Abstract
Nano-encapsulation of poorly soluble anticancer drug was developed with sonication assisted layer-by-layer polyelectrolyte coating (SLbL). We changed the strategy of LbL-encapsulation from making microcapsules with many layers in the walls for encasing highly soluble materials to using very thin polycation / polyanion coating on low soluble nanoparticles to provide their good colloidal stability. SLbL encapsulation of paclitaxel resulted in stable 100-200 nm diameter colloids with high electrical surface ξ-potential (of -45 mV) and drug content in the nanoparticles of 90 wt %. In the top-down approach, nanocolloids were prepared by rupturing powder of paclitaxel using ultrasonication and simultaneous sequential adsorption of oppositely charged biocompatible polyelectrolytes. In the bottom-up approach paclitaxel was dissolved in organic solvent (ethanol or acetone), and drug nucleation was initiated by gradual worsening the solution with the addition of aqueous polyelectrolyte assisted by ultrasonication. Paclitaxel release rates from such nanocapsules were controlled by assembling multilayer shells with variable thicknesses and are in the range of 10-20 hours.
Introduction
More than 40% pharmacologically active compounds exhibit poor solubility1-3 and an emphasis in these drugs delivery has been diverted towards development of their nanoparticle formulations which could be injected and then drug will be slow released in blood. A number of Federal Drug Administration approved low soluble anticancer drugs still do not have formulation for delivery at sufficient concentration, and one of them is paclitaxel. Typical maximal solubility of such drugs is less than 0.01 mg/mL, which is too low for efficient injection.
Micelle nanocarriers for low soluble hydrophobic drugs have appropriate particle size of tens nanometer but have low loading capacity2-4. Drug loaded polymeric coacervates show larger particle size of about 250-300 nm, and also low loading 5-7. Synthetic polymer-drug conjugates have been turn up promising results in drug delivery but still challenges with polymer toxicity, circulation instability, low drug-holding capacity and rapid drug release are unanswered 8-11. Apart from liposome and synthetic polymer-based systems, some drug delivery carriers are composed of biopolymers and their self-assemblies, such as virus-, polysaccharide-, albumin- based nanoparticles, and layer-by-layer (LbL) microcapsules 10-17. The desired characteristics of drug delivery for intravenous formulation are as follows: less than 200 nm particle size, biodegradability, administration at significant amount for efficient action, high drug loading and slow drug release. Described here ultrasonicated layer-by-layer polyelectrolyte encapsulation strategy could allow reaching this goals.
In this paper, we present new development of LbL polyelectrolyte encapsulation method (invented in Max Planck Institute, Germany by H. Möhwald, G. Sukhorukov, F. Caruso, E. Donath, and others15) which allowed twenty time decrease of LbL capsule size, from typically 5 micrometer in their works to 100-200 nm in our approach. With this, we changed the strategy of LbL encapsulation from making capsules with many polyelectrolyte layers in the walls to encase highly soluble materials (which is rather difficult for scaling up) to using very thin LbL shells to provide a sufficient surface charge of drug nanoparticles for good colloidal stability of initially low soluble materials (two-step process of sequential adsorption of polycation / polyanion).
Nanoparticle drug carriers were developed by combination of ultrasonication with encapsulation using biodegradable polyelectrolyte. Two approaches to attain the desired features are “top-down” and “bottom-up” synthetic methods. A common feature of these two related methods is ultrasonication formation of drug nanocores accompanied with simultaneous polycation coating providing aqueous colloidal stability16-17. “Top-down” method was more efficient in preparation of large amount of ca 200 nm diameter paclitaxel capsules, and “bottom-up” approach allowed smaller capsules of ca 100 nm diameter but with less product yield. Combination of these two approaches allowed obtaining new dosage forms of paclitaxel that satisfies the major tasks in making nanocolloids of poorly soluble drugs with concentration up to 2 mg/mL, high drug content and controlled dissolution rate suitable for intravenous drug administration.
