Abstract
Huntington’s disease (HD) is an inherited genetic disorder, characterized by cognitive dysfunction and abnormal body movements called chorea. Quinolinic acid (QA) is an endogenous metabolite of tryptophan in the kynurenine pathway. QA-induced alterations are similar to the symptoms of HD patients. Physical exercise has beneficial effects on the brain functions. Exercise increases production of neurotrophic factors in the brain and improves learning ability and memory function. In the present study, we investigated the effects of treadmill exercise short-term memory on QA-induced HD rats in relation with cell proliferation. For the induction of Huntington’s animal model, 2 μL of 100 nmol QA was intrastriatal injected into the rats. The rats in the treadmill exercise groups were forced to run on a treadmill for 30 min once a day, five times a week for 2 weeks. Step-down avoidance test was conducted for the determination of short-term memory. Cell proliferation in the hippocampal dentate gyrus was determined by 5-bromo-2′-deoxyuridine (BrdU) and doublecortin (DCX) immunohistochemistry. Western blot for brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) were performed. In the present results, treadmill exercise alleviated QA-induced short-term memory impairment in HD rats. Treadmill exercise increased cell proliferation in the hippocampal dentate gyrus through enhancing BDNF expression in the HD rats. These results revealed that treadmill exercise is effective for the symptom improvement in the HD patients.
Keywords: Huntington’s disease, Quinolinic acid, Treadmill exercise, Hippocampus
INTRODUCTION
Huntington’s disease (HD) is originally termed as Huntington’s chorea, because of the characteristic involuntary movements. HD is a chronic neurodegenerative disorder, which is inherited in an autosomal dominant fashion (Schwarcz et al., 2010). The degenerative process primarily involves medium spiny striatal neurons, and to a lesser extent, cortical neurons. Enkephalin neurons in the basal ganglia are the most vulnerable site for HD, and their early dysfunction is responsible for chorea development (Mitchell et al., 1999).
HD is a neurodegenerative hereditary illness originated by the mutation of the gene encoding the huntingtin-protein (htt). Medium spiny striatal neurons are selectively vulnerable to the toxicity of glutamate (excitotoxicity) or its analogues, and excitotoxic neuronal death is suggested to be involved in neurodegeneration associated with HD (Estrada Sánchez et al., 2008). Intrastriatal injection of excitotoxins, such as QA, produces a pattern of neuronal cell death that seen in HD patients (Beal et al., 1986).) Therefore, the QA model has frequently been used in both rodents and primates to examine the ability of neurotrophic factors to prevent or minimize the loss of striatal neurons and associated motor and cognitive deficits (Jørgensen et al., 2011).
The hippocampus is a key structural element for the learning ability and memory function in the limbic system. Neurogenesis in the hippocampal dentate gyrus occurs throughout post-natal life, including adult life, and neurogenesis is influenced by the environment factors. Adult hippocampal neurogenesis is composed of several developmental stages, and various characteristic neuronal markers are expressed during neurogenesis (Kempermann et al., 2004). Among these neuronal markers, doublecortin (DCX) is a brain-specific microtubule-associated protein and DCX is considered as the immature neuronal marker (Kim et al., 2013; Fedele et al., 2011).
Cerebral cortex and striatum are the brain areas vulnerable to HD, and hippocampal dysfunction is also suggested as the pathogenesis of HD (Ransome et al., 2012). Adult hippocampal neurogenesis and synaptic plasticity is disrupted in HD mice (Ransome et al., 2012).
Another molecular pathogenesis related to the hippocampus of HD mice is decrement in brain-derived neurotrophic factor (BDNF) (Zajac et al., 2010). BDNF is associated with hippocampal synaptic plasticity and neurogenesis, and it is implicated in the hippocampal-dependent learning process (Ransome et al., 2012). BDNF dysregulation is one of the main etiological mechanisms in HD and other neurodegenerative diseases (Seo et al., 2014; Zuccato and Cattaneo, 2007). BDNF binds to tyrosine-kinase receptor type 2 (TrkB), and then activates signaling pathways (Jeong et al., 2014).
