Significance
Phylogenomic extrapolations indicate the last common ancestor of sponges and eumetazoans existed deep in the Cryogenian, perhaps 200 million years (Myr) before the Cambrian (541 Ma). This inference implies a long Precambrian history of animals phylogenetically allied with sponges. However, there is yet little unequivocal paleontological evidence of Precambrian sponges. Here, we present a newly discovered 600-Myr-old fossil preserved at cellular resolution, displaying multiple poriferan features. The animal was covered with a dense layer of flattened cells resembling sponge pinacocytes, displaying a hollow tubular structure with apparent water inflow and outflow orifices. Although requiring additional specimens of similar form for confirmation, this finding is consistent with phylogenomic inference, and implies the presence of eumetazoan ancestors by 60 Myr before the Cambrian.
Keywords: Precambrian, sponge fossil, metazoan phylogeny, Doushantuo Formation, synchrotron X-ray tomography
Abstract
An extraordinarily well preserved, 600-million-year (Myr)-old, three-dimensionally phosphatized fossil displaying multiple independent characters of modern adult sponges has been analyzed by SEM and synchrotron X-ray tomography. The fossilized animal (Eocyathispongia qiania gen. et sp. nov.) is slightly more than 1.2 mm wide and 1.1 mm tall, is composed of hundreds of thousands of cells, and has a gross structure consisting of three adjacent hollow tubes sharing a common base. The main tube is crowned with a large open funnel, and the others end in osculum-like openings to the exterior. The external surface is densely covered with flat tile-like cells closely resembling sponge pinacocytes, and this layer is punctuated with smaller pores. A dense patch of external structures that display the form of a lawn of sponge papillae has also survived. Within the main funnel, an area where features of the inner surface are preserved displays a regular pattern of uniform pits. Many of them are surrounded individually by distinct collars, mounted in a supporting reticulum. The possibility cannot be excluded that these pits are the remains of a field of choanocytes. The character set evinced by this specimen, ranging from general anatomy to cell type, uniquely indicates that this specimen is a fossil of probable poriferan affinity. So far, we have only this single specimen, and although its organized and complex cellular structure precludes any reasonable interpretation that its origin is abiogenic, confirmation that it is indeed a fossilized sponge will clearly require discovery of additional specimens.
Extensive phylogenomic analyses have converged on a distant Precambrian vintage for the origin of Metazoa, an evolutionary event that must have long predated the last common ancestor of Porifera (sponges) and Eumetazoa (cnidarians + bilaterians), given the organismal and genomic complexity of these major groups. In modern phylogenomic analyses, elegant computational algorithms are applied to sequence divergence data from very large sets of genomic protein and other sequences so as to establish evolutionary branching order, and thus patterns of relatedness among animal clades. Key branch points can then be referred to real time by use of extrapolations from calibrations afforded by the dated fossil record. Various analyses of this type have been published, as well as an authoritative recent review by Erwin and Valentine (1). All calibrated phylogenomic analyses agree on a remotely Precambrian evolutionary origin of sponges.
Although earlier studies portrayed the four extant sponge classes as polyphyletic, technically sophisticated recent analyses indicate Porifera to be monophyletic, that is, to have had a single common evolutionary origin (2, 3). The result implies a last common ancestor in metazoan phylogeny that gave rise to the sponges [and possible metazoan sister groups (3)] on the one hand and to Eumetazoa [plus possible sister groups (3)] on the other. From the enormous and detailed overlap in the gene toolkit of sponges and eumetazoans (4, 5), there can be little doubt that these two lineages indeed derived from a common ancestor. This sponge/eumetazoan ancestor is predicted by phylogenomics to have lived deep in the Cryogenian (6), the geological period that terminated with the end of the Snowball Earth glaciations at 635 Ma, although how deep is not clear at present (7). The Cryogenian origin of this last common ancestor does not tell us, however, when animals displaying the character complexes that identify modern sponges first arose, only when their stem group ancestors diverged from the stem group ancestors of the eumetazoans.
Tantalizing paleontological claims of the Precambrian fossils that display one or another sponge-like characteristic have appeared in recent years, as critically reviewed by Antcliffe et al. (8). We leave aside isolated objects that are claimed to be fossilized siliceous spicules, because these objects have little morphological information content, and also the numerous claims of fossilized sponge-grade organisms from several parts of the world dating to about 555 Ma, close to the Precambrian/Cambrian boundary. There remain, however, several proposed sponge-like fossils of sufficiently deep temporal provenance to be relevant in principle to the phylogenomic predictions, although it must be noted that none are accepted as fossil sponges in the study by Antcliffe et al. (8). Among these putative fossilized sponges are thin-walled, hollow microfossils, perforated by various openings, found in rocks as old as 760 million years (Myr) old (9); asymmetrical ellipsoid forms that appear to be perforated with interconnecting canals of pre-Marinoan Cryogenian age when tomographically reconstructed from images of serial surfaces (10); and a macroscopic triangular impression fossil interpreted as the remains of a conical sponge-like form of Ediacaran age, about 575 Myr old (11). In addition, the recovery from Cryogenian-aged rocks of an organic sterol biomarker that is synthesized by marine demosponges suggested the early presence of this poriferan clade (12), although the conclusion that the provenance of this fossil sterol indicates the existence of Cryogenian sponges is now also questioned (7). Despite phylogenomic extrapolations that indicate divergence of sponge lineages from eumetazoan lineages deep in Precambrian time, lack of substantial paleontological evidence directly supporting this prediction has thus remained frustrating. Such evidence would require recovery and analysis of much better preserved fossils, displaying identifiable sponge characters but dating to the earlier part of the period following Snowball Earth (i.e., earlier Ediacaran) or even before that, to the Cryogenian period. Because sponges do not display tissue-grade anatomical characters or organs beyond a general gross, sponge-like structure, this requirement would essentially demand preservation of recognizable cell types in the fossil. Unfortunately, none of the currently proposed deep Ediacaran or Cryogenian fossils display any evidence of particular cell types.
