Abstract
Cancer survivors participating in supervised exercise programs learn to exercise safely with oversight from care providers who monitor and facilitate their progress. This study investigated the long-term exercise participation levels and identified exercise barriers for graduates from a specialized cancer exercise and education program. Subjects were graduates from a 12-week supervised exercise program (www.canwellprogram.ca) who participated in a, prospective, long-term evaluation. Measures included: six-minute walk test (6-MWT), STEEP treadmill test, Functional Assessment Cancer Therapy-General (FACT-G), Edmonton Symptom Assessment System (ESAS), Godin Leisure-Time Exercise Questionnaire, and exercise barriers survey. Analysis was performed using the paired t -test. Fifty-seven (55% of eligible cohort) CanWell participants (mean age= 60; 74% females) were included in this study. Post program changes included statistically significant reductions in total min on the treadmill and a trend towards improvements in 6-MWT distance. No significant changes were recorded in total FACT-G or ESAS score, however functional well-being approached statistical significant improvements. The most commonly reported exercise barriers included fatigue, cost, and return to work. While most participants (86%) believed they were able to exercise, only 63% reported being able to progress their exercise. These finding demonstrated that although CanWell graduates have substantial support from exercise specialists and most have early success with exercise, environment-related factors diminish long-term independent adherence to exercise. Providing cancer survivors with the skills needed to monitor and progress their exercise routines, or access to “tune-ups” may increase exercise adherence and maximize benefits.
Keywords: Neoplasms, Oncology, Exercise, Community, Evidence-based
INTRODUCTION
The challenging physical, emotional, and psychological effects of cancer and its treatments have been well documented (Binkley et al., 2012; Courneya and Friedenreich, 1999; Friendenreich and Courneya, 1996; Schmitz et al., 2012). A growing body of research shows that exercise is safe and beneficial for cancer survivors (Courneya and Friedenreich, 1999; Courneya et al., 2005; Dolan et al., 2010; McNeely et al., 2006). Furthermore, exercise is associated with improved survival, fewer new cancers, and earlier detection of some cancers (Barbaric et al., 2010). Despite the evidence, many cancer survivors are not meeting basic recommendations for physical activity (Courneya et al., 2012a; 2012b). Similarly, only 5% of survivors accumulate 150 min of moderate to vigorous exercise a week (Statistics Canada, 2011), 10% exercise at pre-diagnosis level during treatment, and 20–30% resume their pre-diagnosis level of exercise following treatment (Courneya et al., 2007).
Cancer survivors face barriers to starting and adhering to exercise programs, including pain, symptoms related to cancer and its treatments, body image issues, lack of information about exercise and exercise safety, lack of supervised exercise programs (Blaney et al., 2010), and lack of priority (Rogers et al., 2007). Investigated strategies to improve exercise adherence include provision of exercise prescriptions upon study completion and including behavior change counseling (Courneya et al., 2012a). Further, survivors who participated in supervised exercise research reported overall better outcomes and more exercise min than survivors in control groups, lasting for at least six-months following the intervention (Courneya et al., 2007; Courneya et al., 2012b). The long-lasting benefits of exercise are encouraging, however, participation in exercise research programs is often time limited (Courneya et al., 2012b), usually due to limited capacity of exercise facilities and available research funding. In a systematic review by Spark et al. it was concluded that there is a “pressing need” to assess and develop long-term interventions that promote maintenance of physical activity treatments that were provided during participation in clinical studies (Spark et al., 2013).
The CanWell exercise and education program was developed with these recommendations in mind. It provides cancer survivors with a community-based (YMCA), evidence-informed, 12-week supervised program where they can learn to exercise safely, in a “normal” environment, and continue to exercise on a long-term basis (Cheifetz et al., 2013). While CanWell was designed to address several of the exercise barriers discussed above, its effects on long-term physical outcomes and exercise adherence has not been investigated.
The purposes of this study were threefold: 1. Investigate the extent to which exercise levels and health-related quality of life (HRQoL) of CanWell participants changed during a long-term follow-up period; 2. Determine the exercise barriers reported by Can-Well participants who did not continue to exercise; 3. To seek association between the assessed outcomes and on-going exercise participation. Our theory was that CanWell participants would continue and exercise due to the fact that they know the benefits of exercise, learnt to exercise safely, and have access to an exercise facility where exercise and cancer experts are available for consultation on a long-term basis.
MATERIALS AND METHODS
Participants
Participants were recruited from the CanWell cohort who exercised between 2009 and June 2012. Inclusion criteria were adults diagnosed with any type or stage of cancer and at any phase of cancer treatment. Participants in CanWell live in the community, ambulate independently, have no acute medical conditions, and no medical contraindications identified on pre-exercise safety screening (Cheifetz et al., 2013). Excluded were those with unstable cardiac conditions. Prior to contacting CanWell participants, hospital electronic charts were searched to identify those who were deceased. Participants were invited for a one-hour face-to-face assessment session at the YMCA or were asked to complete the surveys over the phone if they were unable, or unwilling, to attend the gym-based testing. Informed, written consent was obtained from all participants.
