Abstract
Fibroblast strains were derived from two regions of the lower genital tract of localized provoked vulvodynia (LPV) cases and pain-free controls. Sixteen strains were derived from four cases and four controls, age and race matched, following pre-sampling mechanical pain threshold assessments. Strains were challenged with six separate stimuli: live yeast species (C. albicans, C. glabrata, C. tropicalis, and S. cerevisiae), yeast extract (zymosan), or inactive vehicle. Production of prostaglandin E2 (PGE2) and interleukin-6 (IL-6) were pro-inflammatory response measures. Highest IL-6 and PGE2 occurred with vestibular strains following C. albicans, C. glabrata, and zymosan challenges, resulting in the ability to significantly predict IL-6 and PGE2 production by genital tract location. Following C. albicans and C. glabrata challenge of all sixteen fibroblast strains, adjusting for dual sampling of subjects, PGE2 and IL-6 production significantly predicted the pre-sampling pain threshold from the genital tract site of sampling. At the same location of pain assessment and fibroblast sampling, in situ immunohistochemical (IHC)(+) fibroblasts for IL-6 and Cox-2 were quantified microscopically. The correlation between IL-6 production and IL-6 IHC(+) was statistically significant yet biological significance is unknown because of the small number of IHC(+) IL-6 fibroblasts identified. A low fibroblast IL-6 IHC(+) count may result from most IL-6 produced by fibroblasts existing in a secreted, extracellular state. Enhanced, site-specific, innate immune responsiveness to yeast pathogens by fibroblasts may be an early step in LPV pathogenesis. Fibroblast strain testing may offer an attractive/objective marker of LPV pathology in women with vulvodynia of inflammatory origin.
Introduction
The most common cause of premenopausal, chronic intercourse pain (dyspareunia) is localized provoked vulvodynia (LPV)[43]. LPV exacts a severe psychosexual and interpersonal toll[48]. The pathogenesis of LPV is unknown, resulting in a disarray of disease classification and a lack of mechanism-specific therapy. Vulvovaginal candidiasis is the most commonly reported antecedent to LPV[1;34;64] and a vulvodynia animal model has been developed by subjecting female mice to recurrent vulvovaginal infection with C. albicans[22]. Through a series of preliminary and published studies[26], we have been testing the premise that the vulvar vestibule of all women possesses a unique, embryologically-defined, inflammatory/immunologic responsiveness and that LPV pain reflects an extreme example of a natural phenomenon.
LPV pain to light touch, also known as allodynia, is indistinguishable from well-documented experimental and clinical examples of neural pain fiber (nociceptor) sensitization. Allodynia can be induced by intradermal or subcutaneous pro-inflammatory factors such as IL-6 and PGE2 [14;19;49]. IL-6 and PGE2 are elevated in chronic pain conditions[8;23]. IL-6 and PGE2 induction elicits allodynia[13;41], while IL-6 and PGE2 suppression reduces allodynia[16;67], and in preclinical animal models of pain, IL-6 and PGE2 factor/receptorknockout mice display reduced allodynia[55]. Following the development of site-specific fibroblast strains*, we have reported that fibroblasts from the vulvar vestibule produce high levels of IL-6, IL-8, and PGE2 following stimulation by prevalent irritants, such as yeast extracts or yeast breakdown products[26]. This heightened pro-inflammatory response was found in the vulvar vestibule of both LPV-afflicted and pain-free woman, but was markedly enhanced in LPV-afflicted cases. We therefore concluded that vulvodynia arises in a region of the genital tract naturally predisposed to inflammation, namely the vulvar vestibule, and is initiated by specific irritants or infectious agents, such as yeast cell wall products or live yeast. Our current study explored in greater depth, the site-specific responsiveness to live “virulent” and “commensal” yeast species compared to yeast byproducts in both LPV cases and pain free controls. The study also investigated the relationship between pain threshold and the site of fibroblast isolation (from pain-associated vestibular regions and vulvar regions not associated with pain). We used clinically validated measures of mechanical pain, regional proinflammatory fibroblast density determined by in situ IHC, and production of pro-inflammatory pain mediators, IL-6 and PGE2, to assess this relationship. A clear understanding of site-specific response differences, including comparisons of the intracellular mechanisms of action, may provide a better understanding of LPV pathogenesis and lead to the discovery of mechanism-based, local therapy.
Materials and Methods
Two sample groups: LPV-afflicted cases and age / race-matched, pain-free controls were recruited from the Division of General Ob/Gyn clinical practice of the University of Rochester between December 2012 and February 2014. All subjects provided informed consent and the research project was approved by the University of Rochester Institutional Review Board (RSRB # 42136). Cases needed to fulfill “Friedrich’s Criteria” for the diagnosis of LPV which included clinical evidence of tenderness localized within the vulvar vestibule confirmed by cotton swab test (CST) using the modified diagnostic criteria of Bergeron et al.[4] Anatomically, the vestibule is situated between the external vulva (consisting of the labia majora, labia minora, perineum, and mons pubis) and the vagina. Anatomic landmarks include Hart’s line, between the external vulva and vestibule and the hymeneal ring between the vestibule and vagina (see further illustration in Figure 1D–E below). This project involved a small number of cases and controls, based upon the need to develop and maintain fibroblast strains (two strains per subject) and the overall complexity of the experimental design. The limitation in subject number necessitated a careful selection and characterization of cases and controls with matching on particular pivotal characteristics such as age and race. A decision to match by age (within 3 years) was based on reports of increased IL-6 production by fibroblasts from older donors[65]. A decision to racially match was based on reported racially-related pain threshold differences[17] and the potential difference in pro-inflammatory mucocutaneous reactivity based on melanin concentration[57]. Other research has found histopathologic differences in vulvar nerve density, hormonal receptors, and inflammatory infiltrate based upon the classification of primary and secondary vulvodynia[30;38]. Primary vulvodynia is characterized by a clinical history of pain experienced at first introital touch and may be recognized during attempted tampon insertion or insertional dyspareunia. Secondary vulvodynia is characterized by insertional pain development following a pain-free time interval[64]. LPV Cases entering the study were categorized as primary and secondary and this study permitted recruitment of either category. All subjects were selected from patients previously scheduled for gynecologic surgery.
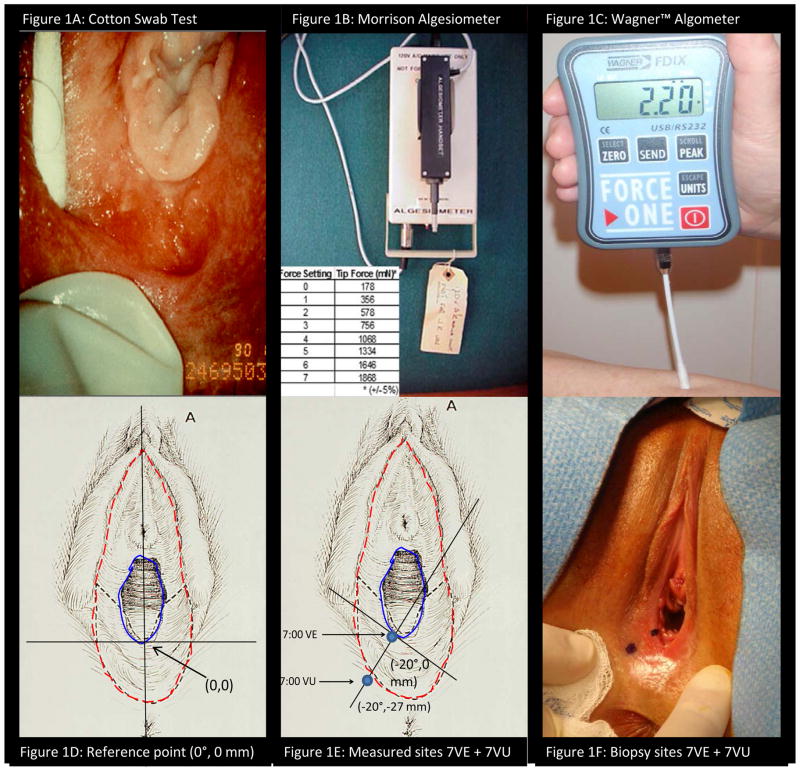
Figure 1.