Neuroprotective effects of exercise on various brain insults are well documented (Kim et al., 2011; Lang et al., 2010; Petrus et al., 2008; Seo et al., 2013). However, the effects of treadmill exercise on HD are not clarified. In the present study, we evaluated the effect of treadmill exercise on short-term memory in relation with neurogenesis in the hippocampus using QA-induced HD rats. For this study, step-down avoidance task, immunohistochemistry, and western blot were conducted.
MATERIALS AND METHODS
Animals and treatments
Female Sprague-Dawley rats (210±10 g, 6 weeks old) were used for this study, and the experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health (NIH) and the Korean Academy of Medical Sciences. The animals were housed under controlled temperature (23±2°C) and lighting (08:00 to 20:00 h) conditions with food and water available ad libitum. The animals were randomly divided into four groups (n=10 in each group): sham-operation group, the sham-operation and treadmill exercise group, the HD-induced group, and the HD-induced and treadmill exercise group.
Induction of HD model
HD was induced using a previously described procedure (Lee et al., 2006). In brief, the rats were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France) and prepared for surgery. When the rats was unresponsive (no ocular or pedal reflexes), the head was shaved and placed into a digital stereotaxic device (stereotaxic frame, Benchmark DeluxeTM; MyNeurolab, St. Louis, MO, USA). They were positioned in a stereotaxic apparatus, and unilateral intrastriatal injection of quinolinic acid (2 μL of 100 nmol) was conducted using a Hamilton syringe according to the following coordinates: AP +0.7 mm, L +2.8 mm, and V −6.0 mm, from bregma. Body temperature was maintained at 37±1°C during the procedure using a rectal probe and a heating pad. The rats were kept warm with heating lamps for 2 h after surgery and then placed in single cages in the postoperative room to recover. Free access to food and water was allowed after recovery from anesthesia. Neurological and health statuses were monitored for 1 week until normal feeding resumed and postoperative weight had been achieved. The rats in the sham-operation group and in the sham-operation and treadmill exercise group were treated identically, but injected with the same volume of normal saline.
Treadmill exercise protocol
The rats in the treadmill exercise groups were subjected to run on a treadmill for 30 min once a day for 14 days. Exercise load for the running group consisted of running at a speed of 2 meters/min for the first 5 min, at a speed of 5 meters/min for the next 5 min, and then at a speed of 8 meters/min for the last 20 min, with the 0° inclination.
Step-down avoidance test
The latency of the step-down avoidance test was determined to evaluate the short-term memory, according to the previously described method (Jin et al., 2014). The rats were placed on a 7×25 cm platform, which was 2.5 cm high. The platform faced a 42×25 cm grid of parallel 0.1 cm-caliber stainless steel bars spaced 1 cm apart. In training session, the animals received a 0.5 mA scramble foot shock for 2 sec immediately upon stepping down. Two hours after training, the latency (sec) was determined. The interval between the rats stepping down and placing all four paws on the grid was defined as the latency time. Latency over 300 sec was counted as 300 sec.
Tissue preparation
The rats were sacrificed 15 days after the starting of experiment, immediately after step-down avoidance task. To begin the sacrifice process, the animals were fully anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). The anesthetized rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4%paraformaldehyde (PFA) in 100 mM phosphate buffer (PB) at pH 7.4. Brains were dissected, post-fixed in the same fixative overnight, and transferred to 30% sucrose for cryoprotection. Coronal sections of 40 μm thickness were made with a freezing microtome (Leica, Nussloch, Germany). The sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount® (Fisher Scientific, Fair Lawn, NJ, USA).
BrdU immunohistochemistry
BrdU immunohistochemistry was used for the detection of newly generated cells in the hippocampal dentate gyrus, as the previously described method (Kim et al., 2010). In brief, the sections were initially permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated with 50% formamide-2×standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 h with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were then incubated for another 1 h with avidin-peroxidase complex (1:100; Vector Laboratories). For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.03% H2O2, 0.02% 3,3′-diaminobenzidine (DAB), and 40 mg/mL nickel chloride (nickel-DAB) for 5 min.
