Abstract
Fecal alpha1-proteinase inhibitor (α1-PI) concentration has been to diagnose enteric protein loss in dogs and cats. Chronic lymphocytic enteritis is commonly seen in the marmoset (C. jaccus) and is characterized by hypoalbuminemia. As a prelude to immunoassay development for detecting enteric protein loss, marmoset serum α1-PI was purified using immunoaffinity chromatography and ceramic hydroxyapatite chromatography. Partial characterization was performed by reducing gel electrophoresis and enzyme inhibitory assays. Protein identity was confirmed with peptide mass fingerprinting and N-terminal amino acid sequencing. Molecular mass, relative molecular mass, and isoelectric point for marmoset α1-PI were 54 kDa, 51677, and 4.8-5.4, respectively. Trypsin, chymotrypsin, and elastase inhibitory activity were observed. N-terminal amino acid sequence for marmoset α1-PI was EDPQGDAAQKMDTSHH. In conclusion, marmoset α1-PI was successfully purified from serum with an overall yield of 12% using a rapid and efficient method. Purified marmoset α1-PI has characteristics similar to those of α1-PI reported for other species.
Keywords: common marmoset, alpha1-proteinase inhibitor, purification, characterization
1. Introduction
The common marmoset (Callithrix jaccus) is a Brazilian new world monkey that has been employed in biomedical research since the early 1960's. Its popularity has increased considerably over the years in North America and Europe (Abbott et al., 2003). Marmosets are utilized because of their small size, reduced cost for maintenance, easy husbandry, rapid reproductive turnover, and decreased susceptibility to certain human pathogens. Currently they are used for research in neuroscience, reproductive biology, infectious diseases, behavioral science, drug development, and safety assessment, as well as models of aging research (Mansfield, 2003). Recently, a transgenic marmoset with germ line transmission has been developed (Sasaki et al., 2009), which holds great potential for further biomedical research.
Inflammatory diseases of the intestinal tract, particularly colitis, have been described in marmosets (Chalmers et al., 1983; David et al., 2009; Tucker, 1984). Inflammatory bowel disease (IBD), particularly chronic lymphocytic enteritis (CLE), has been a consistent finding in C. jacchus colonies as evidenced by necropsy findings at two primate centers, the NEPRCa and SNPRCb. Between 1991 and 2000 approximately 60.5% of all marmosets used as controls in various studies had some degree of IBD at necropsy at the NEPRC. Also, between January 2004 and June 2009, IBD was observed in about 16% of marmosets who were >1year of age at the NEPRC (Tardif et al., 2011). In a similar study at the SNPRC facility from 2002 to 2011, IBD was attributed as the cause of death in 44% of marmosets below the age of 6 years (Ross et al., 2012). While no specific etiology has been identified, many etiological factors such as gluten sensitivity, dietary protein deficiency, and the pancreatic spirurid nematode (Trichospirura leptostoma) have been reported (Ludlage and Mansfield, 2003). The disease is characterized by diffuse to segmental lymphocytic enteritis, and clinically manifests itself by failure to thrive in juveniles, or weight loss in adults, with or without diarrhea. A diagnosis is made based on clinical signs, a history of weight loss, and clinicopathological findings like a decreased serum albumin concentration. No effective treatment exists and a final diagnosis is usually made only at necropsy (Ludlage and Mansfield, 2003).
Alpha1-proteinase inhibitor (α1-PI) is a serum glycoprotein synthesized by the liver (Koj et al., 1978b), released into systemic circulation, and inhibiting a variety of serine proteases that protect various tissues from enzymatic damage. Recently, α1-PI has also been described as being of importance in modulating immunity, inflammation, proteostasis, apoptosis, and possibly cellular senescence (Hunt and Tuder, 2012). Alpha1-proteinase inhibitor has been purified from many species including human (Pannell et al., 1974), dog (Melgarejo et al., 1996), cat (Fetz et al., 2004), sheep (Mistry et al., 1991), goat (Vankan and Bell, 1993), rabbit (Koj et al., 1978a), mouse (Minnich et al., 1984), rat (Kuehn et al., 1984), guinea pig (Suzuki et al., 1990), Rhesus monkey (Berninger and Mathis, 1976), and opossum (Catanese and Kress, 1993). Across species, the protein structure and function remain similar, however, a relative deficiency has been reported in people with certain genetic genotypes (Brebner and Stockley, 2013). Deficiency of α1-PI has also been reported in a single dog (Mellor et al., 2006). However, there is limited cross-immuno-reactivity for α1-PI between species and thus assays for the measurement of α1-PI are species-specific (Roll and Glew, 1981).