Materials and Methods
LbL assembly was carried out using polyallylamine hydrochloride (PAH), protamine sulfate and chitosan, as positively charged polyelectrolytes and bovine serum albumin (BSA), alginic acid, and sodium polystyrene sulphonate (PSS), as negatively charged polyelectrolytes (used at concentration of 2 mg/mL). All polyelectrolytes and albumin were purchased from Sigma-Aldrich and paclitaxel (Scheme 1) - from LC Laboratories Inc (Woburn, MA). Deionized water and PBS buffer were used at pH 7.2. Ultrasonicator UIP1000hd (Heilscher, Germany) with titanium sonotron was used at power of 15 W/cm2. During the experiments, the temperature was controlled at 25-30 ° C.
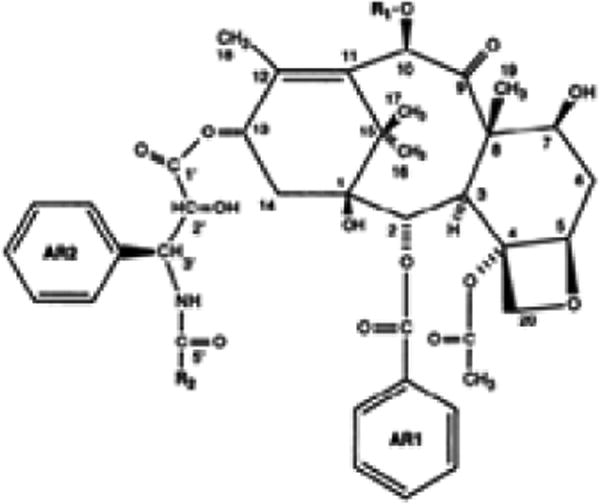
Scheme 1. Chemical structure of paclitaxel18.
Surface potential (ξ-potential) and particle size measurements (by light scattering) were performed using ZetaPlus Microelectrophoresis (Brookhaven Instruments). Field emission scanning electron microscope (Hitachi S 4800) was used for particle imaging. Before SEM imaging, the samples were washed two times with distilled water and diluted ten times. Laser scanning confocal microscope, Leica TCS SP2 (Leica Microsystems Inc) was used to visualize the encapsulated drug with FITC fluorescent tagged polycation layer to confirm the shell formation.
The drug release rates of nanocapsules with different shell composition were measured using standard 1 mL horizontal diffusion chambers with 0.2 μm cellulose acetate membrane. The drug dispersion was placed to the side of the diffusion chamber containing magnetic stirrer and paclitaxel was released against same volume of PBS, pH 7.2 to mimic sink conditions expected in vivo. The concentration of released drugs in PBS was dissolved in ethanol at 1:1 (v/v), centrifuged to remove insoluble polyelectrolytes and measured by with UV spectrophotometer (Agilent 8453) at 227 nm wavelength. Also the same measurements were done to calculate product yield at the end of sonication.
1 Top-down approach
In this method, the drug powder was added into distilled water to attain 0.5 mg/mL concentration followed by 15 W/cm2 ultrasonication. An oppositely charged polyelectrolyte (polycation in this case due to negative surface charge of the used drug) was added for continuous adsorption on the particles during disintegration process (Scheme 2). After approximately one hour of such treatment, 200-nm paclitaxel particles coated with polycation layer were obtained. We used a minimal amount of polycation allowing nanoparticle surface recharging to minimize amount of nonreacted polyelectrolyte in the solution. A series of experiments were carried out to decide a minimal amount of polycation. For example, paclitaxel nanoparticles coated with chitosan and alginic acid showedd variation of amount of polyelectrolytes and corresponding change in particle size.
Scheme 2.
