Significance
Sound frequency discrimination is crucial for daily activities throughout the animal kingdom. This process begins at the auditory peripheral organ known as the organ of Corti in mammals and basilar papilla in birds. This frequency tuning is facilitated by specific anatomical and physiological properties, including gradual changes in the shape of the mechanosensory hair cells and the total number of stereocilia per hair cell along the cochlea. Unlike in birds, the molecular mechanism(s) that establishes this tonotopic organization is not known in mammals. In this study, we provide in vivo evidence that Sonic Hedgehog signaling mediates regional identity of the developing cochlea in both mammals and birds, and this regional identity prefigures the tonotopic organization in the basilar papilla.
Keywords: frequency discrimination, regional identity, Inhba, A2m, Efnb2
Abstract
Sound frequency discrimination begins at the organ of Corti in mammals and the basilar papilla in birds. Both of these hearing organs are tonotopically organized such that sensory hair cells at the basal (proximal) end respond to high frequency sound, whereas their counterparts at the apex (distal) respond to low frequencies. Sonic hedgehog (Shh) secreted by the developing notochord and floor plate is required for cochlear formation in both species. In mice, the apical region of the developing cochlea, closer to the ventral midline source of Shh, requires higher levels of Shh signaling than the basal cochlea farther away from the midline. Here, gain-of-function experiments using Shh-soaked beads in ovo or a mouse model expressing constitutively activated Smoothened (transducer of Shh signaling) show up-regulation of apical genes in the basal cochlea, even though these regionally expressed genes are not necessarily conserved between the two species. In chicken, these altered gene expression patterns precede morphological and physiological changes in sensory hair cells that are typically associated with tonotopy such as the total number of stereocilia per hair cell and gene expression of an inward rectifier potassium channel, IRK1, which is a bona fide feature of apical hair cells in the basilar papilla. Furthermore, our results suggest that this conserved role of Shh in establishing cochlear tonotopy is initiated early in development by Shh emanating from the notochord and floor plate.
The auditory pathway is characterized by its unique ability to discriminate sounds of different frequencies, which is crucial for communication and survival throughout the animal kingdom. Frequency discrimination begins at the organ of Corti in mammals and the basilar papilla (BP) in birds, residing within the cochlea of the inner ear. Cochlear hair cells are specialized to respond to specific frequencies depending on their location along the organ of Corti; hair cells at the base are tuned to high frequency sounds and those at the apex to low frequencies. This functional and spatial arrangement, known as tonotopic organization, is attributable to several features that vary along the longitudinal (tonotopic) axis of the cochlea, including the morphological and physiological properties of the sensory hair cells and their innervating spiral ganglion neurons, as well as extracellular components including the basilar membrane and tectorial membrane (1, 2).
These morphological and physiological features of the cochlea are conferred by a large number of genes that are differentially expressed along the developing cochlea (3–7). Notably, two signaling molecules, bone morphogenetic protein 7 (Bmp7) and retinoic acid (RA), were recently demonstrated to be important for establishing some of the tonotopic features of the BP (6, 7). Sequential apical-to-basal expression gradients of Bmp7 and Raldh3 (encoding the RA synthesizing enzyme, retinaldehyde dehydrogenase 3) appear to confer apical identity in the BP (6) and apical hair cell morphology (7), respectively. However, several important questions remain about the development of the tonotopic organization of the cochlea: (i) What is the signaling pathway(s) upstream of Bmp7 and RA? and (ii) are Bmp7 and RA also required for establishing the tonotopy in the mammalian inner ear?
The Shh signaling pathway is of particular interest in cochlear tonotopic development for several reasons. First, the requirement of Shh emanating from the floor plate and notochord appears conserved between chicken and mouse (8–11). Second, the source of Shh from the ventral midline is thought to generate regional identity of the mouse cochlea along the dorsoventral axis via regulation of Gli activator and repressor activities (10). Thus, it is plausible that this regional identity, generated by a Shh gradient, may also prefigure the tonotopic organization of the mature cochlea in both chicken and mouse (2, 12, 13). However, testing this hypothesis using a loss-of-function approach is not feasible because the cochlea fails to form in the absence of Shh in both species, which precludes tonotopic analyses (8–10). In mice, tonotopic analysis is further confounded because most of the mutant mice do not survive until postnatal day 4, when the morphological differences in sensory hair cells can be first observed (5, 8, 10, 14, 15).
Here, we have examined the roles of Shh in development of tonotopic organization of the cochlea by first using a gain-of-function approach in ovo. Implanting Shh-soaked beads to the developing otocyst causes basal papilla hair cells to exhibit features of their counterparts in the apex such as a decrease in the number of stereocilia and the up-regulation of Kcnj2, which encodes an apical inward rectifier potassium ion channel (IRK1/Kir2.1). These morphological changes are preceded by expansion of apically expressed genes toward the base of the papilla including Bmp7, which has been demonstrated to mediate some of the tonotopic features described here (6). We also examined a gain-of-Shh function mouse model, Pax2Cre/+; SmoM2/+, in which the transducer of Shh is constitutively activated starting at the otic placode stage (16, 17). In this model we also observed expansion of apically associated genes toward the basal cochlea. Furthermore, we provide evidence that the regional cochlear identity is established early by Shh signaling emanating from the ventral midline.
Results
Ectopic Shh Signaling in the Chicken Otocyst Induces Apical Hair Cell Phenotypes in the Basal BP.