After BrdU-specific staining, we performed counter-staining on the same sections using a mouse anti-neuronal nuclei (NeuN) antibody (1:1,000; Chemicon International, Temecula, CA, USA). The sections were then washed three times with PBS, incubated for 1 h with a biotinylated anti-mouse secondary antibody, and processed with the VECTASTAIN® ABC Kit (1:100; Vector Laboratories). For staining, the sections were allowed to react with 0.02% DAB and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.6) for 5 min and the sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount® (Fisher Scientific).
DCX immunohistochemistry
DCX immunohistochemistry was performed, according to a previously described method (Kim et al., 2014). The sections were washed with 0.1 M PBS (pH 7.4), then immersed in a 3% H2O2 and 20% methanol in PBS solution, washed in PBS and incubated for 48 h at 4°C with the α-doublecortin antibody (1 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in a blocking solution of 3% normal donkey serum in PBS. The slices were washed in PBS and in 0.05% Tween 20 in PBS for 15 min before incubation with donkey α-goat biotinylated antibody for 1 h at room temperature (Chemicon) diluted in the blocking solution. The slices were washed in PBS before incubation with ABC Elite kit (Vector Laboratories) for 1 h, washed again in PBS, and incubated with DAB with 0.03% hydrogen peroxide for 5 min. The sections were mounted onto gelatin-coated slides, air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
Western blot analysis BDNF and Trk-B
Western blot was conducted as the previously described method (Kim et al., 2010). The hippocampal tissues were collected, and then were immediately frozen at −70°C. The hippocampal tissues were homogenized on ice, and lysed in a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1 mM EGTA, 1.5 mM MgCl2 6H2O, 1 mM sodium orthovanadate, and 100 mM sodium flouride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad). Protein (30 μg) was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane.
Mouse beta-actin antibody (1:500; Santa Cruz Biotechnology), rabbit BDNF antibody (1:1,000; Santa Cruz Biotech), and rabbit Trk-B antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody for beat-actin and anti-rabbit antibody for BDNF and Trk-B (1:3,000; Vector Laboratories) were used as the secondary antibodies. Experiment was performed in normal lab conditions and at room temperature except membrane transfer. Membrane transfer was performed at 4°C with the cold pack and pre-chilled buffer. Band detection was performed using the enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology).
Data analysis
The number of BrdU-positive and DCX-positive cells in the hippocampal dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and they were expressed as the numbers of cells per mm2 in the hippocampal dentate gyrus. To compare the relative expressions of BDNF and TrkB the detected bands were calculated densitometrically using Image-Pro® Plus software (Media Cybernetics, Silver Spring, MD, USA). Statistical analysis was performed using one-way ANOVA followed by Duncan’s post-hoc test. The results are presented as the mean±standard error of the mean (SEM). Significance was set as P<0.05.
RESULTS
Effect of treadmill exercise on the short-term memory in the step-down avoidance test
The latency time in the step-down avoidance test is presented in Fig. 1. The latency was 131.37±16.41 sec in the sham-operation group, 160.83±12.92 sec in the sham-operation and treadmill exercise group, 22.66±3.77 sec in the HD-induced group, and 71.12±16.74 sec in the HD-induced and treadmill exercise group. The present results indicated that the latency in the HD-induced group was decreased compared to the sham-operation group (P<0.05). However, treadmill exercise significantly increased the latency in the rats of the HD-induced group (P<0.05).
Fig. 1.
