Abstract
BACKGROUND
Well-differentiated gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare tumors with varying metastatic potential. The underlying molecular basis for metastasis by GEP-NETs remains undefined.
METHODS
Quantitative PCR and immunohistochemistry (IHC) staining for ubiquitin carboxyl-terminal esterase L1 (UCHL1) gene and protein expression was performed on a group of localized and metastatic well-differentiated GEP-NET samples acquired from a prospectively maintained tissue bank. The ability of extent of UCHL1 IHC staining to differentiate localized and metastatic tumors was compared to Ki-67 index.
RESULTS
Among 46 total samples, UCHL1 expression at both the gene and protein level was significantly greater among localized GEP-NETs compared with metastatic tumors and metastases (p<0.001). Hyper-methylation of the UCHL1 promoter was commonly observed among metastatic primary tumors and metastases (those with the lowest UCHL1 expression) but not among localized tumors (p<0.001). Poor staining (<50%) for UCHL1 was observed in 27% of localized tumors compared to 87% of metastatic tumors (p=0.001). The presence of <50% staining for UCHL1 was 88% sensitive and 73% specific for identifying metastatic disease. In contrast, there was no association between Ki-67 index and metastatic disease. In multivariable analysis, only UCHL1 staining <50% (OR 24.5, p=0.035) and vascular invasion (OR 38.4, p=0.030) were independent risk factors for metastatic disease at the time of initial surgery.
CONCLUSIONS
Loss of UCHL1 expression by CpG promoter hypermethylation is associated with metastatic GEP-NETs. Extent of UCHL1 staining should be explored as a potentially clinically useful adjunct to Ki-67 index in evaluating GEP-NETs for aggressive features.
Keywords: UCHL1, Neuroendocrine tumors, carcinoid, methylation
Introduction
Gastroenteropancreatic well-differentiated neuroendocrine tumors (GEP-NETs) comprise a relatively rare, heterogeneous group of tumors that demonstrate a wide spectrum of clinical behavior 1–3. Several clinical and pathologic factors have been associated with poorer outcomes of GEP-NETs, such as advanced age, male gender, Caucasian race, higher histological grade, vascular invasion, and high Ki-67 proliferation index4–8. In addition, a striking correlation with poor outcomes has been noted with disease stage. Among patients with well-differentiated (low- and intermediate-grade) GEP-NETs, median survival for patients with localized, regional, and distant disease is 223 months, 111 months, and 33 months, respectively (p<0.001) 9. Interestingly, GEP-NETs from different sites within the gastrointestinal tract vary greatly in their propensity to metastasize for reasons that remain unclear9–11.
At the present time little is known about the underlying molecular changes that differentiate localized and metastatic NETs. Ki-67 proliferation index is the best available biomarker to predict aggressive behavior and is central to current WHO tumor grading12. Ki-67 levels >20% are diagnostic of poorly differentiated GEP-NETs, and have a five-year survival of 0% if the tumor has metastasized. For intermediate-grade (Ki-67 < 20%) and low-grade (Ki-67 <3%) well-differentiated tumors, higher Ki-67 values have been correlated with metastatic disease; however, its utility as a reliable marker is limited by the wide range of values in metastatic tumors as well as controversy over its ideal critical value13. Accordingly, there exists a need for more reliable markers of metastatic disease in well-differentiated GEP-NETs.
Recently, our group performed Next-Generation RNA sequencing on a small group of localized and metastatic GEP-NETs (data unpublished). From this pilot data, we discovered that ubiquitin carboxyl-terminal esterase L1 (UCHL1) gene expression appears to be lost in metastatic primary tumors and metastates. UCHL1 is known to be a regulator of the ubiquitin proteasome pathway and controls intracellular protein stability 14. It is specifically expressed in neurons and in the diffuse neuroendocrine system, and it is a well-known candidate gene in the pathogenesis of Parkinson’s disease 15,16. Recently, UCHL1 has been reported as frequently silenced by CpG promoter hyper-methylation in several tumor types, including breast cancer 17, melanoma 18, nasopharyngeal carcinoma 19, and cholangiocarcinoma 20, but its association with GEP-NETs has not been reported.
Herein, we aimed to evaluate gene expression, immunohistochemistry (IHC) staining, and CpG promoter methylation of UCHL1 among a group of localized and metastatic GEP-NETs, and compared its ability to distinguish localized and metastatic tumors to Ki-67 index.