Because the dorsal–ventral axis of the mouse cochlea is dependent on a gradient of Shh signaling secreted by the floor plate and notochord (10), we first examined whether a similar Shh signaling gradient is present in the developing chicken BP based on the expression patterns of two downstream targets of Shh, Patched 1 (Ptch1) and GLI-Kruppel family member GLI1 (Gli1) (18, 19). Both genes show a ventral-to-dorsal expression gradient that confirms the ventral-to-dorsal gradient of Shh signaling (Fig. S1 B and C). Next, we asked whether this Shh signaling gradient is important for tonotopic organization of the mature BP by perturbing the gradient using beads soaked with Shh protein implanted into chicken otocysts in ovo on 3 consecutive days at embryonic (E) days E2.5, E3.5, and E4.5. The expression domains of Ptch1 and Gli1 were expanded in Shh-implanted ears (Fig. S1 E and F, arrowheads; n = 4/4 for Ptch1, n = 3/3 for Gli1) compared with controls (Fig. S1 B and C). The gross morphology of the inner ear was affected as well, exhibiting severely malformed vestibular structures and a slightly shortened cochlea (Fig. 1 E and I; n = 6/6). The malformed vestibular phenotypes are consistent with previous mouse models of gain-of-Shh functions (8, 10).
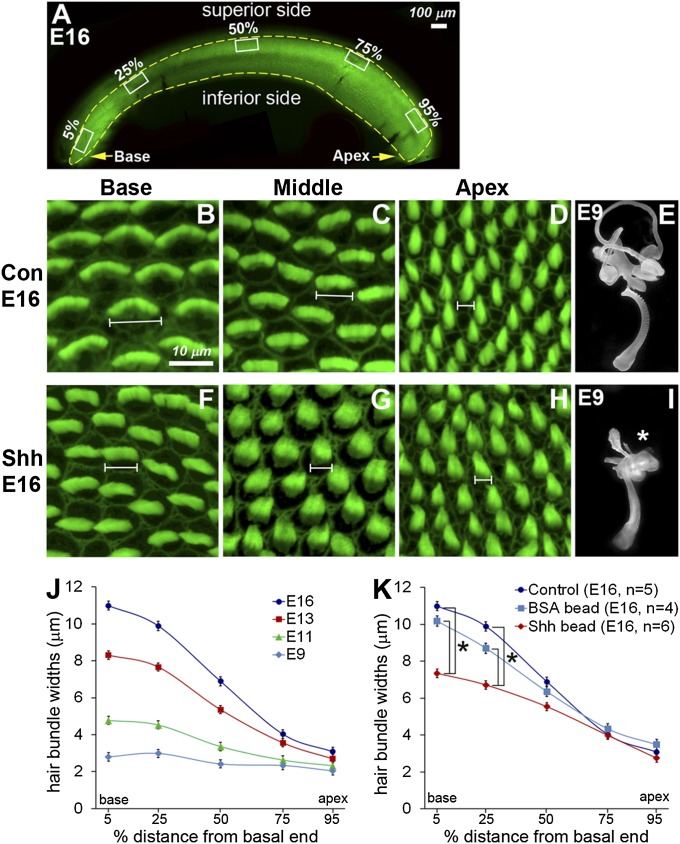
Fig. 1.
Changes in stereociliary morphologies in Shh-bead–implanted BP. (A) E16 BP stained with phalloidin. Rectangles (150 µm × 75 µm) were drawn by the superior edge of the BP at 5%, 25%, 50%, 75%, and 95% positions from the basal end of E9, E11, E13, and E16 control and E16 Shh-bead–implanted ears. Thirty hair cells from each rectangle were randomly selected to measure hair bundle widths. (B–H) Pictures of phalloidin-labeled stereocilia at base (5%), middle (50%), and apex (95%) of E16 control (B–D) and Shh-bead–implanted ears (F–H). (E and I) Paint-filled inner ears of controls (E) and Shh-implanted ears (I) at E9 showing abnormal dorsal vestibular structures (asterisk) and slighlty shorter BP. (J and K) Hair bundle widths of hair cells were plotted as a function of relative distance from the basal end. (J) In controls, the hair bundle width is initially similar along the BP at E9 but gradually increases its width except at the apex. (K) In Shh-bead–implanted ears, hair bundle widths are significantly narrower in the basal regions (5% and 25%) compared with wild-type control or BSA-bead–implanted ears (*P < 0.0001).
To analyze the tonotopic properties of hair cells in the developing BP, we sampled hair cells located in the superior side of the BP along the tonotopic axis (Fig. 1A) (20). Stereocilia bundles along the BP are similar in morphology between E9 and E11 (Fig. 1J and Fig. S2). After E11, the width and length of stereocilia increase at different rates depending on their tonotopic positions such that there is a clear gradation of bundle morphology along the papilla by E16: more numerous short stereocilia on basal hair cells compared with the longer and fewer stereocilia on apical hair cells (Figs. 1 B–D and J and 2 D–F). In Shh-bead–implanted ears, this gradation is disrupted in the base and middle regions of the papilla with narrower stereocilia bundle widths and somewhat taller stereocilia in the middle region (Fig. 1 F–H).
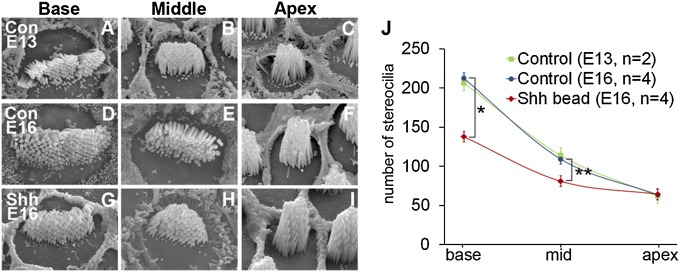
Fig. 2.
