Significance
Herding was the earliest form of African food production and transformed local populations of people and animals. Herders migrated from eastern to southern Africa around 2,000 years ago, but only in small numbers. Zoonotic disease vectors, specifically the tsetse fly, which carries sleeping sickness, are thought to have impeded these movements. Archaeologists have argued that the presence of tsetse flies around Lake Victoria, Kenya, created a barrier that prevented migration and forced subsistence diversification. This study, using stable isotope analysis of animal teeth, reveals the existence of ancient grassy environments east of Lake Victoria, rather than tsetse-rich bushy environments. This overturns previous assumptions about environmental constraints on livestock management in a key area for southward movement of early herders.
Keywords: archaeology, carbon isotopes, food production, East Africa, livestock disease
Abstract
Specialized pastoralism developed ∼3 kya among Pastoral Neolithic Elmenteitan herders in eastern Africa. During this time, a mosaic of hunters and herders using diverse economic strategies flourished in southern Kenya. It has been argued that the risk for trypanosomiasis (sleeping sickness), carried by tsetse flies in bushy environments, had a significant influence on pastoral diversification and migration out of eastern Africa toward southern Africa ∼2 kya. Elmenteitan levels at Gogo Falls (ca. 1.9–1.6 kya) preserve a unique faunal record, including wild mammalian herbivores, domestic cattle and caprines, fish, and birds. It has been suggested that a bushy/woodland habitat that harbored tsetse fly constrained production of domestic herds and resulted in subsistence diversification. Stable isotope analysis of herbivore tooth enamel (n = 86) from this site reveals, instead, extensive C4 grazing by both domesticates and the majority of wild herbivores. Integrated with other ecological proxies (pollen and leaf wax biomarkers), these data imply an abundance of C4 grasses in the Lake Victoria basin at this time, and thus little risk for tsetse-related barriers to specialized pastoralism. These data provide empirical evidence for the existence of a grassy corridor through which small groups of herders could have passed to reach southern Africa.
Herding was the earliest form of food production in Africa, originating in the arid eastern Sahara ∼8 kya. Adoption of food production and increasing mobility in northeastern Africa allowed prehistoric people to manage resource availability amid a drastically changing climate (1) and transformed local populations of people and animals. Saharan herders and hunters spread southward to the Sahel, reaching eastern Africa by 4.5 kya, and eventually reaching southern Africa with sheep and cattle around 2 kya (1–8). The southern African data have been much debated, but recent genetic studies support at least limited movement of early herders from eastern to southern Africa (5, 7). Iron Age Bantu agriculturalist migrations and forager exchange processes also contributed to livestock spread. In contrast to Iron Age farmers, however, the sparse archaeological evidence suggests slow and small-scale southern pastoral migrations (9). Researchers have attributed the limited penetration of southern Africa by stone-using pastoralists to the prevalence of woodland habitats, the distribution of tsetse fly, and the influence of trypanosomiasis on livestock production from Lake Victoria and the Serengeti southward (10, 11). However, site-based paleoenvironmental data have proven difficult to obtain, and although often assumed, the proposition that ancient closed or bushy environments represented a barrier to herding has been seldom examined. Here we present stable isotope analysis of herbivore tooth enamel (n = 86) from Elmenteitan Pastoral Neolithic levels at Gogo Falls (ca. 1.9–1.6 kya) near Lake Victoria, one of the northernmost “woodland tsetse belts” modeled for Africa ca. 2,000 y ago (12, 13). Rather than providing evidence for the Lake Victoria basin as a wooded tsetse fly harboring habitat, these new fine-resolution paleoenvironmental data document extensive C4 grazing by both domesticates and the majority of wild herbivores. A synthesis of lacustrine and terrestrial signals further supports the existence of grassy areas in the Lake Victoria basin at 2,000 B.P., indicating a change in ecology to bushy environments more recently than previously thought, and an ecosystem that would not have supported the tsetse fly around Lake Victoria.
Early herders in northern Kenya relied on sheep, goats, fish, and diverse wild vertebrates (1, 14). Research in western Kenya, and specifically the Lake Victoria basin (Fig. 1), has revealed southwestward movement of Elmenteitan herders into an area populated by complex ceramic-using, fishing hunter-gatherers and a more varied process of adoption of food production. Domestic sheep and goats appear in low numbers starting around 3.7 kya at Wadh Lang’o, and perhaps also at Gogo Falls and Usenge 3 (15), but not at other sites such as Siror (16), suggesting patchy adoption of herding. Specialized dependence on livestock in Africa is first documented in Elmenteitan sites, which date at the earliest to 3.1 kya at Njoro River Cave, east of Lake Victoria (17, 18). Elmenteitan sites occur to the north in Laikipia, on the Mau Escarpment, on the southern end of the Loita Hills and the northern Mara Plains, and at Gogo Falls (the westernmost extent) (18). Despite opportunities for hunting, fauna from Elmenteitan sites on the Mara plains are >90% domestic (19, 20). The exception to this is the site of Gogo Falls, which has a diverse and abundant faunal assemblage, including fish and wild herbivores (21). The unique faunal assemblages from Elmenteitan levels at Gogo Falls and from Prolonged Drift (22), which has a mixed Savanna Pastoral Neolithic stone tool assemblage, have sparked considerable debate over the significance of subsistence variability for understanding environmental risks and the dynamics of frontier pastoral-hunter-gather subsistence during the spread of food production. Foragers in the process of adopting herding, loss of stock by pastoralists, and environmental and disease constraints have all been considered possible factors leading to heavy reliance on wild resources (21–24). South of the Mara plains and Lake Victoria, ancient herders left fewer material traces, leading to arguments regarding the existence of a long-term pastoral-forager frontier, south of which herders did not thrive (25–27).
