Significance
Ricin toxin (RT) is a CDC-designated select agent that can be dispersed as an aerosol. In mammals, aerosolized RT causes rapid and irreversible necrosis of the lung epithelium, multifocal hemorrhagic edema, and death within 24–36 h. A safe and effective recombinant subunit vaccine (RiVax) has been developed and formulated as a thermostable, lyophilized, adjuvant-containing “powder.” This formulation of RiVax elicited neutralizing antibodies against RT, which protected macaques from the lethality of aerosolized RT. The epitope-specific antibody responses in macaques revealed a distinctive profile that was also observed in vaccinated humans. This profile might represent a signature of protection in both species.
Keywords: ricin, vaccine, monoclonal antibody, rhesus macaques, immunoprofiling
Abstract
Ricin toxin (RT) is the second most lethal toxin known; it has been designated by the CDC as a select agent. RT is made by the castor bean plant; an estimated 50,000 tons of RT are produced annually as a by-product of castor oil. RT has two subunits, a ribotoxic A chain (RTA) and galactose-binding B chain (RTB). RT binds to all mammalian cells and once internalized, a single RTA catalytically inactivates all of the ribosomes in a cell. Administered as an aerosol, RT causes rapid lung damage and fibrosis followed by death. There are no Food and Drug Administration-approved vaccines and treatments are only effective in the first few hours after exposure. We have developed a recombinant RTA vaccine that has two mutations V76M/Y80A (RiVax). The protein is expressed in Escherichia coli and is nontoxic and immunogenic in mice, rabbits, and humans. When vaccinated mice are challenged with injected, aerosolized, or orally administered (gavaged) RT, they are completely protected. We have now developed a thermostable, aluminum-adjuvant–containing formulation of RiVax and tested it in rhesus macaques. After three injections, the animals developed antibodies that completely protected them from a lethal dose of aerosolized RT. These antibodies neutralized RT and competed to varying degrees with a panel of neutralizing and nonneutralizing mouse monoclonal antibodies known to recognize specific epitopes on native RTA. The resulting antibody competition profile could represent an immunologic signature of protection. Importantly, the same signature was observed using sera from RiVax-immunized humans.
Ricin toxin (RT) is made by the plant Ricinus communis, which grows worldwide. RT can be easily prepared from pulverized castor beans and is very toxic even in crude form (1). Because of its prevalence and ease of preparation, RT is listed on the CDC Select Agent and Toxins list. RT consists a 32-kDa A chain (RTA) linked by a disulfide bond to a 34-kDa B chain (RTB) (2–4). RTA is a catalytic class II ribosome inactivating protein, RTB is a galactose-specific lectin. The LD50 of RT varies according to the route of exposure. Administered as an aerosol, RT has an LD50 of 5–15 μg/kg (5).
Although both RTA and RTB are immunogenic, most experimental vaccines against RT have used some form of RTA; protection is mediated by antibodies. The leading vaccine candidates at this time are RVEc, developed by the Department of Defense (6, 7), and RiVax, developed at the University of Texas Southwestern (8–11). RiVax is a recombinant RTA with two mutations (V76M, Y80A) that eliminate both its enzymatic activity and its ability to induce vascular leak syndrome in humans (8, 12). The crystal structure of RiVax is virtually identical to that of native RTA, indicating that the two amino acid mutations have a minimal effect on the tertiary structure of the protein (13). The majority of the conformational epitopes should therefore be intact (13). The recombinant protein is a minimum of 103-fold less toxic than RTA and 106-fold less toxic than ricin (8). When injected into mice, rabbits, or humans, RiVax formulated without an adjuvant and RiVax adsorbed to aluminum hydroxide adjuvant (alum) are both immunogenic and safe. The vaccine also protects mice from RT delivered by injection, intragastric gavage, or aerosol (14).
The other vaccine candidate, RVEc, is also a mutant recombinant RTA that has been engineered to remove the hydrophobic carboxyl-terminal region of RTA as well as an N-terminal (residues 34–43) hydrophobic loop, resulting in a smaller molecule with increased solubility and possible thermal stability (6, 7, 15, 16). Although the RVEc mutant protein is truncated, it also elicits protective antibodies in mice (6).
Because an RT vaccine cannot be tested for efficacy in humans, reliable animal models must be used to establish immunological correlates of protection (17, 18). Passive protection studies in mice with RT-neutralizing MAbs have suggested that most of the protective antibodies recognize conformational determinants (19, 20). We have recently developed a lyophilized thermostable adjuvant formulation of RiVax that appears to retain its tertiary structure and, hence, its conformational epitopes (21). In the study reported here, we have tested this formulation of RiVax for its ability to protect rhesus macaques (Macaca mulatta) against lethal doses of aerosolized RT. We have also evaluated both the total and neutralizing antibody titers and the ability of serum antibodies from these animals to competitively inhibit the binding of a small panel of MAbs to RTA. The latter evaluation was carried out to determine whether there was an epitope-specific antibody profile/signature that would correlate with protection. Finally, we have compared this MAb reactivity profile with that from a panel of human sera from a previous clinical trial. Our results demonstrate that this thermostable adjuvanted vaccine formulation protects animals against a lethal aerosol RT challenge. The antibody signature is very similar in all of the protected animals. Furthermore, this profile is virtually identical to that of humans immunized with RiVax, suggesting that humans and macaques respond to similar epitopes on RiVax.
Results
Vaccine.