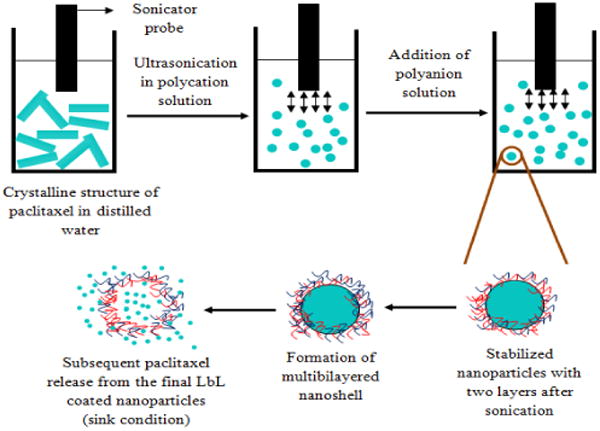
Schematic representation of ultrasonication assisted layer-by-layer assembly using top-down approach. Ultrasonication was applied to break down paclitaxel powder and re-aggregation was prevented by adsorption of polyelectrolytes.
Successively, the second layer of oppositely charged polyelectrolyte (polyanion) was deposited with additional 15 min sonication. As compared to the conventional LbL technique, we have avoided tedious washing step with centrifugation after the first polycation layer adsorption following washless LbL process19. After such two-step polycation / polyanion coating, drug samples were centrifuged at 15,000 rpm for 10 min to remove the excess polyanion, washed and re-suspended in water. Final electrical zeta-potential and size of the particles were recorded to characterize the nanocolloids formed.
2 Bottom-up approach
Paclitaxel was dissolved in “good” solvent (60% ethanol/water and less efficient was acetone/water). After dissolution of the drug at high concentration, ultrasonication was started with aqueous polycation slowly added to the solution. With increase of water concentration, solubility of paclitaxel decreased, reached saturation and the nucleation begin resulting in formation of nano-size particles (scheme 3).
Scheme 3.
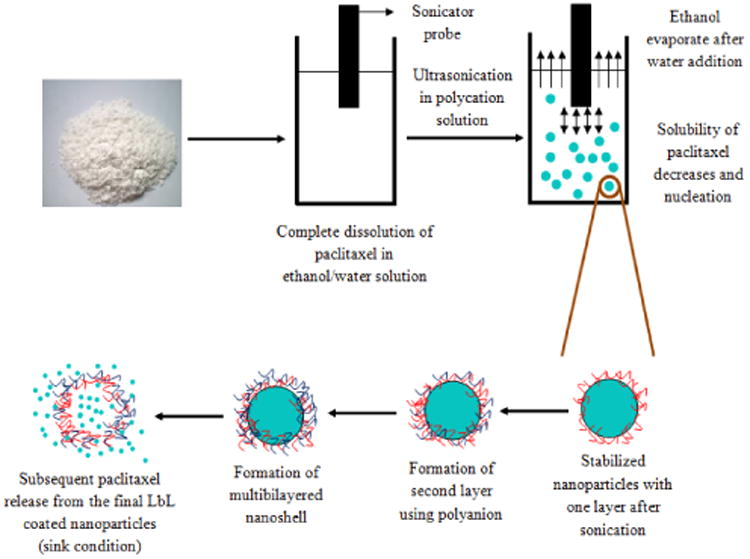
Schematic representation of sonication assisted layer-by-layer assembly using bottom-up approach. Nanoparticulation of paclitaxel dissolved in ethanol solvent was initiated by ultrasonication and with addition of aqueous polycation.
Powerful sonication prevents formation of larger drug particles and polycation adsorption provides surface charge sufficient for colloidal stability. After 45 minutes treatment, the drug particles were centrifuged at 15,000 rpm for 10 minutes and re-suspended in distilled water. The second layer of anionic polyelectrolytes was deposited to maximize the capsule surface ξ-potential. Nonreacted polyanions were removed by centrifugation and paclitaxel nanoparticles with the average particle size of 100 nm were obtained. Additional polyelectrolytes layers may be further built on the drug nanoparticles with traditional LbL process without sonication 15. Paclitaxel nanocolloids were kept in a low volume of saturated solution to prevent drug release
Results and Discussion
1 Top-down SLbL nanocolloids
The particle size of the end products of both top-down and bottom-up approaches were different. Longer time about one and half hour sonication in the top-down approach allowed to attain 200 nm diameter particles. The change in particle size over the sonication time during top-down approach is shown in Fig. 1. At each time interval, the nanoparticles were coated with first polycation layer and followed with 15 min deposition of the second polyanion layer (for example, samples with 45 minutes of sonication time means 30 minutes sonication for cationic layer followed by 15 min sonication for anionic layer). With longer sonication, it was possible to reduce particle size to 200 nm. The nanoformulated product yield in the top-down approach was about of 90%.