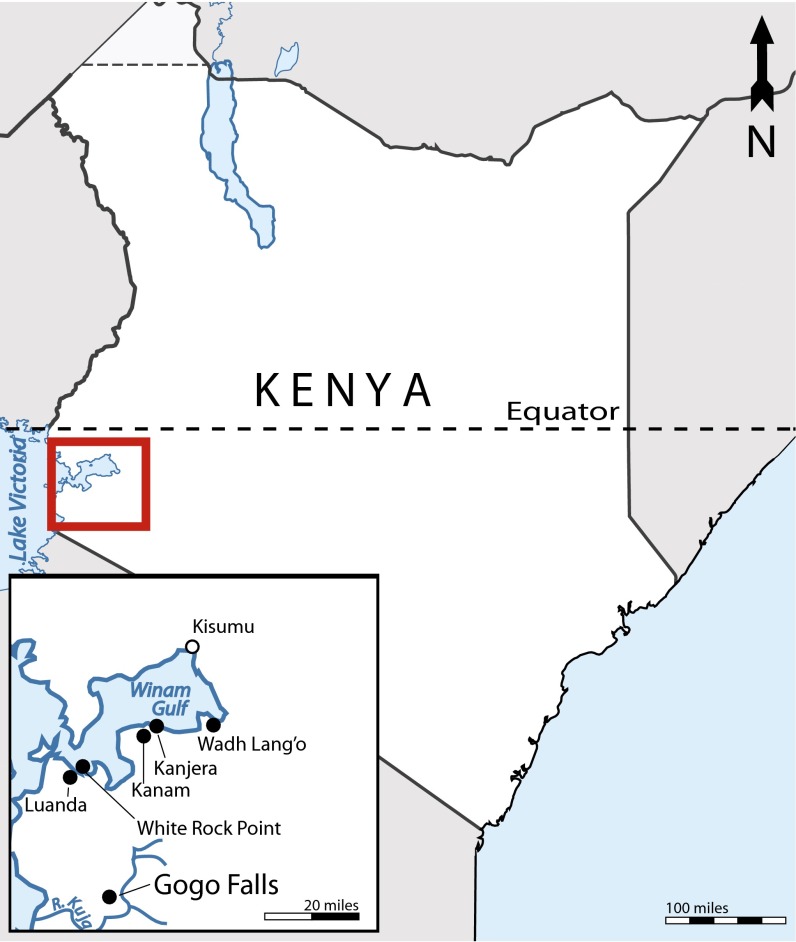
Fig. 1.
Location of Gogo Falls in relation to other Holocene archaeological sites (●) and towns (○) in the Lake Victoria basin.
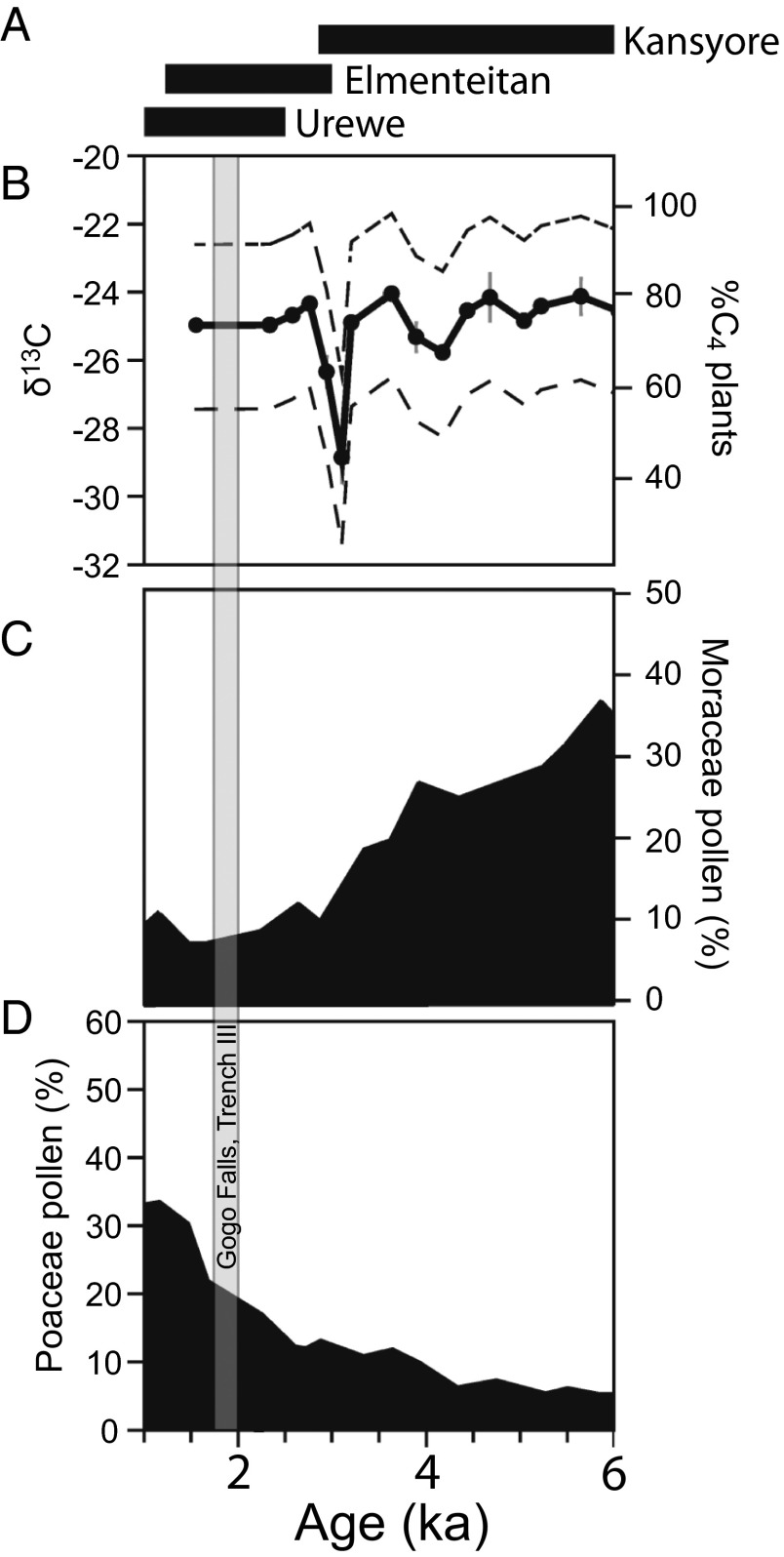
The presence of tsetse flies in eastern Africa has been used as an explanation for observed patterns of subsistence diversity among early pastoralists in the Lake Victoria basin (21, 28) and for limited southward movement (15, 20, 29, 30). A range of zoonoses posed threats for early herders, but trypanosomiasis risks have an especially widespread effect on human communities, even today (10, 12, 13, 30). Mesic, bushy/wooded environments that may have harbored large numbers of tsetse fly (Glossina spp.) would have been poorly suited to extensive cattle stock-keeping. The tsetse fly carries trypanosome parasites that transfer to hosts during blood meals and eventually cause often-fatal sleeping sickness in ungulates (predominately domesticates) and humans. In modern and precolonial times, herders have controlled tsetse fly numbers by heavy grazing, burning and destruction of woody areas, and managed livestock to avoid areas with abundant tsetse (10, 31, 32). It is not unreasonable to assume, then, that bushy, tsetse-rich environments would have impeded heavy reliance on livestock by early herders and may have even created a boundary beyond which it was difficult for large numbers of herders to settle (20, 33). Despite an abundance of archaeological sites, however, terrestrial paleoecological data for the Holocene in Kenya are scarce and have been based primarily on lacustrine archives, rather than sites themselves. Pollen and leaf wax biomarkers from lacustrine records from Lake Victoria yield somewhat conflicting paleoenvironmental interpretations: pollen data indicate decreasing moist tree and shrub presence (indicated by Moraceae pollen) in the Lake Victoria basin until ∼2 kya, when grass pollen (Poaceae) increased (Fig. 2) (34), contrasting with stable carbon isotopes from lacustrine leaf wax biomarkers, which indicate persistence of C4 grasses throughout the last 6 kya, with only a sharp decrease in C4 around 3 kya (Fig. 2) (35). These contrasting results could be explained by differences in spatial integration, with lipids recording a more localized signal within the catchment and pollen recording a more regional vegetation signal (36), and likely also point to varied, fluctuating input from C4 grasses during this period. Even so, pollen evidence suggests only minor (∼10%) moist forest in the region compared with ∼20–30% input from grasses. These records offer the important wider ecological context of regional (pollen) and basinal (leaf waxes) ecology, yet they are too coarse to understand specialized pastoralist expansions, and especially for local evidence (site-based reconstruction) of habitats harboring tsetse.