Under physiologic conditions only minimal amounts of alpha1-proteinase inhibitor are found within the lumen of the gastrointestinal tract. Having a molecular weight similar to albumin, it is believed to be lost into the gastrointestinal tract at a rate comparable to that of albumin. However, unlike albumin, α1-PI is resistant to bacterial degradation or the effects of digestive enzymes within the lumen of the gut, enabling it's detection in fecal samples by an immunoassays (Melgarejo et al., 1998). Fecal α1-PI may be increased even before hypoalbuminemia is observed , making it an early marker for intestinal protein loss (SL Vaden, 2002). Validated assays for measurement of fecal alpha1-proteinase inhibitor for detection of intestinal protein loss are available for humans (Karbach et al., 1983), dogs (Heilmann et al., 2011; Melgarejo et al., 1998), and cats (Burke et al., 2012).
Marmoset α1-PI has not yet been purified or characterized. The objective of this study was to purify and partially characterize α1-PI from the serum of the common marmoset (Callithrix jaccus). The purified marmoset α1-PI will then be utilized for the development of an immunoassay as a noninvasive marker of chronic lymphocytic enteritis and protein loss in marmosets.
2. Materials and methods
2.1 Marmoset serum
Surplus serum submitted to the Gastrointestinal Laboratory for routine testing (NEPRCa), and sera harvested from euthanized common marmosets as part of routine colony management procedures (SNPRCb) were used. These procedures were approved by the animal care and use committee at the respective institution. Serum samples were stored at −80°C and shipped on dry ice.
2.2 Affinity column chromatography
An empty column (XK 16/20, GE Healthcare Life Sciences, Piscataway, NJ) was packed with 11.8 ml of alpha1-antitrypsin select resin (alpha1-antitrypsin select media, GE Healthcare Biosciences, Piscataway, NJ) as per manufacturer's instructions. This column was stored in 20% ethanol at +4°C until further use. Pooled marmoset serum was diluted in a 1:10 dilution with Buffer A; [20 mM Tris(hydroxymethyl)aminomethane hydrochloride (Sigma-Aldrich, St. Louis, MO), 50 mM sodium chloride (Sigma-Aldrich, St. Louis, MO), pH 7.4]. After thorough mixing, the sample was filtered through a 0.2 μm pore size filter and applied to the column coupled with a preparative fast protein liquid chromatography (FPLC) system (ÄKTA, GE Healthcare Biosciences, Piscataway, NJ). Before loading the serum sample, the column was equilibrated with 50 ml of Buffer A at a flow rate of 2 ml/min, and then with 50 ml of Buffer B; [20 mM Tris-HCl, 2 M magnesium chloride hexahydrate (Sigma-Aldrich, St. Louis, MO), pH 7.4] at 4 ml/min. The sample was injected and the absorbance at 280 nm monitored. The peak that resulted from the injection was allowed to return to baseline. Subsequently, buffer B was applied at a flow rate of 4 ml/min. One milliliter fractions were collected during the elution phase and analyzed using sodium dodecyl sulphate electrophoresis polyacrylamide gel electrophoresis (SDS–PAGE) with precast gels under reducing conditions (NuPAGE® Novex® 10% Bis-Tris mini-gels, Life Technologies, Grand Island, NY) and using a known molecular weight standard marker (Mark 12, Life Technologies, Grand Island, NY). The affinity column was cleaned using 50 ml of each: 2 M sodium chloride (Sigma-Aldrich, St. Louis, MO), 6 M guanidine HCl (Sigma-Aldrich, St. Louis, MO), and 10% isopropyl alcohol (Sigma-Aldrich, St. Louis, MO) in succession, and then stored in 20% ethanol (Decon Labs, King of Prussia, PA). All the buffers used were prepared in ultrapure water with 18.2 MΩ.cm resistivity at +25°C (Milli-Q, EMD Millipore Corporation, Billerica, MA), and were filtered (0.2 μm), and degassed before use.
2.3 Buffer exchange and concentration
Eluted fractions that contained protein of the expected molecular weight of marmoset α1-PI (approximately 50 kDa) were pooled. Buffer was exchanged to buffer C [10 mM sodium phosphate (Sigma-Aldrich, St. Louis, MO), pH 6.95] using a dialysis cassette (Slide-A-Lyzer dialysis cassettes, 10K MWCO, Thermo Fisher Scientific, Rockford, IL) as per manufacturer's recommendations. The dialyzed material was concentrated to a final volume of 4 ml using centrifugal filters (Amicon® Ultra 15 ml filter, EMD Millipore Corporation, Billerica, MA) and stored at −80°C.
2.4 Ceramic hydroxyapatite chromatography (CHT)
A 5 ml pre-packed ceramic hydroxyapatite chromatography type II media cartridge (Bio ScaleTM Mini CHT Type II, Bio-Rad, Hercules, CA) was used. The CHT column was connected to the purification system and prepared for injection after equilibration with 25 ml of buffer C, followed by 25 ml of buffer D [400 mM sodium phosphate (Sigma-Aldrich, St. Louis, MO), pH 6.95]. The concentrated protein was injected onto the CHT column at a flow rate of 1 ml/min and the flow-through was collected in 1 ml fractions. These fractions were screened for protein activity using the trypsin inhibitory activity assay (see 2.7.1). Fractions that had the highest activity were pooled, buffer exchanged as before to phosphate buffered saline pH 7.2 (BupH phosphate buffered saline packs, Thermo Fisher Scientific Inc., Rockford, IL), and concentrated to a final protein concentration of 1 mg/ml. The concentrated protein was stored at –80°C for further characterization.