Although ricin holotoxin is stable, isolated RTA is unstable in an aqueous milieu, rapidly forming macromolecular aggregates after disruption of tertiary structure (22). To stabilize RiVax on the surface of alum for potential long-term storage and thermostability, we developed a process to lyophilize the adjuvant and protein complex in the presence of glassifying excipients to preserve its conformation (21).
Vaccination and Aerosol Challenge.
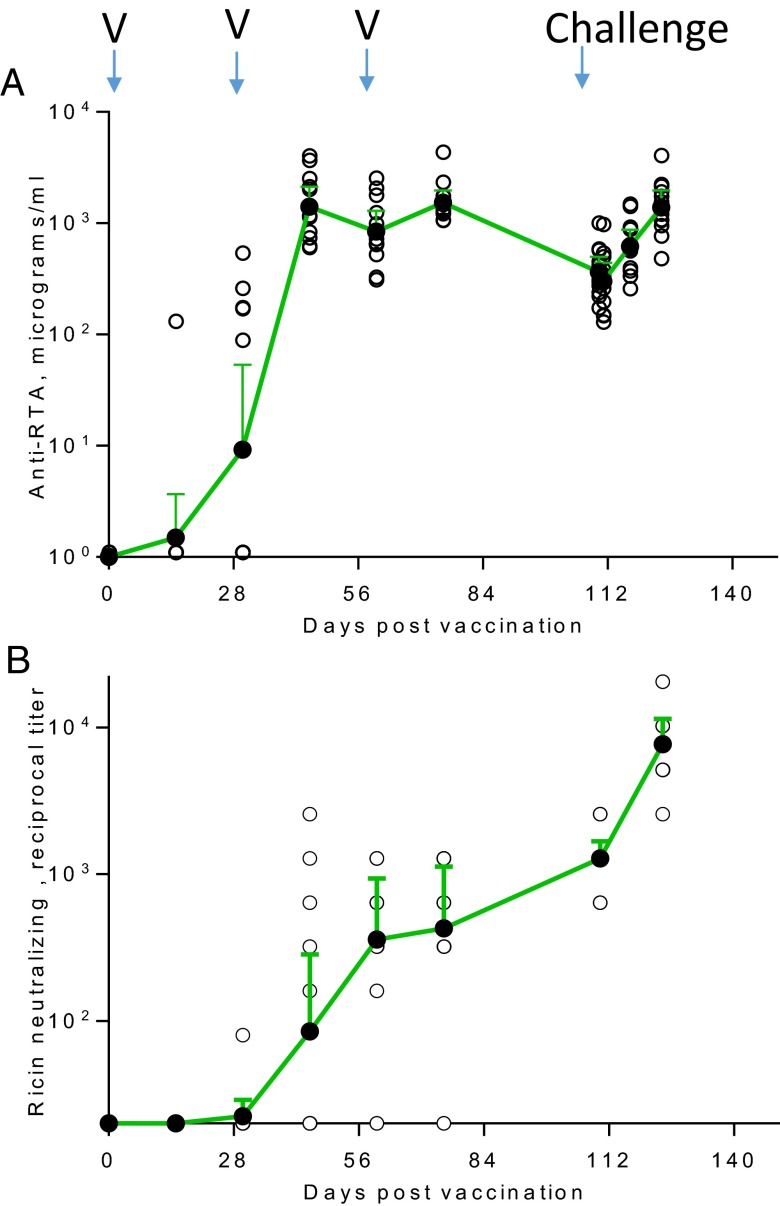
Rhesus macaques were vaccinated three times at monthly intervals with lyophilized vaccine reconstituted with water. Seven weeks after the third vaccine dose, animals were challenged with a target dose of 3 LD50 of RT (18 µg/kg). The actual inhaled dose for all control and vaccinated animals ranged from 12 to 38 µg/kg, and is equivalent to 1.5–6.5 of the nominal aerosol LD50 (Fig. 1A). Twelve to 30 h after aerosol challenge, control animals exhibited one or more of the following clinical signs: progressive anorexia, dyspnea, labored breathing, varying degrees of tachypnea, and hunched posture. Control group animals that exhibited signs consistent with RT intoxication were euthanized; median time to euthanasia was 42 h. Eleven of 12 vaccinated animals survived challenge and none showed any symptoms experienced by animals in the control group (Fig. 1B). One vaccinated animal was euthanized 7 d after exposure because of complications from bacterial pneumonia. The death of this animal might have been related to lung compromise.
Fig. 1.
Vaccination and challenge. Twelve macaques were vaccinated with 0.5 mL of lyophilized RiVax (100 µg) reconstituted with water immediately before injection at 0, 30, and 60 d. Six macaques were sham-vaccinated at the same time. Macaques were challenged 7 wk after the last vaccination with aerosolized RT (day 110). (A) Inhaled RT doses, vaccinated animals, blue symbols; sham-vaccinated animals, red symbols; two tailed t test, P = 0.68. (B) Time to death in vaccinated and sham-vaccinated rhesus macaques, vaccinated animals; sham-vaccinated animals; log-rank test, P < 0.001.
Telemetry of Physiological Function.
Wireless telemetry devices were implanted in 8 of 12 vaccinated animals and 4 of 6 unvaccinated control animals to continuously measure parameters of electrocardiogram, heart rate, and body temperature (23). Significant physiological changes occurred after RT exposure in the four monitored control animals. These changes included increases in core temperature within 12 h of exposure and severe hypothermia within 24 h (Fig. 2A). Control animals also exhibited significant elevations in heart rate as well as a disruption in the normal diurnal patterns within the first 12 h of exposure (Fig. 2B). In contrast, the monitored vaccinated macaques showed less deviation from normal compared with unvaccinated controls. In vaccinated animals, diurnal rhythms returned to normal within 48 h.
