Abstract
Numerous murine models have been developed to study human cancers and advance the understanding of cancer treatment and development. Here, a preclinical, murine pancreatic tumor model of hepatic metastases via a hemispleen injection of syngeneic murine pancreatic tumor cells is described. This model mimics many of the clinical conditions in patients with metastatic disease to the liver. Mice consistently develop metastases in the liver allowing for investigation of the metastatic process, experimental therapy testing, and tumor immunology research.
Keywords: Medicine, Issue 91, Pancreatic Neoplasms, Immunotherapy, Hemispleen, Hepatic Metastases, Pancreatic Cancer, Liver, Preclinical Model, Metastatic, Murine
Introduction
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer related deaths in the United States1. The five year survival for patients with metastatic PDA is 2-12%1. Although adjuvant and neoadjuvant chemotherapy for pancreatic cancer is more effective today than ever, there still remains a desperate need for novel therapies.
The development of novel therapeutics necessitates reliable preclinical animal models. Although subcutaneous tumor models are straightforward to use, drawbacks include the inability of these tumors to metastasize and mimic the tumor progression and microenvironments seen in human cancers. Genetically engineered models, such as the genetically engineered mouse containing Kras and p53 knock-in oncogenic mutations (the KPC model)2 and the KPC model with a YFP lineage tracer (the PKCY model)3 allow for the study of naturally occurring tumors in immunocompetent mice. Additionally, the tumor microenvironment is unaltered and more closely resembles that seen in human tumor progression. Unfortunately, the timing of tumor development is variable within these models, which makes it difficult to study experimental therapies. Additionally, backcrossing of these mice requires a significant amount of time and financial commitment, which is not always feasible. Finally, implanted xenograft PDA mouse models necessitate immunocompromised mice, which have altered or absent tumor microenvironments. As a result, the efficacy of experimental therapies demonstrated in these preclinical models are often not reproducible in human clinical trials4.
This hemisplenectomy mouse model utilizes Panc02 tumor cells, which are a highly tumorigenic, methylcholanthrene induced pancreatic tumor cell line derived from C57BL/6 mice5,6. Additionally, other murine PDA cell lines derived from the KPC mice can be used in this model. Schulick et al. have previously developed a hemisplenectomy technique and created a syngeneic mouse model of colon cancer metastases7. This hemisplenectomy model (Figure 1) offers consistent and equal tumor inoculation in immunocompetent mice lending itself to the investigation of novel therapeutic agents7-10.
Protocol
All animal experiments conformed to the guidelines of the Animal Care and Use Committee of the Johns Hopkins University, and animals were maintained in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Care (AAALAC). The surgical procedure is performed under sterile conditions in an operating room that undergoes a minimum of fifteen air exchanges per hour. All instruments are sterilized by autoclaving prior to the procedure and again with 70% ethanol in between each mouse during the procedure. All mice not expected to survive the surgical procedure are euthanized under CO2 for 3 min followed by cervical dislocation while still under anesthesia.
1. Cell Preparation
Resuspend cultured Panc02 cells in phosphate buffered saline at 2 x107 cells/ml, and keep on ice.
2. Mouse Preparation
Administer Ketamine (100 mg/kg) mixed with Xylazine (10 mg/kg) intraperitoneally to 8-12 week old C57BL/6 female mice in divided doses.
Check to see that the toe pinch withdrawal reflex is abolished to ensure that the mouse is fully anesthetized.
Administer additional doses of Ketamine (100 mg/kg) mixed with Xylazine (10 mg/kg) as needed to fully anesthetize the mice.
Apply Puralube Vet Ointment to the eyes of the mice to prevent drying while the mice are under anesthesia.
Shave the surgical area of the mice to avoid contamination of the wound.
Prep the left subcostal area of the incision with 70% ethanol and then iodine.
Place a surgical drape around the abdomen to maintain sterility.
3. Laparotomy
Make a left subcostal incision in line with the left ear through the skin and through the peritoneum using a scalpel.
Express the spleen through the incision by applying simultaneous digital pressure along the cranial and caudal aspects of the incision.
Locate the splenic blood vessels at the inferior end of the spleen.
Divide the spleen by placing two Horizon medium size ligating clips in the center of the spleen being careful to avoid damaging the splenic blood vessels at the splenic poles.
Place the upper pole of the spleen back into the peritoneum to avoid contamination.