2.5 Gel electrophoresis and purity
Protein purity was determined by reducing SDS-PAGE using precast 10% Bis-Tris mini-gels (NuPAGE® Novex® 10% Bis-Tris mini-gels, Life Technologies, Grand Island, NY) and subsequent staining with a ready-to-use colorimetric stain formulated with Coomassie dye R-250 (Imperial protein stain, Thermo Fisher Scientific, Rockford, IL) as per manufacturer's recommendation.
2.6 Protein concentration
Protein concentration was determined using the Bradford protein assay (Thermo Scientific Pierce, Rockford, IL).
2.7 Proteinase inhibitory activity
2.7.1 Trypsin inhibitory activity
Trypsin inhibitory activity was measured using a previously established assay (Fetz et al., 2004) and was used to monitor the purification process. Briefly, bovine trypsin (Sigma-Aldrich, St. Louis, MO) and Nα- benzoyl-DL-arginine-p nitroanilide (BAPNA, Sigma-Aldrich, St. Louis, MO) were used as the proteinase and the substrate, respectively. The change in absorbance due to the release of p-nitroanilide was used to measure trypsin activity in a 96-well microtitre plate. The activity was measured over a 15 minute interval at a wavelength of 405 nm on a kinetic plate reader. Absorbance of each well was measured every 30 seconds. The maximum rate of change in absorbance was automatically calculated by integrating across the 30 different measurement points and was used for calculating trypsin activity. For the purpose of this study, one arbitrary unit of specific activity was defined as the amount of marmoset α1-PI necessary to reduce the maximum rate of change of absorbance of the test wells to 50% of the negative control well.
2.7.2 Elastase inhibitory activity
Elastase inhibitory activity was assayed as described previously (Stoll et al., 2007). Briefly, methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide was used as the substrate for human neutrophil elastase (SERVA Electrophoresis GmbH, Heidelberg, Germany) in a 96-microwell format. Elastase inhibition was determined by the absence of an increase in the absorbance, measured over 15 minutes at a wavelength of 405 nm in a microwell where marmoset α1-PI was pre-incubated with the enzyme.
2.7.3 Chymotrypsin inhibitory activity
Chymotrypsin inhibitory activity was assayed as described previously (Muharsini et al., 2000). Briefly, inhibitory activity was demonstrated using 0.35 mM succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (SAPNA, Sigma-Aldrich, St. Louis, MO) as substrate with a chymotrypsin solution of 15 U/ml in a microtitre 96 well plate and reading the activity using a plate reader at 405 nm. Similar to other enzyme inhibitory activities, when the enzyme was pre-incubated with marmoset α1-PI, the lack of increase in the absorbance measured over 15 minutes at a wavelength of 405 nm was used to determine chymotrypsin inhibitory activity.
2.8 Determination of molecular weight and relative molecular mass
Molecular weight was estimated by using 10% Bis Tris polyacrylamide gel electrophoresis under reducing conditions, against a standard protein ladder (Mark 12, Life Technologies, Grand Island, NY). The molecular weight was estimated using gel analysis software (Quantity One 1-D Analysis Software, Bio-Rad Laboratories, Hercules, CA). The relative molecular mass (Mr) was estimated using surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF-MS;Protein Chip® SELDI, System, Bio-Rad Laboratories, Hercules, CA) using 6 ng of purified marmoset α1-PI immobilized onto a nonselective normal phase chromatographic array (NP 20 Protein Chip® array, Hercules, CA ).
2.9 Isoelectric point
The pI (isoelectric point) was estimated using native isoelectric focusing with a linear pH gradient from 3 to 10 in a vertical format on a precast polyacrylamide gel (Novex® pH 3-10 IEF protein gel, Life Technologies, Grand Island, NY).
2.10 Specific absorbance
Specific absorbance of marmoset α1-PI was determined by using the absorbance as measured by the spectrophotometer (NanoDrop 1000, NanoDrop products, Wilmington, DE) and the corresponding protein concentration as determined by a Bradford protein assay.
2.11 N-terminal amino acid sequence and tryptic peptide mass fingerprint (PMF)
The purified protein was submitted to the Protein Chemistry Laboratory (Department of Biochemistry and Biophysics, Texas A&M University, TX) for N-terminal amino acid sequencing using automated Edman's protein sequencing on a Model 492 automated protein sequencer (Applied Biosystems, Foster City, CA). This was followed by comparing the sequence against an established database. Homology between species was determined using the percentage of homologue amino acids of the amino acid sequence portion determined.