Study design
The CanWell exercise adherence and barriers study is a, prospective, observational cohort study that was designed as an extension to the CanWell research program (Cheifetz et al., 2013). Included participants completed the same physical and quality of life measures used in the CanWell research program with the addition of an Exercise Barriers questionnaire. For the purpose of the current study, CanWell program completers are those who completed at least two out of three measurement sessions in the original study (Cheifetz et al., 2013). The CanWell study was approved by the Hamilton Health Science (HHS)/McMaster University Research Ethics Board (clinicaltrials.gov ID: NCT00798200).
Physical performance measures
Endurance testing was performed using the standardized exponential exercise protocol (STEEP) as it is useful for participants of varying capabilities (Northridge et al., 1990). It has been shown to produce similar peak VO2 measurements as the modified Bruce treadmill test, but in shorter timeframes (Riley et al., 1992). The STEEP has been used in healthy (Northridge et al., 1990) individuals, patients with congestive heart failure (Riley et al., 1992) and those with lung cancer to predict surgical outcomes (Win et al., 2005). It has demonstrated reliability coefficients ranging from 0.82–0.86 and high test-retest reliability (ICC=0.996) (De Backer et al., 2007).
The six-min walk test (6-MWT) is a functional walk test that has been extensively studied and has been recommended for use in both research and the clinical settings (Solway et al., 2001). It has been used to assess walking performance in people with cancer (Cheville et al., 2008), and has excellent test-retest reliability (ICC 0.82–0.99) (Finch, et al., 2002). The 6-MWT was administered according to a standardized protocol (ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, 2002).
Self-reported quality of life measures
Health-related Quality of Life (HR-QoL) was measured using the Functional Assessment of Cancer Therapy Scale-General (FACT-G) (Cella et al., 1993). This scale has been rigorously investigated for use in assessing HR-QoL of people with different types of cancers and found to be appropriate for both cross-sectional and longitudinal study designs (Cheung et al., 2005). The FACT-G (version 4) is a 27-item self-report measure yielding a total score ranging from zero (0, worst HR-QoL) to 94 (best HR-QoL). This scale also consists of four subscales evaluating physical, emotional, functional, and social well-being. The validity and reliability of FACT-G subscales has been established and the scores are commonly reported in cancer-related literature (Cella et al., 1993).
The Edmonton Symptom Assessment System (ESAS) was used to evaluate the burden of symptoms commonly associated with cancer including pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath (Bruera et al., 1991). The ESAS has been shown to be valid and reliable (ICC=0.65–0.83) (Cheifetz et al., 2014; Watanabe et al., 2012), and has been extensively used throughout the cancer care continuum (Nekolaichuk et al., 2008; Philip et al., 1998; Richardson and Jones, 2009). Currently, the ESAS is also used at Ontario Cancer Centers to monitor disease burden in people during or after cancer treatment.
Self-reported exercise participation measure
The Godin Leisure-Time Exercise Questionnaire was used to evaluate self-reported exercise participation in a 7-day period, prior to the evaluation date (Godin and Shephard, 1985). This questionnaire assesses the frequency of strenuous, moderate, or mild exercises performed for at least 15 min, during a typical week. This tool has been shown to be valid and reliable compared to other self-report measures (Jacobs, et al., 1993) and has been used to evaluate people with cancer (Blanchard et al., 2002).
Exercise barriers measure
Questions evaluating exercise barriers were formulated based on concepts grounded in the Theory of Planned Behavior (TPB) (Ajzen, 1988; 1991). Briefly, the TPB proposes that a person’s intention to change (Ajzen, 1988; 1991) (or in this case incorporate exercise to manage cancer treatment side effects) is based on specific factors. It assumes that intention is formed on the basis of attitudes and beliefs in three areas: behavioral (whether the behavior will achieve the expected outcome; e.g. believe that exercise will improve strength, function, or HR-QoL), subjective norms (what others expect, e.g. family or health-care team expect people with cancer to exercise), and perceived behavioral control (i.e. whether the behavior is under their control, e.g. cancer survivors believe they can exercise) (Ajzen, 1991). It is suggested that by changing these factors there is a greater chance that a person will intend to do an action (Ajzen, 1988; 1991).
Statistical analysis
Descriptive statistics were calculated for baseline demographics, physical characteristics, and all outcome measures utilized. Paired t-test was used to compare change in HR-QoL (ESAS, FACT-G totals and subdomains scores) and physical function (STEEP and 6-MWT) over time (end of CanWell program vs follow-up session) (Portney and Watkins, 2000). Binary logistic regression models were used to itentify end-of CanWell program factors that may have contributed to participation in long-term evaluation, continuation of exercise, the belief that the participant can exercise, or meeting exercise guidelines (Park, 2009). Results are presented as mean±SD with all tests set as 2-sided, and statistical significance set at P<0.05. Statistical analyses were performed using Stata (StataCorp., 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
RESULTS
Fifty-seven (54.8%) of the eligible 104 CanWell program participants consented to take part in this study, with 12 (21%) choosing a phone interview (Fig. 1). While there were no statistically significant differences on demographic variables between current study participants and those who did not, those who declined participation in the current study reported statistically significant lower levels of functional well-being (t(35.39)=2.44, P<0.05) and lower total FACT-G scores approximating statistical significance (t(35.19)=1.89, P=0.068) at the end of the CanWell program.