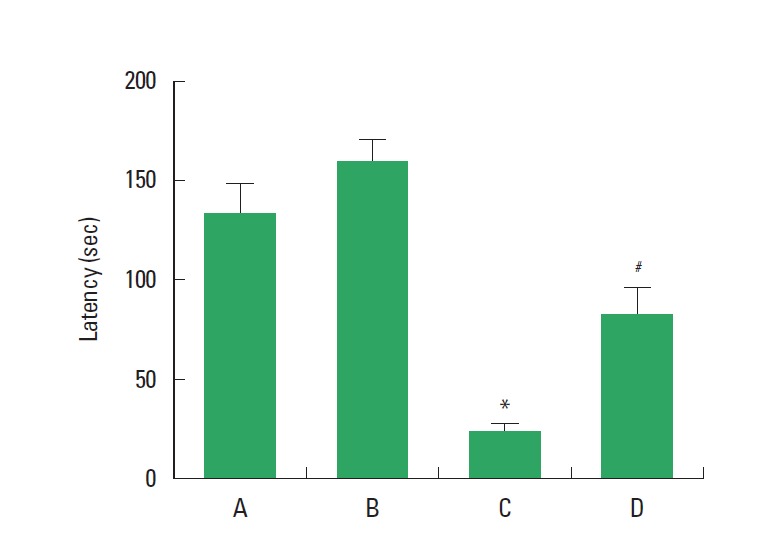
Effect of treadmill exercise on the short-term memory in the step-down avoidance test. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Huntington’s disease (HD)-induced group, and (D) HD-induced and treadmill exercise group. *represents P< 0.05 compared to the sham-operation group. #represents P< 0.05 compared to the HD-induced group. The data are expressed as the mean± standard error of the mean (SEM).
Effect of treadmill exercise on the hippocampal neurogenesis
The effect of treadmill exercise on the neurogenesis in the hippocampal dentate gyrus is presented in Fig. 2. The number of BrdU-positive cells in the hippocampal dentate gyrus was 76.85±6.04 in the sham-operation group, 96.10±11.40 in the sham-operation and treadmill exercise group, 31.29±4.09 in the HD-induced group, and 54.62±4.83 in the HD-induced and treadmill exercise group. The present results indicated that the number of BrdU-positive cells in the hippocampal dentate gyrus was decreased in the HD-induced group compared to the sham-operation group (P<0.05). In contrast, treadmill exercise significantly increased the number of BrdU-positive cells in the rats of the HD-induced group (P<0.05).
Fig. 2.
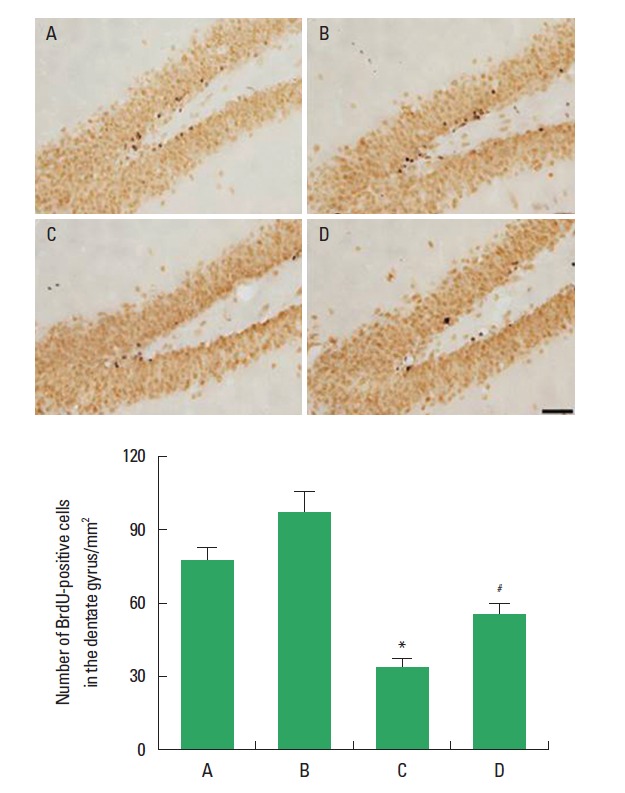
Effect of treadmill exercise on the cell proliferation in the hippocampal dentate gyrus. Upper: Photomicrographs of 5-bromo-2′-deoxyuridine (BrdU)-positive cells. The scale bar represents 50 µm. Lower: Number of BrdU-positive cells in each group. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Huntington’s disease (HD)-induced group, and (D) HD-induced and treadmill exercise group. *represents P< 0.05 compared to the sham-operation group. #represents P < 0.05 compared to the HD-induced group. The data are expressed as the mean± standard error of the mean (SEM).
