Abstract
Background
Although elevated intraocular pressure is a major risk factor for the development of glaucoma, there is increasing evidence that the immune system may be involved in the development of normal-tension glaucoma (NTG). The aim of this study was to determine if NTG is associated with elevated levels of antibodies against human heat shock protein (HSP) 60.
Material/Methods
The study was conducted in 139 subjects (35 subjects with NTG [Group 1], 34 subjects with primary open-angle glaucoma/POAG/[Group 2], 24 subjects with autoimmune rheumatic diseases [Group 3], and 36 healthy controls [Group 4]). All subjects had complete ophthalmologic examination (visual acuity, slit-lamp examination, tonometry, gonioscopy; visual-field examination, and optical coherence tomography/OCT/of the optic nerve head and the macula). Blood samples were collected for the measurements of serum levels of antibodies against human HSP60.
Results
The subjects with rheumatic diseases had the highest median serum level of antibodies against HSP60 – 20.49 ng/mL. The values in the subjects with NTG, POAG, and in controls were 18.79 ng/mL, 18.61 ng/mL and 17.61 ng/mL, respectively (p=0.96).
Conclusions
This study does not confirm the hypothesis that normal-tension glaucoma is associated with elevated blood levels of antibodies against human heat shock protein (HSP) 60.
MeSH Keywords: Autoimmune Diseases, Chaperonin 60, Low Tension Glaucoma
Background
Glaucoma is a group of diseases characterized by chronic progressive optic neuropathy caused by degeneration of retinal ganglion cells and their axons, resulting in the typical appearance of the optic disc, which differentiates it from other optic neuropathies and their visual-field defects [1–4]. There is thinning of the neuroretinal rim and the central depression of the optic disk expands. The causes of optic disk cupping include the loss of retinal ganglion cells, glial cells, and blood vessels [1].
Although elevated intraocular pressure is considered a major risk factor for the development of glaucoma, immune system involvement in the neurodegenerative process has been increasingly emphasized. Numerous studies have been published that confirm the autoimmune component of glaucomatous optic neuropathy, with the immune system responsible for the degeneration of retinal ganglion cells and their axons [5], but other studies contradict this hypothesis [6].
Many of these studies concern the role of heat shock proteins (HSPs) in the pathogenesis of glaucoma. HSPs, also referred to as stress proteins, are named and classified into families according to their molecular mass. Because of considerable antigenic similarities between bacterial and human HSPs, HSPs are highly immunogenic, with the resulting impact on the development of autoimmune diseases via the phenomenon of antigenic mimicry [7–10]. HSPs have a protective role in cells, which start to produce large amounts of these proteins in response to stressors (cellular stress response). In neurological, inflammatory, degenerative, or neoplastic diseases, HSPs accumulate in cells of the nervous system, which confirms the fundamental role of HSPs for neuronal survival [11]. Immune responses to HSPs are involved in a number of human autoimmune diseases, such as atherosclerosis, arthritides, type 1 diabetes mellitus, and multiple sclerosis [10–13]. In glaucomatous eyes, chronic cellular stress has been confirmed in the retina and optic nerve head by the finding of markedly elevated levels of HSP60 and HSP27, which suggests that these proteins are involved in the local defence mechanism in the eye [11].
The few studies conducted to date found elevated levels of antibodies against HSP27, αB-crystalline, HSP60, and HSP70 in patients with glaucoma, especially normal-tension glaucoma (NTG) [14–16]. However, a study published by Boehm et al. does not support those findings, as they observed that although the levels of anti-HSP60 antibodies were elevated in patients with POAG compared to normal controls, the difference was not statistically significant [17].
HSPs are highly immunogenic, but the immune response is not associated with the development of many autoimmune disorders. Although HSP overexpression in glaucomatous eyes initially protects the retinal ganglion cells from further damage, at a later stage it may induce an immune response that contributes to the progression of glaucomatous optic neuropathy. The glial cells of the retina and optic nerve present the antigen. After the glial cells become activated, HSP overexpression may be an immunostimulating factor that leads to breaking of immune tolerance. Elevated levels of antibodies against HSP in patients with glaucoma may reflect a general response to cellular damage, and thus lead to disease progression by attenuating the ability of native HSPs to protect cells [11].
Aim of the study
The aim of the study was to determine if normal-tension glaucoma (NTG) coexists with elevated levels of antibodies against human heat shock protein HSP60.