Fig. 1.
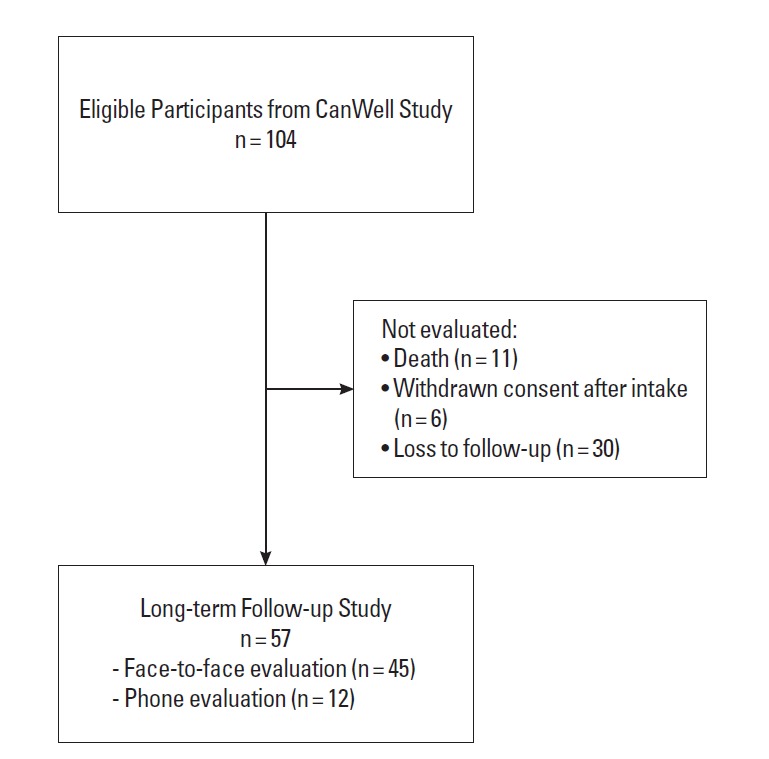
Program participants flow.
The majority of follow-up participants were women (74%) with breast cancer (56%) (Table 1). Participants were evaluated on average 29 months following completion of CanWell.
Table 1.
Participant characteristics at baseline
| Characteristics | CanWell Follow Up (n = 57) | |
|---|---|---|
|
| ||
| Mean (SD) | Min-Max | |
| Age (yr) | 60.2 (8.51) | 43–80 |
| Time from cancer diagnosis to Follow-Up (months) | 63.3 (36.83) | 21.9–173.4 |
| Time from CanWell end to Follow-Up (months) | 28.7 (7.48) | 11.8–41.19 |
| Mass (kg) | 81.1 (17.8) | 54.5–118 |
| Height (m) | 1.69 (0.097) | 1.42–1.88 |
| BMI (kg/m2) | 28.4 (5.61) | 19.7–43.3 |
|
| ||
| Frequency | % of Total | |
|
| ||
| Gender (female) | 42 | 73.7 |
| Cancer Diagnosis | ||
| Breast | 32 | 56.1 |
| Lymphoma | 5 | 8.8 |
| Multiple Myeloma | 4 | 7.0 |
| Prostate | 3 | 5.3 |
| Colon | 3 | 5.3 |
| Brain | 1 | 1.7 |
| Lung | 1 | 1.7 |
| Pancreatic | 1 | 1.7 |
| Leukemia | 1 | 1.7 |
| Ovarian | 1 | 1.7 |
| Other | 5 | 8.8 |
| Treatments | ||
| Surgery | 43 | |
| Chemotherapy | 43 | |
| Radiation | 29 | |
SD, standard deviation; BMI, body mass index.
Outcome measures results are presented in Table 2. Participants had a significant reduction in time on the treadmill (7.9 min vs 7.7 min at follow-up) was observed in the STEEP test (t25=2.51, P<0.05, 95%CI=0.14 to 1.44) with no changes in 6MWT distances. While no statistical significant changes were observed with FACT-G, total ESAS score, or physical, emotional, or social WB (Table 2), functional WB approached statistical significant improvements (t41=−1.9, P=0.059, 95%CI=−2.6 to 0.05).
Table 2.
