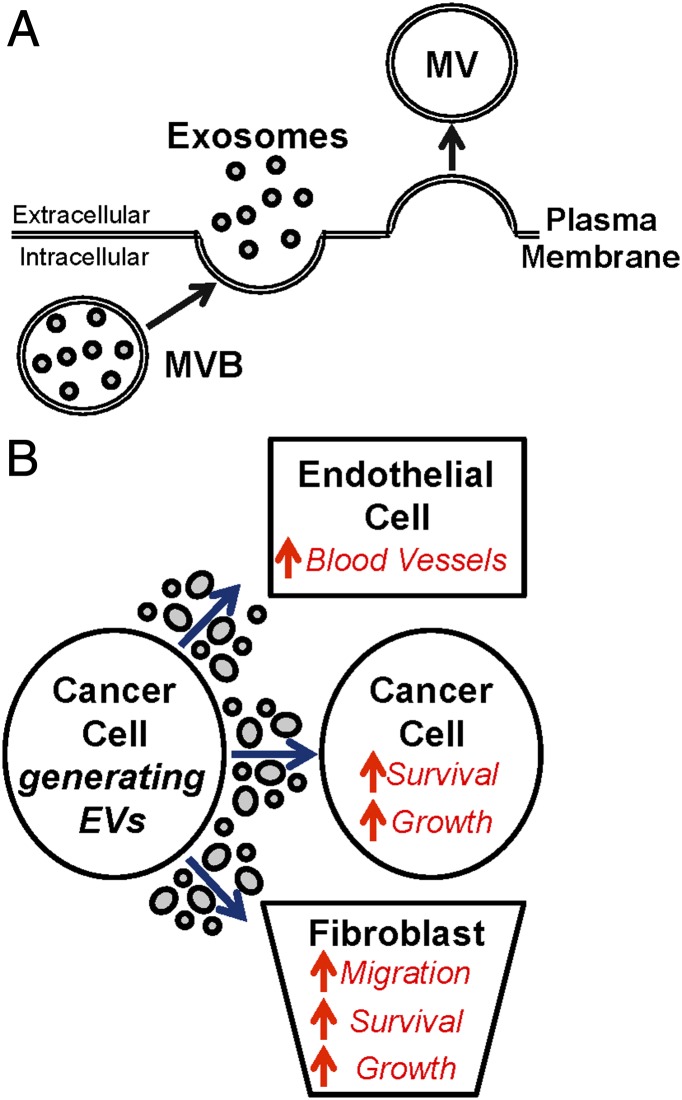
The ability of a cell to receive signals from other cells and then translate them into changes in cell behavior plays crucial roles in development and tissue homeostasis. However, the deregulation of these carefully orchestrated events underlie the onset or progression of several diseases in the adult organism (1, 2), highlighting the need to better understand the mechanisms through which cells communicate with each other. When considering various examples of cell–cell communication, the well-established growth factor receptor signaling paradigm comes to mind (2). In this case, one cell secretes a soluble growth factor into the extracellular environment. The growth factor then binds to its corresponding receptor expressed on the surface of a second cell, resulting in its activation and the initiation of intracellular signaling events that control a myriad of cellular processes ranging from promoting cell growth and differentiation to cell death and migration. In PNAS, Kanada et al. (3) investigate what is emerging as a new and exciting mechanism used by cancer cells to communicate with their environment. It entails the ability of cells to generate and release two types of cargo-containing vesicular structures, collectively referred to as extracellular vesicles (EVs) that are generally believed to differ in their biogenesis and certain physical properties (4–6). One of these types of EVs is exosomes, which are generated as a result of trafficking multivesicular bodies containing endosomes from the cytosol to the cell surface. The multivesicular bodies then fuse with the plasma membrane, releasing the endosomes (now widely referred to as exosomes) into the extracellular space. Most studies that have analyzed exosomes indicate that they range in size from 30 to 80 nm in diameter. Microvesicles (MVs), which represent the other major class of EVs, are considerably larger than exosomes (200–1,500 nm in size) and are generated as an outcome of plasma membrane budding (Fig. 1A). Both exosomes and MVs are able to engage and transfer their cargo to other (recipient) cells, whereupon they significantly influence cellular processes (7–10). In their study, Kanada et al. isolated the different populations of EVs produced by HEK293FT cells and meticulously compared their physical properties, ability to be loaded with different types of cargo, and function. Their findings not only challenged some of the central dogmas in the field but also raised the interesting possibility that EVs might provide an effective delivery mechanism for gene therapy.
Fig. 1.