Figure 1.
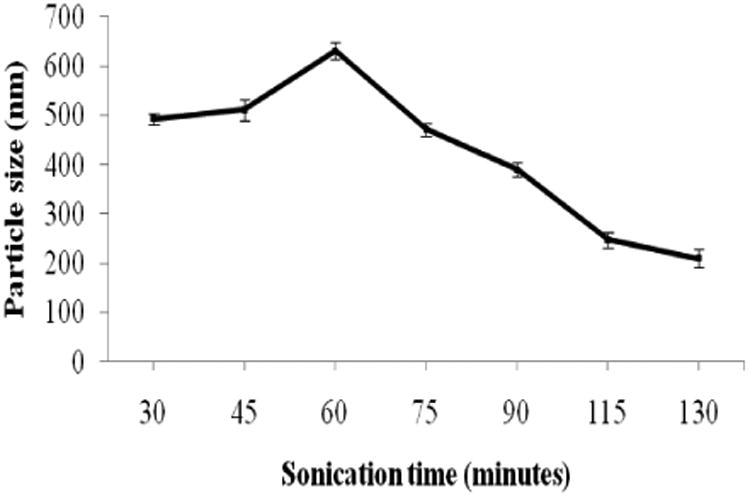
The particle size of paclitaxel in response to ultrasonication time (top-down approach).
An increase in particle size at the beginning of sonication during top-down approach may be due to bridging of larger drug particles with polyelectrolytes. As soon as the uniform layer of polyelectrolyte is spread over the particles, they separate from each other.
2 Bottom-up SLbL Nanocolloids
The particle size formed in this method was about 100 nm, which is smaller than particles obtained with the top-down approach. This treatment was carried out at shorter time of 45 minutes, though the product yield in this approach was about 30%. The experiments were carried out to optimize the factors affecting the drug particle size (alcohol/water ratio for the initial drug solution, primary drug concentration, ultrasonication power and time, and rate of water addition for nucleation initiation). Significant change in particle size due to variation in the rate of water addition and initial drug concentration were observed (Fig. 2). The increase in water addition rate leads to faster crystal nucleation and ultimately increases particle size (Fig. 2a). An increase in the initial drug concentration favored to aggregation during the nucleation which also increased the particle size (Fig. 2b).
Figure 2.
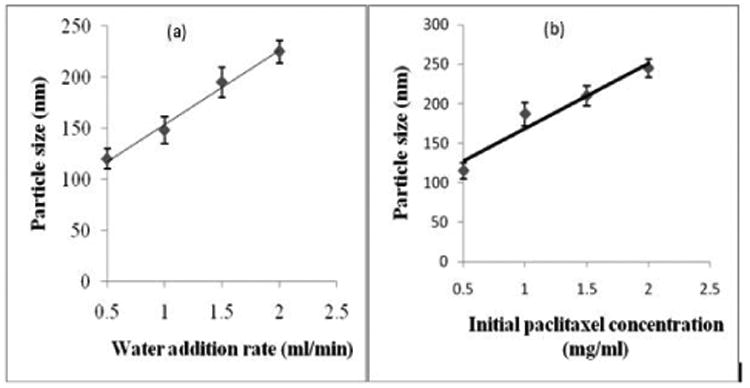
Particle size of paclitaxel during bottom-up SLbL process depending on (a) rate of water addition, and (b) initial paclitaxel concentration.
3 Nanocapsule's Zeta-Potential
Paclitaxel particles ξ-potential was monitored before polyelectrolyte coating and after every step of LbL shell assembly. Since the initial charge of paclitaxel particles was negative, the first layer was coated formed by adsorption of polycation. In the first experiments we used synthetic polyelectrolytes (cationic PAH and anionic PSS) and then switched to biodegradable chitosan, protamine sulfate, alginic acid and bovine serum albumin.