Material and Methods
This was a case-control study carried out in 4 groups of subjects with NTG (Group 1), POAG (Group 2), autoimmune rheumatic diseases (Group 3), and controls (Group 4).
The study was approved by the Bioethics Committee at the Medical Centre of Postgraduate Education. Before entering the study, each subject was informed by a physician and given printed “Patient Information” (which they were told to read carefully) about their participation in a clinical study. Next, subjects gave their informed consent to participation in the study by filling out and signing the Informed Consent for Clinical Studies Form.
All subjects had complete ophthalmologic examination and blood tests. The ophthalmologic examination included visual acuity, intraocular pressure measurements using applanation and pascal tonometry, detailed assessment of the anterior and posterior segments of the eye, pachymetry, gonioscopy, visual-field examination, and optical coherence tomography (OCT) of the optic nerve head and the macula.
Venous blood samples from the study subjects were centrifuged within 30 minutes of collection and the serum was pipetted into separate test tubes and stored in a freezer at −24°C. Serum samples were analyzed by enzyme-linked immunoabsorbent assay (ELISA, StressGen) for antibodies against human HSP60 when blood samples had been collected from all subjects.
The exclusion criteria were as follows: lack of informed consent, ocular diseases that might lead to false results (e.g., corneal disorders with possible impact on visual acuity and visual field, including corneal ulceration, corneal injury, corneal dystrophy, and corneal swelling); disorders of the lens, including mature cataract, brown cataract and congenital cataract with impact on visual acuity and visual field; retinal disorders with possible impact on visual acuity and visual field, including acquired macular disorders (e.g., age-related macular degeneration); retinal vascular disorders (e.g., diabetic retinopathy), retinal detachment; neurological disorders and optic nerve disorders with possible impact on visual acuity and visual field, including status post cerebrovascular accident, optic neuritis, and optic neuropathy); severe systemic disease with possible impact on serum antibody levels including chronic viral hepatitis; chronic inflammatory pulmonary disorders; systemic malignancies, viral infections (e.g., AIDS, infectious mononucleosis, and influenza); bacterial infections (tuberculosis, syphilis, leprosy, brucellosis, salmonellosis, subacute endocarditis); parasitic infestations (e.g., malaria, filariasis, and schistosomiasis); status after vaccination against viral disease; medical treatments affecting autoantibody levels and intraocular pressure; and pregnancy.
Statistical analysis
Statistical analyses were performed using STAT software. The normality of distribution of the study variables in groups was assessed with the Shapiro-Wilk test. Because of departures from normality assumptions, the non-parametric Kruskal-Wallis test was used for comparisons. The chi-square test was used to compare frequencies. The entire statistical analysis was performed at the significance level α=0.05. Results were considered statistically significant at p<0.05.
Results
We included a total of 139 patients in the study. Their demographic data are presented in Table 1.
Table 1.
Demographic data of patients included in the study.
| Number of subjects | Age range | Mean age | |||
|---|---|---|---|---|---|
| % | From | To | |||
| Group 1 | |||||
| Women | 25 | 71 | 37 | 80 | 68.1 |
| Men | 10 | 29 | 54 | 80 | 66.9 |
| Total group 1 | 35 | 37 | 80 | 67.8 | |
| Group 2 | |||||
| Women | 22 | 65 | 51 | 80 | 66.6 |
| Men | 12 | 35 | 43 | 78 | 60.4 |
| Total group 2 | 34 | 43 | 80 | 64.4 | |
| Group 3 | |||||
| Women | 34 | 100 | 23 | 77 | 47.4 |
| Man | 0 | 0 | 0 | ||
| Total group 3 | 34 | 0 | 77 | 47.4 | |
| Group 4 | |||||
| Women | 27 | 75 | 30 | 74 | 52.0 |
| Men | 9 | 25 | 40 | 75 | 59.0 |
| Total group 4 | 36 | 30 | 75 | 53.8 | |
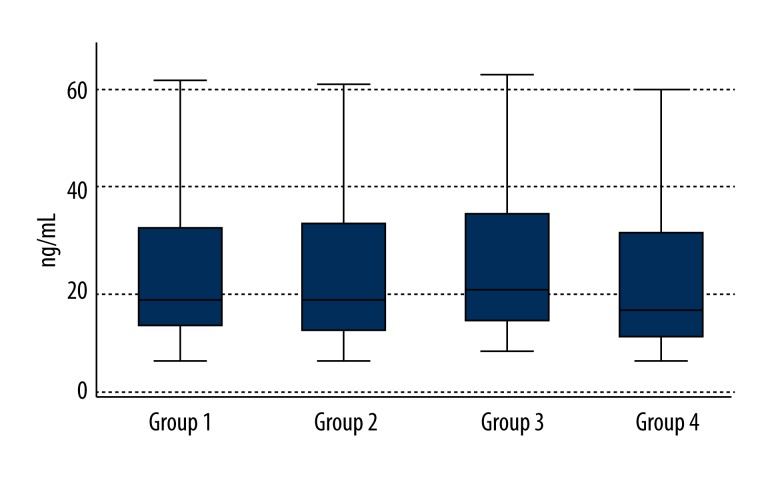
The following levels of antibodies against human HSP60 were measured in the study groups (Figure 1):
Figure 1.