Photographs of the three methods of pain measurement (1A–C): 1A) Cotton Swab Test performed on the vulvar vestibule, 1B) Morrision algesiometer with display of the 8 force settings produced by the apparatus, 1C) Wagner™ Algometer with digital display of force applied by attached swab. Illustration and photograph of method of measurement of vulvar location (1E–F): 1D) Initial reference point defined by vertical line bisecting urethral and anal orifices, 1E) Rotation of axis to 7:00 position (−20 degrees) and measurement out from hymeneal remnants, 1F) Photograph of paired sites of threshold measurement and tissue sampling.
Subjects underwent a structured health history and three published methods of vulvar mechanical pain measurement and assessment (Figure 1A–C): the “cotton swab test” (CST), Morrision algesiometer, and Wagner™ algometry. The CST (Figure 1A), a direct scaling measure, conformed to the technique of Bergeron et al.[4]. The CST applied a consistent, but uncalibrated, force on 12 defined points of the labia majora, minora, and lower vagina. Precise measurement of the defined anatomic sampling sites is described below. Following a consistently “light force”, manually applied by cotton swab, study subjects rated evoked pain on a numeric rating scale (NRS) ranging from 0 = “no pain” to 10 = “worst possible pain”. The Morrison algesiometer method (Figure 1B), a threshold measure using Method of Constant Stimuli, conformed to previously described method[20;25]. Probe force ranged from 176 mN to 1868 mN in 8 increments. A random staircase method was performed on four anatomic sites of the vestibule and external vulva: 5:00 external vulva and vestibule and 7:00 external vulva and vestibule. Based upon the particular paired site (5:00 or 7:00 in position) that displayed the widest threshold differential by Morrison algesiometry, the external vulvar-vestibular pair subsequently underwent threshold assessment by Wagner™ algometry. The Wagner™ algometer method (Wagner Instruments, Greenwich, CT) (Figure 1C), a threshold method using Method of Limits, followed the technique described by Zolnoun et al.[70] and tested the previously defined external vulvar and vestibular sites corresponding those tested by the Morrison algesiometer. Using the Wagner™ algometer, an increasing 0.5 N per second force (range 0 to 5 N) was applied perpendicular to the mucocutaneous surface by a moistened, dacron tipped swab affixed to the Wagner™ algometer. Force was terminated at point of pain development (signaled by hand-held clicker) or when the test reached 5 N force. Consistently increasing 0.5 N per second force change was assured by pre-test practice using MESUR™ gauge software, (Mark-10 Corp. Copiague, NY). The threshold testing alternated between external vulvar and vestibular sites until the median value of 3 tests within each test site varied less than 10%. The interstimulus interval at each paired site approximated 2 minutes. The Wagner™ algometer was considered the primary threshold measure for the study with the CST and the Morrison algesiometer available for comparative construct validity assessment. The identical anatomic locations of the paired sites undergoing pain threshold assessment were re-confirmed by measurement and subjected to biopsy on the day of surgery, usually 3 to 5 days following pre-operative exam. Location of sampling was confirmed by digital photography. The three selected techniques reflected different dimensions of vulvar pain assessment and thereby provided a chance to assess both the relationship of the particular pain assessment method to fibroblast behavior and the relationship to the other pain assessment methods.
As illustrated in Figures 1D–F, pain threshold testing and subsequent biopsies were performed from two sites: a) lower 1/3 of the vulvar vestibule (at 5:00 or 7:00) in close approximation to the hymen and b) the adjacent external vulva. As seen in Figure 1D, a line was created from the midpoint of the glans clitoris to center of the anal orifice with an intersection of the base of hymen to be designated as (0,0). In Figure 1E, sampling sites were measured in mm using the defined (X, Y) axes with X axis rotation tangential to the hymenal remnants. The two biopsy sites were separated by the region of color and reflectance change known as Hart’s line (dotted outer line, Figure 1D and 1E) which is considered the embryologic interface of endodermal derived (vestibule) and ectodermal derived (external vulva) tissue. Figure 1F, following measurement and marking, photographs were taken to document location of threshold testing/sampling. The distance between the center of each of the testing/sampling sites (“painful” vestibule and “pain-free” external vulva) ranged 18 to 30 mm with a mean of 27 mm. At surgery, biopsy sites corresponding to pain threshold sites, were confirmed by repeat measurement. From each of the two sites, two--6 mm biopsies were processed one sample for fibroblast strain development and one sample for in situ IHC study.
Fibroblast strains were established according to our published methods using RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), followed by subsequent passaging in Minimum Essential Medium (MEM) + 10% FBS[36]. Early passage vulvar and vestibular fibroblast strains were seeded at 5 × 104 cells/well. After achievement of confluence, fibroblasts were serum-reduced for 48 h in fresh media containing 0.5% FBS. Fibroblast cellular identity was confirmed by microscopic inspection. Prior confirmation of cells as fibroblasts was performed with cell type-specific markers (vimentin positive, collagen expressing) and epithelial cell marker (cytokeratin negative), smooth muscle and myofibroblast marker (α-smooth muscle actin negative), endothelial cell marker (CD34 negative), and bone marrow derived cell marker (CD45 negative)[3]. The fibroblasts were used for analysis following early passage (4 to 7 passages). Cultures of fibroblast strains were seeded to 24-well tissue culture plates at roughly half confluence and were allowed to grow until completely confluent (~3–4 days) at 37°C and 5% CO2 in Minimal Essential Media (MEM) supplemented with 10% fetal bovine serum (FBS), GlutaMAX, gentamycin, and antibiotic/antimycotic solution (Gibco Life Technologies, Grand Island, NY). Once confluent, cells were transitioned to serum-reduced media (supplemented with 0.05% FBS) and incubated for 48 h.
The evening prior to infection, yeast cells were inoculated into 10 ml cultures of yeast peptone dextrose broth (YPD; Fisher Scientific) from YPD plate cultures less than two weeks old. Yeast cultures were incubated overnight at 37°C and 220 rpm for Candida albicans, Candida glabrata, and Candida tropicalis, while Saccharomyces cerevisiae was incubated at 30°C and 220 rpm. After ~18 h growth, cultures were diluted to OD600 = 1.0 in fresh YPD broth. Inoculums were prepared by diluting these yeast cultures to ~1 × 104 CFU/ml in antibiotic/antimycotic-free MEM supplemented with 0.05% FBS and GlutaMAX. Confluent fibroblast wells were then infected with 1 ml (1 × 104 blastoconidia) of yeast inoculum each and incubated for 24 h at 37°C and 5% CO2. At the same time, zymosan challenged wells were treated with 100 μg/ml zymosan (Sigma-Aldrich, St. Louis, MO), which was diluted in MEM from a 250X stock dissolved in 100% EtOH. Zymosan is a commercially available preparation of the cell wall of Saccharomyces cerevisiae, which is a mixture of beta;-glucan and mannoprotiens, both of which are highly stimulatory[28]. A corresponding vehicle control was also prepared. At the end of the experiment, supernatants were collected to determine fibroblast viability (Cytotox One kit, Promega Corp., Madison, WI) and cytokine release. Standard sandwich ELISAs were performed for IL-6 (BD Biosciences, Franklin Lakes, NJ) and competitive EIA assays were performed for PGE2 quantification (Cayman Chemical Company, Ann Arbor, MI).
The yeast strains, C. albicans SC5314, C. glabrata BG2, C. tropicalis 20336, and S. cerevisiae, were all originally from the American Type Culture Collection (ATCC), provided from the microbiology lab of author C.H. All Candida strains selected are clinical isolates that have been sequenced and are available from the ATCC and these strains have been proven virulent in mouse models (http:/www.candidagenome.org). Although S. cerevisae is regarded as largely non-pathogenic, its cell wall shares greater that 90% similarity with known pathogens C. albicans and C. glabrata[35]. These particular strains have been selected because their genotypic and phenotypic information is available and their behaviors are comparatively predictable versus new uncharacterized clinical isolates. We also cultured the tissues in tandem, with the preparation of fibroblast strains and histological examination, tissues were processed for microbiological testing specifically attempting to isolate relevant yeast strains.