Physical function and HR-QoL outcome measure results
| n | End of CanWell | CanWell Follow Up | P-value | |
|---|---|---|---|---|
|
|
|
|||
| Mean (SD) | Mean (SD) | |||
| STEEP (min) | 26 | 8.97 (1.85) | 8.18 (2.12) | 0.02 |
| 6-MWT (meters) | 35 | 493 (71.98) | 498 (88.45) | 0.6 |
| FACT-G | 42 | 86.7 (12.19) | 87.9 (14.83) | 0.5 |
| Physical WB | 44 | 23.0 (3.65) | 22.5 (4.59) | 0.5 |
| Functional WB | 42 | 20.5 (4.69) | 21.8 (5.15) | 0.059 |
| Emotional WB | 42 | 20.0 (3.00) | 19.5 (3.23) | 0.3 |
| Social WB | 44 | 23.7 (4.35) | 24.4 (5.74) | 0.4 |
| ESAS | 36 | 14.6 (12.16) | 15.5 (14.00) | 0.7 |
STEEP, standardized exponential exercise protocol; 6MWT, 6-min walk test; FACT-G, Functional Assessment of Cancer Therapy Scale-General; WB, well-being; ESAS, Edmonton Symptom Assessment System.
The reported Godin Leisure-Time score averaged 29.8 (SD= 28.04, ranging from 0 to 155), with 26% of participants often engaging in physical activity that works up a sweat. The majority of participants reported that they either sometimes, or never/rarely, participated in physical activities that produce a sweat (39% and 35%, respectively).
In terms of exercise participation and barriers (Table 3), the majority of participants renewed their YMCA yearly memberships (67%) and 74% reported that they were able to complete the 12-week exercise program. The three most common barriers reported included cancer-related fatigue (12%), YMCA membership cost (12%), and return to work (11%).
Table 3.
Exercise participation and barriers reported by CanWell graduates at long term follow-up
| Participation | Frequency | Percent |
|---|---|---|
| Renewed yrly YMCA membership | 38 | 67.8 |
| Completed full CanWell program | 42 | 75 |
| Continue to exercise following CanWell | 47 | 85.4 |
| General Barriers to Exercise | ||
| Injury during CanWell | 3 | 5.3 |
| Returned to work | 6 | 10.5 |
| Family commitments | 2 | 3.5 |
| Exercise takes too long | 2 | 3.5 |
| Further cancer treatment | 3 | 5.3 |
| YMCA too far from home | 3 | 5.3 |
| Feel not safe at the YMCA (to exercise) | 1 | 1.7 |
| Worry about exercise related injury | 1 | 1.7 |
| Did not enjoy exercise | 1 | 1.7 |
| Difficult to motivate myself | 4 | 7.0 |
| YMCA membership too expensive | 7 | 12.3 |
| Cancer Specific Barriers to Exercise | ||
| Cancer did not affect ability to exercise | 6 | 10.5 |
| Fatigue affected ability to exercise | 7 | 12.3 |
| Pain affected ability to exercise | 5 | 8.8 |
| Cancer recurrence affected ability to exercise | 2 | 3.5 |
| Ongoing cancer treatment affected ability to exercise | 4 | 7.0 |
| Location of exercise following CanWell | ||
| At home | 12 | 21.0 |
| Same YMCA as CanWell | 25 | 43.9 |
| Other community gym | 4 | 7.0 |
| Other YMCA facility | 1 | 1.7 |
| Other | 8 | 14.0 |
| Do not exercise | 5 | 8.8 |
| No answer | 2 | 3.5 |
| Frequency of Exercise | ||
| Once a week | 1 | 1.7 |
| 2–3 times a week | 20 | 35.1 |
| Twice a week | 10 | 17.5 |
| More than three times a week | 16 | 28.1 |
| Do not exercise | 8 | 14.0 |
| No answer | 2 | 3.5 |
| Mode of Exercise | ||
| Equal aerobic and strength exercise | 26 | 45.6 |
| Mostly aerobic exercise | 11 | 19.3 |
| Mostly strength exercise | 2 | 3.5 |
| Mostly pool exercise | 2 | 3.5 |
| Other | 7 | 12.3 |
| Not applicable | 6 | 10.5 |
| No answer | 3 | 5.3 |
| Exercise supervision needs | ||
| Comfortable to exercise with no supervision | 27 | 47.4 |
| Occasional supervision of fitness trainer specifically trained to work with cancer survivors | 6 | 10.5 |
| Occasional supervision of fitness trainer with no cancer related training | 11 | 19.3 |
| Regular supervision of fitness trainer specifically trained to work with cancer survivors | 5 | 8.8 |
| Regular supervision of fitness trainer with no cancer related training | 2 | 3.5 |
| No answer | 6 | 10.5 |
Considering exercise prescription parameters, exercise frequency was 2–3 times per week in 53%, and more than three times a week in 28%, of participants. Exercise types included a balance between aerobic and strength exercises (46%) and a subset of participants reported that they no longer required exercise supervision (47%).
The majority of participants expected to continue and exercise in the next 12 months (83%) and 86% wanted to exercise. Additionally, while 72% intended to increase their exercise intensity, 16% were not sure and 7% did not intend to change exercise intensity.
Participants (86%) believed they could exercise and 91% believed exercise would help them. Most participants felt they could stay motivated (84%) and that they are safe to exercise independently (79%). However, only 63% thought they could progress their exercise program independently.