We chose the polyelectrolytes depending on the molecular weight and surface charges. Initial experiments include synthetic polyelectrolytes with higher surface charge in order to stabilize the nanoparticles. This is often appropriate for many industrial applications (such as paint formulation). For pharmaceutical application, we described biodegradable polymers. We did not recognize essential difference in using these two types of polyelectrolytes.
In Fig. 3 one can see the electrical surface potential alternation after each layer of deposited polycation and polyanion. After adsorption of the first polycation potential raised to +21 ± 2 mV and adsorption of the second anionic layer resulted in larger magnitude of ξ-potential of -45 ± 3 mV and its magnitude remained approximately the same during further polyelectrolyte layer deposition. This high surface potential allowed samples to be stable for more than one month at concentration of 1 mg/mL. The shell composed from chitosan and alginic acid gave better colloidal stability than LbL shells based on protamine sulfate and bovine serum albumin multilayers which showed aggregation in 1-2 days after processing. ξ--potential characteristics of paclitaxel nanocapsules were similar for both top-bottom and bottom-up preparation methods.
Figure 3.
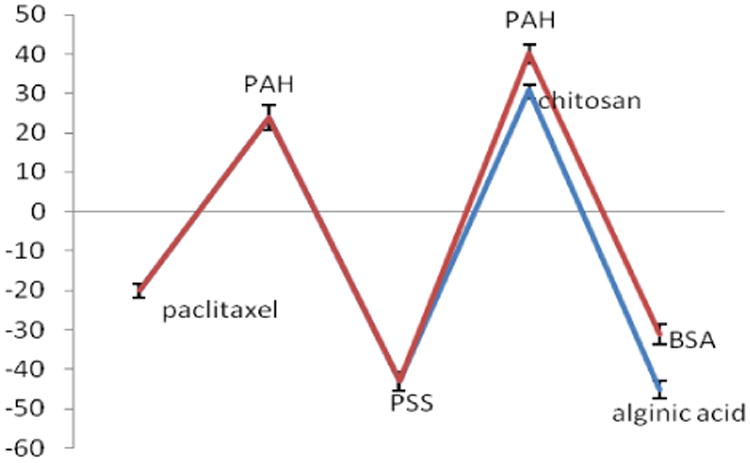
ξ-potential of paclitaxel coated with two different bilayers of PAH-PSS-PAH-BSA and PAH-PSS-chitosan-alginic acid using top-down approach.
4 SEM and Confocal Imaging of Paclitaxel Nanoparticles
Scanning electron microscopy images of the original paclitaxel powder revealed needle-like particles of 10-15 μm length. Two different approaches of SLbL nano/encapsulation showed some difference in nanoparticle size and shape (Fig.4). Top-down approach showed the rectangular particles with the average size of about 220 × 100 nm. Whereas SEM images of paclitaxel prepared using bottom-up approach gave isometric particles with an average size of 100 ± 20 nm.
Figure 4.
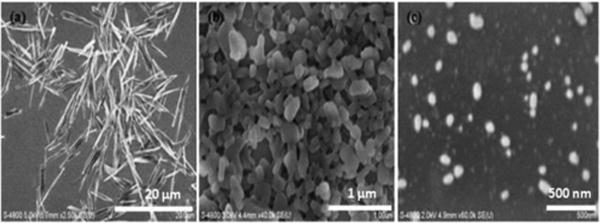
SEM images of paclitaxel in (a) the original form; (b) after SLbL assembly using top-down approach, and (c) after SLbL treatment using bottom-up approach.
To confirm the presence of polyelectrolyte layer on the paclitaxel, we made larger microparticles using shorter sonication time. This allowed to resolve LbL coating labeled with fluorescent FITC (due to the confocal microscopy resolution limit of 250 nm, it was not possible to visualize the shell wall on 200 nm diameter paclitaxel). Fig. 5 gives fluorescent confocal image of micrometer-size capsule cross-section where green shell is the polyelectrolyte capsule wall.
Figure 5.