Materials and Methods
Patient Selection
A prospectively maintained tissue bank was reviewed to identify patients who had undergone surgery for a well-differentiated GEP-NET at a single academic tertiary care referral center between May 2002 and December 2012. Two additional tumor samples were obtained from the Cooperative Human Tissue Network, which is funded by the National Cancer Institute. Only well-differentiated neuroendocrine tumors arising in the pancreas, stomach, small bowel, colon, or rectum from patients aged 18 years and older were included in this study. Moderately- and poorly-differentiated neuroendocrine tumors, as well as those arising from primary sites other than those listed above were excluded. Demographic, clinical, and pathological data, including Ki-67 values, were collected for each patient. Particular attention was paid to the presence of regional nodal or distant metastases at the time of surgery. Informed written consent was obtained from all patients for the future use of tissue for genetic research purposes prior to their initial operation. The Institutional Review Board of Weill Cornell Medical College approved this study.
To elucidate potential markers of metastatic disease, samples of primary tumors that had metastasized and the samples of the metastatic lesions themselves were combined into a single “metastatic” group, which was compared to localized primary tumors.
Tissue Collection
Tumor samples were collected immediately after resection, snap-frozen in liquid nitrogen, and stored at −80°C until further analysis. Unstained 5 μM-thick formalin-fixed paraffin (FFPE) embedded slides of the tumor were obtained for immunohistochemistry. A gastrointestinal pathologist (N.P.) reviewed a hematoxylin and eosin (H&E) stained slide of each tumor to confirm the presence of a GEP-NET in the specimen.
RNA Extraction
RNA was extracted from frozen tissue and cells using the RNeasy Mini Kit and purified with the RNeasy Min Elute Cleanup Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. RNA quality was assessed with a Bioanalyzer (Agilent Technologies, Santa Clara, CA). An RNA Integrity Number (RIN) of ≥ 7 was required for quantitative RT-PCR (qPCR).
Reverse Transcription and Quantitative PCR
First-strand cDNA synthesis was performed as previously described 21. The qPCR was performed with the TaqMan® Gene Expression Assay (Life Technologies, Carlsbad, CA) using predesigned primers for UCHL1 and GUSB according to the manufacturer’s instructions. The following thermal cycling parameters were used: incubation at 50°C for 2 minutes, denaturing at 95°C for 10 minutes, then 40 cycles of the amplification step (denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute). UCHL1 gene expression was normalized relative to the housekeeping gene GUSB. All experiments were performed in triplicate and gene expression values were calculated according to the ΔΔ CT method22.
Immunohistochemistry Staining and Grading
IHC staining of UCHL1 was accomplished using the Bond III Autostainer (Leica Microsystems, Illinois, USA). Formalin-fixed, paraffin-embedded tissue sections were first baked and deparaffinized. Antigen retrieval was followed by heating the slides at 99–100°C in Bond Epitope Retrieval Solution 1 for 30 minutes. Sections were then subjected to sequential incubations with primary antibody (anti-PGP9.5 rabbit polyclonal, 1:200 dilution, Dako, Glostrup, Denmark), post-primary (equivalent to secondary antibody), polymer (equivalent to tertiary antibody), endogenous peroxidase block, diaminobenzidine (DAB) and hematoxylin for 15, 8, 8, 5, 10 and 5 minutes (Bond Polymer Refine Detection; Leica Microsystems), respectively. Finally the sections were dehydrated in 100% ethanol, and mounted in CytosealTM XYL (Richard-Allan Scientific, Kalamazoo, MI).
Grading of UCHL1 staining was performed by a gastrointestinal pathologist (N.P.) based on extent of staining within the tumor. Tumors were divided into quartiles based on percent of tumor cells that stained positive for UCHL1. Tumors with >50% staining were considered positive for UCHL1 expression.
Quantitative DNA Methylation Analysis by Mass Spectrometry
DNA methylation was measured using mass spectrometry (Epityper, Sequenom, San Diego, CA) as previously described 23. A two-kilobase region around the promoter of the UCHL1 gene was probed (chr4: 41,257,820–41,260,026). One μg of DNA was treated with sodium bisulfite using the EZ methylation kit (Zymo-Research, Irvine, CA). CpG islands with variable methylation (standard deviation >1.0) between samples were compared.