Logistic regression analysis (Table 4) revealed the CanWell participants who were able to ambulate farther as evaluated by the 6MWT were more likely to meet exercise guidelines at the follow-up study (OR 1.03, 95% CI=1.00 to 1.07). Further, those who reported higher levels of social WB during the CanWell program showed a strong trend towards being more likely to continue and exercise following the supervised exercise program (P=0.077).
Table 4.
Logistic regression results
| Dependent variables | Participated in long-term follow-up | Meet exercise guidelines | “Believe I can exercise” | Continued to exercise |
|---|---|---|---|---|
|
|
|
|
|
|
| Independent variables | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Gender | 0.83 (0.19–3.45) | 0.46 (0.06–3.39) | 0.02 (0.002–139.2) | 1.14 (0.24–5.32) |
| ESAS score | 1.04 (0.96–1.12) | 0.89 (0.76–1.06) | 0.96 (0.84–1.09) | 0.82 (0.59–1.14) |
| 6MWT distance | 0.99 (0.99–1.01) | 1.03 (1.00–1.07)* | 0.99 (0.97–1.03) | 0.99 (0.96–1.04) |
| STEEEP total min | 1.05 (0.69–1.60) | 0.64 (0.22–1.80) | 1.88 (0.56–6.25) | 3.03 (0.55–16.74) |
| FACT-G total score | 1.03 (0.99–1.07) | 0.99 (0.94–1.06) | 1.23 (0.96–1.57) | 1.04 (0.97–1.12) |
| PWB | 1.11 (0.89–1.39) | 0.85 (0.44–1.67) | 0.82 (0.49–1.35) | 0.98 (0.49–1.98) |
| SWB | 1.00 (8.85–1.19) | 0.73 (0.46–1.16) | 1.19 (0.87–1.66) | 1.52 (0.95–2.45) ** |
| EWB | 0.97 (0.72–1.32) | 1.19 (0.54–2.64) | 1.35 (0.74–2.46) | 1.01 (0.48–2.12) |
| FWB | 1.10 (0.93–1.30) | 1.06 (0.75–1.50) | 1.06 (0.75–1.50) | 0.61 (0.31–1.21) |
| Age at diagnosis | 1.03 (0.92–1.11) | 1.15 (0.97–1.35) | 1.02 (0.84–1.23) | 1.01 (0.78–1.29) |
| Age at CanWell start | 1.01 (0.95–1.08) | 0.98 (0.89–1.08) | 0.93 (0.62–1.39) | 0.96 (0.78–1.19) |
| BMI | 0.98 (0.87–1.09) | 0.97 (0.82–1.15) | 0.54 (0.22–1.32) | 0.59 (0.31–1.13) |
P< 0.05.
P= 0.077.
ESAS, Edmonton Symptom Assessment System; STEEP, standardized exercise protocol; FACT-G, Functional Assessment Cancer Therapy-General; PWB, physical wellbeing; SWB, social wellbeing; EWB, emotional wellbeing; FWB, functional wellbeing; BMI, body mass index.
DISCUSSION
Results from the current study suggest that while cancer survivors have on-going challenges to participate in life-long exercise programs, involvement in supervised exercise and education programs, such as CanWell can lead to improved exercise adherence. Sixty-seven percent of study participants renewed their YMCA membership and 82% reported that they continued to exercise. Linke et al. (2011) investigated attrition and adherence rates of subjects participating in randomized-controlled exercise interventions and found attrition rates ranging from 7–58% and mean adherence above a commonly reported level of 66%. Similar adherence rates of 65% were reported by Jancey et al. (2007) and this level of participation was considered adequate when compared to other exercise studies (Jancey et al., 2007). The higher level of exercise adherence of CanWell graduates may relate to the incorporation of formal education sessions, participation in a supervised (individualized) 12-week exercise program, and availability of regulated health-care professionals from a partner acute care hospital in the community exercise facility (Cheifetz et al., 2013).
For the current study, 104 graduates of the CanWell program were eligible to participate, but only 57 (54.8%) did so. This participation rate is lower than that reported by Courneya et al. (2012b) in a six-month follow-up of cancer survivors with lymphoma (Courneya et al., 2012b) and may be explained by the inclusion of a mixed diagnosis group and older participants in the current study. Conversely, this participation rate is slightly higher than that reported by Haas et al. (2012) in the well-established FitSTEPS community-based exercise program (Haas et al., 2012). It is important to note that attrition rates of 50% are not uncommon in the fitness industry in healthy individuals (Haas et al., 2012). Importantly, almost 29% of the 104 CanWell graduates were lost to follow-up, contributing to the observed adherence rate. Reasons for this loss may include changes in contact information not reported to the hospital, moving away from the HHS catchment area, and possible death not captured in the hospital electronic system.