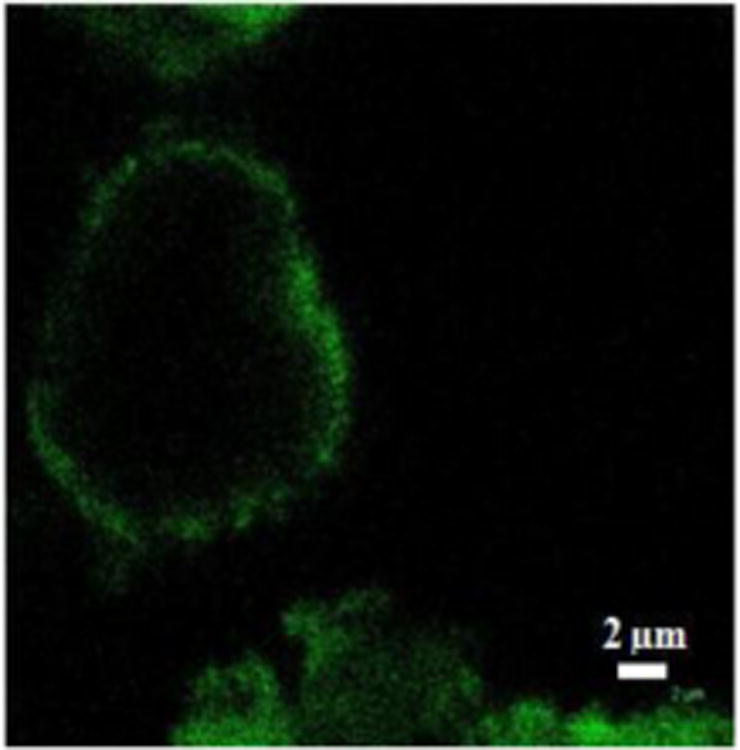
Confocal fluorescent images of 5 micron paclitaxel particle after 10 min SLbL treatment assembly using top-down approach (FITC labelled chitosan was used in the shell wall).
5 Drug release from nanocolloids after SLbL processing
The release profiles of original paclitaxel powder, paclitaxel nanocolloids with one polycation layer and three polycation / polyanion bilayer coating were analyzed in standard sink conditions (initial drug concentration was 2 mg/mL). The release curves fitting were done with exponential Peppas' model19. 70% of original paclitaxel powder was released within eight hours (Fig. 7). Nanoparticulated paclitaxel coated with one polyelectrolyte layer lead to slightly faster release due to smaller particle size in nanoformulation as compared with micrometer size of the original paclitaxel. Paclitaxel nanocolloids coated with three bilayers of PAH / BSA showed lower drug release rate due to increasing thickness of the capsule wall. For example, in eight hours only 40 % of the two layers coated sample was release as compared with 80 % for one layer coated sample. LbL technique allows for control of drug release rate from polyelectrolytes-stabilized nanoparticles by changes the number of coating layers or the shell composition.
Figure 7.
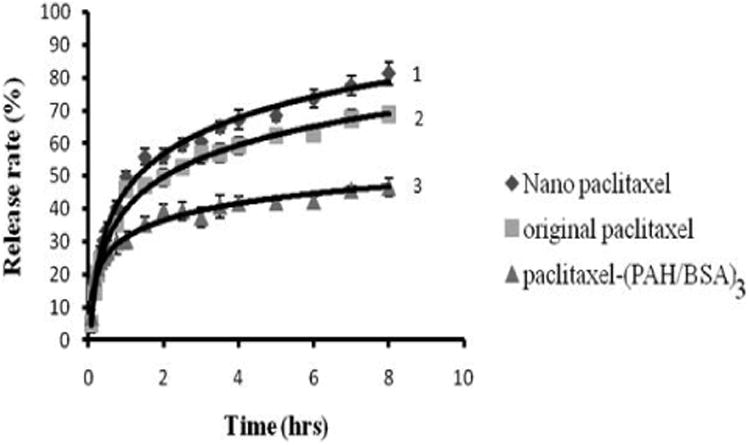
Release from paclitaxel nanocolloidal particles produced with SLbL top-down approach: protamine sulfate coating (PS) – 1, original paclitaxel without coating – 2, and (PS/BSA)3 coating - 3.