Statistical Analysis
P-values were calculated using Pearson’s chi-squared test, student’s T-test, Mann-Whitney U-test, one-way Analysis of Variance, or Kruskal-Wallis test, as appropriate. Continuous variables that followed a normal distribution are presented as mean ± standard deviation (SD), while those that were not normally distributed are presented as median (range). Variables that were significantly associated with metastatic disease on univariable analysis were entered into a multivariable logistic regression model to identify independent risk factors for metastatic disease. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 12.0 (College Station, TX).
Results
Study Cohort
In total, 46 patients with 46 primary GEP-NET tumors were included in this study, including patients whose tumors originated in the pancreas (n=11), stomach (n=6), small bowel (n=19), appendix (n=6), and colon/rectum (n=4). In addition, five distant metastases were analyzed, including three liver metastases and two peritoneal implants. When grouped by location of the primary tumor, there was no difference in gender between the groups, but age, tumor size, pathologic stage, and presence of metastases varied significantly by site of origin (Table 1). Notably, the incidence of metastases at the time of surgery ranged from 0% for appendiceal NETs to 89% for small bowel NETs (P<0.001), which is consistent with previously reported heterogeneity in metastatic potential by site of origin9–11.
Table 1.
Patient Demographics and Histopathologic Features of Well-Differentiated Neuroendocrine Tumors Grouped by Site of Origin
| N=46 | Pancreas (N=11) | Stomach (N=6) | Small Bowel (N=19) | Appendix (N=6) | Colon/Rectum (N=4) | p-value |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 46.2±16.8 | 57.8±16.4 | 60.7±10.6 | 43.5±15.7 | 58.8±5.6 | 0.028* |
|
| ||||||
| Sex | ||||||
| Male | 4 (36%) | 1 (17%) | 6 (35%) | 2 (33%) | 2 (50%) | 0.874 |
| Female | 7 (64%) | 5 (83%) | 11 (65%) | 4 (67%) | 2 (50%) | |
|
| ||||||
| Tumor Size (median (range)) | 4.0 (1.3–6.5) | 1.4 (0.5–5.0) | 2.4 (0.7–11.0) | 0.7 (0.2–2.0) | 2.0 (0.8–4.5) | <0.001* |
|
| ||||||
| Stage | ||||||
| I | 2 (25%) | 1 (20%) | 0 (0%) | 6 (100%) | 2 (50%) | <0.001* |
| II | 3 (38%) | 3 (60%) | 1 (5%) | 0 (0%) | 0 (0%) | |
| III | 1 (12%) | 0 (0%) | 7 (39%) | 0 (0%) | 0 (0%) | |
| IV | 2 (25%) | 1 (20%) | 10 (56%) | 0 (0%) | 2 (50%) | |
|
| ||||||
| Any Mets | 3 (27%) | 1 (17%) | 17 (89%) | 0 (0%) | 2 (50%) | <0.001* |
|
| ||||||
| LN Mets | 2 (18%) | 0 (0%) | 16 (89%) | 0 (0%) | 0 (0%) | <0.001* |
|
| ||||||
| Distant Mets | 2 (18%) | 1 (17%) | 11 (61%) | 0 (0%) | 2 (50%) | 0.022* |
|
| ||||||
| Vasc. invasion | 1 (9%) | 1 (17%) | 10 (53%) | 1 (17%) | 0 (0%) | 0.053 |
|
| ||||||
| Positive Margins | 1 (25%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0.492 |
|
| ||||||
| Ki67 index [median, (range)] | 5% (1–16%) | 4% (1–5%) | 1.5% (1–10%) | 2% (1–5%) | 5.5% (2–10%) | 0.131 |
qPCR = quantitative PCR, IHC = immunohistochemistry, Vasc. = vascular, Mets = metastases, LN = lymph node.
p-value with statistical significance
Among the 46 tumors included, 23 were localized tumors and 23 had either locoregional or distant metastases at the time of surgery. Patients with metastatic disease were significantly older, had larger tumors, and had a higher incidence of vascular invasion compared to those with localized tumors (Table 2).
Table 2.