The purified protein on a gel was submitted to the Protein Chemistry Laboratory at Texas A&M University for tryptic mass fingerprinting. Briefly, the gel was subjected to tryptic digestion and the resulting peptides were extracted and the unfractionated mixture was analyzed by Matrix- assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS). Tandem mass spectra were extracted, charge state deconvoluted and deisotoped by Mascot Distiller version 2.2.1. All MS/MS samples were analyzed using Mascot (Matrix Science, London, UK; version Mascot) and X! Tandem (The GPM, thegpm.org; version CYCLONE (2010.12.01.1)). The spectrum modeler, X! Tandem was set up to search a subset of the NCBInr_20110312 database. Mascot was set up to search the NCBInr_20110312 database (unknown version, 13366630 entries) assuming the digestion with trypsin. Mascot and X! Tandem were searched with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 1.5 Da. Oxidation of methionine and iodoacetamide derivative of cysteine were specified in Mascot and X! Tandem as variable modifications. Scaffold (version Scaffold_3.1.2, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide and protein identifications were accepted as per specifications from the Peptide Prophet algorithm (Keller et al., 2002) and Protein Prophet algorithm (Nesvizhskii et al., 2003).
2.12 Immunologic cross-reactivity and Western blotting
Polyclonal antibodies were raised in a New Zealand white rabbit by inoculation with 200 μg of purified marmoset α1-PI emulsified in Freund's complete, and then by repeated injections of 100 μg of purified marmoset α1-PI in Freund's incomplete adjuvant by a commercial antibody production service (Lampire Biological Laboratories, Pipersville, PA). Specificity of antibodies raised were determined by using a Western blot, using marmoset serum, pooled protein fractions from the A1AT select affinity column (Alpha1-antitrypsin select medium consists of binding ligands made from camelidae-derived single domain antibody fragments, obtained after immunization of lamas with human A1AT protein on an agarose matrix), and purified marmoset α1-PI. A 1:5000 dilution of the primary antibody with approximately a titer of 80%, and a 1:50,000 dilution of secondary antibody (Goat anti-Rabbit IgG, Pierce, Thermo Fisher Scientific Inc., Rockford, IL) were used. Cross-reactivity of sera raised against marmoset α1-PI was assayed using the radial double immunodiffusion against human, dog, cat, mouse, and rat serum. Various non-human primate sera from the Houston zoo and the Phoenix zoo were also tested for cross-reactivity with marmoset α1-PI. The primate species tested included the Geoffrey's marmoset, pied tamarin, cotton topped tamarin, golden lion tamarin, ring tailed lemur, black and white ruffled lemur, red fronted lemur, mandrill, red capped mangabey, Allen's swamp monkey, De Brazza's monkey, Schmidt's monkey, orangutan, Rhesus monkey, pigtail monkey, and the chimpanzee. Marmoset serum was used as a positive control and phosphate buffered saline as negative control.
3. Results
3.1 Purification of marmoset α1-PI
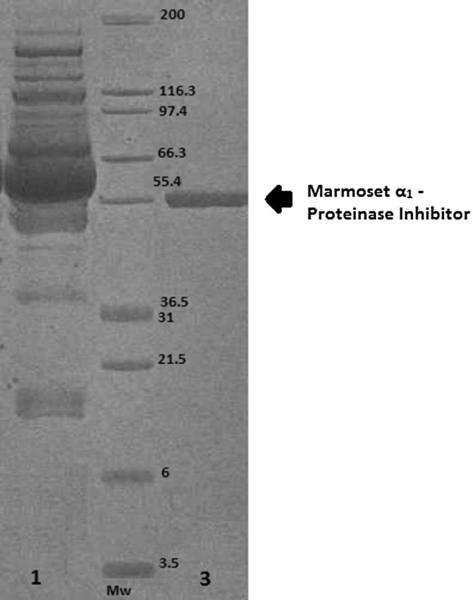
Marmoset α1-PI was successfully purified from marmoset serum. Results from an exemplary purification are summarized in Table 1. Progression of the purification process was monitored by use of SDS-PAGE and the trypsin inhibition assay. Marmoset α1-PI was retained on the A1AT select affinity column. The majority of other serum proteins were observed in the flow through (Fig. 1). Retained marmoset α1-PI and other proteins were eluted with Buffer B. The eluted fractions were visualized on a gel, and fractions with marmoset α1-PI were pooled and concentrated. Proteolytic activity could not be measured during this phase because of the interference from magnesium ions present in the buffer. Marmoset α1-PI was then concentrated, buffer exchanged, and loaded onto the ceramic hydroxyapatite (CHT) column. Marmoset α1-PI did not bind to the column and this step helped to remove other major protein contaminants (Fig. 2). The overall yield of the purification protocol was about 11.9%. There was a 19-fold increase in the purification. After the final concentration step marmoset α1-PI was visualized as a single band on SDS-PAGE after staining (Fig. 3).
Table 1.