Vestibular and external vulvar tissue samples for in situ IHC microscopic study were received in additional separate containers. Tissue samples were oriented, bisected, and formalin-fixed. Paraffin-embedded tissue was cut into 5 micron sections for IHC staining. Monoclonal antibodies to IL-6 (1:1000 dilution), (Leica Biosystems, Buffalo Grove, IL), and Cox-2 (1:200 dilution), (Cell Marque, Rocklin, CA) were used during staining procedures conforming to previously published techniques[18]. During a pro-inflammatory process, like the mucocutaneous assault by pathogenic yeast, arachidonic acid product increases with enzymatic clevage of tissue lipids by cytosolic lipooxygenase A2. Arachidonic acid is, in turn, converted into intermediary Prostaglandin H2 by Cox-2, and finally converted into PGE2 by microsomal Prostaglandin E Synthetase (mPGES-1)[59]. Although PGE2 antibody is available for IHC, Cox-2 IHC is felt to be a better and more widely published marker of inflammation. Density of fusiform-shaped fibroblasts expressing these proteins in tissue sections from both vestibule and external vulva were evaluated by counting total number of cells in 10 high power fields (HPF) at 400x magnification using an Olympus BX-41 microscope,(Olympus Corp. Tokyo, Japan). Evaluation of cell morphology and enumeration was restricted to mesenchymal areas in close proximity to the epithelium, within 50 μm of basal epithelial cell layer, to include primarily the sub-epidermal neural plexus region[63] in the evaluation. Slide identifiers were masked to the evaluator (K.S.) with respect to case/control and vestibule/external vulva.
Statistical Analyses
Demographic data was compiled with calculation of means, standard deviations, medians, or proportions where appropriate. Paired t-tests were performed for some analyses of pro-inflammatory response by location. Linear mixed models were developed to take into account the repeated biopsies from a given individual. Pearson product-moment correlation coefficients characterized the univariate associations between fibroblast responsiveness to yeast products, IHC(+) in situ fibroblast density, and pain assessment methods. Power and sample size calculations were based on location-specific, fibroblast strain production of PGE2 and IL-6 following zymosan challenge. From our previous publication[26] and preliminary studies submitted during grant application (unpublished), differences in site-specific mean production of PGE2 and IL-6 following zymosan provocation were found to be substantially (over 10 fold) higher pg/ml concentrations of the pro-inflammatory factors, vestibule compared to external vulva. We anticipated that a live yeast challenge, particularly by species with greater virulence like C. albicans, should be even more stimulatory than zymosan. We therefore hypothesized that the production of PGE2 and IL-6 would be significantly higher in the vestibular fibroblast strains compared to external vulvar strains following zymosan and even more enhanced by live yeast products, such as C. ablicans. Three observations per group (case/control) for a paired t-test comparing the two correlated means in a two sided test, with alpha = 0.05 would have more than a 90% power to detect the 10 fold difference.
Additional linear mixed models analyzed the ability to predict site-specific pain thresholds based upon fibroblast responsiveness to yeast challenge again adjusting for the repeated biopsies from a given individual. We selected the Wagner™ Algometer pain threshold as the dependent variable and independent variables included: 1) fibroblast PGE2 or IL-6 responsiveness to live C. albicans, 2) fibroblast PGE2 or IL-6 responsiveness to live C. glabrata, and 3) Cox-2 or IL-6 IHC(+) in situ fibroblast count (number per 10 HPF). Live C. albicans and C. glabrata challenges were selected as independent variables for the model based upon robust pro-inflammatory fibroblast responses following these challenges. Mechanical sensation is widely recognized to be perceived logarithmically as defined by Weber’s Law. Based upon Weber’s Law, the Wagner™ algometer pain threshold measurements underwent a log transformation, prior to use as the dependent variable[44]. We studied the univariate associations of the log transformed threshold data with fibroblast responsiveness to yeast challenge and Cox-2 and IL-6 IHC(+) in situ fibroblast count and we studied the multivariate associations of the log transformed threshold data with fibroblast responsiveness to yeast challenge combined with Cox-2 and IL-6 IHC(+) in situ fibroblast count. Each regression procedure was based on a discrete hypothesis; no adjustments for multiple comparisons were made. For all analyses, 2-tailed testing at P < 0.05 was used for statistical significance. Linear Mixed model analyses and statistical graphics were carried out using SAS/STAT software, Version 9.3 of the SAS System (Copyright © July, 2011, SAS Institute Inc) on a Windows 7 platform. Other statistical analyses were completed on STATA software, Release 13, (STATA Corp., College Station, TX).
Results
Table 1 reviews subject demographics and pain assessments of the four cases and four controls, sampled at two sites each, providing a total of 16 fibroblast strains. Cases and controls were age matched with a mean age of 33.5 years, and all were Caucasian, non-Hispanic. The mean ± s.d. duration of vulvodynia in LPV cases was 72 ± 26 months. All subjects denied use of systemic or topical corticosteroids, regular use of NSAID’s, or chronic inflammatory illness with the exception of LPV. No topical vaginal agents had been used within one week of biopsy. One LPV-case reported the intermittent use of an NSAID but did not use within 7 days of surgery. With respect to menstrual status at time of biopsy, one case and one control subject were in the proliferative phase, one control subject was in the luteal phase, two LPV cases and two controls used oral contraceptives (active tablets at time of biopsy), and one LPV case had undergone hysterectomy and bilateral salpingo-ophorectomy and was using topical estrogen cream, vaginally. Maturation indices were similar between cases and controls and showed no evidence of vaginal parabasal cells and a predominance of intermediate cells. Pre-procedure, all subjects had a vaginal pH < 4.5, a negative vaginal yeast culture, negative saline and 10% potassium hydroxide microscopic vaginal smears, and negative Affirm™ probe testing. The proportion of subjects who practiced full vulvar hair removal was 3/4 cases and 2/4 controls. Based upon our pattern of recruitment, all cases in this analysis were clinically classified as having “secondary” LPV. Pain levels to cotton swab test (CST) ranged from 7/10 to 9/10 by NRS at the vestibule of LPV cases and 0/10 at the vestibule for pain-free controls. Both LPV cases and pain-free controls reported a median NRS of 0/10 at the external vulvar sites. Morrison algesiometry found a median pain threshold of 467 mN at the vestibule of LPV cases with all remaining sites: external vulva of LPV cases, vestibule of controls and external vulva of controls found to be pain free up to the maximum pulse force applied (1868 mN). Because pain thresholds for a substantial number of instances exceeded the maximum algesiometer force, the limited analyzable datapoints also limited assessment of construct valitidity. The Wagner™ algometry found lower pain thresholds at vestibular sites relative to external vulvar sites in both LPV cases (0.65 ± 0.16 N; 2.16 ± 1.89 N, respectively) and pain-free controls (2.10 ± 0.37 N; 4.09 ± 0.64 N, respectively). For similar vestibular sites of testing, our pain threshold values were comparable to those first reported by Zolnoun et al.[70].
Table 1.