Levels of antibodies against human heat shock protein HSP60 in all study groups.
Group 1: 18.79 ng/mL (Q1–14.71; Q3–35.52);
Group 2: 18.61 ng/mL (Q1–13.56; Q3–36.20);
Group 3: 20.49 ng/mL (Q1–15.3; Q3–36.67);
Group 4: 17.61 ng/mL (Q1–13.09; Q3–34.03).
The result was not statistically significant (p=0.96).
Discussion
In this study, serum levels of antibodies against human HSP60 were measured in 4 groups of subjects: with NTG, with POAG, with autoimmune rheumatic diseases, and normal controls. In the NTG group, the level of HSP60 antibodies was higher compared to the POAG group and controls, but the difference was not statistically significant. The highest level of antibodies against HSP60 was found in subjects with autoimmune rheumatic diseases, but the difference between this group and the remaining groups was not statistically significant. These findings do not confirm the results of 2 studies performed by Wax et al. [14,16]. In the first study, they demonstrated that patients with glaucoma had elevated levels of antibodies against HSP60, both human and bacterial, based on the comparison between subjects with NTG and POAG, and normal controls. The levels of antibodies against human HSP60 in the NTG subjects and POAG subjects were comparable, but in both groups they were significantly higher compared to controls. The levels of antibodies against bacterial HSP60 were significantly elevated in the NTG subjects compared to the POAG subjects. According to Wax et al., comparable levels of antibodies against human HSP60 in NTG and POAG might suggest a similar mechanism underlying optic disc neuropathy, while elevated levels of antibodies against bacterial HSP60 in the subjects with NTG might indicate a stronger abnormal immune response to bacterial HSP60. In their later study, Wax et al. compared the levels of antibodies against bacterial and human HSP60 in larger groups of patients from the United States and Japan and revealed elevated levels of antibodies against human and bacterial HSP60 in NTG patients compared to POAG patients, who, however, had higher HSP60 antibody levels compared to controls [16]. The results of these 2 studies are not quite consistent, as the earlier study did not find any differences between the levels of antibodies against HSPs in NTG and POAG [14].
The findings by Boehm et al. in Germany do not support the above observations by Wax et al. Boehm et al. measured, among other variables, the levels of antibodies against HSP60 in subjects with POAG and in controls, but did not find elevated HSP60 antibodies in the former compared to the latter [17]. Also, the results of the present study do not confirm the observations by Wax et al. reported from the United States and Japan. We demonstrate lack of significant differences in the levels of antibodies against human HSP60 between NTG and POAG subjects and controls. Subjects’ ages could not have had any impact on this finding, because they were comparable in all studies discussed. The differences may be due to other factors. First of all, antibodies against human HSP60 may also be found in healthy individuals, both neonates and adults [18,19]. Additionally, the international literature now offers increasing evidence for the autoimmune component of atherosclerosis, with an important role of HSPs, including HSP60, in its pathogenesis [13,20,21]. According to recent research, antibodies against HSP60 may play a role in the etiology of cardiomyopathy, including diabetic cardiomyopathy (hence, elevated levels of anti-HSP60 antibodies in diabetes) and atrial fibrillation, but not in arterial hypertension [20]. Another important observation of this study is absence of elevated levels of antibodies against HSP60 in subjects with rheumatic diseases, despite the autoimmune component of their disorder. This finding may be accounted for by the role of antibodies against HSP60 in the pathogenesis of atherosclerosis. Only patients with rheumatic diseases and co-existing atherosclerosis were found to have elevated serum titers of antibodies against HSP60 [22]. Nowadays, rheumatic diseases are increasingly diagnosed in younger patients, and in this study the mean age of the subjects with rheumatic diseases was 47.7 years and none of them had ischemic heart disease. It may be safely assumed that in patients with NTG, HSP60 antibodies would elevate only in patients with coexisting atherosclerosis, which is why these antibodies could not be detected in the sera of the relatively young glaucoma subjects with no history of cardiovascular disease assessed in this study. In the studies from other international centers discussed in this paper, there is no information concerning a possible history of cardiovascular disease in the subjects with NTG and elevated titers of antibodies against HSP60 [14,16].