Characteristics of Localized vs. Metastatic Well-Differentiated Neuroendocrine Tumors
| Localized (N=23) | Metastatic (N=23) | p-value | |
|---|---|---|---|
| Age (mean ± SD) | 49.6±15.3 | 59.1±13.1 | 0.033 |
| Tumor Size [median in cm, (range)] | 1.5 (0.2–6.5) | 3.0 (0.7–11) | 0.019 |
| Vascular invasion (% patients) | 9% | 48% | 0.007 |
| Positive Margins (% patients) | 5% | 5% | 1.000 |
| Ki-67 index [median % (range)] | 2.5% (1–16%) | 3.5% (1–10%) | 0.868 |
| UCHL1 staining <50% (% patients) | 27% | 87% | 0.001 |
UCHL1 Gene Expression in Localized vs. Metastatic GEP-NETs
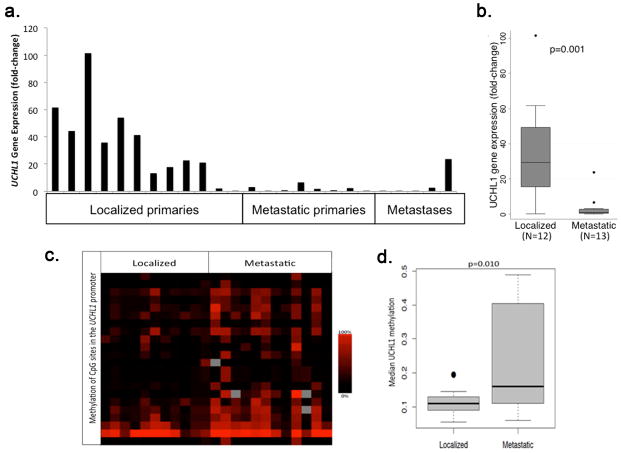
Frozen tumor samples were used to measure UCHL1 transcript levels in 25 tumor samples, including tumors from the pancreas (n=11), stomach (n=4), small bowel (n=5), and distant metastases (n=5) (Figure 1A). Twelve of the tumors were localized while thirteen were metastatic. UCHL1 gene expression was on average 35-fold greater among localized primary tumors compared to metastatic primary tumors and metastases (p<0.001, Figure 1B).
Figure 1.
(A) UCHL1 gene expression in a group of 25 GEP-NETs, including 12 localized primary tumors, eight metastatic primary tumors, and five distant metastases. (B) Boxplot showing that the median UCHL1 transcript level in metastatic tumors and metastases was significantly lower than in localized tumors (p=0.001). (C) Heat map depicting hyper-methylation of many CpG sites (y-axis) around the UCHL1 promoter of metastatic tumors compared to localized tumors. (D) Boxplot showing that the median frequency of methylation among the variable CpG sites in metastatic tumors and metastases was significantly greater than among localized tumors (p=0.010).
CpG Methylation Screening Around the UCHL1 Promoter
To determine whether CpG hypermethylation of the promoter was a potential mechanism for UCHL1 silencing as has been suggested in other tumors, we performed methylation screening of the UCHL1 gene. We focused on the region around the promoter (1 kb downstream and 1kb upstream of the promoter region) in 23 of the 25 samples that we evaluated for UCHL1 gene expression (insufficient tissue prevented analysis of two samples). Among variable CpG regions (i.e., regions with a standard deviation for methylation frequency of ≥ 0.1), metastatic primary tumors and metastases were significantly hyper-methylated compared to localized tumors (p<0.001), which corresponded to the tumors with the lowest UCHL1 transcript levels (Figures 1B and 1C).
Immunohistochemistry Staining for UCHL1 Distinguishes Localized and Metastatic GEP-NETs
We next performed IHC staining for UCHL1 protein on a total of 31 primary tumors, including 15 localized tumors and 16 metastatic tumors. Nine of these tumors were also evaluated for UCHL1 gene expression, while the remaining 22 were independent samples. We observed perfect correlation between UCHL1 gene expression and protein staining in all nine samples for which both were evaluated (data not shown).