Demographics, hormonal / shaving status, and the three measures of pain assessment shown. Each subject produced strains from two anatomic locations resulting in 16 fibroblast strains, in total.
| Case N=4 Ss, 8 strains |
Control N=4 Ss, 8 strains |
|
|---|---|---|
|
|
||
| Age (yrs.) | 33.5 | 33.5 |
| Nulliparity | 1 | 2 |
| Caucasian, non Hispanic | 4 | 4 |
| Mean Maturation Index (Parabasal/Intermediate/Superficial) | 0 / 84 / 16 | 0 / 88 / 12 |
| OC/ERT use | 3 | 2 |
| Duration from LMP (mean days) | 21 | 11 |
| Full vulvar hair removal (shaving) | 3 | 2 |
| Cotton Swab test (median NRS) | ||
| Vestibule | 8 | 0 |
| External vulva | 0 | 0 |
| Algesiometer pain threshold (median mN) | ||
| Vestibule | 467 | 1868 |
| External vulva | 1868 | 1868 |
| Pain threshold algometer (mean N ± sd) | ||
| Vestibule | 0.65 ± 0.16 | 2.10 ± 0.37 |
| External Vulva | 2.16 ± 1.89 | 4.09 ± 0.64 |
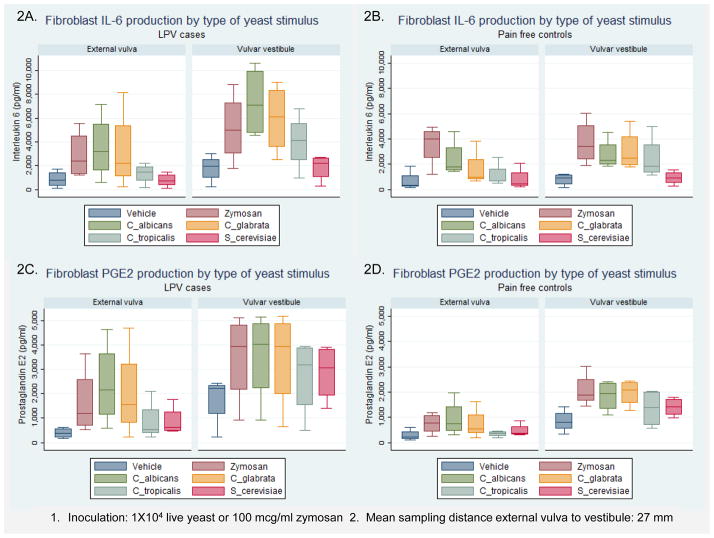
Stratified by presence or absence of LPV and substratified by sampling location, Figures 2A–D display the results of IL-6 and PGE2 production following live yeast challenge by selected yeast species, zymosan, and inactive vehicle. As noted in Figure 2, the mean anatomic sampling distance between vestibular and external vulvar biopsies was 27 mm and all live yeast challenges utilized species-specific 1 × 104 blastoconidia. With respect to the anatomic location of sampling, mean PGE2 production (Figure 2C) was highest following live C. albicans challenge of LPV vestibular fibroblasts (3539 ± 1879 pg/ml), followed by LPV external vulvar fibroblasts (2391 ± 1708 pg/ml), control vestibular fibroblasts (1841 ± 609 pg/ml) (Figure 2D) and control external vulvar fibroblasts (937 ± 726 pg/ml). Mean IL-6 production (Figure 2A) was highest following live C. albicans challenge of LPV vestibular fibroblasts (7361 ± 3046 pg/ml), followed by LPV external vulvar fibroblasts (3528 ±2753 pg/ml), control vestibular fibroblasts (2741 ± 1241 pg/ml) (Figure 2B), and control external vulvar fibroblasts (2396 ± 1483 pg/ml). In controls (Figures 2B and D), zymosan was as effective in producing a IL-6 / PGE2 response as any live yeast species, including C. albicans. Mean PGE2 production by zymosan-challenged control vestibular fibroblasts exceeded control external vulvar fibroblasts over 2-fold, 2065 ± 680 pg/ml vs. 742 ± 410 pg/ml (t=3.46; P=0.04). The mean IL-6 and PGE2 production by control fibroblast strains following zymosan or any live yeast challenge found vestibular fibroblasts consistently exceeded external vulvar fibroblasts. The data displayed in Figure 2 should be interpreted as representing trends rather than demonstrating statistical significance, except where stated. While the differences are clinically meaningful, they failed to achieve statistical significance and if differences this large are found upon addition to the sample size statistical significance will be attained.
Figure 2.
A–D. Box and whisker plots of fibroblast IL-6 pg/ml production (upper row) and PGE2 pg/ml producton (lower row) following challenge by live yeast, yeast products, and yeast product-free vehicle. Comparison of response case vs. control, subdivided by anatomic location, are displayed. Inoculation used a standard yeast count 1×104 CFU live yeast or 100 mcg/ml zymosan. Box represents median value and upper and lower quartiles, whiskers represent upper and lower ranges.
Cox-2 positive fibroblasts were identified in proximity to mucocutaneous layer as illustrated in Figure 4 A-400X and B-200X. Fibroblast counting was restricted to mesenchymal tissue in proximity (approx. 150 μm) to the mucocutaneous basal cell layer in order to incorporate the sub-epithelial neural plexus region. Higher Cox-2 IHC(+) expression in fibroblasts were noted both in vestibular areas with chronic inflammatory infiltrate and in vestibular areas absent of such infiltrate. By location, in situ mean Cox-2 IHC(+) fibroblasts were 29.0 (±16) per 10 HPF in external vulvar biopsies and 46.8 (±27) per 10 HPF in vulvar vestibule biopsies. In situ mean IHC(+) IL-6 fibroblasts were very rarely seen, 0.1 (±0.35) per 10 HPF, in the external vulvar biopsies and 1.4 (±3) per 10 HPF, in the vulvar vestibular biopsies. As illustrated in Figures 4A and B, the Cox-2 IHC(+) signal is evident in spindle shaped mesenchymal cells characteristic of fibroblasts. Vimentin staining had been performed in earlier work confirming these spindle shaped cells were consistent with fibroblasts.
Figure 4.
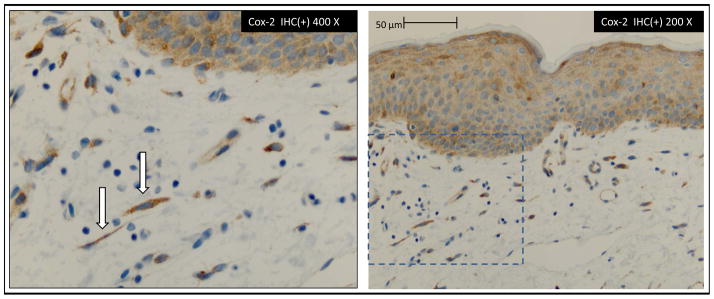
Immunohistochemical (IHC) identification of Cox-2 IHC(+) cells with spindle cell morphology of fibroblasts. IHC(+) fibroblast in close proximity to basal cell layer of epithelium in estimated region of sub-epithelial neural matrix.
Table 2 displays univariate correlations for selected variables. Included in Table 2 are fibroblast production levels following challenge by the two live yeast strains, C. albicans and C. glabrata, shown to evoke highest PGE2 and IL-6 production, the in situ density (number per 10 HPF) of IHC(+) fibroblasts for Cox-2 and IL-6, and the three pain threshold measurements. To compare correlations with earlier reports, only vestibular measures are reported (N=8). If the entire dataset were included (N=16), the introduction into a univariate correlation of tissue specimen pairs derived from the same subject would not permit with the same type of interpretability. Challenge by the identical yeast species, C. albicans or C. glabrata, resulted in significant correlations with IL-6 and PGE2 production (r= 0.89 to 0.87; P < 0.05), respectively. Significant correlations existed between CST pain assessment and the production of PGE2 or IL-6 following either C. albicans or C. glabrata challenge (range of r = 0.84 to 0.94; P < 0.05). Significant correlation was also found between LN(Wagner™ algometer) measure and the production of IL-6 following C. albicans infection (r = −0.70; P < 0.05) and correlation of algometry to PGE2 fibroblast production approached significance (r = −0.68; P < 0.06). In contrast, correlations between Morrison algesiometer and IL-6 and PGE2 production were the weakest of the three pain assessments (r = −0.29 to −0.48). Correlations between fibroblast Cox-2 IHC(+) count and fibroblast response to yeast were poor to moderate (r = 0.35 to 0.53). The correlations between IL-6 production and IL-6 IHC(+) (r = 0.78 to 0.91) were statistically significant but biological significance is questionable because of the extremely small number of IL-6 fibroblasts found per 10 HPF of IHC(+). Correlations between the three methods of mechanical pain assessment were significant (P < 0.05) with the exception of the correlation between CST and Morrison algesiometer. Finally, the correlations displayed in columns 9 and 10 of Table 2 illustrate the improvement in correlation between post-live yeast IL-6 and PGE2 production and the log transformation of Wagner™ algometer threshold values (as an example, r = −0.56 improves to r = −0.68 for PGE2 post-C. albicans), thus supporting Weber’s Law [44].