Not surprisingly, the majority of CanWell graduates continued to exercise at the same facility as the initial exercise program, suggesting that structured programs and familiarity with the exercise facility are factors that may aid exercise adherence. This is important as often cancer and exercise-related research is university or hospital based, limiting the ability of on-going exercise participation in the same location as the initial study.
Study participants exhibited a statistically significant reduction in endurance (STEEP) compared to their ability at the end of Can-Well. This result is different than that reported by Courneya et al. (2009) where participants did not change in aerobic ability at six-month follow-up. Our results may be different due to including a mixed participant group, older participants, and a longer follow-up period. It is also possible that age-related decline, comorbid health conditions, or cancer-related issues have a greater impact on older patients observed over a longer follow-up period.
Another possible contribution to reduction in STEEP times is the fact that participants in the current study may have not changed their exercise intensity between CanWell completion and follow-up leading to a decline in fitness. While exercise intensity was not directly evaluated, approximately 35% did not think they could progress their exercise program without assistance. Furthermore, exercise participation captured by the Godin Questionnaire demonstrates that participants are not exercising at levels similar to healthy individuals (Godin and Shephard, 1985) but at reduced levels consistent with patients who have active myeloproliferative disorder (Mesa et al., 2007) or Parkinson’s disease (Garber and Friedman, 2003). Conversely, participants reported exercising at appropriate frequencies (2–3 times a week or more) and used both aerobic and strength exercise programs, meeting required exercise frequencies and modes to produce physical improvement. The reduced ability and lack of confidence about exercise progression, in the face of maintained commitment to exercise frequency, suggest that re-checks with a physical therapist or exercise specialist, to modify exercise prescriptions might be useful. Further, health teaching should include the importance, and method, to progressing an exercise program based on individual medical status.
Participants in the current study did not report significant changes in overall HR-QoL or in their disease burden. Conversely, FitSTEPS study participants reported improved HR-QoL during long-term follow-up, however physical measurements of performance were not assessed (Haas et al., 2012). Considering the reduced endurance discussed above, it is important for care providers to understand that many cancer survivors’ HR-QoL may not automatically improve with exercise. This is likely due to individual factors such as disease progression, co-morbidities, or other exercise barriers, but that does not necessarily translate to negative effects on HR-QoL.
The top three exercise barriers reported by study participants included fatigue, YMCA membership fees, and return to work. Fatigue is a common exercise barrier reported by many cancer survivors regardless of the underlying disease (Coleman et al., 2011; Courneya et al., 2009; Haas, 2011). “Lack of time” for exercise was also a barrier consistently reported by many people relating to work and family demands (Courneya et al., 2005; Courneya et al., 2008; Haas et al., 2012; Rogers et al., 2007). However, one of the reasons the YMCA was chosen as the location for CanWell is its expanded hours allowing participants to find convenient hours to exercise. The results of this study may reflect that “lack of time” relates more to exercise being a lower priority. This emphasizes the need to educate cancer survivors on the benefits of exercise and help them identify strategies to prioritize exercise over other activities. The financial barrier was surprising, as a not-for-profit organization the YMCA offers generous financial assistance for those in need. A possible explanation for the financial barrier may be lack of knowledge of available resources, or perhaps, participants not wishing to discuss their financial challenges. This suggests a need for more “push-out” of information on financial assistance using discreet, supportive, strategies.
This study has several limitations that need to be considered when interpreting the results. The number of cancer survivors lost to follow-up from the start of CanWell to follow-up may have affected the results of the fitness testing. However, the CanWell levels of adherence are similar, or better, than other exercise intervention studies (Courneya et al., 2005; Haas et al., 2012; Jancey et al., 2007; Linke et al., 2011). Other factors affecting CanWell exercise adherence have been discussed in detail elsewhere, however, briefly included disease progression, injuries not related to exercise (e.g. falls), vacations, and return to work (Cheifetz et al., 2013). These same factors may have contributed to post-program non-adherence and may explain the decline in some health indicators identified with longer follow-up. The small subgroups in cancer diagnostic groups other than breast cancer limited our ability to delve deeper assessing the contribution of cancer diagnosis on exercise behaviours or outcomes. The lack of a full medical examination at final follow-up limited our ability to determine the extent to which cancer reoccurrence/progression or other comorbid health problems may have affected behaviour or performance. Finally, since this work was quantitative, we were unable to fully explain the benefits, barriers or facilitators that contributed to the effects we observed. Future research should include sufficient numbers of patients with different types of cancer to analyze diagnostic subgroup effects, add a qualitative component to determine factors that affect intention and behaviour, and evaluate different variants in the approach that affect follow-up (i.e. tune-up visits) or access (variants in program fee structure or implementation).
In summary, we examined the physical fitness and exercise barriers of a mixed group of cancer survivors who previously exercised in the CanWell program. Overall, participants exhibited slight reductions in endurance, but were able to maintain physical function and HR-QoL. Furthermore, participants believed that they had the skills and knowledge to exercise safely independently in the community. However, opportunities to improve confidence, skills, and accessibility for exercise participation and progression were found. These findings can inform the development of exercise and education programs for cancer survivors.