Fig. 2.
(A) Holocene pottery traditions present at archaeological sites in the Victoria basin. (B) Leaf wax δ13C and modeled %C4 from lacustrine sedimentary cores in Lake Victoria (35). (C) Moraceae (tropical mesic trees and shrubs) pollen counts from lacustrine sedimentary cores in Lake Victoria (data from ref. 34). (D) Poaceae (grass) pollen counts from lacustrine sedimentary cores in Lake Victoria (data from ref. 34). B, C, and D modified with permission from Elsevier; www.sciencedirect.com/science/journal/02773791.
To determine whether or not shifting tsetse-rich environments were present at particular locales during specific periods, direct stable isotope-based paleoecological analysis at archaeological sites can provide a complement to faunal analysis (21, 37–39). Isotopic measurements of enamel carbon (δ13C) and oxygen (δ18O) can be used to understand diet, habitat, and climate (40, 41). Although bone and tooth collagen have been previously used to assess paleoenvironment at Holocene archaeological sites in Kenya (42), tooth enamel is preferred, as it resists digenetic alteration and can be easily compared with fauna from older periods of geologic time (43). In some instances, faunal tooth enamel isotopes may provide our best estimates of environmental change over time, because these signals are less temporally and spatially attenuated than other proxies, such as leaf wax biomarkers (44), which may be influenced by reservoir effects during catchment transport before deposition (45, 46). The environmental context of the Elmenteitan layers of Gogo Falls has been previously interpreted as a grassland/bushland mosaic, based on analysis of wild species diversity and the presence of modern browsing taxa such as roan or sable antelope (Tragelaphini) and bushpig (Potamochoerus), as well as grazers such as oribi (21). The relatively low proportions of domestic stock were interpreted as reflecting environmental constraints on pastoral productivity in the region. The presence of the tsetse fly in a presumed seasonally mesic, bushy/woody environment, it was argued, prevented herders from relying exclusively on domesticates, which resulted in seasonal fishing and hunting of large wild ungulates (21). These interpretations have not yet been ground-truthed with a local, terrestrial paleoecological indicator.
Results
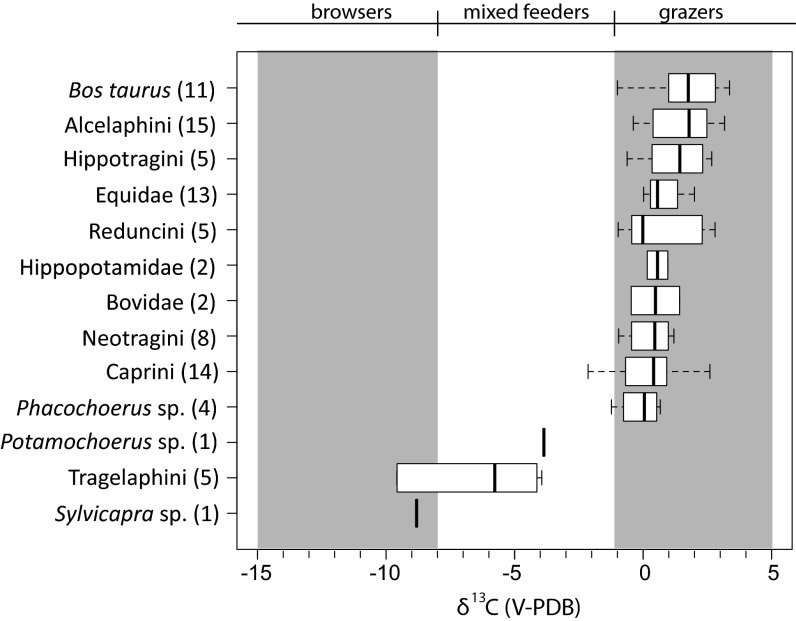
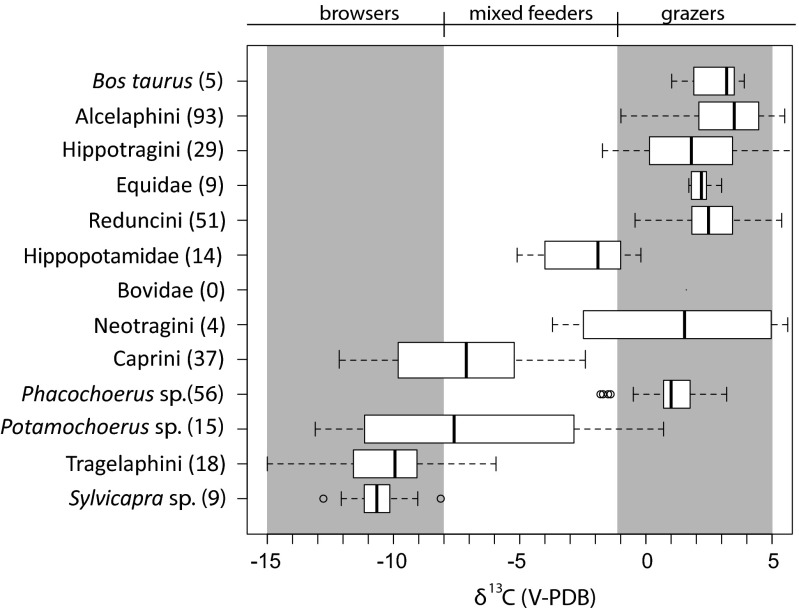
Isotopic data are presented in in Fig. 3 and SI Appendix, Tables S1 and S3. For comparison, a compilation of modern comparable herbivore δ13C1750 values, primarily from southern Kenya and northern Tanzania, are presented in Fig. 4 (SI Appendix, Table S2) (47–53). The average δ13C values indicate a diet with >80% C4 grass for 10 of 13 taxa from Gogo Falls. Potamochoerus sp. (δ13C = −3.86‰; n = 1), Sylvicapra sp. (δ13C = −8.81‰; n = 1), and Tragelaphini (δ13C = −6.6‰; n = 5) are the most 13C-depleted herbivores in the assemblage, but they are more 13C-enriched than their modern counterparts (Fig. 4; SI Appendix, Table S2). Modern hippos average −2.3 ± 1.7‰, whereas hippos at Gogo Falls average 0.6 ± 0.6‰. The presence of both large migratory and small, local bovids provides further evidence for both locally and basinally grassy environments (21).