4. Tumor Injection
Draw up 150 μl of phosphate buffered saline into a 26 G x 5/8” syringe.
Draw up 100 μl of Panc02 (2 x106) cells into the same syringe maintaining the syringe in an upright position at all times during the injection.
Dip the needle in a 70% ethanol solution. The needle is dipped in 70% ethanol to ensure sterility.
Inject the cells slowly into the exposed hemispleen being sure the syringe is kept upright at all times. The bevel of the syringe should be observed at all times through the splenic capsule to avoid injecting the cells under the spleen. A whitening of the spleen and blood vessels should be observed upon injection.
Elevate the spleen and apply one medium Horizon clip under the spleen to occlude the splenic blood vessels.
Apply one small Horizon clip to the most distal aspect of the pancreas and splenic vessels.
Ligate the pancreas and splenic vessels from the hemispleen by cutting above the Horizon clips.
Place the mouse on its’ side, and irrigate the incision with distilled water.
5. Abdominal Closure and Recovery from Anesthesia
Close the peritoneum with a 4-0 running stitch.
Apply two to three skin clips to close the skin incision.
Administer 0.1 mg/kg buprenorphine or 5-10 mg/kg Carprofen subcutaneously to alleviate post surgical pain.
Place the mouse on a heating pad for recovery until mobile and the mouse demonstrates regular breathing patterns. Animals are only returned to the company of other animals after fully recovering from the surgical procedure.
6. Necropsy Examination and Harvesting of the Liver
Between 30 to 60 days following the surgery, mice will begin to show clinical symptoms of metastasis and will require euthanasia. Signs of metastatic disease requiring humane euthanasia include enlarged abdomens and the development of ascites. Euthanize mice by inhalation of CO2.
Following inhalation, perform cervical dislocation.
After the animal has been determined to be non responsive, make an incision through the peritoneum to expose the abdomen using scissors.
Using forceps and scissors, gently cut the liver out of the abdomen.
Place the liver in OCT compound, and flash freeze using liquid nitrogen. Store the liver at -80°C. Alternatively, the liver can be fixed in 16% neutral buffered formalin at RT for 24 hr and paraffin embedded.
Representative Results
Panc02 tumor cells injected into the hemispleen (Figure 1) form liver metastases in 100% of the mice, while lung and peritoneal metastases are not observed if the technique is performed correctly. This technique has been performed on over one hundred mice using Panc02 cells in multiple, independent experiments, and reproducibly, all untreated mice die with liver metastases between 30 and 60 days following the hemisplenectomy procedure (Figure 2). To obtain statistical significance between the untreated and treated groups, ten mice per group are needed. However, this number will vary with respect to the cell line and treatment scheme used. When Panc02 cells are used in this model, multiple liver metastases can be observed upon necropsy (Figure 3).
KPC tumor cells, which are a syngeneic pancreatic tumor cell line derived from transgenic mice having tissue-specific Kras and p53 knock-in mutations11, can also be used in this model. Using these cells results in similar liver metastases (Figure 4). However, when evaluating liver metastasis formation using a different cell line, the number of cells injected should be titrated until all untreated mice develop liver metastases. Panc02 cells can be used as a positive control to ensure that the surgery was performed correctly. Importantly, while the extent of the formation of liver metastases and survival can vary depending on the cell line used, within one experiment, all untreated mice develop liver metastases at a similar burden and die from these metastases reproducibly within a short period of time. Therefore, normalization of tumor burden by ultrasound is not necessary when using this technique.
Failure to induce liver metastasis results in a normal appearing liver upon necropsy, which may indicate that the procedure was not performed properly. However, failed or delayed formation of metastases may result following treatment with therapies that suppress or inhibit metastatic growth (Figure 5), resulting in prolonged survival. Therefore, this model can be used to assess the effects of various therapeutic agents on tumor growth and the formation of metastases.
Because it is technically feasible to evaluate the liver by ultrasound, a small animal ultrasound (Figure 6)12 can be used to assess the in vivo formation of metastases in the liver. In addition, the formation of metastases can be further examined by histopathology following necropsy (Figure 7).