This table shows the sequential purification of marmoset α1-PI from 2 ml of marmoset serum
| Purification stage | Protein content (mg) | Total inhibitory activity (units) | Specific activity (U/mg of protein) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Serum | 111.57 | 250 | 2.2 | 100 | 1 |
| Affinity chromatography | 2.0 | 59 | 29.5 | 23.6 | 13 |
| Ceramic hydroxyapatite chromatography | 0.68 | 30 | 43.7 | 11.9 | 19 |
Fig. 1.
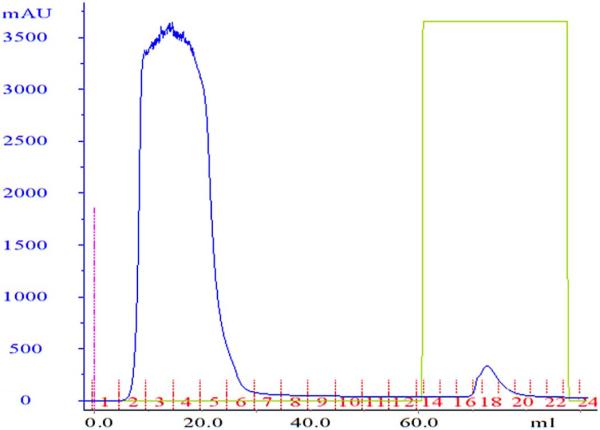
Chromatogram for purification with the A1AT select immunoaffinity column. Diluted marmoset serum was applied onto the column and the column washed with buffer A. Bound marmoset α1-PI was eluted by use of 100% buffer B. Fractions in tubes 17, 18 and 19 on the chromatogram (third peak) showed the area corresponding to the band with the highest concentration of marmoset alpha1-proteinase inhibitor. Protein elution was monitored by measurement of absorbance (in milli-absorbance units [mAU]) at 280 nm.
Fig. 2.
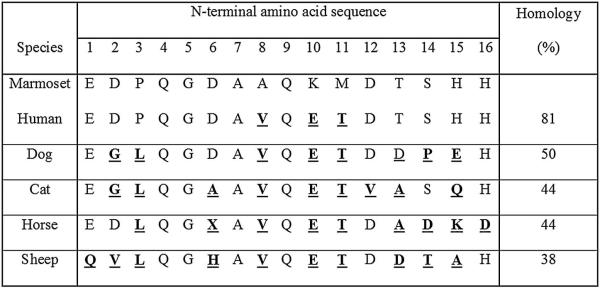
Ceramic hydroxyapatite chromatography of partially purified marmoset α1-PI. Buffer C, at a flow rate of 1 ml/min, was used as the mobile phase. Fractions of the second peak showed the highest trypsin inhibition of 100% and were pooled and concentrated. Protein elution was monitored by absorbance (in milli-absorbance units [mAU]) at 280 nm.
Fig. 3.
This figure shows an Imperial protein stain stained SDS-PAGE of partially purified marmoset α1-PI. The molecular weights of a set of molecular mass markers (Mw) are shown in middle of the gel. The following material was loaded into the lanes: lane 1: marmoset serum, 3: pure α1-PI after ceramic hydroxyapatite chromatography
3.2 Characterization of marmoset α1-PI
The estimated molecular mass on a 10% Bis Tris polyacrylamide electrophoresis gel was about 54 kDA. The relative molecular mass (Mr) was estimated 51,677. The pI (isoelectric point) of marmoset α1-PI was revealed by 6 bands in close proximity between 4.8 and 5.4. The specific absorbance of marmoset α1-PI was 1.6. The N-terminal amino acid sequence of the last 16 amino acids were EDPQGDAAQKMDTSHH, using the single letter code. Homology between the amino acid sequences of marmoset α1-PI and other species like human, dog, cat, horse and sheep were 81%, 50%, 44%, 44%, and 38%, respectively (Fig. 4). This sequence yielded two hits on UNIPROTKB (http://www.uniprot.org) for the common marmoset; one was an unidentified protein (F7GL69_CALJA) and the other alpha1-antitrypsin (U3FMP8_CALJA) with 100% identity. The tryptic peptide mass fingerprint (PMF) and database search of the PMF data showed sequence similarity with alpha1-proteinase inhibitor. Immunologic cross-reactivity determined using hyperimmune serum raised against marmoset α1-PI was observed only with serum from the Geoffrey's marmoset among all of the species tested.
Fig. 4.