Fig. 3.
Box plots of δ13C tooth enamel values from archaeological tooth enamel, Trench III, Gogo Falls.
Fig. 4.
Box plot of δ13C1750 tooth enamel values from modern comparative fauna from Kenya and Tanzania. *Neotragini and Sylvicapra sp. values were calculated to tooth enamel values from keratin, using εkeratin-enamel values of 11.1‰ because of a lack of enamel values in the literature.
Domestic fauna are pure C4 feeders at Gogo Falls. Bos taurus is within the range of pure C4 diet at both Gogo Falls and in the modern environment. Tribe Caprini (Subfamily Caprinae), which includes both goats and sheep, is again overwhelmingly within the range of a pure C4 diet (1.6 ± 1.4‰). Modern goat δ13C1750 values from locations across Kenya range from −2.6‰ to −12.3‰, whereas modern sheep range from −0.3‰ to −1.5‰ (SI Appendix, Fig. S1 and Table S4) because of the different dietary preferences of sheep and goats. Sheep are primarily grazers, whereas goats are mixed feeders that tend to browse, as their highly efficient digestive physiology confers an advantage for consuming low-quality woody forage (50, 54–57). Criteria for distinguishing domestic sheep and goat teeth have not been considered reliable in Africa (58), and Gogo Falls samples, at the time of identification, were simply identified as sheep or goat. Future studies may distinguish teeth and postcranial bones in African samples (59). If both sheep and goats were present at Gogo Falls, then these isotopic results indicate a much higher consumption of C4 resources by modern goats than is typically observed (50, 56, 60). If the isotope data represent values exclusively from sheep, which is more likely, this level of C4 intake is not unusual, yet the presence of sheep only at this site is unusual compared with current herding practices in East Africa.
Discussion
In comparison with the present day, isotopic analyses of herbivores indicate that during the Elmenteitan occupation at Gogo Falls, there were few C3 browsers or mixed C3–C4 feeders, suggesting a landscape dominated by C4 grasses. This interpretation is in general agreement with δ13Cwax values (Fig. 2) from lacustrine cores in Lake Victoria (which likely indicate vegetation locally, within the lake catchment), and to a lesser extent with pollen records, which indicate mixed environments regionally. These three lines of evidence, taken together, strongly imply an abundance of C4 grasses in the basin during this period. Vegetation in and around the Winam Gulf today has been characterized as “evergreen and semi-evergreen bushland and thicket,” “edaphic wooded grasslands and grasslands on drainage impeded or seasonally flooded soils,” and “moist combretum wooded grassland” (which comprises the ecology area around the Kuja River, where the Gogo Falls is located) (61). Faunally based interpretations, primarily the presence of grazers and modern browsers such as Tragelaphini and Potamochoerus sp., of the ancient environment at Gogo Falls suggested a woodland/grassland/bushland mosaic that is climatologically and ecologically similar to today (21), which would have supported tsetse flies. This bushland interpretation is not supported by the isotopic data, which are dominated by C4-grazing taxa with few C3-browsing taxa, contradicting long-held assumptions of modern climate and ecology persisting over the last 3 millennia in most regions of eastern Africa (19, 21, 24).
Given the resolution of data available, it is not yet possible to quantify the relative proportions of grassy and woody vegetation; however, abundant C4 grasses must have been present. Abundant grassy vegetation may have been the result of changes in rainfall seasonality in the later Holocene (62, 63), changes in total rainfall in the Lake Victoria basin, increased burning, or ecological factors, such as heavy grazing by domesticates or wild herbivores. Controlled grazing by cattle, sheep, and goats can stimulate new grass growth where grazing herbivores thrive (64, 65). Overgrazing, however, makes environments more bushy and reduces grass height (66). Burning promotes new grass and may have maintained grasslands if herders were regularly burning savanna for livestock, as is a common practice in modern eastern Africa (67–69). The presence of megaherbivores maintains grasslands as well, and when both burning and elephant or giraffe populations are high, grasslands expand (67, 68, 70). Given such complex environmental interactions in African grasslands today, the question arises whether ancient herders in the Lake Victoria basin helped create grasslands through grazing and fire, or whether they were created and maintained by other ecological and climatological factors (71, 72).
The isotopic data from Gogo Falls and leaf wax data from the Lake Victoria basin reveal an ideal environment for large grazing herds of domesticates in the immediate Lake Victoria basin catchment, one rich in C4 grasses. Such grasslands were not an ideal environment for tsetse flies, which calls into question the maintenance of long-term areas of hunter-gatherer-pastoral interaction and the inhibition of southward movements of herders by stable and extensive bands of tsetse bush (2). This study fits with the findings of recent genetic research including Y-chromosome, lactase persistence, and livestock data that point to some migration of herders from eastern to southern Africa (5–7). Archaeologists arguing for successful early pastoral migrations from eastern to southern Africa have pointed to some similarities between Elmenteitan pottery and early herder ceramics in southern Africa (73). Our data imply that a tsetse-rich barrier preventing herders from moving through the Lake Victoria basin into northern Tanzania and other parts of sub-Saharan Africa (9, 24, 33, 74) was not widespread 2,000 y ago, bolstering the possibility of such connections.