Effect of treadmill exercise on the hippocampal neuronal marker
The effect of treadmill exercise on the neuronal marker in the hippocampal dentate gyrus is presented in Fig. 3. The number of DCX-positive cells in the hippocampal dentate gyrus was 256.30±11.27 in the sham-operation group, 303.91±23.32 in the sham-operation and treadmill exercise group, 154.35±6.82 in the HD-induced group, and 226.33±20.03 in the HD-induced and treadmill exercise group. The present results indicated that the number of DCX-positive cells in the hippocampal dentate gyrus was decreased in the HD-induced group compared to the sham-operation group (P<0.05). In contrast, treadmill exercise significantly increased the number of DCX-positive cells in the rats of the HD-induced group (P<0.05).
Fig. 3.
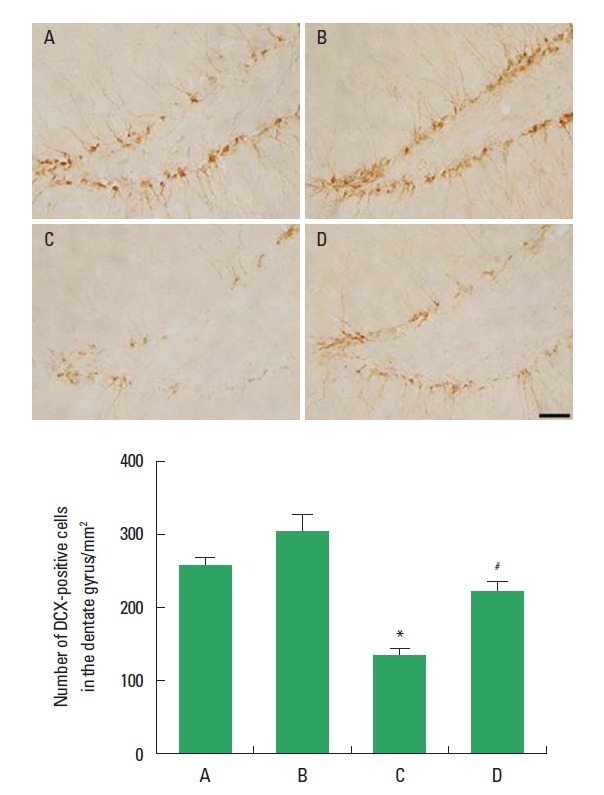
Effect of treadmill exercise on the doublecortin (DCX) expression in the hippocampal dentate gyrus. Upper: Photomicrographs of DCX-positive cells. The scale bar represents 50 µm. Lower: Number of DCX-positive cells in each group. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Huntington’s disease (HD)-induced group, and (D) HD-induced and treadmill exercise group. *represents P< 0.05 compared to the sham-operation group. #represents P< 0.05 compared to the HD-induced group. The data are expressed as the mean ± standard error of the mean (SEM).
Effect of treadmill exercise on the BDNF and TrkB expression in the hippocampus
The effect of treadmill exercise on the level of BDNF and TrkB protein in the hippocampus was analyzed is presented in Fig. 4. When the level of BDNF (14 kDa) in the sham-operation group was set as 1.00, the level of BDNF was 1.28±0.04 in the sham-operation and treadmill exercise group, 0.75±0.03 in the HD-induced group, and 0.92±0.01 in the HD-induced and treadmill exercise group.
Fig. 4.