Fig. 2.
Physiological responses of vaccinated and sham-vaccinated macaques after exposure to RT aerosol. Changes (Δ) in body temperature (C°) (A) and heart rate (B) in vaccinated macaques (blue symbols) and control sham-vaccinated macaques (red symbols) from baseline following challenge with aerosolized RT. Inset of A is fever intensity between vaccinated and control macaques (P < 0.03).
Pathology.
Gross lesions were confined primarily to the lung and tracheobronchial lymph nodes of all of the nonvaccinated control animals. Their lungs were heavy and wet, weighing 80–152 g (normal = 20–40 g) with fluid in the large airways and mottled with patchy, dark red, firm hemorrhagic zones, and peripheral pale hyperinflated zones. No discernible gross lesions were noted in the nasal cavity. Peribronchial nodes and the mediastinum were edematous.
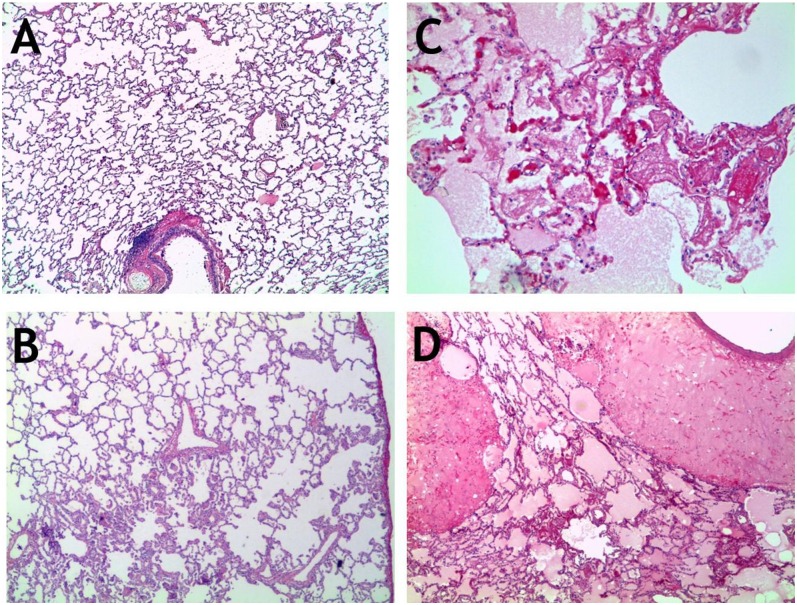
Histologically, lungs of the nonvaccinated animals exhibited severe extensive perivascular and alveolar edema, variable amounts of fibrin deposition, and neutrophillic infiltration mixed with smaller numbers of macrophages in and around terminal bronchioles (Fig. 3). In a few of the control animals, scattered areas of bronchiolar epithelial necrosis were noted. The most prominent pathological feature in the control animals was flooding of alveolar, perivascular, and peribronchial/bronchiolar spaces with large amounts of eosinophillic, proteinaceous fluid (edema) containing abundant fibrin strands admixed with large numbers of degenerate and viable neutrophils and lesser numbers of lymphocytes, plasma cells, and alveolar macrophages (Fig. 3). These spaces frequently contained erythrocytes (hemorrhage). The terminal bronchioles, alveolar epithelium, and alveolar spaces were the most affected. The alveolar septa were often expanded by fibrin, fluid, and aforementioned inflammatory infiltrate. The cellular debris and degenerate neutrophils were frequently present in the alveolar spaces admixed with fluid. The upper respiratory tract showed mild inflammatory infiltration. Medullary sinuses of peribronchial lymph nodes were edematous and occasionally infiltrated with neutrophils.
Fig. 3.
Histopathology. Sections of lungs from animals either vaccinated with RiVax (A and B) or sham-vaccinated (C and D) and then exposed to a lethal dose of aerosolized RT. (A) Otherwise unremarkable normal lung; (B) mild hyperplasia and focal inflammation; (C) marked edema and lung fibrin accumulation; (D) massive edema and associated inflammation. (Magnification: A, B, and D, 20×; C, 100×.)
In contrast to the lungs of control animals, animals surviving until necropsy at 14 d after exposure to RT were grossly normal, except for occasional mild multifocal proliferation of fibroblasts with collagen deposition around terminal respiratory bronchioles, which were often lined by hyperplasic epithelium. These areas of the lung were occasionally infiltrated with small numbers of lymphoid cells, suggesting resolving epithelial damage and an ongoing immune response (Figs. 2 and 3 A and B). Consistent with this finding, peribronchial lymph nodes were hyperplasic.
All other tissues examined were free of significant lesions in all vaccinated and control animals.
Immunogenicity.
Antibody responses against RiVax were measured by ELISA. Fig. 4 shows the levels of IgG anti-RiVax 2 wk after each immunization, and for the 14-d period following RT exposure. Five of 12 animals seroconverted 30 d after the first vaccination and all seroconverted 2 wk after the second vaccination [geometric mean titer (GMT) = 140 µg/mL, range 60–4,026 µg/mL]. Anti-RTA antibodies declined following the third immunization (on day 60). Seven weeks after the third vaccination (day 110), antibody titers were ∼20% of the peak level determined on day 75 (2 wk after the third vaccination) (Fig. 4A and Table 1). The challenge with RT boosted the anti-RTA antibody responses so that they were comparable to peak pre-exposure levels.
Fig. 4.