Changes in the number of stereocilia in Shh-bead–implanted BP. (A–I) Scanning electron microscopy images taken from base, middle, and apex of E13 (A–C) and E16 control (D–F), and E16 Shh-implanted (G–I) BP. (J) The number of stereocilia per hair cell was plotted as a function of the relative distance from the basal end. Stereocilia numbers are greatest in the base and decrease gradually toward the apex. These graded patterns are similar between E13 and E16 control BPs (P > 0.9999 for all three regions). In Shh-bead implanted ears, stereocilia numbers are significantly reduced in the base and middle regions of the BP compared with wild-type controls (J, *P < 0.001, **P < 0.05).
The stereocilia phenotype was first quantified by comparing the width of the entire stereocilia bundle between control and Shh-bead–implanted ears. The bundle width of basal hair cells in Shh-treated ears is significantly narrower than that of control hair cells and is comparable to that of stereocilia located in the middle region of controls (Fig. 1K), suggesting that ectopic Shh activation in the otocyst resulted in basal hair cells adapting more apical characteristics. Furthermore, we counted the total number of stereocilia per hair cell using scanning electron micrographs of hair cells from three different regions of the papilla (base, mid, and apex). At E16, hair cells in Shh-bead–implanted ears had significantly fewer stereocilia in the base and middle regions than controls (Fig. 2 D–J). These morphological changes of stereocilia in Shh-treated ears likely represent a tonotopic change rather than a developmental delay, because stereocilia number is reportedly established by E10 (21), and in support of this finding, we observed no difference in stereocilia number between E13 and E16 of controls (Fig. 2J). Together, these results indicate that ectopic Shh signaling starting in the otocyst stage can lead to tonotopic changes in hair bundle morphologies in the mature BP.
Shh Signaling Positively Regulates Bmp7 Expression in the Developing BP.
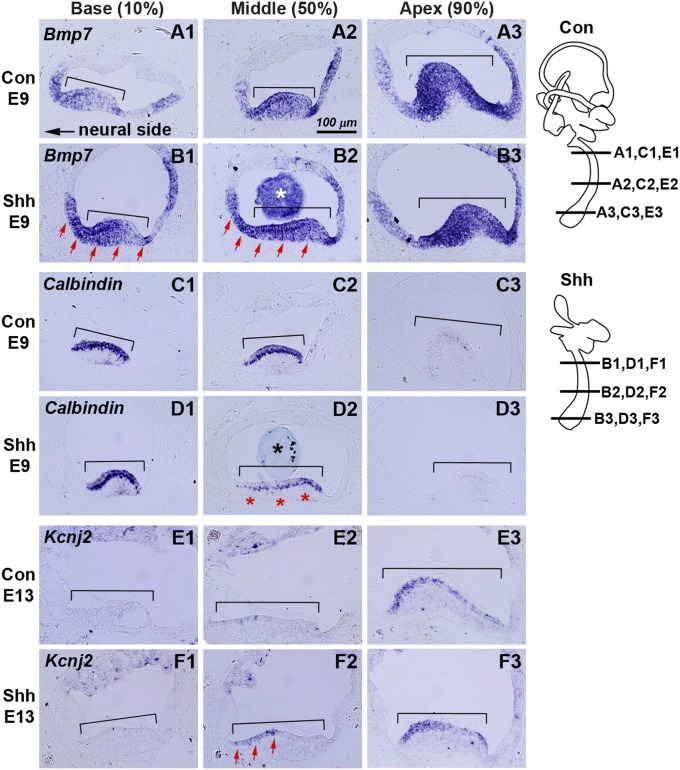
The apical to basal gradient of Bmp7 signaling was recently shown to determine tonotopic organization of hair cells in explants of BP (6). We investigated Bmp7 expression in our in vivo Shh model and found that Bmp7 expression levels are evidently increased in the basal and middle regions in Shh-bead–implanted ears (Fig. 3B, arrows; n = 12/14) compared with controls (Fig. 3A). In contrast, Bmp7-bead–implanted ears did not demonstrate changes in expression of Ptch1, a readout for Shh signaling (Fig. S3 A and B; n = 5/6). Together, these results suggest that the ventral-to-dorsal gradient of Shh signaling is upstream of Bmp7 in mediating tonotopic specification of the BP.
Fig. 3.
Changes of gene expression patterns in Shh-implanted BP. Expression patterns of chicken Bmp7 (A and B), Calbindin (C and D), and Kcnj2 (E and F) in sham-operated control (A, C, and E) and Shh-bead–implanted (B, D, and F) BP at E9 (A–D) or E13 (E and F). Bmp7 expression shows an increasing basal–apical gradient in controls (A1–A3) but this expression is up-regulated in the basal and middle regions of Shh-bead–implanted ears (B1 and B2, red arrows). White and black asterisks in B2 and D2 indicate the implanted beads. Calbindin, which normally shows strong expression in the sensory epithelium (brackets) of basal and middle regions of BP (C1–C3), is decreased in the middle region of Shh-bead–implanted ears (D2, asterisks). Kcnj2 expression, which is restricted to the apical sensory epithelium in controls (E1–E3), is up-regulated in the middle region of Shh-bead–implanted ears (F2, arrows).
Ectopic Shh Signaling Induces Apical Properties in Basal Hair Cells.