Table 2.
Zero order Pearson product-moment correlations adjusted for repeated measures (N=8 vestibular strains)
Zero order correlation for fibroblast measures selected for linear regression model in addition to values found for each of the three methods of pain assessment. Calculated Log transformation of Wagner™ algometer values also displayed in column 10 to demonstate improved correlation to fibroblast measures.
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|
|
|||||||||
| Fibroblast measures | |||||||||
| 1 PGE2 post-C. albicans | 0.99* | 0.87* | 0.89* | 0.52 | 0.78 | 0.94* | −0.48 | −0.56 | −0.68 |
| 2 PGE2 post-C. glabrata | 0.87* | 0.89* | 0.59 | 0.78 | 0.92* | −0.43 | −0.50 | −0.63 | |
| 3 IL-6 post-C. albicans | 0.97* | 0.55 | 0.91 | 0.90* | −0.47 | −0.61 | −0.70* | ||
| 4 IL-6 post-C. glabrata | 0.57 | 0.84 | 0.84* | −0.29 | −0.47 | −0.56 | |||
| 5 Cox-2 IHC(+) count | 0.54 | 0.37 | −0.07 | 0.19 | 0.03 | ||||
| 6 IL-6 IHC(+) count | 0.85* | −0.55 | −0.60 | −0.71* | |||||
| Threshold measures | |||||||||
| 7 CST (Median) | −0.63 | −0.77* | −0.86* | ||||||
| 8 Morrison algesiometer | 0.81* | 0.84* | |||||||
| 9 Wagner algometer | 0.98* | ||||||||
| 10 LN(Wagner algometer) | |||||||||
|
|
|||||||||
P < 0.05
A linear regression model was developed to determine if sampling location (external vulva or vestibule) could predict IL-6 or PGE2 production, adjusting for presence or absence of LPV. We utilized a linear mixed model format to statistically adjust for dual sampling from a given individual. PGE2 production was significantly higher in vestibular fibroblasts when challenged with any stimuli: vehicle (t= 3.64; P= 0.08), zymosan (t= 3.17; P= 0.02), C. albicans (t= 2.58; P= 0.04), C. glabrata (t= 2.73; P= 0.03), C. tropicalis (t= 3.1; P= 0.02), and S. cerevisiae (t= 4.51; P= 0.003). IL-6 production in vestibular fibroblasts was significantly higher following C. glabrata (t= 2.73; P= 0.03) and C. tropicalis (t= 3.1; P= 0.02) challenge.
Table 3 displays the results of the univariate and multivariate mixed effects models for the regression of log transformed Wagner™ algometry measurements on PGE2 and IL-6 production by fibroblast strains and in situ IHC(+) count. Wagner™ algometry was the threshold measure of choice based on good construct validity and reliability[70]. Following C. albicans and C. glabrata challenge, PGE2 and IL-6 production by fibroblast strains, significantly predicted pain threshold variation by Wagner™ algometry in the univariate analyses; it also predicted pain threshold in the multivariate models, while the in situ IHC(+) count did not. Post C. glabrata challenge IL-6 production approached statistical significance, adjusting for in situ IHC(+) IL-6 count,(t= −2.35; P=0.07). Table 3 includes the Akaike Information Criterion (AICC), with a correction for small n. Given a number of models, the preferred model is the one with the minimum AICC. This rewards goodness of fit and penalizes the fitting of too many parameters. For example, the simpler univariate models for C. albicans and C. glabrata in each circumstance would be viewed as preferred over the multivariate models that included the in situ IHC(+) count because they afforded simplicity and parsimony, as evidenced by the smaller AICC values. According to the univariate models, a 1 N elevation (improvement) in pain threshold predicted a 1000 pg/ml decline in PGE2 fibroblast response to C. albicans and a 1300 pg/ml decline in fibroblast response to C. glabrata. It should be noted that Beta coefficients displayed in Table 3, were adjusted to 1 ng/ml unit for both PGE2 and IL-6 production to improve readability of the regression display. Figure 3A and B displays scatter plots, fitted mixed effects regression lines, 95% confidence intervals (represented by the blue shaded area), and statistical significance for the ability of PGE2 and IL-6 production, following C. albicans infection, to predict pain threshold. As is evident, values appear to fall close to the fitted regression line particularly at the higher PGE2 and IL-6 production levels that are also associated with greater mechanical allodynia (lower thresholds). Although the scatterpoints displayed in Figure 3A and B includes all samples (N=16), the points located on the right lower corners of Figures 3A and B (high PGE2 and IL-6 production and low pain threshold), represent entirely the vestibular strains derived from LPV cases.
Table 3.
Mixed effects† models predicting pain threshold by Wagner Algometer (log transform)
Hierarchical regression analyses of fibroblast measures (independent variables) predicting log transform pain threshold by Wagner™ Algometer (dependent variable). N=16.
(β = beta coefficient; SEβ = standard error of beta coefficient; t = Student’s t test; P = probability; AICC = Akaike Information Criterion)
| Model | Independent Variables | N=16 Strains | P | AICC¥ | ||
|---|---|---|---|---|---|---|
| 3‡ | SEβ | t | ||||
| C. albicans stimulus of PGE2 pathway | ||||||
| 1 | Fibroblast=> PGE2 post C. albicans | −0.38 | 0.08 | −4.76 | 0.002 | 29.7 |
| 2 | Fibroblast=> PGE2 post C. albicans | −0.38 | 0.08 | −4.56 | 0.004 | 33.3 |
| Fibroblast Cox-2(+)count in situ | 0.001 | 0.006 | 0.26 | ns | ||
| C. glabrata stimulus of PGE2 pathway | ||||||
| 1 | fibroblast=> PGE2 post C. glabrata | −0.36 | 0.08 | −4.57 | 0.003 | 30.5 |
| 2 | fibroblast=> PGE2 post C. glabrata | −0.38 | 0.08 | −4.39 | 0.005 | 33.9 |
| Fibroblast Cox-2(+)count in situ | 0.002 | 0.006 | 0.40 | ns | ||
| C. albicans stimulus of IL-6 pathway | ||||||
| 1 | Fibroblast=> IL-6 post C. albicans | −0.18 | 0.05 | −3.86 | 0.006 | 33.3 |
| 2 | Fibroblast=> IL-6 post C. albicans | −0.19 | 0.08 | −2.46 | 0.05 | 36.9 |
| Fibroblast IL-6(+)count in situ | 0.004 | 0.11 | 0.04 | ns | ||
| C. glabrata stimulus of IL-6 pathway | ||||||
| 1 | fibroblast=> IL-6 post C. glabrata | −0.18 | 0.05 | −3.41 | 0.01 | 35.1 |
| 2 | fibroblast=> IL-6 post C. glabrata | −0.14 | 0.07 | −2.11 | ns | 38.1 |
| Fibroblast IL-6(+)count in situ | −0.076 | 0.10 | −0.79 | ns | ||
| Quantified IHC(+) measures alone | ||||||
| 1 | Fibroblast Cox-2(+)count in situ | −0.007 | 0.008 | −0.88 | ns | 46.5 |
| 1 | Fibroblast IL-6(+)count in situ | −0.21 | 0.08 | −2.54 | 0.04 | 38.4 |
Mixed effects adjusting for sampling two sites of same subject
Beta coefficient PGE2 and IL-6 expressed in ng/ml
Akaike Information Criterion
Figure 3.