Total IgG and neutralizing antibodies in vaccinated macaques. (A) Total anti-RTA antibodies determined by ELISA against RiVax at indicated times following primary immunization. Time of vaccination is designated by “V”. (B) Representation of reciprocal dilutions of neutralizing antibody titers. In both A and B, the titers of individual animals are depicted by unfilled circles; the filled circles are the geometric mean titers with 95% confidence interval (CI).
Table 1.
Anti-RTA responses in vaccinated animals
| Days after first vaccination | 15 | 30 (v) | 45 | 60 (v) | 75 | 110 (c) | 124 |
| ELISA, total IgG, µg/mL–GMT (95% CI) range | 1.5 (0.6–3.7) 0–130 | 9.2 (1.6–53) 0–259 | 1401 (920-2137) 60–4026 | 839 (545-1293) 311–2534 | 1534 (1190-1977) 1216–4360 | 364 (267-499) 173–1004 | 1383 (967-1979) 482–4043 |
| Seroconversion rate* | 1/12 | 5/12 | 12/12 | 12/12 | 12/12 | 12/12 | 12/12 |
| Neutralizing antibodies–GMT† (95% CI) range | 20 NA | 22 (20-29) 20–80 | 85 (24-285) 20–2560 | 359 (139-933) 20–1280 | 427 (163-1132) 20–1280 | 1280 (982-1669) 640–2560 | 7670 (5160-11404) 2560–20480 |
| Seroconversion rate‡ | 0/12 | 1/12 | 5/12 | 10/12 | 10/12 | 12/12 | 12/12 |
Seroconversion rates were defined as antibodies above 0 µg/mL, the background of the assay.
The background value was 20.
Seroconversion to neutralizing antibodies is defined as titers greater than 4× background at 1:20, the dilution yielding 50% survival of Vero cells cultured with RT.
Neutralizing Antibody Responses.
Neutralizing antibodies were induced in all of the vaccinated animals. However, the appearance of these antibodies lagged slightly behind that of total antibodies such that fewer than 50% (5 of 12) of the animals had detectable neutralizing antibodies 2 wk after the second vaccination (Fig. 4B), whereas 10 of 12 of the animals had neutralizing antibodies at the time of the third vaccination on day 60 and all of the animals had neutralizing antibodies before RT challenge (Table 1). These results suggest that either the ELISA for total antibodies was more sensitive than the neutralization assay, that neutralization required higher levels of antibodies, or that neutralizing antibodies developed later. None of the nonvaccinated control macaques developed anti-RiVax antibodies. Finally, following exposure to RT, anti-RTB antibodies might have been made, and these could have contributed to neutralization.
Antibodies Against Known Epitopes on RTA.
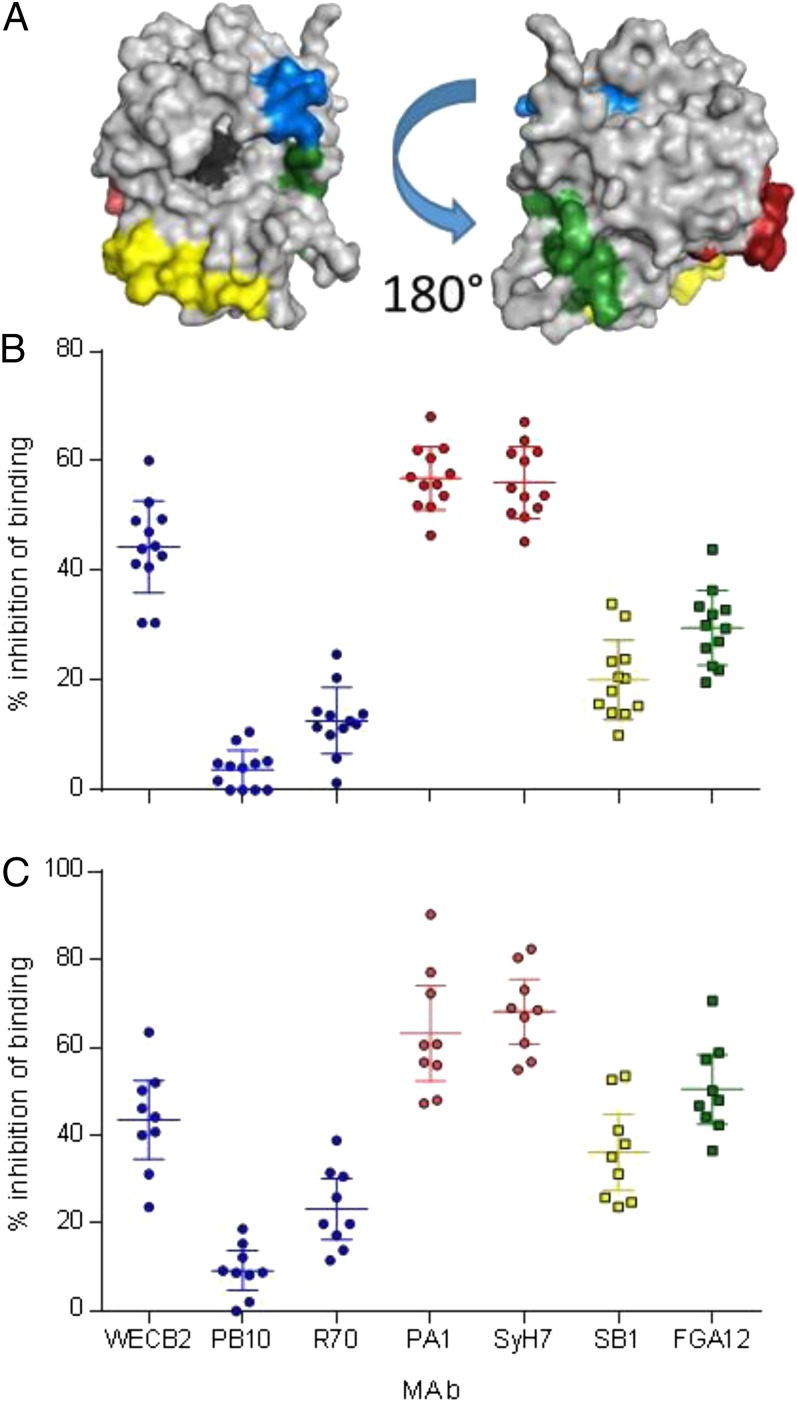
We have previously described a collection of MAbs that recognize both toxin-neutralizing and nonneutralizing B-cell epitope clusters on RTA (19, 20). For example, two nonneutralizing MAbs, FGA12 and SB1, recognize linear epitopes in the N terminus and C terminus of RTA, respectively (20). Neutralizing MAbs WECB2, PB10, and R70 recognize epitopes localized around residues 98–108 (cluster 1), whereas neutralizing MAbs PA1 and SyH7 recognize epitopes around residues 187–198 (cluster 2) (Fig. 5A).
