059 [DOI] [PubMed] [Google Scholar]
- 92.Hayoun D, Kapp T, Edri-Brami M, Ventura T, Cohen M, Avidan A, et al. HSP60 is transported through the secretory pathway of 3-MCA-induced fibrosarcoma tumour cells and undergoes N-glycosylation. FEBS J (2012) 279(12):2083–95. 10.1111/j.1742-4658.2012.08594.x [DOI] [PubMed] [Google Scholar]
- 93.Hightower LE, Guidon PT, Jr. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol (1989) 138(2):257–66. 10.1002/jcp.1041380206 [DOI] [PubMed] [Google Scholar]
- 94.Mansilla MJ, Costa C, Eixarch H, Tepavcevic V, Castillo M, Martin R, et al. Hsp70 regulates immune response in experimental autoimmune encephalomyelitis. PLoS One (2014) 9(8):e105737. 10.1371/journal.pone.0105737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia (2002) 18(6):576–85. 10.1080/0265673021000017109 [DOI] [PubMed] [Google Scholar]
- 96.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol (2005) 5(4):318–30. 10.1038/nri1593 [DOI] [PubMed] [Google Scholar]
- 97.Ibrahim EC, Morange M, Dausset J, Carosella ED, Paul P. Heat shock and arsenite induce expression of the nonclassical class I histocompatibility HLA-G gene in tumor cell lines. Cell Stress Chaperones (2000) 5(3):207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J (1999) 18(21):5943–52. 10.1093/emboj/18.21.5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shkoda A, Ruiz PA, Daniel H, Kim SC, Rogler G, Sartor RB, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology (2007) 132(1):190–207. 10.1053/j.gastro.2006.10.030 [DOI] [PubMed] [Google Scholar]
- 100.Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology (2013) 144(2):357–368e9. 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 101.Cheng SB, Sharma S. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol (2014). 10.1111/aji.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One (2010) 5(3):e9575. 10.1371/journal.pone.0009575 [DOI] [PMC free article] [PubMed] [Google Scholar]