Conclusions
This study does not confirm the hypothesis that normal-tension glaucoma is associated with elevated blood levels of antibodies against human heat shock protein (HSP) 60. Future studies with larger groups of patients are needed to further investigate the role HSP60 plays in the pathogenesis of glaucoma.
Footnotes
Disclosure of the conflict of interest
The authors declare that there are no conflicts of interest.
Source of support: This study was funded from the science budget in the years 2010–2014 as our own research project under the title “Is there any association between elevated levels of antibodies occurring in autoimmune diseases and normal tension glaucoma?” (No. N N402 283139)
References
- 1.Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Primary open-angle glaucoma. N Eng J Med. 2009;360:1113–24. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA. Glaucoma. Lancet. 2011;377:1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 4.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tezel G, Wax MB. Glaucoma. Chem Immunol Allergy. 2007;92:221–27. doi: 10.1159/000099273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skonieczna K, Grabska-Liberek I, Terelak-Borys B, Jamrozy-Witkowska A. Selected autoantibodies and normal-tension glaucoma. Med Sci Monit. 2014;20:1201–9. doi: 10.12659/MSM.890548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pache M, Flammer J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv Ophthalmol. 2006;51:179–212. doi: 10.1016/j.survophthal.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Young DB. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992;4:396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- 9.Wax MB. The case for autoimmunity in glaucoma. Exp Eye Res. 2011:187–90. doi: 10.1016/j.exer.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Wax MB. Is there a role for the immune system in glaucomatous optic neuropathy? Curr Opin Ophthalmol. 2000;11:145–50. doi: 10.1097/00055735-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Tezel G, Hernandez R, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 2000;118:511–18. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- 12.Tezel G, Yang J, Wax MB. Heat shock proteins, immunity and glaucoma. Brain Res Bull. 2004;62:473–80. doi: 10.1016/S0361-9230(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 13.Grundtman C, Kreutmayer SB, Almanzar G, et al. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:960–68. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wax MB, Tezel G, Saito I, et al. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1998;125:145–57. doi: 10.1016/s0002-9394(99)80084-1. [DOI] [PubMed] [Google Scholar]
- 15.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–87. [PubMed] [Google Scholar]
- 16.Wax MB, Tezel G, Kawase K, Kitazawa Y. Serum autoantibodies to heat shock proteins in glaucoma patients from Japan and the United States. Ophthalmology. 2001;108:296–302. doi: 10.1016/s0161-6420(00)00525-x. [DOI] [PubMed] [Google Scholar]
- 17.Boehm N, Wolters D, Thiel U, et al. New insight into autoantibody profiles from immune privileged sites in the eye: A glaucoma study. Brain Beh Immun. 2012;26:96–102. doi: 10.1016/j.bbi.2011.07.241. [DOI] [PubMed] [Google Scholar]
- 18.Quintana FJ, Cohen IR. The HSP60 immune system network. Trends Immunol. 2011;32:89–95. doi: 10.1016/j.it.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Quintana FJ, Getz G, Hed G, et al. Cluster analysis of human autoantibody reactivities in health and in type 1 diabetes mellitus: a bio-informatic approach to immune complexity. J Autoimmun. 2003;21:65–75. doi: 10.1016/s0896-8411(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo M, Macario AJ, de Macario EC, et al. Heat shock protein-60 and risk for cardiovascular disease. Curr Pharm Des. 2011;17:3662–68. doi: 10.2174/138161211798220981. [DOI] [PubMed] [Google Scholar]
- 21.Foteinos G, Xu Q. Immune-mediated mechanisms of endothelial damage in atherosclerosis. Autoimmunity. 2009;42:627–33. doi: 10.1080/08916930903002529. [DOI] [PubMed] [Google Scholar]
- 22.Murdaca G, Colombo BM, Cagnati P, et al. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. 2012;224:309–17. doi: 10.1016/j.atherosclerosis.2012.05.013. [DOI] [PubMed] [Google Scholar]