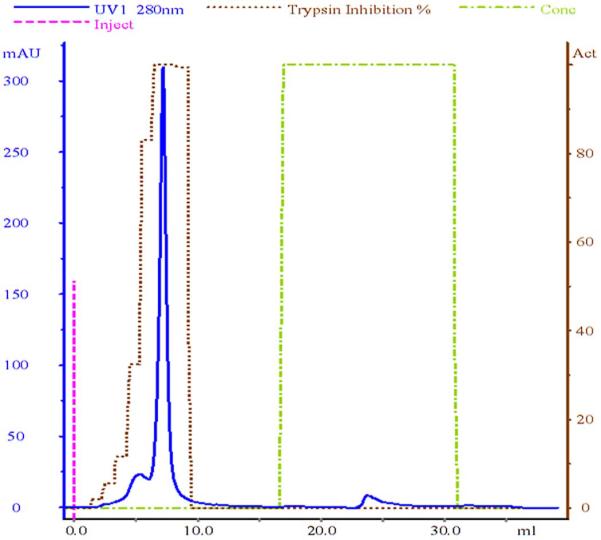
N-terminal amino acid sequences of α1-PI from different species. This table shows the N-terminal amino acid sequence of the last 16 amino acid residues of α1-PI in the common marmoset, cat, dog, human being, sheep, and horse. Amino acid residues in bold and underlined indicate differences in the sequence, with respect to marmoset α1-PI.
4. Discussion
No cross-reactivity was observed between marmoset samples on human and canine assays and purification of marmoset α1-PI was needed to develop a suitable fecal immunoassay. In this study, we report the successful purification of α1-PI from marmoset serum using a two-step chromatographic procedure. The identity of marmoset α1-PI was confirmed by tryptic peptide mass fingerprinting, primary sequence determination of the N-terminus of the first 16 amino acids, and by enzyme inhibition assays.
The described protocol is quick, reproducible, and has a good yield. Purified marmoset α1-PI was sufficiently pure as indicated by a single band on SDS-PAGE. Marmoset α1-PI purification has not been reported elsewhere in literature. Serum availability for purification is a major limitation in marmosets because of their small body size and thus limited blood volume; hence a process with a high yield was necessary for purification. Though a variety of methods have been employed in the purification of serum alpha1-proteinase inhibitors from other species, the use of immunoaffinity chromatography has only been described for human sera. Alpha1-antitrypsin (A1AT) select medium consists of A1AT binding ligands attached to an agarose matrix. A1AT binding ligands are made of camelidae-derived single domain antibody fragments, which were obtained from lamas after immunization with human A1AT protein. Very recently, using the same immunoaffinity media, human alpha1-antitryspin select was purified with a 60% yield in the initial step, with an overall yield of 42% for the entire purification protocol (Zhang et al., 2013). Our yield was lower at ~12%. This difference may be attributable to the difference in affinity of marmoset α1-PI for the A1AT select resin. During the purification process, we observed that after repeated usage of the A1AT Select column, a thorough multistep cleaning in-place protocol was necessary to maintain column performance. Trypsin inhibitor assay was also found to be altered by high magnesium concentrations in the elution buffers during the immunoaffinity purification, thus a buffer exchange was necessary.
The characteristics of marmoset α1-PI are similar to those of α1-PI from other species. The pI between 4.8-5.4 is close to that reported in the cat (4.3-4.7), rat (4.3-4.8), guinea pig (4.5-4.9), rabbit (4.8-5.0), human (4.55-4.47), dog (4.7-4.9), and sheep (4.95) (Fetz et al., 2004; Koj et al., 1978a; Melgarejo et al., 1996; Mistry et al., 1991; Pannell et al., 1974; Suzuki et al., 1990). Purified marmoset α1-PI did retain its ability to inhibit the proteolytic activity of bovine trypsin, chymotrypsin, and human elastase. Similar enzyme inhibitory activity has been reported for α1-PI purified from sheep (Gupta et al., 2008). The observed molecular mass of marmoset α1-PI is similar to that reported for other species [range: 47 – 72 kDa] (Berninger and Mathis, 1976; Fetz et al., 2004; Melgarejo et al., 1996; Pannell et al., 1974; Roll and Glew, 1981). The protein identity was confirmed by N-terminus amino acid sequencing and PMF which are widely accepted methods for protein identification (Gevaert and Vandekerckhove, 2000). A homology of 81.3% was observed between human and marmoset α1-PI. No immunological cross reactivity was observed across other species tested, except for the Geoffrey's marmoset. This limited cross-reactivity was expected and has been reported earlier (Roll and Glew, 1981).
In summary, this is the first report of the successful purification and partial characterization of marmoset alpha1-proteinase inhibitor. The purified protein showed considerable homology to alpha1-proteinase in other species with regard to activity, Mr, and pI. The purification of the protein and development of the hyperimmune serum described here would serve as a prelude to the development of an immunoassay for the measurement of fecal marmoset alpha1-proteinase inhibitor concentrations.
Research Highlights.
Marmoset alpha1-proteinase inhibitor was purified from serum using immunoaffinity chromatography and ceramic hydroxyapatite chromatography.
Partial characterization was performed by reducing gel electrophoresis and enzyme inhibitory assays.
Protein identity was confirmed with peptide mass fingerprinting and N-terminal amino acid sequencing.
Purified marmoset α1-PI has characteristics similar to those of α1-PI reported for other species.