Next we examined whether ectopic Shh signaling alters expression of genes associated with physiological properties of hair cells along the tonotopic axis. Calbindin, encoding a calcium binding protein, is expressed strongly in basal hair cells and this expression gradually becomes weaker in hair cells toward the apex of the BP (Fig. 3C) (22). Graded levels of Calbindin expression in hair cells are postulated to be required for calcium-dependent, frequency-specific responses along the tonotopic axis of the BP (22). In Shh-bead–implanted ears, the Calbindin expression domain is more restricted at the base compared with control ears, and Calbindin message levels are reduced in the middle region of the BP (Fig. 3D, asterisks; n = 11/13). Similar reduction of Calbindin in the middle region of the BP was observed in the Bmp7-bead–implanted ears in ovo (Fig. S3D; n = 3/4), suggesting that ectopic Shh may repress Calbindin indirectly via up-regulation of Bmp7 in ovo. In addition, Kcnj2 (encoding IRK1) is normally expressed specifically in the apex of the BP (Fig. 3E) (23), whereas this expression is up-regulated in the middle regions of the BP in Shh-bead–implanted ears (Fig. 3F, arrows; n = 5/5). Taken together, the Calbindin and Kcnj2 data suggest that ectopic Shh treatments may lead to alterations in the physiological properties of hair cells in the mature BP.
Constitutively Activated Shh Signaling Affects Regional Identity Along the Tonotopic Axis of the Mouse Cochlea.
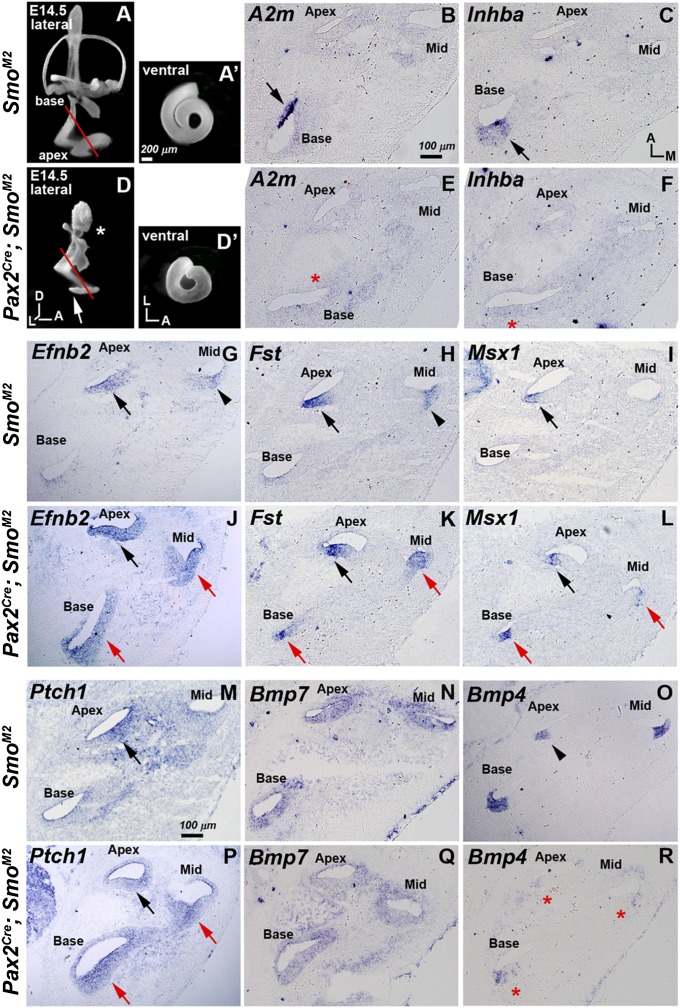
Our previous analyses of compound mutants of Gli2 and Gli3 (downstream transcriptional mediators of Shh signaling) revealed that the mouse cochlea is specified by a gradient of Shh signaling with the apical region requiring the highest level of Shh and the basal region the lowest (10). However, the downstream targets of Shh signaling involved in this specification are not fully understood. Here, we focused on the developmental profiles of five genes that are differentially expressed in the developing cochlea: follistatin (Fst), ephrin B2 (Efnb2), and msh homeobox 1 (Msx1) in the apical cochlea and alpha-2-macroglobulin (A2m) and inhibin beta-A (Inhba) in the basal cochlea (5, 10). These five genes were selected based on their early onset of differential expression patterns in the cochlear anlage, with Fst preferentially expressed in the apex as early as E10.5, followed by the sequential expression of Efnb2 and Msx1 in the apex at E11.5, and A2m and Inhba expression in the base at E11.5 and E13.5, respectively (Figs. S4, S5, and S6). We tested whether these expression gradients are disrupted in cochleas of Pax2Cre/+; SmoM2/+ mice expressing a constitutively active form of the Shh transducer, Smoothened (SmoM2), which is activated starting at the otic placode stage (16, 17). Pax2Cre/+; SmoM2/+ mutants die around E14.5, and their inner ears are severely malformed with a stunted endolymphatic duct/sac and absent vestibular structures (Fig. 4 A and D, asterisk). In addition, the cochlea is shortened, reaching only one turn compared with one and a half turns in SmoM2/+ control embryos at E14.5 (Fig. 4 A′ and D′).
Fig. 4.