Here, we present a new, remarkably preserved, phosphatized fossil, recovered from rocks of the early Ediacaran Doushantuo Formation in South China, which are dated to about 600 Myr old (stratigraphy and geological context are presented below). The fossilized animal, about 2–3 mm3 in size, was composed of easily recognizable cells. It displays the unmistakable gross anatomy of an adult sponge-grade animal, but beyond this finding, several distinct cell types and cellular structures can be clearly recognized, as displayed in the figures accompanying this report. The Doushantou Formation has been the source of a remarkable series of fossils that similarly reveal cellular level preservation. These phosphatized microfossils include metazoan as well as other forms, such as acritarchs (13), multicellular algae, and Volvox-like forms displaying cellular detail (14–17). In the present context, the most important Doushantuo microfossils are cleavage stage and later embryonic forms that apparently represent a variety of different animal lineages at diverse developmental stages (14, 18–23). Adult forms have been reported only rarely, which has increased the difficulty of interpreting the putative fossilized embryos. However, reported Doushantuo microfossils include small tubular cnidarians (24, 25) and a small bilaterian form, Vernanimalcula (26), of which multiple fossils have been recovered (27). Alternative interpretations have been proffered for virtually all of the Doushantuo microfossils (28–31). The present report, which describes an unmistakable adult animal form, will alter the structure of this debate, although the full force of the implications will not be realized until more than this single specimen becomes available.
Observations and Biological Structure
Geology and Fossil Preservation.
Two contextual aspects are important for interpretation of this new fossil, namely, its geological setting and the obviously biological origin of its predominant features. The fossil derives from the middle Doushantuo Formation in central Guizhou (the exact location and Doushantuo stratigraphy are shown in Fig. S1). The Doushantuo Formation of South China includes deposits representing most of the Ediacaran period, from Snowball Earth at 635 Ma to 551 Ma (32). This period includes many climatic transients, as summarized for the whole period in Fig. S2. The exact age of the deposit from which the fossil was obtained, as established by absolute age dating and integrated stratigraphic correlations across South China, is about 600 Ma (Fig. S3). The fossil came from a shallow sea environment where phosphorite grains containing microfossils have been redistributed through wave and current action (33, 34). As a consequence of a high-energy environment, only small fossiliferous particles have survived, which is possibly one of the critical reasons why adult specimens have been difficult to recover in this unique Precambrian deposit rich in phosphatized microfossils with cellular preservation.
The specimen that is the subject of this paper was released from its carbonate matrix by acid maceration and then studied by SEM and by propagation phase-contrast synchrotron radiation X-ray microtomography (PPC-SR-μCT) at the European Synchrotron Radiation Facility. These high-resolution observations yielded specific evidence of the biological origins of its morphology, which cannot be explained as an artifactual consequence of diagenetic postmortem changes. Fig. S4 provides a convincing example. The specimen is covered with hundreds of thousands of flat membrane-bounded “sacs,” the fossilized remnants of the outer cellular layers of the specimen as we detail below. Measurements of the diameter of these structures display two different classes, one round and one oval. However, contrary to any but the result of biological origin, the sizes of the sacs within each class are very narrowly distributed. A further feature indicating a biogenic origin comes from close-up views of these sacs, in which it can be seen that the membranes consist of tiny apatite crystals apparently randomly oriented without any parallel alignment (Fig. S5). The feature excludes a diagenetic artifact, such as an apatite crystalline lining, which generally exhibits regular alignment of crystals (35).
Overall Anatomy.
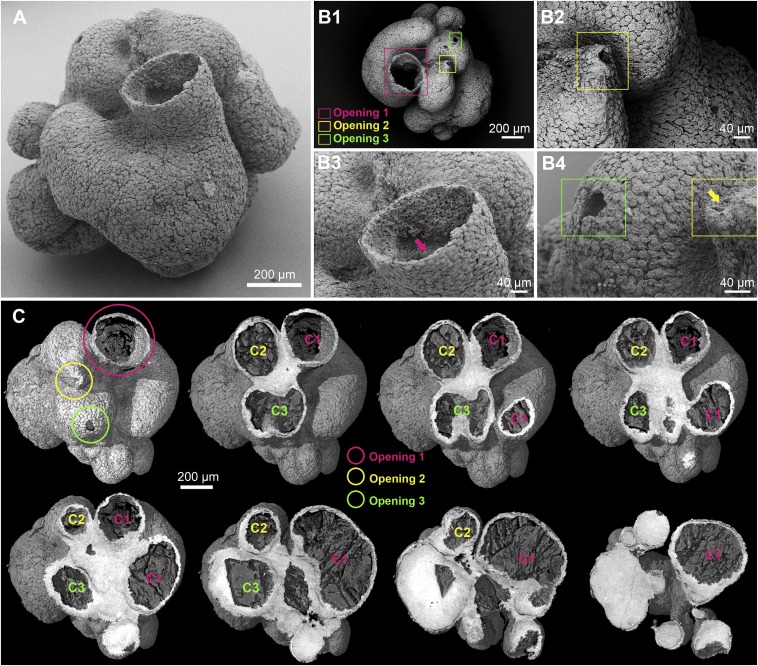
An external SEM view of the fossil is shown in Fig. 1A. As seen from the scale bar (Fig. 1A), the organism was somewhat over 1.2 mm in width and depth and about 1.1 mm high, and high-resolution SEM images show that it consisted of hundreds of thousands of cells. As we see below (Fig. 2), its walls were several cell layers deep. Although it lacks an overt anterior/posterior or dorsal/ventral axis, the fossilized organism has a clear basal/apical polarity, terminating in a major funnel-like opening at the end facing up in Fig. 1A, which we here term “apical.” The overall morphology is best described as follows. The animal consisted of three independent tubular chambers adjacent to one another and emergent from a common base, which the light (false) color of the PPC-SR-μCT image indicates to be of higher mineral density in the fossil (Fig. 1C). The largest chamber gives rise to a probable outflow funnel, which is ∼300 μm in diameter at its exit, but each of the other two chambers also resolves into apical, osculum-like openings. The opening in Fig. 1 B, 2 lies at the peak of a well-defined raised projection, but this projection-like structure cannot be seen in the osculum of Fig. 1 B, 4, if it was originally present, because the structure appears to have been subterminally broken. The successive tomographic sections (Fig. 1C) confirm that the largest of the three chambers is the one opening into the funnel. This chamber is curved and bulbous, whereas the other two emerge more directly up from the common base of the specimen. In the fossil, the interior of the tubes is packed with a low-density postmortem fill, except for the apical domain of the funnel, which remains exposed (e.g., Fig. 1A).