Acknowledgements
The authors would like to thank Dr. Steven N. Austad and Dr. Corrina Ross from the Barshop Institute for Longevity & Aging Studies University of Texas Health Science Center at San Antonio for their support and the supply of Marmoset serum for this study. We acknowledge the help of Dr. Katja Weber, for her technical assistance with the SELDI-TOF. The authors would like to thank Ms. Ellen Syris and Dr. Hui Xie, GE Healthcare for their assistance and test sample of A1AT select medium. We would also like to thank Dr. Larry Dangott, Director of the Protein Chemistry Laboratory, Texas A&M University for his services and assistance in the protein mass fingerprinting and N-Terminal sequencing. Lastly, we would like to thank Dr. Lynnette Waugh from the Phoenix Zoo and Dr. Lauren Howard from the Houston Zoo, for providing the primate serum samples for testing cross-reactivity. The study was funded in part by a grant from the National Institutes of Health (NIH R24 # 1R24RR023344-01A2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The New England Primate Research Center, Harvard Medical School, Boston, Massachusetts
Southwest National Primate Research Center, Texas Biomedical Research Institute, San Antonio, Texas
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comparative Medicine. 2003;53:339–350. [PubMed] [Google Scholar]
- Berninger RW, Mathis RK. Isolation and characterization of alpha1-antitrypsin from rhesus-monkey serum. The Biochemical Journal. 1976;159:95–104. doi: 10.1042/bj1590095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner JA, Stockley RA. Recent advances in alpha1-antitrypsin deficiency-related lung disease. Expert Review of Respiratory Medicine. 2013;7:213–229. doi: 10.1586/ers.13.20. [DOI] [PubMed] [Google Scholar]
- Burke KF, Ruaux CG, Suchodolski JS, Williams DA, Steiner JM. Development and analytical validation of an enzyme-linked immunosorbent assay (ELISA) for the measurement of alpha1-proteinase inhibitor in serum and faeces from cats. Research in Veterinary Science. 2012;93:995–1000. doi: 10.1016/j.rvsc.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Catanese JJ, Kress LF. Opossum serum alpha1-proteinase inhibitor: purification, linear sequence, and resistance to inactivation by rattlesnake venom metalloproteinases. Biochemistry. 1993;32:509–515. doi: 10.1021/bi00053a015. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Murgatroyd LB, Wadsworth PF. A survey of the pathology of marmosets (Callithrix jacchus) derived from a marmoset breeding unit. Laboratory Animals. 1983;17:270–279. doi: 10.1258/002367783781062217. [DOI] [PubMed] [Google Scholar]
- David JM, Dick EJ, Jr., Hubbard GB. Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). Journal of Medical Primatology. 2009;38:347–359. doi: 10.1111/j.1600-0684.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz K, Ruaux CG, Steiner JM, Suchodolski JS, Williams DA. Purification and partial characterization of feline alpha1-proteinase inhibitor (alpha1-PI) and the development and validation of a radioimmunoassay for the measurement of feline alpha1-PI in serum. Biochimie. 2004;86:67–75. doi: 10.1016/j.biochi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Gevaert K, Vandekerckhove J. Protein identification methods in proteomics. Electrophoresis. 2000;21:1145–1154. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1145::AID-ELPS1145>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Appu Rao AG, Gowda LR. Purification and biochemical characterization of ovine α1-proteinase inhibitor: Mechanistic adaptations and role of Phe350 and Met356. Protein Expression and Purification. 2008;57:290–302. doi: 10.1016/j.pep.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Heilmann RM, Paddock CG, Ruhnke I, Berghoff N, Suchodolski JS, Steiner JM. Development and analytical validation of a radioimmunoassay for the measurement of alpha1-proteinase inhibitor concentrations in feces from healthy puppies and adult dogs. Journal of Veterinary Diagnostic Investigation. 2011;23:476–485. doi: 10.1177/1040638711404152. [DOI] [PubMed] [Google Scholar]
- Hunt JM, Tuder R. Alpha1-antitrypsin: one protein, many functions. Current Molecular Medicine. 2012;12:827–835. doi: 10.2174/156652412801318755. [DOI] [PubMed] [Google Scholar]
- Karbach U, Ewe K, Bodenstein H. Alpha1-antitrypsin, a reliable endogenous marker for intestinal protein loss and its application in patients with Crohn's disease. Gut. 1983;24:718–723. doi: 10.1136/gut.24.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical Chemistry. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Koj A, Hatton MW, Wong KL, Regoeczi E. Isolation and partial characterization of rabbit plasma alpha1-antitrypsin. The Biochemical Journal. 1978a;169:589–596. doi: 10.1042/bj1690589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A, Regoeczi E, Toews CJ, Leveille R, Gauldie J. Synthesis of antithrombin III and alpha1-antitrypsin by the perfused rat liver. Biochim Biophys Acta. 1978b;539:496–504. doi: 10.1016/0304-4165(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Kuehn L, Rutschmann M, Dahlmann B, Reinauer H. Proteinase inhibitors in rat serum. Purification and partial characterization of three functionally distinct trypsin inhibitors. The Biochemical Journal. 1984;218:953–959. doi: 10.1042/bj2180953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comparative Medicine. 2003;53:369–382. [PubMed] [Google Scholar]