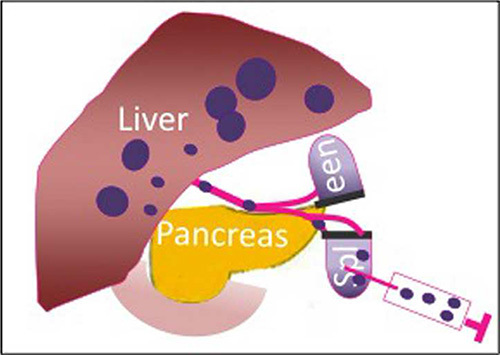 Figure 1.
Schematic of the hemisplenectomy model, which is used to create pancreatic hepatic metastases with Panc02 tumor cells.
Figure 1.
Schematic of the hemisplenectomy model, which is used to create pancreatic hepatic metastases with Panc02 tumor cells.
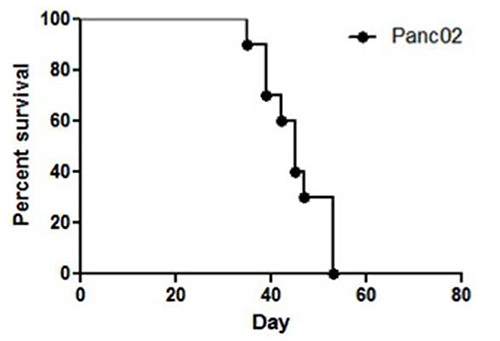 Figure 2.Representative survival of C57BL/6 mice after undergoing Panc02 hemisplenectomy on day 0 is shown in the Kaplan-Meier curve (n=10). Mice injected with Panc02 tumor cells in this model will all die between 30 and 60 days following the procedure if anti neoplastic therapies are not administered.
Figure 2.Representative survival of C57BL/6 mice after undergoing Panc02 hemisplenectomy on day 0 is shown in the Kaplan-Meier curve (n=10). Mice injected with Panc02 tumor cells in this model will all die between 30 and 60 days following the procedure if anti neoplastic therapies are not administered.
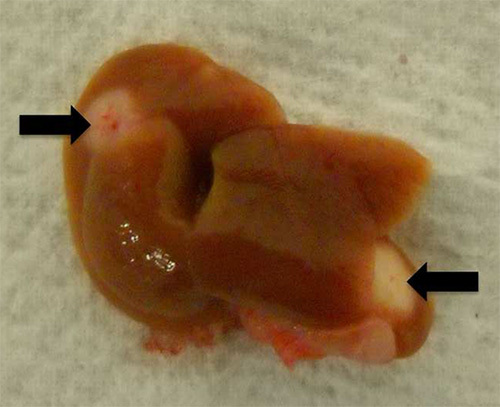 Figure 3.
Gross examination of the liver following injection of Panc02 cells into the hemispleen reveals liver metastases (indicated by arrows).
Please click here to view a larger version of this figure.
Figure 3.
Gross examination of the liver following injection of Panc02 cells into the hemispleen reveals liver metastases (indicated by arrows).
Please click here to view a larger version of this figure.
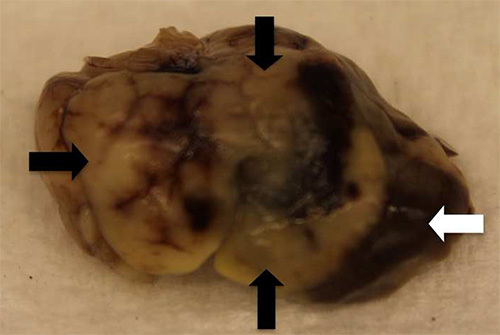 Figure 4.
Gross examination of the liver following hemispleen injection of KPC tumor cells into the hemispleen reveals liver metastases (black arrows) that replace the normal hepatic parenchyma (white arrow).
Please click here to view a larger version of this figure.
Figure 4.
Gross examination of the liver following hemispleen injection of KPC tumor cells into the hemispleen reveals liver metastases (black arrows) that replace the normal hepatic parenchyma (white arrow).
Please click here to view a larger version of this figure.