Promotion of apical identity and suppression of basal identity in Pax2Cre/+; SmoM2/+ cochlea. (A, A′, D, and D′) Lateral (A and D) and ventral (A′ and D′) views of paint-filled SmoM2/+ (A and A′) and PaxCre/+; SmoM2/+ (D and D′) inner ears at E14.5. In PaxCre/+; SmoM2/+ inner ears, vestibular structures failed to develop except for a rudimentary endolymphatic duct (D, asterisk) and the cochlea is shortened (D and D′, arrow) compared with SmoM2/+ controls (A′). (B, C, E, and F) Inhba and A2m are restricted to the basal region in controls (B and C, arrows), but are completely down-regulated in PaxCre/+; SmoM2/+ mutants (E and F, red asterisks). (G–L) Apical-expressed genes, Efnb2, Fst, and Msx1, (G–I, arrows and arrowheads), are ectopically up-regulated in the entire PaxCre/+; SmoM2/+ cochlea (J–L, red arrows). (M and P) Ptch1 expression, which is strong in the apical turn and weaker toward the base in SmoM2/+ controls (M, arrow), is expanded to the entire cochlea in PaxCre/+; SmoM2/+ mutants (P, red arrows). (N and Q) Bmp7 is broadly expressed in the cochlea without an obvious gradient along the tonotopic axis in SmoM2/+ controls (N), whereas Bmp7 expression is reduced in Pax2Cre/+; SmoM2/+ mutants (Q). (O and R) Bmp4 is strongly expressed in the lateral cochlear region of the basal and middle turns and weakly in the apical turn of SmoM2/+ controls (O, arrowhead). Bmp4 expression is down-regulated in Pax2Cre/+; SmoM2/+ mutants (R, asterisks).
Constitutive activation of Shh signaling in Pax2Cre/+; SmoM2/+ ears resulted in ectopic up-regulation of Ptch1 expression along the entire length of the cochlea in mutant mice (Fig. 4P, red arrows) relative to SmoM2/+ controls (Fig. 4M). In the Pax2Cre/+; SmoM2/+ cochlea, expression levels of the apical genes, Efnb2, Fst, and Msx1, are expanded along the entire cochlea (Fig. 4 G–L, red arrows). In contrast, the basal genes, Inhba and A2m, are completely down-regulated in the mutant cochlea (Fig. 4 E and F, asterisks), compared with controls (Fig. 4 B and C, arrows). Otx2, a ventral otic gene that may be regulated by Shh but shows no gradient of expression in the cochlea (10), as well as Sox2, a prosensory marker, are largely unaffected by ectopic Shh signaling (Fig. S7). These results indicate that ectopic Shh activation is sufficient to expand the apical region of the cochlea at the expense of the basal region.
Downstream Targets of Shh Signaling Are Not Conserved Between the Mammalian Cochlea and Avian Basilar Papilla.
Because Bmp7 appears to be a major mediator of Shh signaling that establishes the tonotopic organization of the BP, we asked (i) whether there is a similar apical-to-basal gradient of Bmp7 in the developing mouse cochlea, and (ii) whether this expression is affected in the Pax2Cre/+; SmoM2/+ mutant cochlea. In contrast to the chicken BP, the mouse cochlea does not display a gradient of Bmp7 expression along the longitudinal axis at E14.5 or earlier, when other regional genes are differentially expressed (Fig. 4N). In addition, Bmp7 expression is slightly down-regulated in Pax2Cre/+; SmoM2/+ mutant cochleas (Fig. 4Q), indicating that Shh signaling negatively regulates Bmp7 expression in the developing mouse cochlea. Another Bmp family member, Bmp4, is expressed in the lateral compartment of the cochlea with a weaker expression in the apical turn (Fig. 4O, arrowhead), where Fst, an antagonist of TGFβ signaling, is strongly expressed (Fig. 4H). In Pax2Cre/+; SmoM2/+ mutants, Fst expression is ectopically up-regulated toward the base (Fig. 4K, red arrow), and Bmp4 expression is down-regulated (Fig. 4R, asterisk). These results suggest that, whereas Shh up-regulates Bmp7 in the BP, Bmp pathways are down-regulated by Shh in the developing mouse cochlea, indicating that downstream targets of Shh signaling may not be conserved between the avian BP and mammalian cochlea.
Regional Cochlear Identity Is Specified Early by Shh Emanating from the Ventral Midline.
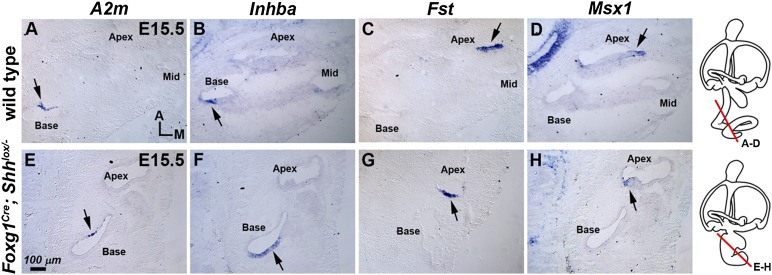
Shh required for cochlear development is sequentially secreted by two different sources: initially from the floor plate and notochord in the ventral midline and later from the spiral ganglion neurons starting at E11.75 (15, 24). We thus asked which source(s) of Shh mediate the specification of regional cochlear identity. Some of the regional cochlear markers such as Fst and A2m are already differentially expressed in the cochlear primordium (Fig. S4), before the detection of Shh transcripts in the spiral ganglion (15, 24), suggesting that Shh signaling from the ventral midline may be more important for regional cochlear specification. To test this hypothesis, we examined the cochlea of Foxg1Cre; Shhlox/− mutants, in which Shh signaling from the spiral ganglion but not the ventral midline is selectively abolished (15). The cochlea of Foxg1Cre; Shhlox/− mutants is severely shortened, reaching only a half turn (15). Despite this shortened length, the relative expression domains of apical genes such as Fst and Msx1 and basal genes such as A2m and Inhba are not altered in Foxg1Cre; Shhlox/− mutants (Fig. 5), suggesting that Shh secreted by the spiral ganglion is not required to establish regional identity of the cochlea.
Fig. 5.