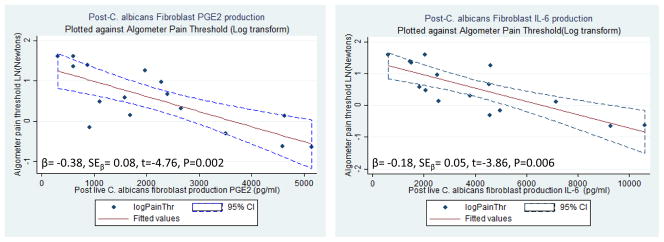
Scatterplots with fitted values and 95% CI’s (dotted) for PGE2 (Fig 3a.) and IL-6 (Fig 3b.) production versus mechanical pain threshold (Log Transform)
Scatterplots of fibroblast production of PGE2 (right graph) and IL-6 (left graph) plotted against log transform of mucocutaneous pain threshold, done prior to tissue sampling, from the identical anatomic sites (N=16). Central line represents fitted values of linear regression delimited by 95% confidence intervals (dotted lines).
Discussion
In LPV, pain to light touch is found to be highly delineated to the vestibule with painful and non-painful sites separated by a matter of millimeters. Specific live yeast stimuli, particularly species with a greater tendency for virulence: (e.g. C. albicans and C. glabrata), elicit enhanced IL-6 and PGE2 production in vestibular fibroblasts compared to external vulvar fibroblasts. Anatomically, these fibroblasts exist in situ, a mere 27 millimeters apart, but arise from embryologically distinct anlage. The urethra and vulvar vestibule in the human embryo arises from endoderm of the cloacal membrane [51;52;66]. Fetal human and pig urogenital sinus development are quite similar[51,52]. Following birth, the visible line of change of external vulvar skin (ectodermal derivative) and vestibular mucocutaneous tissue (endodermal derivative) is identified as Hart’s line. In our research, Hart’s line separates the two tissue sampling sites, (red dashed line, Figure 1E). Fibroblasts arising from various anatomic sites carry location specific “stromal address codes”[11;36;58] characterized by specific mixes of cell surface marker expression and vastly different functional properties, including immunological response. By comparatively testing the ability of these location-specificfibroblast strains to induce a pro-inflammatory response (e.g. production of IL-6 and PGE2), we can begin to “tease-out” factors involved in yeast virulence (e.g. hyphae, secretion of pro-inflammatory molecules, expression of specific cell surface-associated moieties), and site-specific fibroblast characteristics that cause differential proinflammatory responses’ leading to differential pain thresholds.
The spectrum of LPV etiology is likely to be multifactorial and yeast vulvovaginitis may be a common, but not necessarily sole antecedent[1;34;42;64]. Extensive research on the pathogenesis of vulvovaginal candidiasis has focused on mucocutaneous, dendritic cell, and macrophage responses to the mucosal assault mounted by pathogenic yeast[40;54]. Solubilized candidal products diffusing into the submucosa[2], may also serve as a potential activating agent for fibroblasts [39]. In contrast to the epithelial layer, the underlying proximal connective tissue matrix in close proximity to subepidermal neural plexus[63] is the prime location for generating cytokine mediated perineural inflammation and associated neuropathic pain[14;19;27]. In this project we have shown that anatomic location defines a difference in the mesenchymal response to live yeast and yeast products in both LPV cases and pain-free controls. LPV cases differ from pain-free controls by the degree of this pro-inflammatory response.
C. albicans and C. glabrata are two of the most commonly virulent yeast species associated with genital tract infection while C. tropicalis has been identified less frequently[35;56]. The immune response linked to invasiveness, although incompletely understood from an immunological perspective, more invasive strains, specifically strains that penetrate and invade mucosa tissue (e.g. C. albicans), tend to elicit a greater immune response[21;45;62]. Currently, very little has been established in regards to the phenotypic or genetic composition of yeast species associated with LPV, their relative virulence, and/or the natural history of yeast in both symptomatic and asymptomatic women. [7;9;15;69]. Therefore, we have selected strains with known virulence, as a relative model of infection. In the future we plan to evaluate clinical isolates shown to be specific to LPV development in our established fibroblast model. In addition to using a live infection model, we have established that yeast components (e.g. zymosan or cell wall fractions can elicit a powerful response in fibroblasts from vulvodynia patients.
Zymosan, as an alternative to live yeast, is a commercially available preparation of the cell wall of Saccharomyces cerevisiae, which is a mixture of β-glucan and mannoprotiens, both of which are highly stimulatory[10;32;37;53]. Mannoprotein may be found in the outermost layers of the yeast cell wall and will readily contact immune mediators (e.g. PAMP receptors) during infection, while 3-glucan is exposed at bud scar during cell division and is actively secreted as a part of the biofilm matrix associated with chronic/persistent infection[6;10;32;37;46;47;53;60]. In previous studies we have demonstrated that fibroblast strains from vulvodynia patients respond strongly to zymosan without a significant reduction in cell (fibroblast) viability[26]. Zymosan treatments are also often easier to standardize than a live infection model. Furthermore, zymosan may have utility for applications where the incorporation of live yeast is not feasible. For example, zymosan may be a useful instrument in studies involving protein extracts, where there are no established methods for separating human and yeast proteins. Together, these are two valuable tools (live infection and zymosan stimulation) that can be used to further dissect the pathogenesis of pain in vulvodynia patients with the ultimate aim of improving therapeutic approaches.
Of the three published clinical methods of mechanical pain assessment, the Wagner™ algometry has undergone the most extensive reliability and validity testing [70]. We also found that the other two techniques: CST and Morrison algesiometry, carried additional methodological and design limitations. Although the CST demonstrates good inter-rater reliability[4], the user-dependent manual application of the cotton swab limits the generalizability and precision with respect to predicting fibroblast behavior. Morrison algesiometry had been designed to generate seven mechanical pulse levels within the vulvar vestibule threshold range of most LPV cases. As a result, the maximal algesiometer level of 1868 mN fell well below threshold for the vestibule and external vulva of controls and the external vulvar threshold of cases thus limiting the testable threshold range. Using our preferred method, Wagner™ algometry we were able to predict mechanical pain threshold by assessment of fibroblast strain IL-6 and PGE2 production following challenges with selected species of live yeast. In spite of our preference for the Wagner™ instrument, all three methods correlated significantly(Table 2), thus demonstrating good construct validity between methods.
Our LPV pathogenesis model developed in this project conforms to several biological “realities” of LPV but complexities of disease phenotype, immune response, and vulvodynia pain will necessitate further research. First, based upon recruitment, this study was limited to secondary LPV cases. Secondary LPV has been proposed to be based on an acquired “irritative” etiology[71] in contrast to primary LPV cases which may have pre-existing extragential sensory / motor sensitivity[33;71] and may be influenced hormonally[31]. Our model involving live yeast perturbation of fibroblasts is consistent with an “irritative” pathogenesis. A search for fibroblast response differences in primary and secondary LPV should be included in future work. Second, the immune response of the vestibule is undoubtedly more complex than our model suggests. We have shown that species of yeast with greater virulence, (C. albicans and C. glabrata), produce a more dramatic pro-inflammatory, pain-inducing response in the vulvar vestibule of LPV cases than commensal species. The intracellular mechanism of such a response will be the subject of future work. Future work also needs to consider amplification of innate immunity by a myriad of environmental factors, and the contribution of cells of epithelial, dendritic, lymphocytic, mast, and neural origin on both innate and acquired immune responses of the vestibule. Finally, our proposed model is focused on peripheral sensitization in development of LPV pain at the expense of de-emphasizing CNS factors. We do not intend to fully explain the complexity of vulvodynia. Instead, this physiologically simple model demonstrates that mucocutaneous pain threshold is predictable by assessment of fibroblast “responsiveness (IL-6 and PGE2 production) irrespective of anatomic location or presence/absence of LPV. The addition of in situ Cox-2 and IL-6 IHC(+) fibroblast count into the analysis resulted no improvement in model prediction. The low count of pro-inflammatory IHC(+) fibroblasts may be based upon a secreted extracellular status of pro-inflammatory factors or may be based upon insensitive in situ IHC techniques. Additional markers of in situ fibroblast activity will be sought in future studies in addition to the use of confocal technology.