Fig. 5.
Evaluation of epitope utilization in rhesus macaques and humans vaccinated with RiVax. Macaques were vaccinated with reconstituted lyophilized RiVax as described, and human volunteers were vaccinated with liquid suspension RiVax freshly adsorbed to alum (11). (A) PyMol diagram of epitope localization, blue circular symbols, neutralizing cluster 1 epitopes (19); red circular symbols, neutralizing cluster 2 (19); square yellow symbols nonneutralizing epitope residues 223–240; square green symbols, nonneutralizing epitope residues 37–48. (B) Competition of macaques sera pre challenge (day 110) against mouse MAb panel, with color coded symbols as in A, WECB2 vs. PB10/R70, P < 0.001. (C) Competition of human sera with MAb panel, with color coded symbols as in A, two-tailed t test, WECCB2 vs. PB10/R70 PA1/SyH7 vs. PB10/R70, P < 0.001 for both human and macaque comparisons.
To determine whether vaccinated animals elicited antibodies against these epitopes, we performed competitive ELISAs in which prechallenge sera were tested for their ability to block the binding of the panel of MAbs to RiVax (19, 20). Sera from RiVax-vaccinated macaques were relatively effective (∼60%) at inhibiting cluster 2 neutralizing MAbs PA1 and SyH7 from binding to plate-bound RiVax, and slightly less effective at inhibiting the cluster 1 MAb WECB2 (∼40%). The sera were largely ineffective at inhibiting the binding of MAbs PB10 and R70 to RiVax (Fig. 5B). Sera from vaccinated animals also partially inhibited (∼40–50%) the two nonneutralizing MAbs, FGA12 and SB1. These results indicate that the animals elicited antibodies against known neutralizing and nonneutralizing B-cell epitopes on RTA.
We also performed competitive MAb binding assays with serum samples from nine humans who had been vaccinated with RiVax adsorbed to alum as part of a recent clinical trial (11). The human sera displayed virtually the same MAb inhibition profile as observed in the RiVax-vaccinated macaques (Fig. 5C). These results support the use of macaques as an animal model for determining the efficacy of RiVax according to the Food and Drug Administration animal rule (24).
Discussion
The development of a prophylactic ricin vaccine has been a long-term objective of the military. However, no vaccine has been approved and added to the National Vaccine Stockpile. We have previously described a recombinant subunit vaccine called RiVax, which is safe and immunogenic in mice, rabbits, and humans. It protects mice against lethal doses of injected, aerosolized, and gavaged RT (14, 25, 26). The vaccine has been formulated as a thermostable lyophilized adjuvanted “cake” that is dissolved in water before immunization. The objective of this study was to examine the efficacy of this vaccine in rhesus macaques and to determine whether there was a signature of immunity in protected macaques that might be extrapolated to humans.
The findings to emerge from this study are as follows: (i) three doses of the vaccine elicited neutralizing antibodies that completely protected macaques against a lethal challenge of aerosolized RT; (ii) all animals seroconverted and neutralizing antibodies were present after three vaccinations; (iii) before aerosol challenge, the sera from all animals showed a distinctive antibody profile against epitopes defined by a previously characterized panel of MAbs; and (iv) the antibody profile was almost identical to that observed using sera from humans immunized with RiVax/alum in a previous clinical trial (11). To our knowledge, this is the first published report demonstrating protection of nonhuman primates against aerosolized RT by vaccination with a recombinant RTA vaccine. Importantly the antibody signature in the protected macaques was also observed in sera from humans who received RiVax. This finding suggests that rhesus macaques represent an effective model for defining a correlate of protection in vaccinated humans.
The current vaccine is an alum-adsorbed vaccine, but lyophilized with a glassifying excipient and reconstituted with water immediately before use (21). Lyophilization results in long-term stability that will be important for stockpiling. Lyophilization also results in the preservation of conformational epitopes on RTA. This formulation of RiVax is biochemically stable at 40 °C for at least 1 y (21). When used in mice, it remained fully immunogenic and protective (21).