Specification of regional cochlear identity in the absence of ganglionic Shh signaling. Expression patterns of region-specific genes in wild type (A–D) and Foxg1Cre; Shhlox/− mutant cochleas at E15.5 (E–H). Expression domains of basal genes, A2m and Inhba (E and F, arrows; n = 4) and apical genes, Fst (n = 4) and Msx1 (G and H, arrows; n = 5/6) are located in the basal and apical regions of the shortened cochlea of Foxg1Cre; Shhlox/− mutants, respectively. The schematics indicate the level of sections. A, anterior; M, medial.
Discussion
Shh Gradient Regulates Regional Cochlear Identity Along the Tonotopic Axis in both Birds and Mammals.
In this study, we examined the relationship between the ventral-to-dorsal gradient of Shh signaling and the development of tonotopic organization in both the chicken BP and mouse cochlea. Disruption of the Shh gradient by implanting Shh beads into the chicken otocyst in ovo or by constitutive activation of Shh signaling in the otic epithelium of Pax2Cre/+; SmoM2/+ mouse mutants resulted in up-regulation of apex-specific genes in the BP/cochlea and a loss of basal identity (Fig. 6). These gene expression changes led to morphological changes in the basal hair cells that are associated with tonotopic organization in the chicken BP (Fig. 6). Additionally, Kcnj2 is up-regulated in the basal BP. Because Kcnj2 expression is one of the few bona fide properties of apical hair cells (25), the ectopic up-regulation of this K+ channel's transcripts in response to ectopic Shh is likely to change frequency response properties of the hair cells. We would predict similar changes in tonotopic organization in the mutant mouse models if they were to survive.
Fig. 6.
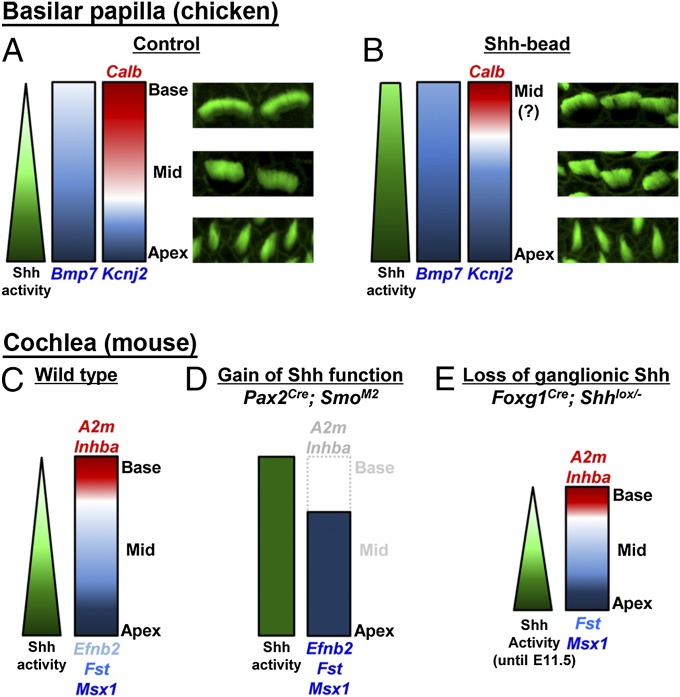
Summary of Shh-mediated regional specification in avian BP and mammalian cochlea. (A) In the chicken BP, an apical-to-basal gradient of Shh signaling induces an apical-to-basal gradient of Bmp7 expression, which in turn acts as an intrinsic cue for establishing regional identity in the developing BP. Expression of Kcnj2 is restricted to the apex, whereas Calbindin (Calb) expression shows a basal-to-apical gradient. (B) When Shh signaling is ectopically activated in the otocyst by Shh-bead implantation in ovo, apical genes (Bmp7 and Kcnj2) are expanded toward the base, whereas calbindin expression is restricted to the base of the BP. (C) In the mouse cochlea, an apical-to-basal gradient of Shh signaling regulates expression of both basal (A2m and Inhba) and apical (Efnb2, Fst, and Msx1) genes along the developing cochlea. (D) Constitutive activation of Shh signaling causes an expansion of apical genes and a loss of basal genes. (E) In the absence of Shh signaling from the spiral ganglion, the regional identity was acquired normally in the shortened cochlea, suggesting that Shh signaling from the ventral midline is sufficient for specification of the tonotopic axis of the cochlea.
The tonotopic axis of the chicken BP is specified by an apical–basal gradient of Bmp7 expression (6). This signaling is later reinforced by a graded apical–basal Raldh3 gradient, which functions to control region-specific hair cell morphologies (7). Our in ovo studies here support a role for Bmp7 in establishing tonotopy in the papilla and further show that Shh functions upstream of Bmp7. Taken together, these results suggest that tonotopic organization is established by a temporal cascade of molecular events that begins with Shh gradient emanating from the ventral midline and is later refined by Bmp7 and RA gradients within the BP.
Downstream Mediators of Shh Signaling in Tonotopy Are Not Conserved Between Birds and Mammals.
Despite the conserved role of Shh in mediating regional identity of both the chicken BP and mouse cochlea, the downstream targets of Shh do not appear to be conserved between these two species. Whereas Bmp7 expression is positively regulated by Shh in the developing BP, it is neither graded along the tonotopic axis nor positively regulated by Shh signaling in the developing mouse cochlea (Figs. 3 and 4). Instead, both Bmp7 and Bmp4 are down-regulated by constitutively activated Shh signaling in the mouse cochlea (Fig. 4), indicating that Shh regulates Bmps differently between avian BP and mouse organ of Corti.