Fig. 1.
Overall anatomy of the specimen. (A) Scanning electron micrograph showing the main tubular chamber with a large opening and additional chambers viewed from the exterior. (B) Outflow orifices. (B, 1) Scanning electron micrograph of a top-down view of A with three openings marked by colored frames; opening 1 is the large opening in the upper center of A. (B, 2–4) Scanning electron micrograph close-up views of putative outflow openings located by frames in B, 1. Note the dense covering of flattened surface cells in B, 2–4. The arrow in B, 3 shows the location of images in Fig. 2 B, 1 and 2; the arrow in B, 4 shows the orifice highlighted in B, 2. (C) Three-dimensional digital PPC-SR-μCT reconstructions with a surface rendering (upper left) showing the location of three openings and the remaining digital sections (left to right, upper to lower) showing increasing depth indicating three separate chambers [chamber 1 (C1), chamber 2 (C2), and chamber 3 (C3)] emerging from a common base that appears white in the image.
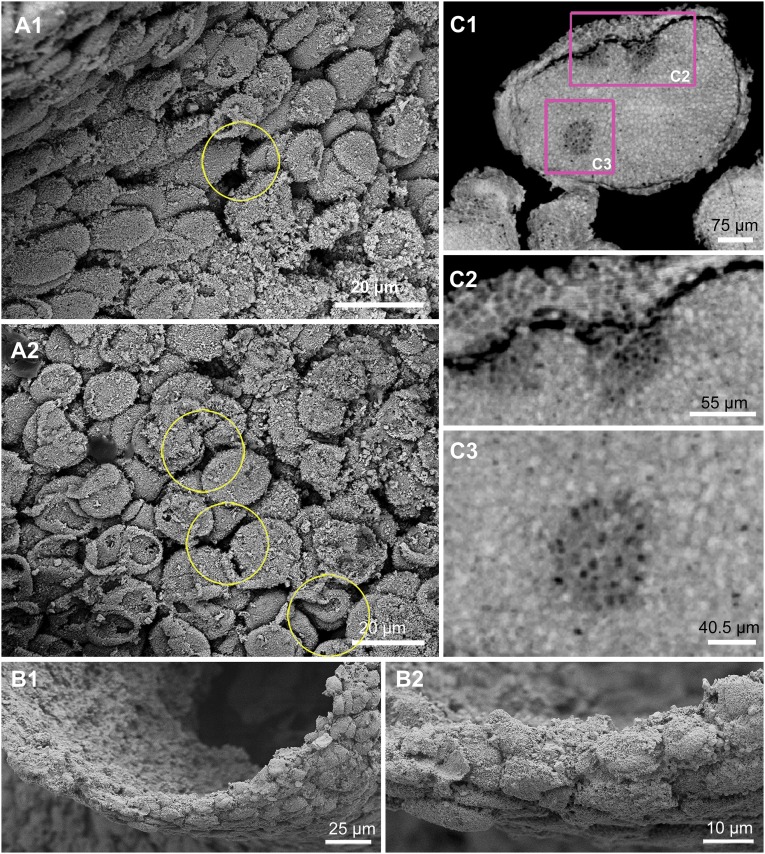
Fig. 2.
Flattened surface cells and base or holdfast. (A, 1 and 2) Scanning electron micrograph close-up views showing flattened surface cells; the yellow circles indicate the gaps and pores between cells. (B) External edge of the funnel. (B, 1 and 2) Scanning electron micrograph close-up views of the rim of the funnel indicated by the arrow in Fig. 1 B, 3, showing that body wall is three to six cells thick. (C) Base or holdfast of specimen. (C, 1) Digital PPC-SR-μCT section showing regionally distinctive areas (indicated by pink frames) within the basal portion of the specimen. (C, 2) Digital PPC-SR-μCT section close-up of the area indicated in C, 1 showing the boundary between the outer layer of the cells and the inner structural material. (C, 3) Digital PPC-SR-μCT section close-up of the area indicated in C, 1 showing the differentially structured domain within the parenchymal material, which appears in the computational cross-section as a disk containing low-density dark spots that may be cavities or cells.
External Covering of the Fossilized Organism.
Fig. 1 B, 2–4 displays the structures interpreted as flattened cells, which densely and evenly cover the surface of the specimen, and these cells can be seen at higher magnification in Fig. 2 A, 1 and 2. These cells have a narrow size range of 8–12 μm across (Fig. S4) but are not uniform in shape or orientation. They do not seem to have been biologically connected as in a typical animal epithelium, but close inspection shows that their contours often fit reciprocally, suggesting that they grew coordinately. The external layer does not have a morphological continuity that would have allowed it a sealing function; furthermore, it is perforated with numerous pore-like openings (yellow circles in Fig. 2 A, 1 and 2).
The features thus far considered assign the fossilized organism to a clade that includes modern sponges. Thus, its main tubes open to the outside apically and the walls are perforated (we can neither exclude nor suggest the presence of porocytes, which, in modern sponges, are the specialized cells constituting the pores within the sponge body walls through which nutrient-bearing waters are taken in). The general form that these features take in a simple modern sponge is shown for comparison in Fig. 3A. The external flattened cells of the fossilized animal bear an uncanny resemblance to the pinacocytes of several groups of modern sponges, as illustrated, for example, in Fig. 3B. As would have been the case in the external layers of the fossilized specimen, the pinacoderm of modern sponges is “leaky.” Furthermore, as in modern sponges that use pinacoderm coverings, no basement layer or interface can be discerned in the fossil. The high-magnification images of the rim of the funnel in Fig. 2 B, 1 and 2 show that the pinacoderm of the fossilized organism is three to six cells thick. As we see below, in the one place where internal structures are, at least to some extent, preserved other than the base, there is clear evidence that the pinacocyte layers do not constitute the whole of the body wall. Thus, an entirely different cellular structure is present in the inner surface of the wall of the funnel.