(A) Exosomes and MVs are two forms of EVs generated through distinct mechanisms. Exosomes form as a result of multivesicular bodies (MVBs) containing endosomes being trafficked from the cytosol to the cell surface. The MVBs then fuse with the plasma membrane, releasing exosomes into the extracellular space. In contrast, MV biogenesis occurs through the outward budding and fission of the plasma membrane. (B) Cancer cells use EVs to communicate with other cells in its environment to drive tumor progression. The transfer of EVs between cancer cells promotes their growth and survival. However, EVs also can stimulate endothelial cells to form blood vessels, as well as promote the growth, survival, and migration of tumor-associated fibroblasts.
Although the study of EVs is in many ways still in its infancy, it is attracting a good deal of attention for several reasons. The first, and perhaps most important of these, is because of the contents of EVs. Exosomes and MVs have each been shown to contain a variety of cargo not typically thought to be released by viable cells, including cell surface receptors, cytosolic and nuclear proteins, metabolic enzymes, RNA transcripts, microRNAs, and even DNA (4–6). Although the contents of EVs often contain distinguishing signatures that allow them to be traced back to their cell of origin, it is worth emphasizing that the contents of EVs are specific and not simply a random sampling of the proteins and nucleic acids that comprise the cell (7, 9). Indeed, how certain proteins and different types of nucleic acids are routinely incorporated into EVs by cells, whereas others are apparently excluded, is just one of the many intriguing questions surrounding this new area of cell–cell communication. In all likelihood, specific intracellular mechanisms are in place to ensure that the proper protein and nucleic acid cargo is incorporated into the different classes of EVs (6).
Another reason EVs are attracting a great deal of interest in both the basic research and pharmaceutical/biotech communities is because they are being implicated in a number of different physiological and pathological contexts, ranging from pregnancy to heart disease (11, 12). However, by far, the context in which EVs have been most extensively studied is cancer biology (4–10). It is now widely held that most types of cancer cells generate EVs to some degree. It also appears that highly aggressive and advanced stage forms of cancer cells generate more EVs than lower-grade cancer cells, suggesting that their biogenesis may be up-regulated during disease progression. The EVs from cancer cells are taken up by other cancers cells, promoting their growth and survival (7), as well as their invasive and metastatic activity (10). Different lines of evidence also suggest that EVs can manipulate the normal tissue that surrounds developing tumors, i.e., the tumor microenvironment, as a means of enhancing cancer progression. For example, the addition of MVs released by the highly aggressive human MDAMB231 breast cancer cell line to cultures of nontransformed fibroblasts and mammary epithelial cells, two major cell types found within the microenvironment of breast tumors, caused them to acquire some of the characteristics of cancer cells, including the ability to grow under nutrient-limiting conditions and survive apoptotic challenges (9). Thus, a tumor burden may not be solely due to the expansion of the cancer cells but may also include those normal cells that have been engaged by EVs and thus undergone changes that enable them to exhibit phenotypes reminiscent of cancer cells. Moreover, other studies have further implicated EVs in shaping the tumor microenvironment through the recruitment of stroma and immune cells to the tumor, as well as inducing tumor vascularization (8, 13, 14). Both of these outcomes are important steps for supplying the tumor with the necessary nutrients and oxygen to sustain its growth.
These findings have allowed us to start piecing together a picture of how cancer cells use this unconventional form of cell communication to drive tumor progression (Fig. 1B). Not surprisingly, they have also motivated cancer researchers and pharmaceutical companies to explore the potential clinical applications of EVs. Numerous proteomics and microarray analyses performed on EVs collected from cancer cells of various types and/or grades have led to the identification of literally hundreds of differentially expressed proteins, RNA transcripts, and microRNAs (4–7). Now, this information is being expanded on to determine whether the therapeutic targeting of specific EV cargo could be used as a strategy to block the cancer-promoting effects of EVs (5). Moreover, cancer cell-derived EVs have been detected in blood samples taken from cancer patients (7). Thus, the idea that they could potentially be used as a source of diagnostic information is being aggressively pursued (5).