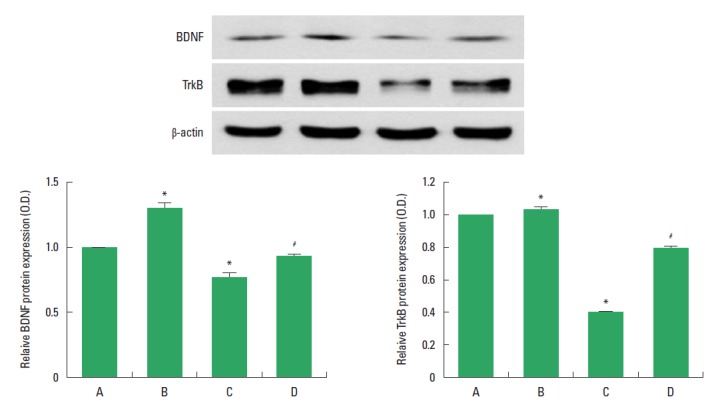
Effect of treadmill exercise on the brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) expression in the hippocampus. Upper: Western blot analysis of BDNF and TrkB in the hippocampus. Left lower: Mean optical density of BDNF expression in each group. Right lower: Mean optical density of TrkB expression in each group. (A) Sham-operation group, (B) sham-operation and treadmill exercise group, (C) Huntington’s disease (HD)-induced group, and (D) HD-induced and treadmill exercise group. *represents P< 0.05 compared to the sham-operation group. #represents P< 0.05 compared to the HD-induced group. The data are expressed as the mean± standard error of the mean (SEM).
When the level of mature TrkB (95 kDa) in the sham-operation group was set as 1.00, the level of TrkB was 1.04±0.01 in the sham-operation and treadmill exercise group, 0.39±0.00 in the HD-induced group, and 0.74±0.01 in the HD-induced and treadmill exercise group. The present results indicated that BDNF and TrkB expression in the hippocampus was significantly decreased in the HD-induced group compared to the sham-operation group (P<0.05). In contrast, treadmill exercise significantly increased the BDNF and TrkB expression in the rats of the HD-induced group (P<0.05).
DISCUSSION
HD is a dominantly inherited disorder, characterized by progressive neurodegeneration in the striatum and other brain regions (Kent, 2004). Excitotoxicity has been suggested to induce neurodegeneration associated with HD (Estrada Sánchez et al., 2008). QA-induced HD animal model showed HD-like symptoms, such as deficits in learning ability and rota-rod performance (Kalonia et al., 2011). In the present results, short-term memory was deteriorated in the QA-induced HD rats.
Reduced level of BDNF in the hippocampus was observed in the HD mice (Pang et al., 2006). Reduced BDNF expression in the HD brain accompanied with the shrinkage of hippocampus in the late-stage HD patients (Zajac et al., 2010). Decrement of hippocampal volume in the mice model of HD was caused by suppression of cell proliferation (Gil et al., 2005; Zajac et al., 2010). In the present results, cell proliferation in the hippocampal dentate gyrus was decreased, furthermore BDNF and TrkB expression was also suppressed in the HD rats.
Treadmill exercise increases production of neurotrophic factors in the brain, and through this mechanism, treadmill exercise improves learning ability and memory function (Baek et al., 2012; Kim et al., 2010; Sim, 2014). Voluntary wheel running was reported to be effective in delaying the onset of motor and cognitive deficits in the mouse model of HD (Pang et al., 2006; Zajac et al., 2010).