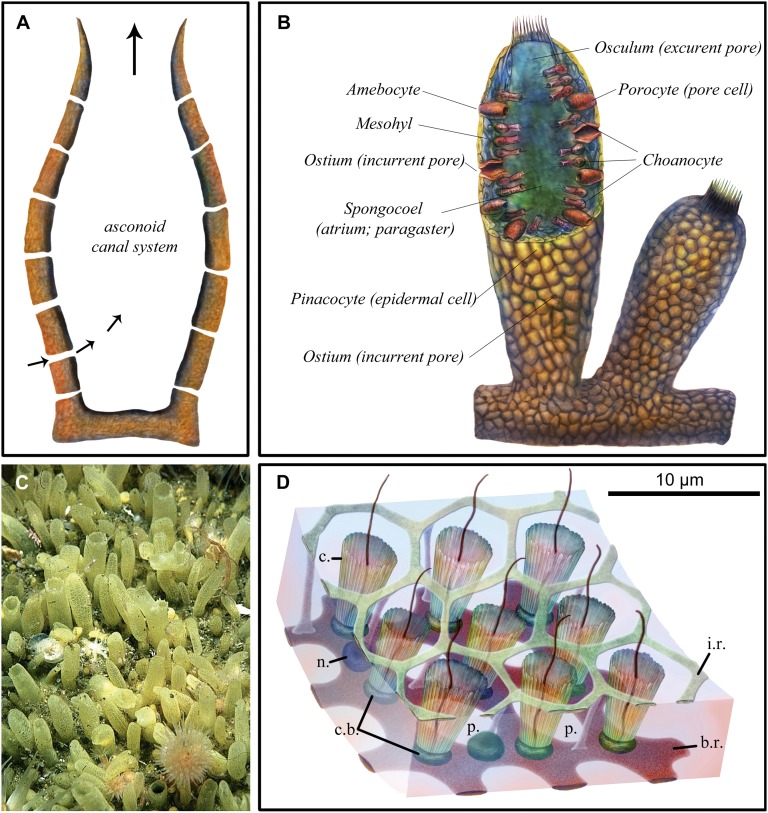
Fig. 3.
Modern sponge anatomy. (A) Schematic cross-section of simple asconoid sponge morphology with a central cavity and base. Pores (ostia) in body wall carry water inflow, and the large orifice (osculum) is used for outflow (arrows indicate direction of water flow). (B) Schematic cross-section showing three layers of the body wall, including external pinacocyte cells, internal choanocyte cells, and mesohyl separating them; incurrent pores in the body wall (ostia); and an excurrent opening (osculum) to the body chamber (spongocoel), as well as additional cell types (porocyte and amebocyte). (C) Surface of the demosponge Polymastia penicillus showing hollow papillae up to 1 cm long. Image courtesy of Bernard Picton (Department of Natural Sciences, National Museums Northern Ireland, Cultra, Holywood, United Kingdom). (D) Schematic of a small portion of the flagellated chamber wall of a hexactinellid sponge, based on light microscopy of several species and EM of Rhabdocalyptus dawsoni. Modified from data from ref. 36. From the surface, the inner reticulum of the trabecular syncytium provides a matrix-like structure surrounding the collars, from within which extend choanocyte flagellae. b.r, basal reticulum of trabecular syncytium; c, collar; c.b., collar body; i.r., inner or secondary reticulum of trabecular syncytium; n., nucleus of trabecular syncytium; p., prosopyle.
Base or Holdfast.
Higher magnification PPC-SR-μCT sections through the high-density base material from which all three tubes appear to arise are reproduced in Fig. 2 C, 1–3. In modern sponges, the structures at the equivalent basal end of the organism are used as a holdfast, locking these sedentary organisms in place on the sea floor. In the fossilized specimen, there were clearly two layers in this region. An outer cellular wall several cells thick entirely surrounds a solid inner mass that might have had a cellular structure, although the resolution of the microtomographic image is not sufficient to ascertain this cellular structure (Fig. 2C). Thus, the base is sharply different from the more apical structures, because the tubular form of the whole apical portion of the organism in this region has given way to a parenchymal material that underlies all three tubes. The gap between the outer layer and the inner mass, which appears as a dark band (Fig. 2 C, 1 and 2), is unlikely to represent a basement membrane, because it is several microns wide, although this breadth could be a taphonomic artifact. Whatever its nature, the inner parenchymal mass is not homogeneous. The sections in Fig. 2C reveal several round patches of lower density material, each containing about 20 very dark spots or cavities that could be the remains of channels or possible cells a few microns in diameter (close-up view in the center of Fig. 2 C, 3) or due to variations in taphonomic conditions. Despite these uncertainties, the minimum conclusions regarding the basal structures are that the fossilized organism generated a distinct basal material, as do modern sponges. This region thus also displays a likely primary differentially organized structure both peripherally vs. internally.
Regional External Specialization: The Papillae.