Under sink conditions, 50% of non-coated paclitaxel crystals (without sonication) were solubilized within two hours, while three bilayer LbL coating extended this time to more than ten hours (extrapolation). Similar release rate results were obtained for bottom-up approach in paclitaxel nanoparticulation. Paclitaxel coated with one layer of PAH after bottom-up approach (particle size was 100 ± 20 nm) showed slightly faster release than top-down approach (particle size is 220 ± 20 nm) due to the smaller particle size.
In the proposed paclitaxel formulation, we obtained not only nano size of the drug particles but our nanocapsules contained high drug content of 80-90 % due to very thin capsule walls (of ca 10 nm, as it was estimated from Quartz Crystal Microbalance measurements of the LbL multilayers of corresponding compositions). Deposition of three bilayer film of PAH/BSA on silver coated 9 MHz-QCM resonator resulted in 485 ± 20 Hz frequency shift which corresponds to the thickness of 9.7 ± 0.5 nm.
Discussion
We carried out SLbL nano-encapsulation of paclitaxel with synthetic and biodegradable polyelectrolytes (PAH, PSS, chitosan, bovine serum albumin, protamine sulfate, and alginic acid). The assembly based on protamine sulfate (as polycation) and bovine serum albumin (as polyanion) leads to soon precipitation of the formed drug nanoparticles due to insufficient surface potential of the capsules. Using another pair of polyelectrolytes (natural chitosan and alginic acid) allowed formation of highly charged stable paclitaxel nanocolloid with particle sizes of 100 to 200 nm and concentration up to 1 mg/mL. Reached nano-particulated paclitaxel concentration is of 200 times higher than solubility of paclitaxel in water (which makes our formulation perspective for medical injection. Observed rectangular shape of paclitaxel particles after SLbL treatment may reflect its crystalline unite cell (this compound has orthogonal unite cell). XRD was performed to confirm the preservation of paclitaxel crystal structure after long-time sonication.
In both “top-down” and “bottom-up” approaches, ca 10% of the particles were flashed out under powerful ultrasonication. Besides, bottom-up approach leads to increased losses due to formation of large particles with fast nucleation rate which were then removed by centrifugation. Thus, the efficiency of top-down approach is higher than bottom-up approach.
In the last 12 years hundreds research paper were published on formation of polyelectrolyte LbL microcapsules with diameters of ca 5 μm. Many capsule aspects were discussed in details, such as optimal shell composition and its structure, permeability in dependence on the number of layers in the shell21, influence of external factors on shell permeability and release rate, penetration of the LbL capsules to biological cells, and other, as it was well summarize in recent review15. Novelty of our work consists in drastic decrease of the LbL capsule diameter (from microns to 200 nm) due to simultaneous sonication and LbL assembly. We assume that many features of polyelectrolyte shells will be preserved for these much smaller capsules, however it needs further studying21. Further improvement of the both techniques can be achieved by increasing the sonication power. Ultrasonication efficiency depends on the shock wave power released on the drug particle surface due to gas microbubbles collapsing. One can choose additives which will increase in number of bubbles and decompose without damaging the original drug product. Another approach to increase sonication power is working under elevated pressure which may be reached with compressed gas atmosphere22. The free reactive groups of biocompatible polyelectrolytes (amine or acidic) at the outermost layer may help to bind PEG for longer particle circulation in blood or receptors for targeted drug delivery.
Conclusion
Sonication layer-by-layer nano-encapsulation is a promising technique to produce stable 1-2 mg/mL concentration nanocolloids of poor water soluble drugs, like paclitaxel. The particle size in such paclitaxel formulation is reduced from initial tens of micrometers to 100-200 nanometers which allow intravenues injection, increases drug circulation time and availability at tumour site. The particle size can be optimized using either top-down or bottom-up SLbL-treatment. Layer-by-layer encapsulation was carried out with biodegradable polyelectrolytes protamine sulfate, alginic acid, chitosan and bovine serum albumin.