On a local level, our data show that Elmenteitan herders at Gogo Falls were not forced into hunting and fishing because of ecological constraints on stock-keeping. The Elmenteitan mammalian fauna at Gogo Falls are made up of 53.5% domestic livestock comprising cattle and sheep (based on isotopes). Fishing was also a significant activity (21). Recent research in the Lake Victoria basin has shown that the nearby site of Wadh Lang’o shares similar dates of 1,950 ± 35 y B.P. for early Elmenteitan levels (27, 75) and a number of similarities with Gogo Falls: Wadh Lang’o is also a large open-air site with a long sequence containing Kansyore, Elmenteitan, and Iron Age horizons (27, 75). It is situated in a similar environmental setting, on the banks of a river flowing into Lake Victoria. There are as yet no direct paleoecological proxies from this site, but according to lacustrine paleoenvironmental archives (Fig. 2), it was also likely situated in a grassy environment. Fishing is a significant activity at both sites; however, the mammals in the Elmenteitan faunal horizons in Trench 1, Wadh Lang’o (below datum 140–190 cm), are predominantly (89–91%) domestic, suggesting limited hunting (75). Alcelaphines are represented, but the zebra and oribi present at Gogo Falls are absent at Wadh Lang’o. Herding strategies also differed between Wadh Lang’o and Gogo Falls, with cattle making up 1% or less of the domestic fauna at Wadh Lang’o (n = 88 below datum 140–190 cm; n = 156 below datum 140–160 cm; SI Appendix, Table S5) compared with 32% (n = 328) at Gogo Falls. In strong contrast with these sites in the Lake Victoria basin, there is no fishing and very little hunting in Elmenteitan sites on the plains of southern Kenya (19, 20). Cattle, sheep, and goats make up 97–100% of the fauna at the open air settlement sites in the Lemek-Mara, including Ngamuriak, Sugenya, and Oldorotua, as well as the Central Rift Valley rock shelter of Maasai Gorge (SI Appendix, Table S5). Reliance on cattle and some small stock, combined with fishing and significant hunting at Gogo Falls, is an unexpected contrast with nearby Elmenteitan sites and does not fit a typical Elmenteitan subsistence pattern. Nor do the fauna, material culture, and organization of this site suggest hunter-gatherers in the process of adopting herding. Gogo Falls appears to have been populated by a unique group of people, potentially a small group of herders, who took flexible approaches to subsistence opportunities and interactions with hunter-gatherer-fishers as they followed grassy corridors in eastern Africa.
Conclusions
Our results provide much-needed late Holocene paleoenvironmental data for the Lake Victoria basin and demonstrate that substantial ecological changes occurred over the last 2,000 y. Isotopic data reveal that the fauna were dominated by C4 grazers with very few C3 browsing taxa, suggesting a grassy paleoecological setting for the unique Elmenteitan site of Gogo Falls. This challenges interpretations based on macrofaunal remains and models of disease-depressed livestock production (21). The isotopic findings also draw attention to herder relations with hunter-gatherers, rather than ecology, in consumption of large wild ungulates in western Kenya. This suggests that social factors may have played a greater role than previously thought in subsistence diversity during the spread of pastoralism in Eastern Africa. High levels of hunting at Gogo Falls can no longer be attributed to closed or bushy habitats that inhibited livestock production and southward movement of herders (21) and contributed to long-term frontier interactions between pastoralists and hunter-gatherers (25, 27, 74). These data raise interesting questions about the interplay among herders, environmental management and adaptation, and ecological drivers for grassland maintenance. Finally, in light of the isotope data from Gogo Falls and lacustrine leaf wax data (35), the Lake Victoria basin may have been the setting for grassy corridors through which demic migration of pastoralists occurred out of eastern Africa and toward southern Africa (30).
Materials and Methods
Site Description.
The archaeological site of Gogo Falls is located on the banks of the Kuja River by a dam that bears the same name (34°21′E, 0°34′S; Fig. 1). The area receives ∼1,060 mm/y in rainfall, with bimodal rainfall seasonality, with rains occurring in March–May and October–November (28). At this time, the area surrounding the site has been ecologically altered by farming, but natural vegetation is characterized as a wooded grassland (61). Nearby local wild fauna include roan antelope, zebra, and giraffe, and historically, a diverse mammalian population including elephant, lion, cheetah, and rhinoceros (61). Browsers are rare, and thus woodland and thicket encroachment has been occurring in the area (76).
Gogo Falls was excavated by Robertshaw in 1980 and 1983 (28, 77), and teeth analyzed for this article were uncovered during the 1983 excavation. Five noncontinuous trenches were dug in the study area, each yielding abundant faunal remains, artifacts, and potsherds spanning three cultural traditions: Kansyore, Elmenteitan, and Urewe (28). The stratigraphic details of Trench III is presented by Robertshaw (28), but the highlight of this excavation is a 1.5-m ash and dung midden layer containing Elmenteitan pottery exclusively, which Robertshaw interpreted as a stock-keeping area. Radiocarbon dating of charcoal near the top of Trench III gave an uncalibrated age of 1,770 ± 80 y B.P., and another date from the bottom of the trench gave an age of 1,990 ± 80 y B.P. (28), with no evidence of stratigraphic mixing. These dates calibrate to 1,646 calibrated y B.P. (calBP) ± 96 and 1,900 calBP ± 100 at 95.4% confidence using ShCal13 (78).
Teeth were selected from faunal analysis by Marshall and Stewart (21). All the faunal material excavated from the 1.5-m ash midden is in excellent preservation. The fauna from this trench are diverse, containing fish, avian, and mammalian remains. Domestic caprines (goat and sheep) constitute the largest proportion of the assemblage (36.4%), followed by cattle (17.1%), topi/hartebeest (presented here at the tribal level of identification, “Alcelaphini”; 15.2%), oribi (presented as “Neotragini”; 11.8%), zebra (11.3%), reedbuck (“Reduncini”; 2.8%), warthog (1.8%), and eland and roan/sable (“T. oryx” and “Hippotragini”, respectively; 1.1%) (21). Stable isotope analysis was carried out on a subset of these teeth, including samples from rarer fauna as well (i.e., Potamochoerus sp. and Sylvicapra sp.).
Laboratory Analysis.