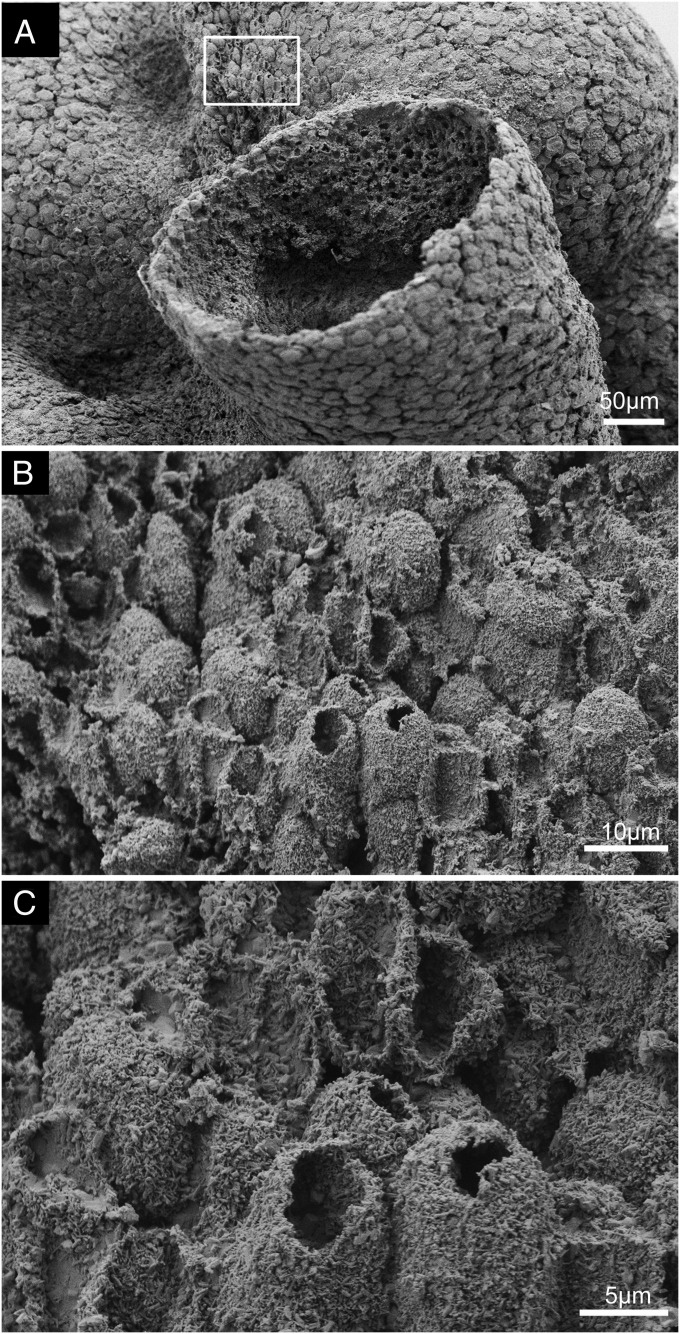
Another structural feature illustrating the differentiated structural complexity of this fossilized animal is illustrated in Fig. 4. A longitudinal patch of unique surface structures is seen in Fig. 4A, localized to a region of the surface that roughly extends from the large funnel to near the secondary osculum labeled “opening 2” in Fig. 1 B, 1. No other region of the exterior surface of the specimen displays this feature. Here, in place of the dense layers of flat pinacocytes seen everywhere else, the external surface is covered with a lawn of very closely packed, protruding, short, hollow tubes, many of which are open but some of which are not. These short, hollow tubes can be seen at two different magnifications in Fig. 4 B and C. Some are >10 μm tall, and they are typically 5–8 μm in diameter. They are arrayed at maximum packing density. Broken examples reveal no surviving internal contents, whether in tubes that from their regularly shaped orifices were open at their apices in life or were apparently originally closed and then irregularly broken either near the tips or more basally. There is no indication of multicellular construction in the tube walls; instead, these structures would appear to consist of elongated, hollow, cylindrical projections of single cells; the tubes are not notably different in size from the pinacocytes. Again, these structures are reminiscent of inhalant tubular extensions of the surface seen in some modern sponges (namely, the papillae), as illustrated in the photographic image of a surface region of the demosponge Polymastia shown in Fig. 3C; thus, it is likely that they served a function related to water intake in the studied specimen.
Fig. 4.
Specialized surface structures. (A) Scanning electron micrograph of the exterior with the framed area showing the location of a patch of short, hollow tubes. (B and C) Scanning electron micrograph close-up views showing details of these surface-specialized tube structures.
Inside the Funnel.
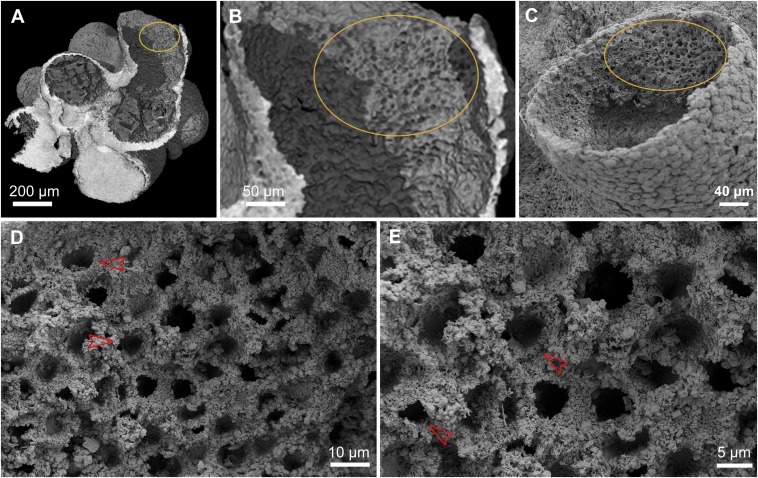
Another unique aspect of this fossil is the only preserved interior surface discovered where organized structures survive, and where both PPC-SR-μCT and the higher resolution SEM examination could be applied. This region is on one side of the interior, toward the mouth of the main funnel. The PPC-SR-μCT images shown in Fig. 5 A and B are of immediate interest, because they indicate that this inner surface was lined with an array of spaced pits, arranged nonrandomly in rows, pairs, and sometimes rosettes. The high-magnification SEM views of the same region shown in Fig. 5 C–E reveal several very striking features of this interior pit array. First, the pits are all of about the same diameter, except where one side is manifestly caved in; that is, the pits remaining circular in form are about the same size as one another (Fig. 5D). In other words, the pits are the remains of homologous structures, about 8–10 μm in diameter. Second, many of the pits show a prominent raised collar immediately surrounding the aperture. At high magnification (Fig. 5 D and E), these collars can be seen to consist of taurus-like structures clearly separated from the surrounding matrix or set into this matrix (red arrowheads). Third, many of the pits have been deformed, appearing squeezed on one side, indicating that the whole pit-bearing layer was soft tissue and not rigid. Fourth, the impression from the PPC-SR-μCT image of the patterned disposition of the pits is confirmed at higher magnification, as illustrated, for example, by the pit rosette highlighted in Fig. 5D. Fifth, the pits and their collars appear to be inserted in a geometrical, reticular mesh that appears to provide their structural support and regularly spaced disposition.