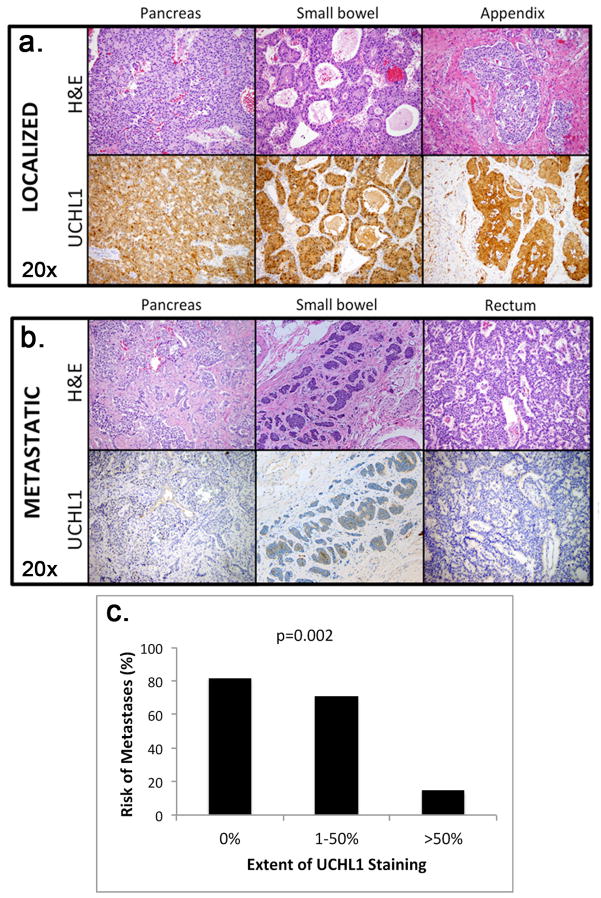
Localized primary tumors consistently showed significantly more extensive staining for UCHL1 protein than metastatic primary tumors (p<0.001, Figures 2A and 2B). The incidence of metastatic disease at the time of surgery was inversely proportional to the extent of UCHL1 staining of the primary tumor: 82% of tumors with no UCHL1 staining had metastasized compared to 71% of tumors with 1–50% of the tumor staining, and only 15% of tumors with >50% staining (p=0.002, Figure 2C). Poor staining for UCHL1 (≤50% of tumor cells) was 88% sensitive and 73% specific for the presence of metastatic disease (either lymph node or distant) at the time of surgery, and it had positive and negative predictive values of 78% and 85%, respectively.
Figure 2.
Representative images of hemotoxylin and eosin (H&E) stains (20×) and corresponding UCHL1 immunohistochemistry (IHC) stains (20×) of three localized primary tumors (A) and three metastatic primary tumors (B) from various locations within the gastrointestinal tract. UCHL1 showed very strong tumor-specific nuclear and cytoplasmic staining (brown) in localized tumors but was significantly weaker in metastatic tumors. (C) Extent of UCHL1 staining was inversely proportional to the risk of having metastatic disease at the time of initial surgery (p=0.002).
Ki-67 Proliferation Index Fails to Distinguish Localized and Metastatic Well-Differentiated GEP-NETs
The well-differentiated GEP-NETs included in this study had similar Ki-67 indices when compared by site of origin, despite significant differences in disease stage (Table 1). Furthermore, there was no difference in median Ki-67 values when comparing localized and metastatic tumors (2.5% vs. 3.5%, respectively, p=0.868) (Table 2). When using a Ki-67 index of >2% as a marker for increased metastatic potential, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 56%, 50%, 45%, and 61%, respectively. The specificity increased to 91% when the critical Ki-67 value was increased to >10%, however, this decreased the sensitivity to 0%.
Independent Risk Factors for Metastatic Disease at the Time of Surgery
Factors that were identified on univariable analysis as being significantly associated with metastatic disease (age, tumor size, vascular invasion, and UCHL1 staining <50%) were entered into a multivariable logistic regression analysis with metastatic disease set as the dependent variable. UCHL1 staining <50% (OR 24.5, 95% CI 1.2–482.2) and vascular invasion (OR 38.4, 95% CI 1.4–1035.5) were both identified as independent risk factors for metastatic disease, but age and tumor size were no longer significant (Table 3).
Table 3.
Multivariate Logistic Regression For Risk Factors Associated With Metastatic Disease at the Time of Surgery
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.0 | 0.9–1.2 | 0.423 |
| Vascular invasion | 38.4 | 1.4–1035.5 | 0.030 |
| Size | 1.8 | 0.8–3.8 | 0.149 |
| UCHL1 staining <50% | 24.5 | 1.2–482.2 | 0.035 |
CI – confidence interval
Discussion
The most recent classification system proposed by the World Health Organization in 2010 classifies neuroendocrine tumors by differentiation and location3. This system represents a substantial improvement over its predecessors that were based upon presumed embryological origin (foregut/midgut/hindgut) and biological activity (functional vs. non-functional), which provided little insight into the expected behavior of the tumor 24. However, much about what differentiates indolent and more aggressive GEP-NETs at both the molecular and population levels remains poorly understood. Herein, we sought to address the clinical problem of elucidating key molecular features that distinguish less aggressive localized NETs from highly aggressive metastatic NETs.