In these studies we used a severe model of aerosolized RT intoxication (LD50 = 5.8 µg/kg) that resulted in a lethal outcome in sham-vaccinated animals within 28–48 h of aerosol exposure (5). Control animals had distinct elevations in heart rate accompanied by elevated core temperature following aerosolization. Before death, the animals developed severe, diffuse, necrotizing bronchiolitis and alveolitis with massive alveolar, perivascular and peribronchial/bronchiolar edema shortly after the challenge. In contrast, animals that received vaccine generally experienced less fever and fewer aberrations in heart rate after RT challenge; other adverse events were also minimal, suggesting only low levels of RT-induced pulmonary inflammation. However, one vaccinated macaque was euthanized 7 d after RT aerosol exposure; follow-up clinical pathology on this animal indicated bacterial pneumonia. To what extent this was related to lung compromise is unknown.
Importantly, in an attempt to define an immunological correlate of protection, we determined whether vaccinated animals made antibodies against known protective epitopes on RTA. It has been estimated that the panel of neutralizing and nonneutralizing MAbs used in this study define epitopes that span roughly 50% of the surface of RTA and ∼75% of the total number of predicted B-cell epitopes, based on programs such as Emini Surface Accessibility, Discotope, and ElliPro (19, 20). Our studies revealed that macaques responded to two neutralizing conformational determinants recognizing cluster 1 and cluster 2 (defined by MAbs WECB2, and PA1), implying that the conformational regions were preserved and both immunogenic and protective. Surprisingly, macaques generated only low levels of antibodies against known linear epitopes overlapping the cluster 1 conformational epitope (WECB2). Both cluster 1 MAbs PB10 and R70 recognize an α-helical linear determinant (residues 97–108) on the surface of RTA, but macaques made only low levels of antibodies that competed effectively for binding to these epitopes. In mice MAb, R70 defines an immunodominant epitope. In addition, PB10 has been humanized and developed in a plant expression system and shown to passively protect mice against exposure to aerosolized RT (27). In the case of cluster 2 antibodies, macaques developed similar responses between SyH7 (residues187–198) and its cognate conformational MAb as well as antibodies against nonneutralizing linear epitopes in residues 37–48 (FGA12) and residues 223–240 (SB1). Although residues 98–108 might be immunodominant in mice, this epitope might not be immunodominant in macaques or humans.
Perhaps more striking is the observation that humans develop anti-RTA with an epitope utilization pattern identical to that observed in macaques. The RiVax used in the human study was similar to the one used here except that it was an aqueous formulation freshly prepared and adsorbed to alum immediately before each vaccination (11). Further clinical development of RiVax will involve experiments aimed at dose-finding for optimal protection of the lungs and further dissecting the immunologic correlate of protection observed in these studies.
Materials and Methods
Preparation of Vaccine.
RiVax was derived from a single lot of 100 L of an E. coli fermentation and stored in 50% (vol/vol) glycerol at −20 °C (lot 190-FF-100L-090105, Lonza). The stock was dialyzed before adsorption of the antigen to aluminum hydroxide (Alhydrogel, E.M. Sergeant Adjuvants). RiVax was prepared by adsorbing 200 µg of antigen to 1 mg/mL alum equivalents as hydroxide in 10 mM histidine and 144 mM NaCl (pH 6.0) with 8% (wt/vol) trehalose and lyophilized, as described previously (21). The final vaccine was manufactured by Nanotherapeutics (Alachua, FL). A single lot of lyophilized vaccine consisting of >500 3-mL vials for reconstitution with 1 mL of WFI was used for vaccination.
Toxin.
Purified RT, derived from R. communis, was produced from castor beans at the University of Texas Southwestern (8) and was obtained from BEI resources.
Animal Husbandry and Telemetry.
Rhesus macaque were born and housed at the Tulane National Primate Research Center (Covington, LA), which is US Department of Agriculture-licensed and fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Subcutaneous radiotelemetry transmitters combined with sensors capable of detecting biopotential signals of an electrocardiogram as well as thermistor-type sensors capable of detecting temperature signals (T34G-8; Konigsberg Instruments) were surgically implanted under aseptic conditions in 8 of the 12 vaccinated macaques and 4 of 6 control macaques before the start of the study. Animals determined to be in respiratory distress and those that survived for 14 d after exposure to RT were euthanized by an overdose of sodium pentobarbital, consistent with the recommendation of the American Veterinary Medical Association’s Panel on Euthanasia, and submitted for necropsy. All methods were approved by the Tulane Institutional Animal Care and Use Committee.
Vaccination of Macaques.
Animals received three intramuscular vaccinations of 100 µg of reconstituted RiVax (n = 12) or PBS (n = 6) in 500 μL at monthly intervals. The vaccinated animals were observed for signs of adverse reactions after each vaccination. Animals were bled before treatment, 2 or 3 wk after each vaccination, just before the next vaccination, and just before RT challenge. Following aerosol challenge, blood was also collected when the animals either succumbed to intoxication or 14 d after challenge when the experiment was terminated.
RT Aerosolization, Dosing, and Calculation.
Aerosolization, dosing and delivery of RT were performed as described previously (28). Inductive plethysmography that measures volume of air breathed by each individual animal per minute was performed just before the RT exposure. RT was dissolved in 10 mL sterile phosphate buffer saline to the desired concentration for each animal based on plethysmography data obtained 2 d before the exposure. The aerosol were generated directly in the head-only chamber using a Collision three jet-nebulizer (BGI) with fully automated management control system (Biaera) all within a Class III biological safety cabinet housed within the Tulane National Primate Research Center high-containment (BSL-3) laboratories. The nebulizer operated at 18 lb/inch2 equating to a flow of 7.51 L/min and produced 3.0E + 04 particles per cc with a mass median aerodynamic diameter of ∼1.4 µm. The aerosol exposure lasted 10 min. Air samples were continuously obtained during the exposure and the protein concentrations of these samples were determined using a micro-BSA protein assay kit (Thermo Scientific). The aerosol concentrations were determined and the inhaled dose of RT for each animal was calculated by multiplying the empirically determined aerosol exposure concentration (microgram per liter of air) in the chamber by volume of air estimated to have been breathed by the animal (via results of plethysmography just before exposure). The LD50 of RT was 5.8 µg/kg body weight (5) and the target dose for this experiment was set at the equivalent of three LD50s (18 µg/kg). The mean inhaled dose of RT across all animals was 4.4 ± 1.4 LD50s.