Acknowledgments
This study was supported by a research grant from the Juravinski Cancer Center Foundation, Hamilton Health Sciences, Ontario, Canada. Guidance and assistance provided by Professor Paul Stratford, Dr. Linda Woodhouse, Dr. Julie Richardson, and Dr. Sara McEwen. This study could not be completed without CanWell participants and the Les Chater YMCA staff. Oren Cheifetz was supported by Hamilton Health Sciences, Hematology Program in his role as a Clinical Specialist-Physiotherapy/Oncology and Mc-Master University, School of Rehabilitation Sciences.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Ajzen I. Attitudes, personality, and behavior. Chicago: Dorsey Press; 1988. [Google Scholar]
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50(2):179–211. [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-min walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: a systematic literature review. Physiother Can. 2010;62(1):25–34. doi: 10.3138/physio.62.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley JM, Harris SR, Levangie PK, Pearl M, Guglielmino J, Kraus V, Rowden D. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl):2207–2216. doi: 10.1002/cncr.27469. [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Courneya KS, Rodgers WM, Murnaghan DM. Determinants of exercise intention and behavior in survivors of breast and prostate cancer: an application of the theory of planned behavior. Cancer Nurs. 2002;25(2):88–95. doi: 10.1097/00002820-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Blaney J, Lowe-Strong A, Rankin J, Campbell A, Allen J, Gracey J. The cancer rehabilitation journey: barriers to and facilitators of exercise among patients with cancer-related fatigue. Phys Ther. 2010;90(8):1135–1147. doi: 10.2522/ptj.20090278. [DOI] [PubMed] [Google Scholar]
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cheifetz O, Packham TL, Macdermid JC. Rasch analysis of the Edmonton Symptom Assessment System and research implications. Curr Oncol. 2014;21(2):e186–194. doi: 10.3747/co.21.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz O, Park Dorsay J, Hladysh G, MacDermid J, Serediuk F, Wood-house LJ. CanWell: meeting the psychosocial and exercise needs of cancer survivors by translating evidence into practice. Psychooncology. 2013;23:204–215. doi: 10.1002/pon.3389. [DOI] [PubMed] [Google Scholar]
- Cheung YB, Goh C, Thumboo J, Khoo KS, Wee J. Variability and sample size requirements of quality-of-life measures: a randomized study of three major questionnaires. J Clin Oncol. 2005;23(22):4936–4944. doi: 10.1200/JCO.2005.07.141. [DOI] [PubMed] [Google Scholar]
- Cheville AL, Troxel AB, Basford JR, Kornblith AB. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008;26(16):2621–2629. doi: 10.1200/JCO.2007.12.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman EA, Goodwin JA, Coon SK, Richards K, Enderlin C, Kennedy R, Stewart CB, McNatt P, Lockhart K, Anaissie EJ, Barlogie B. Fatigue, sleep, pain, mood, and performance status in patients with multiple myeloma. Cancer Nurs. 2011;34(3):219–227. doi: 10.1097/NCC.0b013e3181f9904d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Vallance JK, Fairey AS. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med. 2005;29(2):147–153. doi: 10.1207/s15324796abm2902_9. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Karvinen KH, Campbell KL, Pearcey RG, Dundas G, Cap-stick V, Tonkin KS. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Karvinen KH, Vallance JKH. Exercise Motivation and Behavior Change. In: Feuerstein M, editor. Handbook of Cancer Survivorship. Springer; US: 2007. pp. 113–132. [Google Scholar]
- Courneya KS, McKenzie DC, Reid RD, Mackey JR, Gelmon K, Friedenreich CM, Ladha AB, Proulx C, Lane K, Vallance JK, Segal RJ. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med. 2008;35(1):116–122. doi: 10.1007/s12160-007-9009-4. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, Proulx C, Lane K, Ladha AB, Vallance JK, Liu Q, Yasui Y, McKenzie DC. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2572–2578. doi: 10.1158/1055-9965.EPI-07-0413. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Frieden-reich CM, Tankel K, Basi S, Chua N, Mazurek A, Reiman T. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Stevinson C, McNeely ML, Sellar CM, Friedenreich CM, Peddle-McIntyre CJ, Chua N, Reiman T. Effects of supervised exercise on motivational outcomes and longer-term behavior. Med Sci Sports Exerc. 2012a;44(3):542–549. doi: 10.1249/MSS.0b013e3182301e06. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Stevinson C, McNeely ML, Sellar CM, Friedenreich CM, Peddle-McIntyre CJ, Chua N, Reiman T. Predictors of follow-up exercise behavior 6 months after a randomized trial of supervised exercise training in lymphoma patients. Psychooncology. 2012b;21(10):1124–1131. doi: 10.1002/pon.2010. [DOI] [PubMed] [Google Scholar]