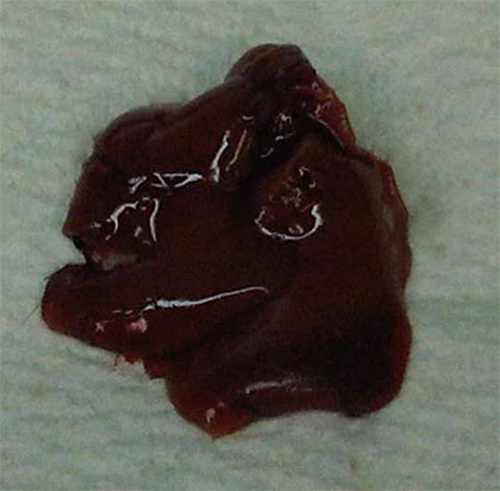 Figure 5.
Gross examination of the liver following effective therapeutic intervention. Please click here to view a larger version of this figure.
Figure 5.
Gross examination of the liver following effective therapeutic intervention. Please click here to view a larger version of this figure.
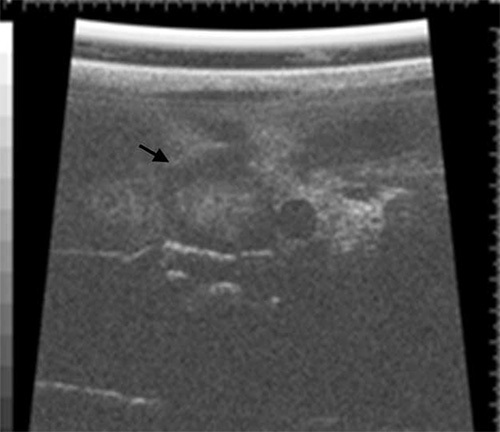 Figure 6. Liver ultrasonography using a Vevo 660 small animal ultrasound on a mouse with a large metastatic lesion in the liver (black arrow) following the hemisplenectomy procedure.
Please click here to view a larger version of this figure.
Figure 6. Liver ultrasonography using a Vevo 660 small animal ultrasound on a mouse with a large metastatic lesion in the liver (black arrow) following the hemisplenectomy procedure.
Please click here to view a larger version of this figure.
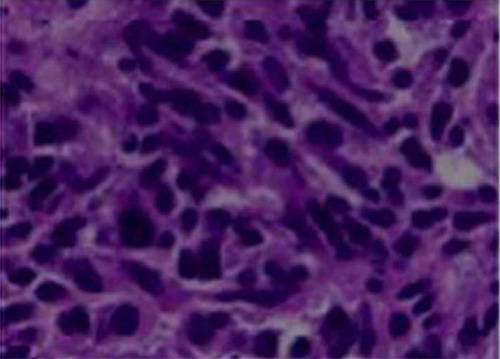 Figure 7.
H&E staining of paraffin embedded liver metastases formed by Panc02 cells in the hemisplenectomy model.
Please click here to view a larger version of this figure.
Figure 7.
H&E staining of paraffin embedded liver metastases formed by Panc02 cells in the hemisplenectomy model.
Please click here to view a larger version of this figure.
Discussion
This preclinical murine model of pancreatic cancer metastasis to the liver via a hemispleen injection technique is the first model described that mirrors many of the clinical and immunological conditions of patients with metastatic disease to the liver. This model offers several advantages over other murine models. First, unlike transgenic models of pancreatic cancer in which only 30% of the mice develop metastases to the liver and the timing of metastases formation is quite variable, this model offers the advantage of consistently obtaining metastasis to the liver in 100% of mice at a uniform time, enabling researchers to study metastatic disease in this setting. Additionally, this model retains a naive hemispleen to allow for peripheral blood immune analysis in a reproducible model. Furthermore, in the absence of anti-neoplastic therapies, mice die from tumor progression at a consistent time point depending on the initial tumor load, and survival is consistent from one experiment to the next. Finally, this model has a variety of applications including the study of liver metastasis13, tumor immunology10, and experimental therapy testing9.