The exact mechanism by which UCHL1 regulates tumor development remains uncertain, although recent data suggest that UCHL1 increases the half-life of p53 and p14ARF, decreases the half-life of Mdm2, and activates the p53 signaling pathway 19,25. This is the first report of loss of UCHL1 expression in metastatic GEP-NETs. Additional in vitro studies are needed to determine whether the mechanisms involved in GEP-NET pathogenesis are similar to those described in other tumors.
Several recent studies have suggested that UCHL1 promoter hypermethylation is an epigenetic modulator that results in decreased UCHL1 expression in various types of cancer17–20. Our findings build upon this existing data by suggesting that silencing of UCHL1 by CpG hyper-methylation is also associated with metastatic disease among GEP-NETs. Additional studies are needed to determine whether epigenetic modification of UCHL1 can be exploited to treat advanced disease.
Currently, Ki-67 index is one of the most commonly used markers used to identify aggressive behavior in GEP-NETs. However, controversy over its optimal critical value for identifying aggressive behavior has lead to inconsistent sensitivities and specificities26,27. The limited utility of Ki-67 among well-differentiated neuroendocrine tumors may also be explained by the fact that by definition they all have a Ki-67 index <20%. Despite this, it is known that even well-differentiated GEP-NETs can metastasize early and widely. Therefore, additional molecular markers that may identify tumors at risk for more aggressive behavior would be clinically beneficial. For this reason, we limited this study to only well-differentiated tumors so that level of differentiation could be excluded as a confounder when searching for factors associated with aggressive behavior. We found that ≤50% staining of UCHL1 among GEP-NETs tumors performed markedly better than Ki-67 index at identifying tumors with such aggressive behavior, and therefore should be explored as a clinically useful adjunct to Ki-67 index.
The <50% cut off mark for UCHL1 staining was initially chosen arbitrarily, but post-hoc analysis using a wide range of cut points showed that 50% provides the best combination of sensitivity and specificity (data not shown). If these data are validated in a larger prospective study, the optimal cut point may be refined further and UCHL1 may then be incorporated into a clinical prognostic algorithm.
The clinical data that were used in this study to compare UCHL1 expression to clinical features were limited to variables that were known at the time of surgery, including the presence of lymph node and/or distant metastases. One limitation to this study is that long-term follow-up data were unavailable for many of the patients, and thus we were unable to correlate UCHL1 expression to long-term outcomes, such as recurrence and mortality. Additional studies, incorporating larger samples of patients, are required to determine if UCHL1 staining can be used to predict future behavior of the tumor.
In conclusion, we found that silencing of UCHL1 by CpG hyper-methylation is associated with locally advanced and metastatic tumors, but not localized tumors. Furthermore, the extent of primary tumor IHC staining for UCHL1 is inversely proportional to the incidence of metastatic disease, and therefore should be explored as an adjunct to Ki-67 index for identifying more aggressive tumors. Additional studies are warranted to investigate the cellular changes induced by UCHL1 silencing as well as the feasibility of targeting UCHL1 as a potential novel treatment for advanced disease.
Synopsis.
Loss of UCHL1 expression by CpG promoter hypermethylation is associated with metastatic GEP-NETs but not with localized tumors. Extent of UCHL1 staining should be explored as a potentially clinically useful adjunct to Ki-67 index in evaluating GEP-NETs for aggressive features.
Acknowledgments
This study was funded by a grant from the Raymond and Beverly Sackler Foundation, grant TL1RR024998 of the Clinical and Translational Science Center at Weill Cornell Medical College, and by a donation from the Dancers Care Foundation.
Footnotes
There are no financial disclosures from any authors.