In the present study, treadmill exercise alleviated QA-induced short-term memory impairment in HD rats. Treadmill exercise increased cell proliferation in the hippocampal dentate gyrus through enhancing BDNF expression in the HD rats. These results revealed that treadmill exercise is effective for the symptom improvement in the HD patients.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-327-G00099).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Baek SS, Jun TW, Kim KJ, Shin MS, Kang SY, Kim CJ. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain Dev. 2012;34:45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Estrada Sánchez AM, Mejía-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res. 2008;39:265–276. doi: 10.1016/j.arcmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Fedele V, Roybon L, Nordström U, Li JY, Brundin P. Neurogenesis in the R6/2 mouse model of Huntington’s disease is impaired at the level of NeuroD1. Neuroscience. 2011;173:76–81. doi: 10.1016/j.neuroscience.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Gil JM, Mohapel P, Araujo IM, Popovic N, Li JY, Brundin P, Petersen A. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol Dis. 2005;20:744–751. doi: 10.1016/j.nbd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Jeong HI, Ji ES, Kim SH, Kim TW, Baek SB, Choi SW. Treadmill exercise improves spatial learning ability by enhancing brain-derived neurotrophic factor expression in the attention-deficit/hyperactivity disorder rats. J Exerc Rehabil. 2014;10:162–167. doi: 10.12965/jer.140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JJ, Ko IG, Kim SE, Shin MS, Kim SH, Jee YS. Swimming exercise ameliorates multiple sclerosis-induced impairment of short-term memory by suppressing apoptosis in the hippocampus of rats. J Exerc Rehabil. 2014;10:69–74. doi: 10.12965/jer.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen JR, Emerich DF, Thanos C, Thompson LH, Torp M, Bintz B, Fjord-Larsen L, Johansen TE, Wahlberg LU. Lentiviral delivery of meteorin protects striatal neurons against excitotoxicity and reverses motor deficits in the quinolinic acid rat model. Neurobiol Dis. 2011;41:160–168. doi: 10.1016/j.nbd.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Kalonia H, Kumar P, Kumar A. Licofelone attenuates quinolinic acid induced Huntington like symptoms: possible behavioral, biochemical and cellular alterations. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:607–615. doi: 10.1016/j.pnpbp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kent A. Huntington’s disease. Nurs Stand. 2004;18:45–51. doi: 10.7748/ns2004.04.18.32.45.c3596. [DOI] [PubMed] [Google Scholar]
- Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10:2–8. doi: 10.12965/jer.140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW, Kim CJ. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504:35–39. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerotol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Park CY, Shin MS, Kim CJ, Jee YS. Treadmill and wheel exercise alleviate lipopolysaccharide-induced short-term memory impairment by enhancing neuronal maturation in rats. Mol Med Rep. 2013;7:31–36. doi: 10.3892/mmr.2012.1160. [DOI] [PubMed] [Google Scholar]
- Lang R, Koegel LK, Ashbaugh K, Regester A, Ence W, Smith W. Physical exercise and children with autism spectrum disorders: A systematic review. Res Autism Spectr Disord. 2010;4:565–576. [Google Scholar]
- Lee ST, Park JE, Lee K, Kang L, Chu K, Kim SU, Kim M, Roh JK. Noninvasive method of immortalized neural stem-like cell transplantation in an experimental model of Huntington’s disease. J Neurosci Methods. 2006;152:250–254. doi: 10.1016/j.jneumeth.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Cooper AJ, Griffiths MR. The selective vulnerability of striatopallidal neurons. Prog Neurobiol. 1999;59:691–719. doi: 10.1016/s0301-0082(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience. 2006;141:569–584. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Petrus C, Adamson SR, Block L, Einarson SJ, Sharifnejad M, Harris SR. Effects of exercise interventions on stereotypic behaviours in children with autism spectrum disorder. Physiother Can. 2008;60:134–145. doi: 10.3138/physio.60.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransome MI, Renoir T, Hannan AJ. Hippocampal neurogenesis, cognitive deficits and affective disorder in Huntington’s disease. Neural Plast. 2012;2012:874387. doi: 10.1155/2012/874387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Guidetti P, Sathyasaikumar KV, Muchowski PJ. Of mice, rats and men: revisiting the quinolinic acid hypothesis of Huntington’s disease. Prog Neurobiol. 2010;90:230–245. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo TB, Cho HS, Shin MS, Kim CJ, Ji ES, Baek SS. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil. 2013;9:220–229. doi: 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo TB, Kim TW, Shin MS, Ji ES, Cho HS, Lee JM, Kim TW, Kim CJ. Aerobic exercise alleviates ischemia-induced memory impairment by enhancing cell proliferation and suppressing neuronal apoptosis in hippocampus. Int Neurourol J. 2014;18:187–197. doi: 10.5213/inj.2014.18.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats. J Exerc Rehabil. 2014;10:81–88. doi: 10.12965/jer.140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, Craig JM, Saffery R, Hannan AJ. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington’s disease mice. Hippocampus. 2010;20:621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]