During greater than 25 years of vulvodynia research, exquisite pain to a myriad of stimuli highly localized to the vulvar vestibule has been a consistent observation[5;50;70]. During this time, there has been little progress in understanding the etiopathogenesis of vulvodynia and the contribution to provoked pain originating from this small anatomic zone. Indeed, localized vestibular pain is not unique to LPV. Recently, the chronic pain of atrophic vaginitis has been shown by sensory testing to be localized to the vulvar vestibule rather than the vagina [30]. Based upon our research and that of others[71], we can propose the following mechanism for secondary LPV, and potentially other subtypes. There exists in all women, pain-free and LPV afflicted alike, an enhanced innate immune mechanism localized to the vulvar vestibule and controlled, in part, by site-specific fibroblasts. Upon provocation by environmental factors operating through innate immunity, pro-inflammatory cytokines and neurokines increase. Changes in peripheral nerve structure and function ensues through several processes leading to peripheral sensitization[12;61]. Following a series of environmental triggers, the genetically predisposed woman develops progressively higher levels of inflammation and allodynia leading to the LPV condition. Extragenital sensory threshold changes and motor dysfunctiondevelop thought to be based upon the phenomenon of “central sensitization”[24;29;68;71]. Additional psycho-affective alterations, such as depression, catastrophizing, and hypervigilance evolve as the secondary LPV condition persists[71]. Assuming that our proposal and observations are valid, the phenomenon of site-specific, enhanced responsiveness to innate immune mediators may precede LPV onset and promote early LPV pathogenesis. Therapeutic intervention at this early stage may raise the hope of effective new options and, ideally, primary prevention.
Acknowledgments
Supported by National Institutes of Health grant: HD069313 (NICHD)
Footnotes
Strains are defined as cellular collections derived from different anatomic sites of different human beings.
DF, MF, CW, SP, KS, AB, CH, SC, SM, MI, and RP have no conflicts of interest to disclose in relation to this article.
References
- 1.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics & Gynecology. 2006;107(3):617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley C, Morhart M, Rennie R, Ziola B. Release of Candida albicans yeast antigens upon interaction with human neutrophils in vitro. Journal of Medical Microbiology. 1997;46(9):747–755. doi: 10.1099/00222615-46-9-747. [DOI] [PubMed] [Google Scholar]
- 3.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, Phipps RP. Isolation and phenotypic characterization of lung fibroblasts. Methods in Molecular Medicine. 2005;117:115–127. doi: 10.1385/1-59259-940-0:115. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI. Vulvar vestibulitis syndrome: reliability of diagnosis and evaluation of current diagnostic criteria. Obstetrics & Gynecology. 2001;98(1):45–51. doi: 10.1016/s0029-7844(01)01389-8. [DOI] [PubMed] [Google Scholar]
- 5.Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain. 2001;94:177–183. doi: 10.1016/S0304-3959(01)00352-9. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Frontiers in cellular and infection microbiology. 2012;2:142. doi: 10.3389/fcimb.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CJ, Wong M, Davis CC, Kanti A, Zhou X, Forney LJ. Preliminary characterization of the normal microbiota of the human vulva using cultivation-independent methods. J Med Microbiol. 2007;56(Pt 2):271–276. doi: 10.1099/jmm.0.46607-0. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli F, Mucci MP, Cociancich L, Micheli W, Bacarini L, Cisternino M. Prostaglandin E2 liberation in the synovial fluid induced by organo-iodinated contrast media. Interrelations with the genesis of post-arthrographic pain [Italian] Radiologia Medica. 1987;74(6):512–515. [PubMed] [Google Scholar]
- 9.Chaban B, Links MG, Jayaprakash TP, Wagner EC, Bourque DK, Lohn Z, Albert AY, van Schalkwyk J, Reid G, Hemmingsen SM, Hill JE, Money DM. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome. 2014;2:23. doi: 10.1186/2049-2618-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaffin WL. Candida albicans cell wall proteins. Microbiology and molecular biology reviews: MMBR. 2008;72(3):495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2002;99(20):12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1. 9 in normal and inflamed rats. Neuroscience Letters. 2004;355(1–2):45–48. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88(3):239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 14.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. British Journal of Pharmacology. 1992;107(3):660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danielsson D, Teigen PK, Moi H. The genital econiche: focus on microbiota and bacterial vaginosis. Ann N Y Acad Sci. 2011;1230:48–58. doi: 10.1111/j.1749-6632.2011.06041.x. [DOI] [PubMed] [Google Scholar]
- 16.Daymond TJ, Rowell FJ. Reduction of prostaglandin E2 concentrations in synovial fluid of patients suffering from rheumatoid arthritis following tiaprofenic acid or indomethacin treatment. Drugs. 1988;35(Suppl-8) doi: 10.2165/00003495-198800351-00004. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosomatic Medicine. 2001;63(2):316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Elias JM. Technical considerations in immunocytochemistry. Cell Vision. 1998;5(1):35–36. [PubMed] [Google Scholar]
- 19.Eliav E, Benoliel R, Herzberg U, Kalladka M, Tal M. The role of IL-6 and IL-1beta in painful perineural inflammatory neuritis. Brain, Behavior, & Immunity. 2009;23(4):474–484. doi: 10.1016/j.bbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Eva LJ, Reid WM, MacLean AB, Morrison GD. Assessment of response to treatment in vulvar vestibulitis syndrome by means of the vulvar algesiometer. American Journal of Obstetrics & Gynecology. 1999;181(1):99–102. doi: 10.1016/s0002-9378(99)70442-4. [DOI] [PubMed] [Google Scholar]
- 21.Falgier C, Kegley S, Podgorski H, Heisel T, Storey K, Bendel CM, Gale CA. Candida species differ in their interactions with immature human gastrointestinal epithelial cells. Pediatric research. 2011;69(5 Pt 1):384–389. doi: 10.1203/PDR.0b013e31821269d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, Bennett GJ, Ribeiro-da-Silva A, Binik YM, Mogil JS. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Science Translational Medicine. 2011;3(101):101ra191. doi: 10.1126/scitranslmed.3002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. [Erratum appears in J Immunol 1990 Aug 1;145(3):1037 Note: Alvaro-Garcia JM [corrected to Alvaro-Gracia JM]] Journal of Immunology. 1990;144(9):3347–3353. [PubMed] [Google Scholar]
- 24.Foster DC, Dworkin RH, Wood RW. Effects of intradermal foot and forearm capsaicin injections in normal and vulvodynia-afflicted women. Pain. 2005;117(1–2):128–136. doi: 10.1016/j.pain.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Foster DC, Kotok MB, Huang LS, Watts A, Oakes D, Howard FM, Stodgell CJ, Dworkin RH. The tampon test for vulvodynia treatment outcomes research: reliability, construct validity, and responsiveness. Obstetrics & Gynecology. 2009;113(4):825–832. doi: 10.1097/AOG.0b013e31819bda7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. American journal of obstetrics and gynecology. 2007;196(4):346 e341–348. doi: 10.1016/j.ajog.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 27.Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Research. 1994;#19;657(1–2):133–140. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Howe N, Volk K, Tati S, Nickerson KW, Petro TM. Candida albicans cell wall components and farnesol stimulate the expression of both inflammatory and regulatory cytokines in the murine RAW264. 7 macrophage cell line. FEMS immunology and medical microbiology. 2010;60(1):63–73. doi: 10.1111/j.1574-695X.2010.00717.x. [DOI] [PubMed] [Google Scholar]
- 29.Giesecke J, Reed BD, Haefner HK, Giesecke T, Clauw DJ, Gracely RH. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstetrics & Gynecology. 2004;104(1):126–133. doi: 10.1097/01.AOG.0000129238.49397.4e. [DOI] [PubMed] [Google Scholar]