What are the downstream effectors of Shh signaling that establish tonotopy in the mouse organ of Corti? One candidate effector is Activin, another member of TGFβ superfamily. In the developing mouse cochlea, Inhba (encoding a subunit of the Activin complex), is expressed in a basal-to-apical gradient, whereas Fst (encoding an antagonist for TGFβ/BMP signaling) is expressed in an opposing apical-to-basal gradient (Fig. 4). This relationship could generate a basal-to-apical gradient of Activin signaling that is complementary to and under the regulation of the apical-to-basal Shh signaling gradient. In support of this notion, Fst has been shown to be a direct target of Gli2 (a transcriptional activator of Shh signaling) in epidermal cells (26). In addition, Activin induces neuronal differentiation by inhibiting Shh signaling and provides positional cues to promote a specific cortical interneuron identity (27). Furthermore, our preliminary microarray data indicate that Fst is strongly down-regulated in Shh null otocysts compared with wild-type controls. The direct involvement of Activin/Fst in mediating tonotopic organization of the mouse cochlea will require further investigation. Nevertheless, the aforementioned model implies that, whereas members of the TGFβ superfamily are downstream of Shh in mediating tonotopic organization in both birds and mammals, the required TGFβ gradient is inverted between the developing BP and cochlea: the chicken BP demonstrates an increasing basal–apical Bmp7 gradient, whereas the mammalian cochlea demonstrates a decreasing basal–apical Activin gradient. Perhaps this finding is not surprising because the BP and organ of Corti are structurally different, and there are many precedents for differences along the tonotopic axes between these two organs. For example, Calbindin is expressed at higher levels in the base of chicken BP (Fig. 3) (22), whereas it is expressed at higher levels in the apex of guinea pig and gerbil organ of Corti (28, 29). In addition, A2m, which is strongly expressed in the base of the mouse cochlea (Fig. 4), is expressed at much higher levels in the apical half of the chicken BP (4). The significance of these opposing expression patterns and their relationship to tonotopic organization will likely be better understood when the functions of these proteins are defined within the hair cells that express them.
Temporal Requirements of Shh Signaling in Tonotopic Organization.
Several lines of evidence suggest that the regional identity that prefigures the tonotopic organization is established early in development by Shh secreted by the floor plate and notochord rather than by the spiral ganglion. First, differential gene expression patterns in the cochlear auditory anlage are evident early in the cochlea, slightly earlier than Shh expression is detectable in the ganglion (Fig. S4). Second, perturbation of Shh signaling in ovo before E4.5 is sufficient to cause a shift in the tonotopic organization of the BP. In vitro results indicate that the regional identity of the BP is established by E6.5 (6), at which time there is no detectable Shh expression in the auditory ganglion yet. Third, regional cochlear patterning does not appear to be affected in mouse mutants with only the absence of the later spiral ganglion source of Shh (Fig. 5). How the differential regional expression patterns translate into a tonotopically organized organ remains unclear. Whereas the later spiral ganglion source of Shh in mice regulates the timing of cell cycle exit and differentiation of cochlear hair cell precursors (15, 30), the earlier perturbation of Shh signaling conducted here in chicken did not seem to affect the timing of hair cell differentiation based on Atoh1 expression (Fig. S8).
It is also unclear whether the later source of Shh secreted by the spiral ganglion contributes to the tonotopic organization. There are several examples of systems in which the initial exposure to a Shh gradient specifies regional identity in the primordial structure, which is then reinforced by prolonged exposure to Shh. For example, in the neural tube, Shh proteins are initially provided by the notochord, and after distinct neuronal subtypes have been specified along the dorsoventral axis of the neural tube, a second Shh source is established in the floor plate, which supports ongoing neural tube development such as maintaining specific neural progenitor cell numbers and regulating gliogenesis (31, 32). Similarly, Shh-mediated digit specification along the anteroposterior axis in the limb bud occurs within the first 12 h of initial Shh production at the zone of polarizing activity, yet graded Shh signaling is continuously required to generate the required number of cartilage progenitor cells (33). Besides Shh signaling, transient exposure to FGF signaling is sufficient to specify the entire proximodistal axis during limb–bud outgrowth, yet the progressive expansion into specific limb segments requires prolonged FGF signaling (34, 35). Thus, there is precedence for a model in which sequential signaling from the midline and spiral ganglion sources of Shh is required for tonotopic patterning of the cochlea. The requirement for consecutive days of Shh delivery in ovo to elicit a modulation of the normal tonotopic patterning supports this hypothesis (Figs. 1 and 2). Alternatively, it is equally likely that other signaling molecules, yet to be identified, are involved in tonotopic organization.
This study provides in vivo evidence for an extrinsic role of Shh in mediating tonotopic organization of the chicken BP and mammalian organ of Corti. The cascade of molecular events leading to the mature tonotopically organized organ is complex. Identification of direct downstream effectors that mediate distinct roles for Shh signaling in context- and stage-dependent manners will facilitate our understanding of how the “pitch-perfect” peripheral auditory organ is constructed across species.
Materials and Methods
The details on mice, bead implantation, paint-fill injection, in situ hybridization, phalloidin staining, and scanning electron microscopy are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Lisa Cunningham for critical reading of the manuscript; Dr. HongKyung Kim, Mr. Tae-Jun Kwon, and Mr. Michael Mulheisen for technical assistance; and Dr. Jinae Lee for statistical analyses. This work is supported by the National Research Foundation of Korea Grant 2013R1A1A2007622 (to E.J.S.), Grants 2014R1A2A1A11051024 and 2013M3A9D5072551 (to J.B.), Faculty Research Grant 6-2014-0024 of Yonsei University College of Medicine (to J.B.), and the National Institute on Deafness and Other Communication Disorders Intramural Program (to D.K.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417856112/-/DCSupplemental.