However, despite their exciting potential, EVs will likely not be used in the clinics until a more rigorous definition of what exactly comprises an exosome vs. a MV is established. Currently, the lack of such detail is largely due to the EV field being relatively young and fluid, as there is an overwhelming amount of information that needs to be carefully scrutinized. Some groups batch collect exosomes and MVs together and then refer to them as only exosomes or MVs. However, even when efforts are made to separate exosomes and MVs from biological fluids (i.e., conditioned medium or blood), the current state-of-the-art approaches are less than ideal. Most rely on differential ultracentrifugation to isolate the more dense MVs from the less dense exosomes (15). Small variations in how the isolation procedures are carried out often result in preparations containing a mixture of exosomes and MVs, making accurate determinations of the physical properties, cargo, and functions of each type of EV difficult to achieve. Nonetheless, much of what we currently know about exosomes and MVs are based on these findings. The work carried out by Kanada et al. underscores just how much more we still need to learn about these vesicles. This is perhaps best exemplified by their determination that the sizes of the exosomes and MVs generated by HEK293FT cells, using several different sizing methods, were similar, ∼150–200 nm in diameter. Although this size range is larger than that typically reported for exosomes (i.e., ∼30–80 nm in diameter), but smaller than that of most MVs (which are ∼200–1,500 nm in diameter), the finding that the sizes of the different forms of EVs are comparable is particularly noteworthy, given that size has been one of the most common and widely accepted characteristics used to distinguish exosomes from MVs. In fact, this concept has been so entrenched in the EV field that approaches to separate exosomes and MVs based on their size is an extremely active area of research (15, 16). Thus, it will be important to see if other cell lines generate exosomes and MVs of overlapping sizes or whether HEK293FT cells are unique in this regard.
The authors went on to compare the abilities of different biomolecules to be loaded in exosomes and MVs and then determined their fate after being transferred to other cells. They found that ectopically expressed reporter proteins, mRNA, and siRNA were more efficiently incorporated into MVs than exosomes, whereas reporter plasmid DNA (pDNA) was only trafficked into MVs. These findings lend further credence to the hypothesis that there is machinery in place within a cell that regulates the loading of distinct types of cargo into EVs (6). The fate of the various reporter molecules expressed in exosomes and MVs were then monitored. Both exosomes and MVs could be taken up by cells. However, all of the cargo in the exosomes, as well as the reporter mRNA and siRNA in the MVs, were rapidly degraded by the recipient cells and thereby failed to exhibit functional effects. In contrast, the reporter proteins and pDNA could be detected in the recipient cells for extended periods of time, suggesting that proteins and pDNA are the functional molecular cargo delivered to cells by MVs.
Therefore, in light of the findings reported by Kanada et al., we are now left with some potentially interesting implications to consider, and a number of questions that will need to be addressed in future studies. One implication in particular is that the ability of pDNA to be functionally transferred from MVs into their recipient cells now means that the potential for such a transfer must be considered when evaluating the results from experiments involving EVs shed by transfected cells. We also now need to consider what these findings might imply for the possibility of transferring endogenous DNA via MVs into cells. Moreover, it will also be important to better understand how the findings reported by Kanada et al. can be reconciled with the many other studies showing that both exosomes and MVs are capable of transferring all types of cargo, including proteins, RNA transcripts, and microRNAs, into recipient cells and in a manner that retains their functional activities (4–14). Admittedly, we have a long way to go in achieving a comprehensive picture of the functional roles of EVs. What is clear is that this work now adds to the growing evidence and emerging roles of EVs as important mechanisms for cell–cell communication that are likely to have broad sweeping biological and biomedical consequences.
Footnotes
The authors declare no conflict of interest.
See companion article on page E1433.
References
- 1.Schauer IG, Sood AK, Mok S, Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia. 2011;13(5):393–405. doi: 10.1593/neo.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218(3):460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 3.Kanada M, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci USA. 2015;112:E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon YJ, Kim OY, Gho YS. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014;47(10):531–539. doi: 10.5483/BMBRep.2014.47.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 6.Antonyak MA, Cerione RA. Microvesicles as mediators of intercellular communication in cancer. Methods Mol Biol. 2014;1165:147–173. doi: 10.1007/978-1-4939-0856-1_11. [DOI] [PubMed] [Google Scholar]
- 7.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonyak MA, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci USA. 2011;108(12):4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2014;111(31):E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Record M. Intercellular communication by exosomes in placenta: A possible role in cell fusion? Placenta. 2014;35(5):297–302. doi: 10.1016/j.placenta.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Danielson KM, Das S. Extracellular vesicles in heart disease: Excitement for the future? Exosomes Microvesicles. 2014;2(1) doi: 10.5772/58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webber JP, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 14.Voloshin T, Fremder E, Shaked Y. Small but mighty: Microparticles as mediators of tumor progression. Cancer Microenviron. 2014;7(1-2):11–21. doi: 10.1007/s12307-014-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia S, et al. Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Rev Mol Diagn. 2014;14(3):307–321. doi: 10.1586/14737159.2014.893828. [DOI] [PubMed] [Google Scholar]
- 16.Santana SM, Antonyak MA, Cerione RA, Kirby BJ. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed Microdevices. 2014;16(6):869–877. doi: 10.1007/s10544-014-9891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]