- Mansfield K. Marmoset models commonly used in biomedical research. Comparative Medicine. 2003;53:383–392. [PubMed] [Google Scholar]
- Melgarejo T, Williams DA, Asem EK. Enzyme-linked immunosorbent assay for canine alpha1-protease inhibitor. American Journal of Veterinary Research. 1998;59:127–130. [PubMed] [Google Scholar]
- Melgarejo T, Williams DA, Griffith G. Isolation and characterization of alpha1-protease inhibitor from canine plasma. American Journal of Veterinary Research. 1996;57:258–263. [PubMed] [Google Scholar]
- Mellor PJ, Fetz K, Maggi RG, Haugland S, Dunning M, Villiers EJ, Mellanby RJ, Williams D, Breitschwerdt E, Herrtage ME. Alphal-proteinase inhibitor deficiency and Bartonella infection in association with panniculitis, polyarthritis, and meningitis in a dog. Journal of Veterinary Internal Medicine. 2006;20:1023–1028. doi: 10.1892/0891-6640(2006)20[1023:aidabi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Minnich M, Kueppers F, James H. Alpha1-antitrypsin from mouse serum isolation and characterization. Comparative Biochemistry and Physiology. B, Comparative Biochemistry. 1984;78:413–419. doi: 10.1016/0305-0491(84)90051-8. [DOI] [PubMed] [Google Scholar]
- Mistry R, Snashall PD, Totty N, Guz A, Tetley TD. Isolation and characterization of sheep alpha1-proteinase inhibitor. The Biochemical Journal. 1991;273(Pt 3):685–690. doi: 10.1042/bj2730685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muharsini S, Sukarsih, Riding G, Partoutomo S, Hamilton S, Willadsen P, Wijffels G. Identification and characterisation of the excreted/secreted serine proteases of larvae of the old world screwworm fly, Chrysomya bezziana. International Journal for Parasitology. 2000;30:705–714. doi: 10.1016/s0020-7519(00)00055-2. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Analytical Chemistry. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Pannell R, Johnson D, Travis J. Isolation and properties of human plasma α1-proteinase inhibitor. Biochemistry. 1974;13:5439–5445. doi: 10.1021/bi00723a031. [DOI] [PubMed] [Google Scholar]
- Roll DE, Glew RH. Isolation and characterization of rat alpha1-antitrypsin. The Journal of Biological Chemistry. 1981;256:8190–8196. [PubMed] [Google Scholar]
- Ross CN, Davis K, Dobek G, Tardif SD. Aging phenotypes of common marmosets (Callithrix jacchus). Journal of Aging Research 2012. 2012 doi: 10.1155/2012/567143. doi:10.1155/2012/567143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Vaden SL, A.V., Levine JF, Harris T, Steiner JM, Williams DA. Fecal a1-proteinase inhibitor activity in soft coated wheaten terriers. Journal of Veterinary Internal Medicine. 2002;16:382. [Google Scholar]
- Stoll A, Suchodolski JS, Ruaux CG, Steiner JM. Purification and partial characterization of canine neutrophil elastase and the development of an immunoassay for the measurement of canine neutrophil elastase in serum obtained from dogs. American Journal of Veterinary Research. 2007;68:584–591. doi: 10.2460/ajvr.68.6.584. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshida K, Ichimiya T, Yamamoto T, Sinohara H. Trypsin inhibitors in guinea pig plasma: isolation and characterization of contrapsin and two isoforms of alpha1-antiproteinase and acute phase response of four major trypsin inhibitors. Journal of Biochemistry. 1990;107:173–179. doi: 10.1093/oxfordjournals.jbchem.a123021. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The Marmoset as a Model of Aging and Age-Related Diseases. Institute for Laboratory Animal Research Journal. 2011;52:54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MJ. A survey of the pathology of marmosets (Callithrix jacchus) under experiment. Laboratory Animals. 1984;18:351–358. doi: 10.1258/002367784780865397. [DOI] [PubMed] [Google Scholar]
- Vankan DM, Bell K. Caprine plasma proteinase inhibitors--I. Partial characterization. Comparative Biochemistry and Physiology. B, Comparative Biochemistry. 1993;104:101–108. doi: 10.1016/0305-0491(93)90344-5. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hou Y, Ding X, Ye S, Cao H, Wang Z, Du X, Xie Y.-w., Li C. Purification and analysis of human alpha1-antitrypsin concentrate by a new immunoaffinity chromatography. Preparative Biochemistry and Biotechnology. 2013;44:725–737. doi: 10.1080/10826068.2013.868358. [DOI] [PubMed] [Google Scholar]