- De Backer IC, Schep G, Hoogeveen A, Vreugdenhil G, Kester AD, van Breda E. Exercise testing and training in a cancer rehabilitation program: the advantage of the steep ramp test. Arch Phys Med Rehabil. 2007;88(5):610–616. doi: 10.1016/j.apmr.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Dolan LB, Gelmon K, Courneya KS, Mackey JR, Segal RJ, Lane K, Reid RD, McKenzie DC. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2826–2832. doi: 10.1158/1055-9965.EPI-10-0521. [DOI] [PubMed] [Google Scholar]
- Finch E, Brooks D, Stratford P, Mayo N. Physical rehabilitation outcome measures: a guide to clinical decision making. Hamilton, Ontario: BC Decker Inc; 2002. [Google Scholar]
- Friendenreich CM, Courneya KS. Exercise as rehabilitation for cancer patients. Clin J Sport Med. 1996;6(4):237–244. doi: 10.1097/00042752-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson’s disease. Neurology. 2003;60(7):1119–1124. doi: 10.1212/01.wnl.0000055868.06222.ab. [DOI] [PubMed] [Google Scholar]
- Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- Haas BK. Fatigue, self-efficacy, physical activity, and quality of life in women with breast cancer. Cancer Nurs. 2011;34(4):322–334. doi: 10.1097/NCC.0b013e3181f9a300. [DOI] [PubMed] [Google Scholar]
- Haas BK, Kimmel G, Hermanns M, Deal B. Community-based FitSTEPS for life exercise program for persons with cancer: 5-yr evaluation. Journal of oncology practice/American Society of Clinical Oncology. 2012;8(6):320–4. doi: 10.1200/JOP.2012.000555. 2 p following 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Ainsworth B, Hartman T, Leon A. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Jancey J, Lee A, Howat P, Clarke A, Wang K, Shilton T. Reducing attrition in physical activity programs for older adults. Journal of aging and physical activity. 2007;15(2):152–165. doi: 10.1123/japa.15.2.152. [DOI] [PubMed] [Google Scholar]
- Linke SE, Gallo LC, Norman GJ. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Ann Behav Med. 2011;42(2):197–209. doi: 10.1007/s12160-011-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, Tan AD, Atherton PJ, Sloan JA, Tefferi A. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76. doi: 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-yr retrospective review of validation studies (1991–2006) Palliat Med. 2008;22(2):111–122. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- Northridge DB, Grant S, Ford I, Christie J, McLenachan J, Connelly D, McMurray J, Ray S, Henderson E, Dargie HJ. Novel exercise protocol suitable for use on a treadmill or a bicycle ergometer. Br Heart J. 1990;64(5):313–316. doi: 10.1136/hrt.64.5.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HM. Regression models for binary dependent variables using Stata, SAS, R, LIMDEP, and SPSS. 2009 Working Paper. The University Information Technology Services Center for Statistical and Mathematical Computing, Indiana University. http://www.indiana.edu/~stat-math.
- Philip J, Smith WB, Craft P, Lickiss N. Concurrent validity of the modified Edmonton Symptom Assessment System with the Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer. 1998;6(6):539–541. doi: 10.1007/s005200050212. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of Clinical Research, Application for Practice. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton Symptom Assessment System. Curr Oncol. 2009;16(1):55. doi: 10.3747/co.v16i1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M, Northridge DB, Henderson E, Stanford CF, Nicholls DP, Dargie HJ. The use of an exponential protocol for bicycle and treadmill exercise testing in patients with chronic cardiac failure. Eur Heart J. 1992;13(10):1363–1367. doi: 10.1093/oxfordjournals.eurheartj.a060067. [DOI] [PubMed] [Google Scholar]
- Rogers L, Courneya K, Shah P, Dunnington G, Hopkins-Price P. Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: a pilot study. Eur J Cancer Care. 2007;16(1):55–66. doi: 10.1111/j.1365-2354.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Speck RM, Rye SA, DiSipio T, Hayes SC. Prevalence of breast cancer treatment sequelae over 6 yr of follow-up: the Pulling Through Study. Cancer. 2012;118(8 Suppl):2217–2225. doi: 10.1002/cncr.27474. [DOI] [PubMed] [Google Scholar]
- Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- Spark LC, Reeves MM, Fjeldsoe BS, Eakin EG. Physical activity and/or dietary interventions in breast cancer survivors: a systematic review of the maintenance of outcomes. J Cancer Surviv. 2013;7(1):74–82. doi: 10.1007/s11764-012-0246-6. [DOI] [PubMed] [Google Scholar]
- Statistics Canada Canadian Health Measures Survey: physical activity of youth and adults. 2011 Secondary Canadian Health Measures Survey: physical activity of youth and adults. http://www.statcan.gc.ca/daily-quotidien/110119/dq110119b-eng.htm.
- Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21(9):977–985. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- Win T, Jackson A, Sharples L, Groves AM, Wells FC, Ritchie AJ, Laroche CM. Cardiopulmonary exercise tests and lung cancer surgical outcome. Chest. 2005;127(4):1159–1165. doi: 10.1378/chest.127.4.1159. [DOI] [PubMed] [Google Scholar]