Fig. 5.
Interior surface structures. (A) Digital PPC-SR-μCT section slicing vertically through the main funnel opening shown in Fig. 1A. (B) Magnification of the marked area in A, showing interior surface pit structures. (C) Scanning electron micrograph of the big opening, showing the same interior surface pit structures as in B. (D and E) Close-up views of these interior surface structures of C, showing details of pits and surrounding features. A reticular structure surrounds the regularly spaced pits, which often display clearly preserved circular collars (red arrowheads).
A definitive feature of crown group sponges is their inner linings of choanocytes, each of which produces a classic 9 + 2 flagellum surrounded by a circular microvillar collar. Clear evidence of choanocytes could be regarded as a required characteristic for designation of an organism as poriferan. Given the conventional argument that choanocytes are evolutionary derivatives of their metazoan sister group, the free-living, single-celled choanoflagellates, then even stem group sponges should have had choanocytes. The fossilized organism that is the subject of this discussion fails the test of unequivocal evidence of choanocytes. However, neither can the possibility be excluded that the region of the fossil illustrated in Fig. 5 is the remains of a choanocyte layer. Some modern sponges mount their choanocytes in a syncytial reticular structure, as illustrated in Fig. 3D. The relevant parts of this distinctive structure are the reticulum, the collars, and the recessed flagellum plus bulb. The possibility cannot be excluded that the pits are the loci of the delicate flagellum and the bulbs, which were uniformly lost from their recesses, whereas the remaining structures, the collars and the reticulum, were fossilized and preserved. Thus, we regard the issue of whether choanocytes were present in this specimen to be moot, and interpretation of the fossil must therefore turn on the other characteristics it manifests.
Discussion
In its overall construction, the remarkably well-preserved fossil organism, of which Fig. 1A provides the best overview, displays definitive poriferan characteristics. The fossilized animal was asymmetrical, lacking body axes but presenting a differentiated basal-to-apical structure. Its basic organization is tubiform, consisting of three relatively thick reflexed tubes all emerging from a common basal apparatus in this case, and all opening apically to the outside. The largest tube possessed a prominent funnel-like outflow structure, and each of the others terminates in specialized oscula. Additionally, as in modern sponges, the fossilized organism had numerous pore-like openings in the main body walls of the tubes, through which water could flow in. The animal was apparently sessile with a specialized, dense basal tissue, perhaps functioning like a sponge holdfast, and all of the walls of body tubes were covered with a sponge-like, multicellular external wall. The cellular structure of this wall is strikingly similar to the cellular structure of sponges of some modern classes in displaying distinct, plate-like pinacocytes. These pinacocytes, as in modern sponges, lacked continuous epithelial connections; cross-sectional tomographic observations do not reveal any signs of basement membranes, which are also lacking in modern sponges. In the funnel area and also the holdfast, the inner and outer layers of the body were distinct, again similar to the basic diploblastic organization of sponges. In addition, this animal possessed a sharply delimited external patch of differentiated, hollow tubular structures, perhaps highly modified pinacocytes, resembling the papillae of demosponges. There is no sign of mesohyl, but the gelatinous nature of this material in modern sponges is unlikely to have provided a sufficiently structured substrate for mineralization. More problematic is the absence of clear evidence of internal choanocytes, although the arrays of collared pits seen within the funnel render absence of choanocytes a moot point, as noted above. Even aside from this last issue, the characteristic complex displayed by this fossil does not resemble in detail any one of the modern crown group sponge classes. Thus, for example, hexactinellid sponges, but not demosponges, mount their choanocytes in syncytial reticular structures such as portrayed in Fig. 3D (36), whereas demosponges generate papillae, and the overall essentially simple, smooth-walled tubular structure of this organism also resembles simpler demosponges, such as asconoids. Phylogenetically, this animal is perhaps to be placed on the stem lineage of siliceous poriferans (hexactinellids plus demosponges). A formal description of this fossil, which is named Eocyathispongia qiania, is presented in SI Text (Systematic Paleontology).
Due to its state of preservation, the multiplicity of its recognizable features, and the fortunate circumstance that these features extend to cell-type level characteristics, the informational content of this fossil is unique with respect to any putative sponge of the Ediacaran vintage that has been previously presented. Its age (600 Ma) torques the skeptical evidential landscape of Precambrian sponge evolution. Thus, the discovery of this adult form in the early Ediacaran Doushantuo strata could be said to fulfill the prediction, on the basis of uniquely poriferan cleavage stage embryo forms described earlier, that adult sponges must have existed in that environment (21). Similarly, this specimen provides an independent argument in evaluating the discovery of putative demosponge biomarkers of earlier as well as coeval age (12), and perhaps the same can be said of the paleontological claims of the Cryogenian sponge-like body fossils (9, 10). More to the point with which we began, the age of the elegantly structured poriferan described here provides independent support, for the first time to our knowledge, for the phylogenomic conclusion that the last common poriferan/eumetazoan ancestor must have existed much earlier, deep in the Cryogenian (1). However, important as they are, these conclusions rest on the single specimen described here, and they will require confirmation from additional Doushantuo fossils with the same characteristics. For example, discovery of additional specimens of Eocyathispongia would render inescapable the remote antiquity of the common ancestor of sponges and eumetazons.