Following the sampling procedures outlined by the National Museums of Kenya, teeth were photographed and sampled along broken edges, using a Dremel tool and diamond drill bit. About 1 mg enamel powder was drilled from each sample. Enamel powder was prepared and analyzed at the University of Utah. Enamel powders were treated with 0.1 M buffered acetic acid (pH ∼5.3) for 30 min to remove labile carbonates (79, 80). After acid treatment, powders were rinsed three times with distilled water and dried at 60 °C overnight. Sample powders were weighed into silver capsules and dried under vacuum at 200 °C for 2 h before analysis. Samples were analyzed for δ13C and δ18O on a Finnigan MAT 252 coupled to a Carboflo dual-inlet carbonate device (common phosphoric acid bath at 90 °C for 15 min reaction time). Stable isotope ratios are reported as delta (δ) values relative to the international carbon isotope standard, Vienna Pee Dee Belemnite, using the standard permil (‰) notation, where δ13C = (Rsample/Rstandard − 1) × 1,000 and Rsample and Rstandard are the 13C/12C ratios of the sample and standard, respectively. SD of an internal carbonate standard (Carrara marble) was ±0.1‰.
Dietary designations for herbivores are based on estimated consumption of C4 plants (tropical grasses) and C3 plants (trees, shrubs, herbs), which is calculated using a hypothetical 100% C4 diet and an isotope enrichment (ε*) between diet and herbivore tooth enamel of 14.1‰ (81). Estimated carbon isotope composition of tooth enamel for a 100% C4 diet is based on the average isotopic value of modern C4 plants in eastern and central Africa (−12.9‰; n = 764) (82). Changes in the isotopic composition of atmospheric CO2 resulting from the combustion of fossil fuels between modern environments and preindustrial Holocene (presented here as δ 13C1750) were accounted for by adding 1.6‰ to the modern average δ13C of C4 plants (81, 83, 84).
Supplementary Material
Acknowledgments
We thank P. Robertshaw for permission to conduct stable isotope analysis on excavated material and S. Blumenthal for comments on drafts of this manuscript. We are also grateful to the editor and two anonymous reviewers for helpful suggestions. We thank E. Mbua, P. Kiura, the National Museums of Kenya, and the Kenya National Council for Science and Technology for research permission. This research was supported by funds awarded to K.L.C. through the National Science Foundation’s Graduate Research Fellowship program, the Geological Society of America, and the Global Change and Sustainability Center at the University of Utah.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423953112/-/DCSupplemental.
References
- 1.Marshall F, Hildebrand E. Cattle Before Crops: The Beginnings of Food Production in Africa. J World Prehist. 2002;16(2):99–143. [Google Scholar]
- 2.Smith AB. Origins and spread of pastoralism in Africa. Annu Rev Anthropol. 1992;21:125–141. [Google Scholar]
- 3.Sadr K. The First Herders at the Cape of Good Hope. Afr Archaeol Rev. 1998;15(15):101–132. [Google Scholar]
- 4.Pleurdeau D, et al. “Of sheep and men”: Earliest direct evidence of caprine domestication in southern Africa at Leopard Cave (Erongo, Namibia) PLoS ONE. 2012;7(7):e40340. doi: 10.1371/journal.pone.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranciaro A, et al. Genetic origins of lactase persistence and the spread of pastoralism in Africa. Am J Hum Genet. 2014;94(4):496–510. doi: 10.1016/j.ajhg.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stock F, Gifford-Gonzalez DP. Genetics and African Cattle Domestication. Afr Archaeol Rev. 2013;30:51–72. [Google Scholar]
- 7.Henn BM, et al. Y-chromosomal evidence of a pastoralist migration through Tanzania to southern Africa. Proc Natl Acad Sci USA. 2008;105(31):10693–10698. doi: 10.1073/pnas.0801184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweitzer FR. Archaeological evidence for sheep at the Cape. The South African Archaeological Bulletin. 1974;29(115-116):75–82. [Google Scholar]
- 9.Smith AB. The Origins of Herding in Southern Africa: Debating the “Neolithic” model. Lambert Academic Publishing; Saarbrucken: 2014. [Google Scholar]
- 10.Gifford-Gonzalez DP. Animal disease challenges to the emergence of pastoralism in sub-Saharan Africa. Afr Archaeol Rev. 2000;17(3):95–139. [Google Scholar]
- 11.Smith AB. African Herders: Emergence of Pastoral Traditions. AltaMira Press; Walnut Creek, CA: 2005. [Google Scholar]
- 12.Ford J. The Role of the Trypanosomaises in African Ecology: A Study of the Tsetse Fly Problem. Clarendon Press; Oxford: 1971. [Google Scholar]
- 13.Cecchi G, Mattioli RC, Slingenbergh J, de la Rocque S. Land cover and tsetse fly distributions in sub-Saharan Africa. Med Vet Entomol. 2008;22(4):364–373. doi: 10.1111/j.1365-2915.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- 14.Marshall F, Stewart K, Barthelme J. Early domestic stock at Dongodien in northern Kenya. Azania. 1984;19(1):120–127. [Google Scholar]
- 15.Prendergast ME. Hunters and herders at the periphery: The spread of herding in eastern Africa. In: Jousse H, Lesur J, editors. People and Animals in Holocene Africa Recent Advances in Archaeozoology. Africa Magna Verlag; Frankfurt: 2011. pp. 43–58. [Google Scholar]
- 16.Dale D, Ashley CZ. Holocene hunter-fisher-gatherer communities: new perspectives on Kansyore Using communities of Western Kenya. Azania. 2010;45(1):24–48. [Google Scholar]
- 17.Merrick HV, Monaghan MC. The date of the cremated burials in Njoro River Cave. Azania. 1984;19(1):7–11. [Google Scholar]
- 18.Robertshaw P. The elmenteitan: An early food‐producing culture in East Africa. World Archaeol. 1988;20:57–69. [Google Scholar]
- 19.Marshall F. Origins of specialized pastoral production in East Africa. Am Anthropol. 1990;92:873–894. [Google Scholar]
- 20.Marshall F, Grillo K, Arco L. In: Prehistoric Pastoralists and Social Responses to Climatic risk in East Africa. Sustainable Lifeways: Cultural Persistence in an Ever- changing Environment. Miller NF, Moore KM, Ryan K, editors. University of Pennsylvania Press; Philadelphia: 2011. pp. 74–105. [Google Scholar]
- 21.Marshall F, Stewart K. Hunting, fishing and herding pastoralists of western Kenya: The fauna from Gogo Falls. Archaeozoologia. 1994;7:7–27. [Google Scholar]
- 22.Gifford-Gonzalez DP, Isaac GL, Nelson CM. Evidence for predation and pastoralism at Prolonged Drift: a Pastoral Neolithic site in Kenya. Azania. 1980;15(1):57–108. [Google Scholar]
- 23.Prendergast ME, Mutundu KK. Late Holocene zooarchaeology in East Africa: Ethnographic analogues and interpretive challenges. Documenta Archaeobiologiae. 2009;7:203–232. [Google Scholar]