- 30.Goetsch MF, Lim JY, Caughey AB. Locating pain in breast cancer survivors experiencing dyspareunia: a randomized controlled trial. Obstetrics & Gynecology. 123(6):1231–1236. doi: 10.1097/AOG.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 31.Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: a prospective study. American journal of obstetrics and gynecology. 2010;202(6):614 e611–618. doi: 10.1016/j.ajog.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nature reviews Microbiology. 2012;10(2):112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granot M, Friedman M, Yarnitsky D, Tamir A, Zimmer EZ. Primary and secondary vulvar vestibulitis syndrome: systemic pain perception and psychophysical characteristics. American Journal of Obstetrics & Gynecology. 2001;(1) doi: 10.1016/j.ajog.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 34.Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital tract discomfort. American Journal of Obstetrics & Gynecology. 2001;185(3):545–550. doi: 10.1067/mob.2001.116748. [DOI] [PubMed] [Google Scholar]
- 35.Klis FM, Boorsma A, De Groot PW. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23(3):185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- 36.Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(-) human female reproductive tract fibroblasts. American Journal of Pathology. 2001;159(3):925–935. doi: 10.1016/S0002-9440(10)61768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latge JP. Tasting the fungal cell wall. Cellular microbiology. 2010;12(7):863–872. doi: 10.1111/j.1462-5822.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 38.Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstetrics and gynecology. 2011;117(6):1307–1313. doi: 10.1097/AOG.0b013e31821c33dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lev-Sagie A, Nyirjesy P, Tarangelo N, Bongiovanni AM, Bayer C, Linhares IM, Giraldo PC, Ledger WJ, Witkin SS. Hyaluronan in vaginal secretions: association with recurrent vulvovaginal candidiasis. American Journal of Obstetrics & Gynecology. 2009;201(2):206–205. doi: 10.1016/j.ajog.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Chen Q, Shen Y, Liu W. Candida albicans phospholipomannan triggers inflammatory responses of human keratinocytes through Toll-like receptor 2. Experimental Dermatology. 2009;18(7):603–610. doi: 10.1111/j.1600-0625.2008.00832.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin CR, Amaya F, Barrett L, Wang H, Takada J, Samad TA, Woolf CJ. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. Journal of Pharmacology & Experimental Therapeutics. 2006;319(3):1096–1103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 42.Mann MS, Kaufman RH, Brown D, Adam E. Vulvar vestibulitis: significant clinical variables and treatment outcome. Obstetrics & Gynecology. 1992;79(1):122–125. [PubMed] [Google Scholar]
- 43.Meana M, Binik YM, Khalife S, Cohen DR. Biopsychosocial profile of women with dyspareunia. Obstetrics & Gynecology. 1997;90(4 Pt 1):583–589. doi: 10.1016/s0029-7844(98)80136-1. [DOI] [PubMed] [Google Scholar]
- 44.Mills C, Leblond D, Joshi S, Zhu C, Hsieh G, Jacobson P, Meyer M, Decker M. Estimating efficacy and drug ED50’s using von Frey thresholds: impact of weber’s law and log transformation. Journal of Pain. 2012;13(6):519–523. doi: 10.1016/j.jpain.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Netea MG, Kullberg BJ. Epithelial sensing of fungal invasion. Cell host & microbe. 2010;8(3):219–220. doi: 10.1016/j.chom.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrobial agents and chemotherapy. 2007;51(2):510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nobile CJ, Nett JE, Hernday AD, Homann OR, Deneault JS, Nantel A, Andes DR, Johnson AD, Mitchell AP. Biofilm matrix regulation by Candida albicans Zap1. PLoS biology. 2009;7(6):e1000133. doi: 10.1371/journal.pbio.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nunns D, Mandal D. Psychological and psychosexual aspects of vulvar vestibulitis. Genitourinary Medicine. 1997;73(6):541–544. doi: 10.1136/sti.73.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96(1–2):57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 50.Peckham BM, Maki DG, Patterson JJ, Hafez GR. Focal vulvitis: a characteristic syndrome and cause of dyspareunia. Features, natural history, and management. American Journal of Obstetrics & Gynecology. 1986;154(4):855–864. doi: 10.1016/0002-9378(86)90472-2. [DOI] [PubMed] [Google Scholar]
- 51.Penington EC, Hutson JM. The cloacal plate: the missing link in anorectal and urogenital development. BJU International. 2002;89(7):726–732. doi: 10.1046/j.1464-410x.2002.02655.x. [DOI] [PubMed] [Google Scholar]
- 52.Penington EC, Hutson JM. The urethral plate--does it grow into the genital tubercle or within it? BJU International. 2002;89(7):733–739. doi: 10.1046/j.1464-410x.2002.02656.x. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Garcia LA, Diaz-Jimenez DF, Lopez-Esparza A, Mora-Montes HM. Role of Cell Wall Polysaccharides during Recognition of Candida albicans by the Innate Immune System. Glycobiology. 2011;1:1. [Google Scholar]
- 54.Ramirez-Ortiz ZG, Means TK. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans) [Review] Virulence. 2012;3(7):635–646. doi: 10.4161/viru.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinold H, Ahmadi S, Depner UB, Layh B, Heindl C, Hamza M, Pahl A, Brune K, Narumiya S, Muller U, Zeilhofer HU. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. Journal of Clinical Investigation. 2005;115(3):673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2014;33(5):673–688. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- 57.Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis [Review] [156 refs] Journal of Theoretical Biology. 1993;164(1):103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 58.Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. Journal of Clinical Endocrinology & Metabolism. 2002;87(1):385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 59.Suram S, Silveira LJ, Mahaffey S, Brown GD, Bonventre JV, Williams DL, Gow NA, Bratton DL, Murphy RC, Leslie CC. Cytosolic phospholipase A(2)alpha and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation. PLoS ONE [Electronic Resource] 2013;8(7):e69002. doi: 10.1371/journal.pone.0069002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS pathogens. 2012;8(8):e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tympanidis P, Casula MA, Yiangou Y, Terenghi G, Dowd P, Anand P. Increased vanilloid receptor VR1 innervation in vulvodynia. European Journal of Pain: Ejp. 2004;8(2):129–133. doi: 10.1016/S1090-3801(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 62.Villar CC, Kashleva H, Mitchell AP, Dongari-Bagtzoglou A. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infection and immunity. 2005;73(8):4588–4595. doi: 10.1128/IAI.73.8.4588-4595.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wendelschafer-Crabb G, Kennedy WR, Walk D. Morphological features of nerves in skin biopsies. Journal of the Neurological Sciences. 2006;242(1–2):15–21. doi: 10.1016/j.jns.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 64.Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. American Journal of Obstetrics & Gynecology. 2002;187(3):589–594. doi: 10.1067/mob.2002.125889. [DOI] [PubMed] [Google Scholar]
- 65.Wolf J, Weinberger B, Arnold CR, Maier AB, Westendorp RG, Grubeck-Loebenstein B. The effect of chronological age on the inflammatory response of human fibroblasts. Experimental Gerontology. 2012;47(9):749–753. doi: 10.1016/j.exger.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodruff JD, Parmley TH. Atlas of Gynecologic Pathology. Philadelphia, PA: J.B Lippincott Co; 1988. The Vulva; pp. 1.2–1.6. [Google Scholar]
- 67.Zanjani TM, Sabetkasaei M, Mosaffa N, Manaheji H, Labibi F, Farokhi B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. European Journal of Pharmacology. 2006;538(1–3):66–72. doi: 10.1016/j.ejphar.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Zolnoun DA, Francisco EM, Holden JK, Dennis RG, Tommerdahl M. Altered central sensitization in subgroups of women with vulvodynia. Clinical Journal of Pain. 2011;27(9):755–763. doi: 10.1097/AJP.0b013e31821c98ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou X, Westman R, Hickey R, Hansmann MA, Kennedy C, Osborn TW, Forney LJ. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infection and immunity. 2009;77(9):4130–4135. doi: 10.1128/IAI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. Journal of Pain. 2012;13(9):910–920. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zolnoun D, Hartmann K, Lamvu G, As-Sanie S, Maixner W, Steege J. A conceptual model for the pathophysiology of vulvar vestibulitis syndrome. Obstetrical & Gynecological Survey. 61(6):395–401. doi: 10.1097/01.ogx.0000219814.40759.38. [Review] [81 refs] [DOI] [PubMed] [Google Scholar]