References
- 1.Davis RL. Gradients of neurotrophins, ion channels, and tuning in the cochlea. Neuroscientist. 2003;9(5):311–316. doi: 10.1177/1073858403251986. [DOI] [PubMed] [Google Scholar]
- 2.Mann ZF, Kelley MW. Development of tonotopy in the auditory periphery. Hear Res. 2011;276(1-2):2–15. doi: 10.1016/j.heares.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Frucht CS, Uduman M, Kleinstein SH, Santos-Sacchi J, Navaratnam DS. Gene expression gradients along the tonotopic axis of the chicken auditory epithelium. J Assoc Res Otolaryngol. 2011;12(4):423–435. doi: 10.1007/s10162-011-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowalik L, Hudspeth AJ. A search for factors specifying tonotopy implicates DNER in hair-cell development in the chick’s cochlea. Dev Biol. 2011;354(2):221–231. doi: 10.1016/j.ydbio.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son EJ, et al. Developmental gene expression profiling along the tonotopic axis of the mouse cochlea. PLoS ONE. 2012;7(7):e40735. doi: 10.1371/journal.pone.0040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann ZF, et al. A gradient of Bmp7 specifies the tonotopic axis in the developing inner ear. Nat Commun. 2014;5:3839. doi: 10.1038/ncomms4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiede BR, et al. Retinoic acid signalling regulates the development of tonotopically patterned hair cells in the chicken cochlea. Nat Commun. 2014;5:3840. doi: 10.1038/ncomms4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16(18):2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132(9):2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- 10.Bok J, et al. Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development. 2007;134(9):1713–1722. doi: 10.1242/dev.000760. [DOI] [PubMed] [Google Scholar]
- 11.Brown AS, Epstein DJ. Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development. 2011;138(18):3967–3976. doi: 10.1242/dev.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bok J, Chang W, Wu DK. Patterning and morphogenesis of the vertebrate inner ear. Int J Dev Biol. 2007;51(6-7):521–533. doi: 10.1387/ijdb.072381jb. [DOI] [PubMed] [Google Scholar]
- 13.Groves AK, Fekete DM. Shaping sound in space: The regulation of inner ear patterning. Development. 2012;139(2):245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driver EC, et al. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28(29):7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bok J, Zenczak C, Hwang CH, Wu DK. Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc Natl Acad Sci USA. 2013;110(34):13869–13874. doi: 10.1073/pnas.1222341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie J, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38(4):195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124(13):2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 19.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10(3):301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 20.Tilney LG, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol. 1983;96(3):807–821. doi: 10.1083/jcb.96.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilney LG, Tilney MS, Saunders JS, DeRosier DJ. Actin filaments, stereocilia, and hair cells of the bird cochlea. III. The development and differentiation of hair cells and stereocilia. Dev Biol. 1986;116(1):100–118. doi: 10.1016/0012-1606(86)90047-3. [DOI] [PubMed] [Google Scholar]
- 22.Hiel H, Navaratnam DS, Oberholtzer JC, Fuchs PA. Topological and developmental gradients of calbindin expression in the chick’s inner ear. J Assoc Res Otolaryngol. 2002;3(1):1–15. doi: 10.1007/s101620010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navaratnam DS, Escobar L, Covarrubias M, Oberholtzer JC. Permeation properties and differential expression across the auditory receptor epithelium of an inward rectifier K+ channel cloned from the chick inner ear. J Biol Chem. 1995;270(33):19238–19245. doi: 10.1074/jbc.270.33.19238. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Owen T, Zhang L, Zuo J. Dynamic expression pattern of Sonic hedgehog in developing cochlear spiral ganglion neurons. Dev Dyn. 2010;239(6):1674–1683. doi: 10.1002/dvdy.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- 26.Eichberger T, et al. GLI2-specific transcriptional activation of the bone morphogenetic protein/activin antagonist follistatin in human epidermal cells. J Biol Chem. 2008;283(18):12426–12437. doi: 10.1074/jbc.M707117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cambray S, et al. Activin induces cortical interneuron identity and differentiation in embryonic stem cell-derived telencephalic neural precursors. Nat Commun. 2012;3:841. doi: 10.1038/ncomms1817. [DOI] [PubMed] [Google Scholar]
- 28.Imamura S, Adams JC. Immunolocalization of peptide 19 and other calcium-binding proteins in the guinea pig cochlea. Anat Embryol (Berl) 1996;194(4):407–418. doi: 10.1007/BF00198543. [DOI] [PubMed] [Google Scholar]
- 29.Pack AK, Slepecky NB. Cytoskeletal and calcium-binding proteins in the mammalian organ of Corti: Cell type-specific proteins displaying longitudinal and radial gradients. Hear Res. 1995;91(1-2):119–135. doi: 10.1016/0378-5955(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 30.Tateya T, et al. Hedgehog signaling regulates prosensory cell properties during the basal-to-apical wave of hair cell differentiation in the mammalian cochlea. Development. 2013;140(18):3848–3857. doi: 10.1242/dev.095398. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135(6):1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- 32.Yu K, McGlynn S, Matise MP. Floor plate-derived sonic hedgehog regulates glial and ependymal cell fates in the developing spinal cord. Development. 2013;140(7):1594–1604. doi: 10.1242/dev.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, et al. Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell. 2008;14(4):624–632. doi: 10.1016/j.devcel.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418(6897):501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 35.Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418(6897):539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.