- 24.Gifford-Gonzalez DP. Early pastoralists in East Africa: Ecological and social dimensions. J Anthropol Archaeol. 1998;17(2):166–200. [Google Scholar]
- 25.Prendergast ME, et al. Pastoral Neolithic sites on the southern Mbulu Plateau, Tanzania. Azania. 2013;48(4):498–520. [Google Scholar]
- 26.Bower J. The pastoral neolithic of East Africa. J World Prehist. 1991;5(1):49–82. [Google Scholar]
- 27.Lane P. The “moving frontier” and the transition to food production in Kenya. Azania. 2004;39(1):243–264. [Google Scholar]
- 28.Robertshaw P. Gogo Falls: Excavations at a complex archaeological site east of Lake Victoria. Azania. 1991;26(1):63–195. [Google Scholar]
- 29.Hanotte O, et al. African pastoralism: genetic imprints of origins and migrations. Science. 2002;296(5566):336–339. doi: 10.1126/science.1069878. [DOI] [PubMed] [Google Scholar]
- 30.Smith AB. Environmental limitations on prehistoric pastoralism in Africa. Afr Archaeol Rev. 1984;2:99–111. [Google Scholar]
- 31.Kjekshus H. Ecology Control and Economic Development in East African History: The Case of Tanganyika, 1850-1950. Ohio University Press; Athens, OH: 1996. [Google Scholar]
- 32.Lamprey R, Waller R. In: The Loita-Mara Region in Historical Times: Patterns of Subsistence, Settlement and Ecological Change. Early Pastoralists of South-Western Kenya. Robertshaw P, editor. British Institute of Eastern Africa; Nairobi: 1990. pp. 16–35. [Google Scholar]
- 33.Smith AB. Origins and spread of pastoralism in Africa. Annu Rev Anthropol. 1992;21:125–141. [Google Scholar]
- 34.Kendall RL. An ecological history of the Lake Victoria basin. Ecol Monogr. 1969;39(2):121–176. [Google Scholar]
- 35.Berke MA, et al. Molecular records of climate variability and vegetation response since the Late Pleistocene in the Lake Victoria basin, East Africa. Quat Sci Rev. 2012;55(8):59–74. [Google Scholar]
- 36.Farrimond P, Flanagan RL. Lipid stratigraphy of a Flandrian peat bed (Northumberland, UK): Comparison with the pollen record. Holocene. 1996;6(1):69–74. [Google Scholar]
- 37.Lane P, et al. The transition to farming in eastern Africa: new faunal and dating evidence from Wadh Lang'o and Usenge, Kenya. Antiquity. 2007;81(311):62–81. [Google Scholar]
- 38.Gifford-Gonzalez DP. Faunal Assemblages from Masai Gorge Rockshelter and Marula Rockshelter. Azania. 1985;20(1):69–88. [Google Scholar]
- 39.Marean CW. Hunter to herder: Large mammal remains from the hunter-gatherer occupation at Enkapune Ya Muto rock-shelter, Central Rift, Kenya. Afr Archaeol Rev. 1992;10(1):65–127. [Google Scholar]
- 40.Lee-Thorp JA, Sponheimer M, Luyt J. Tracking changing environments using stable carbon isotopes in fossil tooth enamel: an example from the South African hominin sites. J Hum Evol. 2007;53(5):595–601. doi: 10.1016/j.jhevol.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. A stable isotope aridity index for terrestrial environments. Proc Natl Acad Sci USA. 2006;103(30):11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrose SH, DeNiro MJ. Climate and habitat reconstruction using stable carbon and nitrogen isotope ratios of collagen in prehistoric herbivore teeth from Kenya. Quat Res. 1989;31(3):407–422. [Google Scholar]
- 43.Kohn MJ, Cerling TE. Stable isotope compositions of biological apatite. Rev Mineral Geochem. 2002;48:455–488. [Google Scholar]
- 44.Kingston JD. Shifting adaptive landscapes: Progress and challenges in reconstructing early hominid environments. Am J Phys Anthropol. 2007;50(Suppl 45):20–58. doi: 10.1002/ajpa.20733. [DOI] [PubMed] [Google Scholar]
- 45.Uchida M, et al. Age discrepancy between molecular biomarkers and calcareous foraminifera isolated from the same horizons of Northwest Pacific sediments. Chem Geol. 2005;218(1-2):73–89. [Google Scholar]
- 46.Matsumoto K, Kawamura K, Uchida M. Radiocarbon content and stable carbon isotopic ratios of individual fatty acids in subsurface soil: Implication for selective microbial degradation and modification of soil organic matter. Geochem J. 2007;41:483–492. [Google Scholar]
- 47.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris JM, Cerling TE. Dietary adaptations of extant and Neogene African suids. Journal of Zoology. 2002;256(1):45–54. [Google Scholar]
- 49.Cerling TE, Harris JM, Passey BH. Diets of East African Bovidae based on stable isotope analysis. J Mammal. 2003;84(2):456–470. [Google Scholar]
- 50.Balasse M, Ambrose SH. Distinguishing sheep and goats using dental morphology and stable carbon isotopes in C4 grassland environments. J Archaeol Sci. 2005;32(5):691–702. [Google Scholar]
- 51.Van Der Merwe NJ. Isotopic ecology of fossil fauna from Olduvai Gorge at ca 1.8 Ma, compared with modern fauna. S Afr J Sci. 2013;109(11-12):1–14. [Google Scholar]
- 52.Bocherens H, Koch PL, Mariotti A, Geraads D, Jaeger JJ. Isotopic biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene hominid sites. Palaios. 1996;11(4):306–318. [Google Scholar]
- 53.Kingston JD. In: Stable isotopic analyses of Laetoli fossil herbivores. Geology, Geochronology, Paleoecology and Paleoenvironment, Paleontology and Geology of Laetoli: Human Evolution in Context. Harrison T, editor. Vol 1. Springer, Dordrecht; The Netherlands: 2011. pp. 293–328. [Google Scholar]
- 54.Hofmann RR. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia. 1989;78:443–457. doi: 10.1007/BF00378733. [DOI] [PubMed] [Google Scholar]
- 55.Silanikove N. The physiological basis of adaptation in goats to harsh environments. Small Rumin Res. 2000;35(3):181–193. [Google Scholar]
- 56.Ambrose SH, DeNiro MJ. The isotopic ecology of East African mammals. Oecologia. 1986;69:395–406. doi: 10.1007/BF00377062. [DOI] [PubMed] [Google Scholar]
- 57.Migongo-Bake W, Hansen RM. Seasonal diets of camels, cattle, sheep, and goats in a common range in eastern Africa. J Range Manage. 1987;40(1):76–79. [Google Scholar]
- 58.Badenhorst S, Plug I. The archaeozoology of goats, Capra hircus (Linnaeus, 1758): their size variation during the last two millennia in southern Africa (Mammalia: Artiodactyla: Caprini) Ann Transvaal Mus. 2003;40:91–121. [Google Scholar]
- 59.Zeder MA, Pilaar SE. Assessing the reliability of criteria used to identify mandibles and mandibular teeth in sheep, Ovis, and goats, Capra. J Archaeol Sci. 2010;37:252–242. [Google Scholar]
- 60.Balasse M, Ambrose SH. Mobilité altitudinale des pasteurs néolithiques dans la vallée du Rift (Kenya) : Premiers indices de l’analyse du δ13C de l’émail dentaire du cheptel domestique. Anthropozoologica. 2005;40(1):147–166. [Google Scholar]
- 61. Lillesø J-PB, et al. (2011) The atlas. Potential Natural Vegetation of Eastern Africa (Ethiopia, Kenya, Malawi, Rwanda, Tanzania, Uganda and Zambia), Forest and Landscape Working Papers (Forest & Landscape, University of Copenhagen, Frederiksberg, Denmark), Vol 1, No 61/2011.