The critical steps of this technique are the cell suspension preparation, the tumor cell injection, the placement of the Horizon clips, and the placement of the skin clips. To ensure a successful surgery and avoid potential skewing of the results, the following modifications of the critical steps may be necessary. First, during cell suspension preparation, it is imperative that a single suspension of cells be obtained and injected into the hemispleen. Minor clumping of tumor cells will occlude the splenic vessels and result in premature death during surgery. This can be avoided by resuspending the cells in a declumping agent and by preparing each syringe for injection immediately prior to the injection. Additionally, during the preparation of the syringe, it is imperative that the cells not mix with the phosphate buffered saline flush. This can be achieved by first drawing up the phosphate buffered saline and then very slowly drawing up the cells to avoid mixing, maintaining the syringe in an upright position at all times. Second, during the tumor cell injection step, the bevel of the syringe should be observed at all times through the splenic capsule to avoid injecting the cells under the spleen. A whitening of the spleen and blood vessels should be observed upon injection, which indicates that the tumor cells have been successfully injected into the spleen. In younger mice with smaller spleens, it may be necessary to inject a smaller volume of flush than risk the cells leaking from the spleen. If any leaking is suspected, lay the mice on their side and wash the spleen with distilled water before suturing the mice to help remove any cells that may have leaked during the injection step. Failure to remove these cells will result in tumor growth at the injection site, which may cause death prior to achieving adequate metastases. Third, the placement of the Horizon clips under the hemispleen is important for ensuring that there is no bleeding following removal of the hemispleen. Additional Horizon clips may be used to occlude vessels as necessary. The final critical step of this technique is the placement of the skin clips. If the mice are being following for survival, it may be necessary to suture the skin of the mice rather than using skin clips. Occasionally, the skin clips will fall off prior to the full healing of the skin wound, which could render the mice susceptible to infection. Suturing of the skin, separate from the peritoneum, will prevent this problem.
While this technique is invaluable for studying the metastatic process and metastatic disease, this model has a few limitations. First, the reproducible nature of this model makes it preferred over transgenic and orthotopic models for studying the metastatic process and the tumor microenvironment at metastatic sites. However, because of the nature of the model, only the final steps of the metastatic process, extravasation and colonization, can be studied. This limits the utility of this model for studying the full metastatic process and does not allow for investigation into the signals that stimulate intravasation of tumor cells. Second, while it is important to retain full immune function in these mice by retaining half of the hemispleen, analysis of lymphocytes in the liver include both lymphocytes from the normal liver as well as lymphocytes from the tumor microenvironment, which could result in a biased representation of the true immune status within the tumor microenvironment. The use of more aggressive tumor cell lines that lead to more extensive metastases to the liver could help overcome this limitation of the model. Additionally, if the metastases are visualized upon necropsy, it may be possible to dissect the metastases from the normal liver and analyze only the lymphocytes found in the metastases further circumventing this problem. In summary, while this model has several disadvantages, it also offers many advantages over other models and should therefore be used complement these models when studying metastatic disease to the liver.
Disclosures
The authors have no relevant conflicts of interest to disclose.
Acknowledgments
KF and KO are co first authors along with KS
This work was supported in part by the AHPBA Fellowship (K.S.), NIH K23 CA148964-01 (L.Z.), Johns Hopkins School of Medicine Clinical Scientist Award (L.Z.), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (E.M.J. and L.Z.), the National Pancreas Foundation (L.Z.), the Lefkofsky Family Foundation (L.Z.), the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (E.M.J., L.Z.), the Lustgarten Foundation (L.Z.), and the Sol Goldman Pancreatic Cancer Center grants (K.S., B.H.E., L.Z.).
References
- American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2013. [Google Scholar]
- Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer letters. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese KK, Tuveson DA. Maximizing mouse cancer models. Nature reviews. Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- Corbett TH, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer research. 1984;44:717–726. [PubMed] [Google Scholar]
- Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clinical and translational science. 2008;1:228–239. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, et al. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Annals of surgical oncology. 2003;10:810–820. doi: 10.1245/aso.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, et al. Selective targeting of antitumor immune responses with engineered live-attenuated Listeria monocytogenes. Cancer research. 2006;66:1096–1104. doi: 10.1158/0008-5472.CAN-05-2307. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, et al. Live attenuated Listeria monocytogenes effectively treats hepatic colorectal cancer metastases and is strongly enhanced by depletion of regulatory T cells. Cancer research. 2007;67:10058–10066. doi: 10.1158/0008-5472.CAN-07-0573. [DOI] [PubMed] [Google Scholar]
- Olino K, et al. Tumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Annals of surgical oncology. 2012;19(3):597–607. doi: 10.1245/s10434-011-2037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Marion A, Aoudi W, Basarab A, Delachartre P, Vray D. Blood flow evaluation in high-frequency, 40 MHz imaging: a comparative study of four vector velocity estimation methods. Ultrasonics. 2010;50:683–690. doi: 10.1016/j.ultras.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Zheng L, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PloS one. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]