Recently, transcriptomes from eight species of sponge representing all four extant poriferan classes were analyzed by Riesgo et al. (4). Taken together with the genomes now available for two sponges [Amphimedon, a demosponge (5, 37) and Oscarella, a homoscleromorph sponge (37)], these data show overwhelmingly that the genetic heritage of all four classes is very similar in regard to several sets of metazoan genes of developmental importance. This similarity includes genes encoding the intracellular apparatus needed for Wnt, Hedgehog, and Tgfβ signal transduction; for epithelial junction and focal adhesion formation; and for innate immune response. This result is obviously consistent with the conclusion of poriferan monophyly (2, 3). Because the fossil sponge cannot be assigned uniquely to any of the extant poriferan classes but rather displays characters distributed among the modern sponge classes, it is to be regarded as a stem group descendant of the common ancestor of all sponges. It is of great interest that free-living choanoflagellates (of two different species) share very few of the genes that define the pan-metazoan gene set revealed in the transcriptome and genome sequence studies, whereas, on the other hand, in respect to this portion of their toolkit, the sponges lack only occasional members of the complete eumetazoan repertoire. The fossil stem group sponge described here must lie relatively far up in the phylogenetic tree that encompasses the four sponge classes and had to have shared this same pan-metazoan genomic repertoire.
It is interesting to consider the implications of the 600-Myr-old fossil sponge from the vantage point of our own eumetazoan genomic heritage. Sponge genomes totally lack essential eumetazoan developmental regulatory apparatuses, such as hox genes, and yet share with us an enormously detailed pan-metazoan gene set. Therefore, this plesiomorphic genomic toolkit was being used in the last common ancestor of sponges and eumetazoans, whereas after the divergence of these lineages, additional derived eumetazoan-specific genomic regulatory apparatuses augmented the developmental capacities that would characterize the bilaterians and their sister groups. Thus, just as implied by the current temporal extrapolations of phylogenomics, the “calibration point” afforded by this fossil suggests that the shared metazoan genetic toolkit must have originated in the Cryogenian. Furthermore, if a relatively advanced sponge existed 600 Ma, then so did coeval animals of the eumetazoan lineage that also descended from the same last common poriferan/eumetazoan ancestor. Thus, it is a clear prediction that fossilized organisms of eumetazoan affinity from similarly deep in time await paleontological discovery, and some such may already have been seen in the Doushantuo animal microfossils cited above.
Materials and Methods
The fossil specimen for this study was collected from the gray oolitic, phosphatized dolostone of the Upper Phosphate Member of the Doushantuo Formation at Weng’an phosphate mining area in Guizhou Province, southwest China (Fig. S1). We used acetic acid digestion to liberate the microfossils from the rock samples. All of the microfossils were examined by SEM, and well-preserved specimens were scanned at the European Synchrotron Radiation Facility (Grenoble, France) with high resolution. We used an undulator source, which can deliver a single harmonic X-ray with energy of 17.68 keV, at beamline ID19. The relative monochromaticity of the beam is so good that use of a monochromator was unnecessary. A CCD-based high-resolution detector with isotropic voxel sizes of 0.75 μm was used, and 1,800 projections over 180° were obtained for each scan. To get a phase-contrast effect, 10 mm was adopted as the propagation distance. In addition to the simple edge detection mode, we applied a single distance phase retrieval process for the fossils. This process permits retrieval of high-quality differential contrast very similar to the high-quality differential contrast achieved by holotomography but requiring far more simple acquisition and reconstruction protocols. Three-dimensional volume data processing was performed using VGstudio Max 2.1 software (Volume Graphics). The specimen described in this paper is housed at the Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences.
Supplementary Material
Acknowledgments
We thank beamlines ID19, BM5, and ID22 of the European Synchrotron Radiation Facility for providing beam time. We thank Gang Li from the Institute of High Energy Physics, Chinese Academy of Sciences, for help in the synchrotron scanning experiments. This work was supported by funding from the Ministry of Science and Technology of China (Grant 2013CB835000), the National Natural Science Foundation of China (Grant 41302003), and the Chinese Academy of Sciences (Grant KZZD-EW-02-2). E.H.D. and D.J.B. were supported by US National Science Foundation Grant IOS1240626.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414577112/-/DCSupplemental.
References
- 1.Erwin D, Valentine J. The Cambrian Explosion. Roberts and Company Publishers; Greenwood Village, CO: 2012. [Google Scholar]
- 2.Ryan JF, et al. NISC Comparative Sequencing Program The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science. 2013;342(6164):1242592. doi: 10.1126/science.1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nosenko T, et al. Deep metazoan phylogeny: When different genes tell different stories. Mol Phylogenet Evol. 2013;67(1):223–233. doi: 10.1016/j.ympev.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Riesgo A, Farrar N, Windsor PJ, Giribet G, Leys SP. The analysis of eight transcriptomes from all poriferan classes reveals surprising genetic complexity in sponges. Mol Biol Evol. 2014;31(5):1102–1120. doi: 10.1093/molbev/msu057. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava M, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466(7307):720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erwin DH, et al. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science. 2011;334(6059):1091–1097. doi: 10.1126/science.1206375. [DOI] [PubMed] [Google Scholar]