References
- 1.Pasieka JL. Carcinoid tumors. Surg Clin North Am. 2009 Oct;89(5):1123–1137. doi: 10.1016/j.suc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Arnold R. Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol. 2005 Aug;19(4):491–505. doi: 10.1016/j.bpg.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Bosman FT World Health Organization., International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.Ellison TA, Wolfgang CL, Shi C, et al. A Single Institution’s 26-Year Experience With Nonfunctional Pancreatic Neuroendocrine Tumors: A Validation of Current Staging Systems and a New Prognostic Nomogram. Ann Surg. 2013 May 12; doi: 10.1097/SLA.0b013e31828f3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strosberg JR, Weber JM, Feldman M, Coppola D, Meredith K, Kvols LK. Prognostic validity of the American Joint Committee on Cancer staging classification for midgut neuroendocrine tumors. J Clin Oncol. 2013 Feb 1;31(4):420–425. doi: 10.1200/JCO.2012.44.5924. [DOI] [PubMed] [Google Scholar]
- 6.Volante M, Daniele L, Asioli S, et al. Tumor staging but not grading is associated with adverse clinical outcome in neuroendocrine tumors of the appendix: a retrospective clinical pathologic analysis of 138 cases. Am J Surg Pathol. 2013 Apr;37(4):606–612. doi: 10.1097/PAS.0b013e318275d1d7. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008 Jan;9(1):61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995–2012) in South China. BMC Endocr Disord. 2012;12:30. doi: 10.1186/1472-6823-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008 Jun 20;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 10.Anthony LB, Strosberg JR, Klimstra DS, et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010 Aug;39(6):767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 11.Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010 Aug;39(6):753–766. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]
- 12.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010 Aug;39(6):707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 13.Ohike N, Morohoshi T. Pathological assessment of pancreatic endocrine tumors for metastatic potential and clinical prognosis. Endocr Pathol. 2005 Spring;16(1):33–40. doi: 10.1385/ep:16:1:033. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002 Oct 18;111(2):209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 15.Miyake Y, Tanaka K, Fukushima W, et al. UCHL1 S18Y variant is a risk factor for Parkinson’s disease in Japan. BMC Neurol. 2012;12:62. doi: 10.1186/1471-2377-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragland M, Hutter C, Zabetian C, Edwards K. Association between the ubiquitin carboxyl-terminal esterase L1 gene (UCHL1) S18Y variant and Parkinson’s Disease: a HuGE review and meta-analysis. Am J Epidemiol. 2009 Dec 1;170(11):1344–1357. doi: 10.1093/aje/kwp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang T, Li L, Yin X, et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One. 2012;7(1):e29783. doi: 10.1371/journal.pone.0029783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonazzi VF, Nancarrow DJ, Stark MS, et al. Cross-platform array screening identifies COL1A2, THBS1, TNFRSF10D and UCHL1 as genes frequently silenced by methylation in melanoma. PLoS One. 2011;6(10):e26121. doi: 10.1371/journal.pone.0026121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Tao Q, Jin H, et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res. 2010 Jun 1;16(11):2949–2958. doi: 10.1158/1078-0432.CCR-09-3178. [DOI] [PubMed] [Google Scholar]
- 20.Seol MA, Chu IS, Lee MJ, et al. Genome-wide expression patterns associated with oncogenesis and sarcomatous transdifferentation of cholangiocarcinoma. BMC Cancer. 2011;11:78. doi: 10.1186/1471-2407-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiman DA, Buitrago D, Crowley MJ, et al. Thyroid stimulating hormone increases iodine uptake by thyroid cancer cells during BRAF silencing. J Surg Res. 2013 Jun 1;182(1):85–93. doi: 10.1016/j.jss.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Ehrich M, Nelson MR, Stanssens P, et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):15785–15790. doi: 10.1073/pnas.0507816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberg K. Neuroendocrine tumors of the digestive tract: impact of new classifications and new agents on therapeutic approaches. Curr Opin Oncol. 2012 Jul;24(4):433–440. doi: 10.1097/CCO.0b013e328353d7ba. [DOI] [PubMed] [Google Scholar]
- 25.Bheda A, Shackelford J, Pagano JS. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One. 2009;4(8):e6764. doi: 10.1371/journal.pone.0006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Fujimori T, Tomita S, et al. Clinical validation of the gastrointestinal NET grading system: Ki67 index criteria of the WHO 2010 classification is appropriate to predict metastasis or recurrence. Diagn Pathol. 2013;8:65. doi: 10.1186/1746-1596-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamali M, Chetty R. Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: an overview and the value of Ki-67 immunostaining. Endocr Pathol. 2008 Winter;19(4):282–288. doi: 10.1007/s12022-008-9044-0. [DOI] [PubMed] [Google Scholar]