Statistics.
Statistical analysis was carried out with GraphPad Prism 6 (GraphPad Software). The difference in immunological responses and outcomes between groups was determined by Fisher’s exact test (two-tailed) and the mean survival times after exposure to RT were compared by log-rank analysis of Kaplan–Meier survival curves.
Tissue Collection, Histological Analysis, and Special Stains.
After gross necropsy, tissues were collected in neutral buffered zinc-formalin solution (Z-Fix Concentrate, Anatach). Tissues were processed, sectioned, and stained as previously described (5).
Neutralization Assays.
Sera were individually tested for their ability to protect Vero cells against RT intoxication in vitro using an adaptation of the XTT cell proliferation assay (ATCC). Neutralization was measured based upon the degree inhibition of RT-associated cell killing (10 ng/mL) from the presence of dilutions of serum. Cut-off values were set at the sum of the average absorbance readings from sera from naïve (prevaccinated) animals + three stand deviations of the values.
Purification of Anti-RTA Antibodies from Pooled Macaque Sera.
Sera from seropositive animals were pooled and centrifuged before use. The Ig was purified on protein G-Sepharose (GE Healthcare Bio-Sciences) (29). The specific anti-RTA antibodies were affinity purified on RTA-Sepharose. The eluted protein was concentrated, 0.22 μM filtered, and stored under sterile conditions at 4 °C. All Ig preparations were tested for purity under both nonreduced and reduced conditions by SDS/PAGE using 4–15% gels (30). The percentage of IgG in the Protein G-eluate that was specific antibody was determined by ELISA using the affinity purified anti-RTA The IgG anti-Rivax was then used as a standard curve in all subsequent experiments and values obtained from the standard curve were adjusted for the percentage of the total antibody preparation that was specific antibody.
ELISAs to Measure the Activity of Purified Rhesus Anti-RTA.
Wells of 96-well ELISA plates (Corning) were coated with10 µg/mL of RiVax. The plates were washed with PBS and blocked with Starting Block 100% (Thermo Scientific). Antibody samples, a positive control MAb anti-RTA (R70 clone, IgG1, k) (31) and an isotype-matched negative control, MOPC-21 (Sigma), were diluted in 1% Starting Block in PBS containing 0.01% Tween 20 (Sigma) (PBST20) and incubated for 1 h at room temperature. After washing the wells, the bound antibodies were detected with HRP-conjugated goat anti-rhesus macaque IgG (Southern Biotech) or a HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch). Plates were washed and developed with substrate 3,3′,5.5′ tetramethylbenzine (TMB) (Thermo Scientific) and the reaction was stopped with 2M sulfuric acid (Macron Fine Chemicals). The color intensities were measured using an ELISA plate reader (Molecular Devices) at a wavelength of 450 nm. Each sample was tested in triplicate and the experiments were carried out three to five times.
ELISA to Measure the Titer of Anti-RTA in Sera.
ELISAs were performed described as above. Serial dilutions of sera were prepared in 1% Starting Block in PBST20. The levels of anti-RTA antibodies in experimental sera were determined from a standard curve using the purified anti-RTA. Each sample was tested in triplicate and the experiments were carried out three times.
ELISA Competition Assay Using the Monoclonal Mouse Anti-RTAs.
Wells of microtiter plates were coated with RiVax and blocked as described above. Different concentrations of the MAbs were added and the plates were washed and developed. The 50% maximal binding concentration of each MAb was calculated from the plotted curve. The following MAb clones used were: R70 (IgG1), WECB2 (IgG1), PA1 (IgG1), PB10 (IgG2b), SyH7 (IgG1), SB1(IgG2a), and FGA12 (IgG1) (19, 20, 31). The 50% binding concentration for each MAb was then mixed for 15 min at room temperature with serum containing a 500× concentration of anti-RTA antibody as determined by ELISA or an equal concentration of normal rhesus macaque IgG (Innovative Research). In initial experiments, equal volumes of prevaccination sera from each animal were also included as controls. In the experiments presented here, we chose to present the experimental values minus the matched concentrations of normal rhesus IgG. After a 1-h incubation at room temperature, the bound mouse MAbs were detected with HRP-conjugated goat anti-mouse IgG. The plates were washed, developed, and read as described above. As a further negative control, buffer was used. The percentage blocking of the 50% binding of each MAb by the rhesus or human sera to RiVax was then calculated. This ELISA was performed in triplicate and the assay was repeated two to three times.
Epitope Modeling.
PyMOL (The PyMOL Molecular Graphics System, v1.3, Schrödinger) was used to model B-cell epitopes on RTA with Protein Data Bank ID code 2AAI from the Research Collaboratory for Structural Bioinformatics.
Acknowledgments
We thank Joanne O’Hara, Yinghui Rong, and Greta VanSlyke (Wadsworth Center) for technical assistance. This project was supported by National Institutes of Health Grants AI-082210 (to R.N.B.; Soligenix), A1-070236 (to E.S.V.), Contract HHSN272201400021C (to N.J.M.), and OD-011104-53 (Tulane National Primate Research Center Base Grant; to C.J.R.); and by the Horchow Foundation (E.S.V.).