- 7.Antcliffe J. Questioning the evidence of organic compounds called sponge biomarkers. Paleontology. 2013;56(5):917–925. [Google Scholar]
- 8.Antcliffe JB, Callow RH, Brasier MD. Giving the early fossil record of sponges a squeeze. Biol Rev Camb Philos Soc. 2014;89(4):972–1004. doi: 10.1111/brv.12090. [DOI] [PubMed] [Google Scholar]
- 9.Brain C, Prave A, Hoffman K-H, Fallick A, Botha A. The first animals: Ca. 760 my sponge sponge-like fossils from Namibia. S Afr J Sci. 2012;108(1/2):658–666. [Google Scholar]
- 10.Maloof AC, et al. Possible animal body fossils in pre-Marinoan limestones from S. Australia. Nat Geosci. 2010;3(9):653–659. [Google Scholar]
- 11.Sperling EA, Peterson KJ, Laflamme M. Rangeomorphs, Thectardis (Porifera?) and dissolved organic carbon in the Ediacaran oceans. Geobiology. 2011;9(1):24–33. doi: 10.1111/j.1472-4669.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- 12.Love GD, et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature. 2009;457(7230):718–721. doi: 10.1038/nature07673. [DOI] [PubMed] [Google Scholar]
- 13.Xiao SH, Zhou CM, Liu PJ, Wang D, Yuan XL. Phosphatized acanthomorphic acritarchs and related microfossils from the Ediacaran Doushantuo Formation at Weng’an (South China) and their implications for biostratigraphic correlation. J Paleontol. 2014;88(1):1–67. [Google Scholar]
- 14.Xiao S, Zhang Y, Knoll AH. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature. 1998;391(6667):553–558. [Google Scholar]
- 15.Xiao S, Knoll AH, Yuan X, Pueschel CM. Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae. Am J Bot. 2004;91(2):214–227. doi: 10.3732/ajb.91.2.214. [DOI] [PubMed] [Google Scholar]
- 16.Chen J. The Dawn of Animal World. Jiangsu Science and Technology Publishing House; Nanjing, China: 2004. [Google Scholar]
- 17.Chen L, Xiao S, Pang K, Zhou C, Yuan X. Cell differentiation and germ-soma separation in Ediacaran animal embryo-like fossils. Nature. 2014;516(7530):238–241. doi: 10.1038/nature13766. [DOI] [PubMed] [Google Scholar]
- 18.Hagadorn JW, et al. Cellular and subcellular structure of neoproterozoic animal embryos. Science. 2006;314(5797):291–294. doi: 10.1126/science.1133129. [DOI] [PubMed] [Google Scholar]
- 19.Chen JY, et al. Phosphatized polar lobe-forming embryos from the Precambrian of southwest China. Science. 2006;312(5780):1644–1646. doi: 10.1126/science.1125964. [DOI] [PubMed] [Google Scholar]
- 20.Chen JY, et al. Complex embryos displaying bilaterian characters from Precambrian Doushantuo phosphate deposits, Weng’an, Guizhou, China. Proc Natl Acad Sci USA. 2009;106(45):19056–19060. doi: 10.1073/pnas.0904805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, et al. Phase contrast synchrotron X-ray microtomography of Ediacaran (Doushantuo) metazoan microfossils: Phylogenetic diversity and evolutionary implications. Precambrian Res. 2009;173(1-4):191–200. [Google Scholar]
- 22.Yin Z, et al. Early embryogenesis of potential bilaterian animals with polar lobe formation from the Ediacaran Weng’an Biota, South China. Precambrian Res. 2013;225:44–57. [Google Scholar]
- 23.Yin Z, Liu PJ, Li G, Tafforeau P, Zhu M. Biological and taphonomic implications of Ediacaran fossil embryos undergoing cytokinesis. Gondwana Research. 2014;25(3):1019–1026. [Google Scholar]
- 24.Xiao S, Yuan X, Knoll AH. Eumetazoan fossils in terminal proterozoic phosphorites? Proc Natl Acad Sci USA. 2000;97(25):13684–13689. doi: 10.1073/pnas.250491697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JY, et al. Precambrian animal life: Probable developmental and adult cnidarian forms from Southwest China. Dev Biol. 2002;248(1):182–196. doi: 10.1006/dbio.2002.0714. [DOI] [PubMed] [Google Scholar]
- 26.Chen JY, et al. Small bilaterian fossils from 40 to 55 million years before the cambrian. Science. 2004;305(5681):218–222. doi: 10.1126/science.1099213. [DOI] [PubMed] [Google Scholar]
- 27.Petryshyn V, Bottjer D, Chen JY, Gao F. Petrographic analysis of new specimens of the putative microfossil Vernanimalcula guizhouena (Doushantuo Formation, South China) Precambrian Res. 2013;255:58–66. [Google Scholar]
- 28.Bailey JV, Joye SB, Kalanetra KM, Flood BE, Corsetti FA. Evidence of giant sulphur bacteria in Neoproterozoic phosphorites. Nature. 2007;445(7124):198–201. doi: 10.1038/nature05457. [DOI] [PubMed] [Google Scholar]
- 29.Huldtgren T, et al. Fossilized nuclei and germination structures identify Ediacaran “animal embryos” as encysting protists. Science. 2011;334(6063):1696–1699. doi: 10.1126/science.1209537. [DOI] [PubMed] [Google Scholar]
- 30.Bengtson S, Cunningham JA, Yin C, Donoghue PCJ. A merciful death for the “earliest bilaterian,” Vernanimalcula. Evol Dev. 2012;14(5):421–427. doi: 10.1111/j.1525-142X.2012.00562.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu P, Yin C, Chen S, Tang F, Gao LZ. Affinity, distribution and stratigraphic significance of tubular microfossils from the Ediacaran Doushantuo Formation in South China. Acta Palaeontologica Sin. 2010;49(3):308–324. [Google Scholar]
- 32.Condon D, et al. U-Pb ages from the neoproterozoic Doushantuo Formation, China. Science. 2005;308(5718):95–98. doi: 10.1126/science.1107765. [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Zhang J, Yang A. Integrated Ediacaran (Sinian) chronostratigraphy of South China. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;254(1-2):7–61. [Google Scholar]
- 34.Zhu M, et al. Carbon isotope chemostratigraphy and sedimentary facies evolution of the Ediacaran Doushantuo Formation in western Hubei, South China. Precambrian Res. 2013;225:7–28. [Google Scholar]
- 35.Xiao S, Schiffbauer JD. Microfossil phosphatization and its astrobiological implications. In: Seckbach J, Walsh M, editors. From Fossils to Astrobiology. Springer, Berlin; 2008. pp. 89–117. [Google Scholar]
- 36.Reiswig HM, Mackie GO. Studies on hexactinellid sponges. III. The taxonomic status of Hexactinellida within the Porifera. Philos Trans R Soc Lond B Biol Sci. 1983;301(1107):419–428. [Google Scholar]
- 37.Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/β-catenin complex. Proc Natl Acad Sci USA. 2012;109(32):13046–13051. doi: 10.1073/pnas.1120685109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.