- 62.Tierney JE, Lewis SC, Cook BI, LeGrande AN, Schmidt GA. Model, proxy and isotopic perspectives on the East African Humid Period. Earth Planet Sci Lett. 2011;307(1-2):103–112. [Google Scholar]
- 63.Thompson LG, et al. Kilimanjaro ice core records: evidence of holocene climate change in tropical Africa. Science. 2002;298(5593):589–593. doi: 10.1126/science.1073198. [DOI] [PubMed] [Google Scholar]
- 64.Odadi WO, Karachi MK, Abdulrazak SA, Young TP. African wild ungulates compete with or facilitate cattle depending on season. Science. 2011;333(6050):1753–1755. doi: 10.1126/science.1208468. [DOI] [PubMed] [Google Scholar]
- 65.Veblen KE, Young TP. Contrasting effects of cattle and wildlife on the vegetation development of a savanna landscape mosaic. J Ecol. 2010;98(5):993–1001. [Google Scholar]
- 66.Treydte AC, Baumgartner S, Heitkönig IM, Grant CC, Getz WM. Herbaceous forage and selection patterns by ungulates across varying herbivore assemblages in a South African Savanna. PLoS ONE. 2013;8(12):e82831. doi: 10.1371/journal.pone.0082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dublin HT. Vegetation Dynamics in the Serengeti-Mara Ecosystem: The Role of Elephants, Fire, and Other Factors. In: Sinclair ARE, Acrese P, editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press; Chicago: 1995. pp. 71–90. [Google Scholar]
- 68.Dublin HT, Sinclair ARE, McGlade J. Elephants and Fire as Causes of Multiple Stable States in the Serengeti-Mara Woodlands. J Anim Ecol. 1990;59(3):1147–1164. [Google Scholar]
- 69.Archibald S, Bond WJ. Grazer movements: Spatial and temporal responses to burning in a tall-grass African savanna. Int J Wildland Fire. 2004;13(3):377–385. [Google Scholar]
- 70.Lamprey RH, Reid RS. Expansion of human settlement in Kenya's Maasai Mara: what future for pastoralism and wildlife? J Biogeogr. 2004;31(6):997–1032. [Google Scholar]
- 71.Good SP, Caylor KK. Climatological determinants of woody cover in Africa. Proc Natl Acad Sci USA. 2011;108(12):4902–4907. doi: 10.1073/pnas.1013100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sankaran M, et al. Determinants of woody cover in African savannas. Nature. 2005;438(7069):846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
- 73.Smith AB. Pastoralism in the Western Cape Province, South Africa: A retrospective review. Journal of African Archaeology. 2009;7(2):239–252. [Google Scholar]
- 74.Clark JD. 1980. Early human occupation of African savanna environments. Human Ecology in Savanna Environments, ed Harris DR (Academic Press, London), pp 41-72.
- 75.Prendergast ME. Kansyore fisher-foragers and transitions to food production in East Africa: The view from Wadh Lang'o, Nyanza Province, Western Kenya. Azania. 2010;45(1):83–111. [Google Scholar]
- 76.Muriuki GW, Njoka TJ, Reid RS. Tsetse, wildlife and land-cover change in Ruma National Park, South-western Kenya. J Hum Ecol. 2003;14(4):229–235. [Google Scholar]
- 77.Collett DP, Robertshaw P. Early Iron Age and Kansyore Pottery: Finds from Gogo Falls, South Nyanza. Azania. 1980;15(1):133–145. [Google Scholar]
- 78.Hogg AG, et al. SHCal13 Southern Hemisphere calibration, 0–50,000 years cal BP. Radiocarbon. 2013;55(2):1–15. [Google Scholar]
- 79.Lee-Thorp JA, Van der Merwe NJ. Carbon isotope analysis of fossil bone apatite. S Afr J Sci. 1987;83:712–715. [Google Scholar]
- 80.Koch PL, Tuross N, Fogel M. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J Archaeol Sci. 1997;24(5):417–430. [Google Scholar]
- 81.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120(3):347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 82.Cerling TE. 14.12 Stable Isotope Evidence for Hominin Environments in Africa. In: Cerling TE, editor. Treatise on Geochemistry. 2nd Ed. Elsevier; Amsterdam: 2014. pp. 157–167. [Google Scholar]
- 83.Francey RJ, et al. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B Chem Phys Meterol. 1999;51(2):170–193. [Google Scholar]
- 84.Keeling CD, Piper S, Bollenbacher AF, Walker SJ. 2010 Monthly atmospheric 13C/12C isotopic ratios for 10 SIO stations. Available at cdiac.ornl.gov/trends/co2/iso-sio/iso-sio.html. Accessed October 1, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.