Footnotes
Conflict of interest statement: E.S.V. is an inventor on patents concerning RiVax, which is owned by the University of Texas Southwestern and licensed exclusively to Soligenix, Inc.
References
- 1.Franz DR, Jaax NK. Ricin toxin. In: Sidell FR, Takafuji ET, Franz DR, editors. Textbook of Military Medicine. Office of the Surgeon General, Department of the Army; Washington, DC: 1997. pp. 631–642. [Google Scholar]
- 2.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987;262(12):5908–5912. [PubMed] [Google Scholar]
- 3.Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262(17):8128–8130. [PubMed] [Google Scholar]
- 4.Montfort W, et al. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987;262(11):5398–5403. [PubMed] [Google Scholar]
- 5.Bhaskaran M, et al. Pathology of lethal and sublethal doses of aerosolized ricin in rhesus macaques. Toxicol Pathol. 2014;42(3):573–581. doi: 10.1177/0192623313492248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carra JH, et al. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine. 2007;25(21):4149–4158. doi: 10.1016/j.vaccine.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 7.McLain DE, et al. Protective effect of two recombinant ricin subunit vaccines in the New Zealand white rabbit subjected to a lethal aerosolized ricin challenge: Survival, immunological response, and histopathological findings. Toxicol Sci. 2012;126(1):72–83. doi: 10.1093/toxsci/kfr274. [DOI] [PubMed] [Google Scholar]
- 8.Smallshaw JE, et al. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002;20(27-28):3422–3427. doi: 10.1016/s0264-410x(02)00312-2. [DOI] [PubMed] [Google Scholar]
- 9.Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine. 2005;23(39):4775–4784. doi: 10.1016/j.vaccine.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Vitetta ES, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci USA. 2006;103(7):2268–2273. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitetta ES, Smallshaw JE, Schindler J. Pilot phase IB clinical trial of an alhydrogel-adsorbed recombinant ricin vaccine. Clin Vaccine Immunol. 2012;19(10):1697–1699. doi: 10.1128/CVI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smallshaw JE, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21(4):387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 13.Legler PM, Brey RN, Smallshaw JE, Vitetta ES, Millard CB. Structure of RiVax: A recombinant ricin vaccine. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 9):826–830. doi: 10.1107/S0907444911026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007;25(42):7459–7469. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLain DE, Horn TL, Detrisac CJ, Lindsey CY, Smith LA. Progress in biological threat agent vaccine development: A repeat-dose toxicity study of a recombinant ricin toxin A-chain (rRTA) 1-33/44-198 vaccine (RVEc) in male and female New Zealand white rabbits. Int J Toxicol. 2011;30(2):143–152. doi: 10.1177/1091581810396730. [DOI] [PubMed] [Google Scholar]
- 16.Porter A, et al. Evaluation of a ricin vaccine candidate (RVEc) for human toxicity using an in vitro vascular leak assay. Toxicon. 2011;58(1):68–75. doi: 10.1016/j.toxicon.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin SA. Vaccines: Correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47(3):401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 18.Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56(10):1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 19.O’Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. Localization of non-linear neutralizing B cell epitopes on ricin toxin’s enzymatic subunit (RTA) Immunol Lett. 2014;158(1-2):7–13. doi: 10.1016/j.imlet.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hara JM, et al. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine. 2010;28(43):7035–7046. doi: 10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassett KJ, et al. Stabilization of a recombinant ricin toxin A subunit vaccine through lyophilization. Eur J Pharm Biopharm. 2013;85(2):279–286. doi: 10.1016/j.ejpb.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peek LJ, Brey RN, Middaugh CR. A rapid, three-step process for the preformulation of a recombinant ricin toxin A-chain vaccine. J Pharm Sci. 2007;96(1):44–60. doi: 10.1002/jps.20675. [DOI] [PubMed] [Google Scholar]
- 23.Roy CJ, et al. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014;209(12):1891–1899. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FDA (2014) Guidance for Industry: Product Development Under the Animal Rule. Available at www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf. Accessed January 15, 2015.
- 25.Smallshaw JE, Vitetta ES. A lyophilized formulation of RiVax, a recombinant ricin subunit vaccine, retains immunogenicity. Vaccine. 2010;28(12):2428–2435. doi: 10.1016/j.vaccine.2009.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smallshaw JE, Vitetta ES. Ricin vaccine development. Curr Top Microbiol Immunol. 2012;357:259–272. doi: 10.1007/82_2011_156. [DOI] [PubMed] [Google Scholar]
- 27.Sully EK, et al. Chimeric plantibody passively protects mice against aerosolized ricin challenge. Clin Vaccine Immunol. 2014;21(5):777–782. doi: 10.1128/CVI.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy CJ, Song K, Sivasubramani SK, Gardner DJ, Pincus SH. Animal models of ricin toxicosis. Curr Top Microbiol Immunol. 2012;357:243–257. doi: 10.1007/82_2011_173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng R, et al. The evaluation of recombinant, chimeric, tetravalent antihuman CD22 antibodies. Clin Cancer Res. 2004;10(4):1274–1281. doi: 10.1158/1078-0432.ccr-1154-03. [DOI] [PubMed] [Google Scholar]
- 30.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10(10):3542–3551. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 31.McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun. 2006;74(6):3463–3470. doi: 10.1128/IAI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]