Table 1. A list of experiments for the optimization of polyelectrolytes amount for paclitaxel nanoparticles.
| Concentration of paclitaxel (mg/ml) | Amount of chitosan as 1st layer (mg/ml) | Sonication time (hr) | Amount of alginic acid as 2nd layer (mg/ml) | Sonication time (mins) | Particle size (nm) | Zeta potential (mV) |
| 0.5 | 0.1 | 1 | 0.1 | 20 | 887 ± 7 | -30 ± 5 |
| 0.5 | 0.1 | 1 | 0.2 | 20 | 240 ± 6 | -35 ± 8 |
| 0.5 | 0.15 | 1 | 0.2 | 20 | 190 ± 8 | -33 ± 3 |
| 0.5 | 0.2 | 1 | 0.3 | 20 | 260 ± 9 | -30 ± 7 |
Acknowledgments
This work was supported by Award R01CA134951 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Notes and references
- 1.Edwards G, Krishna S. Eur J Microbio Inf Dis. 2004;23:233. doi: 10.1007/s10096-004-1113-9. [DOI] [PubMed] [Google Scholar]
- 2.Szczepanowicz K, Hoel HJ, Szyk-Warszynska L, Bielanska E, Bouzga AM, Gaudernack G, Simon C, Warszynski P. Langmuir. 2010;26:12592. doi: 10.1021/la102061s. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin V. Adv Drug Delivery Rev. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.A. Domb, Y. Tabata, M. R. Kumar, S. Farber, American Scientific Publishers: Stevenson Ranch, CA, 2007, 5-158
- 5.V. Shabner, J. Collings, Lippincott: Philadelphia, PA, 1990, 12, 320
- 6.Shutava T, Balkundi S, Vangala P, O'Neal P, Steffan J, Bigelow R, Cardelli J, Lvov Y. ACS Nano. 2009;3:2564. [Google Scholar]
- 7.Hobbs SK, Monsky WL, Yuan F. Proc Natl Acad Sci USA. 1998;95:4607. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teicher BA. Clinical Trials and Approval. Humana; Totowa, New Jersey: 1997. [Google Scholar]
- 9.Fernandez AM, Van Derpoorten K, Dasnois L, Lebtahi K, Dubois V, Lobl TJ, Gangwar S, Oliyai C, Lewis ER, Shochat D, Trouet A. J Med Chem. 2001;44 doi: 10.1021/jm0108754. [DOI] [PubMed] [Google Scholar]
- 10.Yalkowsky SH. Techniques of Solubilization of Drugs. Marcel Dekker; New York: 1981. [Google Scholar]
- 11.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clin Pharmaco and Ther. 2008;83:5. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 12.Ariga K, Hill J, Ji Q. Phys Chem Chem Phys. 2007;9:2319. doi: 10.1039/b700410a. [DOI] [PubMed] [Google Scholar]
- 13.Ariga K, Hill JP, Lee MV, Vinu A, Charvet R, Acharya S. Sci Technol Adv Mater. 2008;9:014109. doi: 10.1088/1468-6996/9/1/014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida O, Maruyama K, Sasaki K, Iwatsuru M. Int J Pharmaceutics. 1999;190:49–56. doi: 10.1016/s0378-5173(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 15.De Geest B, Sukhorukov G, Möhwald H. Expert Opin Drug Deliv. 2009;6:613. doi: 10.1517/17425240902980162. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Lvov Y, Sawant R, Torchilin V. J Controlled Release. 2008;128:255. doi: 10.1016/j.jconrel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Zheng, Zhang X, Clark C, Nathan CA, Lvov Y. Langmuir. 2010;26:8236. doi: 10.1021/la101246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastropaolo D, Camerman A, Lu Y, Brayer G, Camerman N. Procendings of the Nat Acad Sci. 1995;92:6920. doi: 10.1073/pnas.92.15.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bantchev G, Lu Z, Lvov Y. J Nanosci Nanotech. 2009;9:396. doi: 10.1166/jnn.2009.j055. [DOI] [PubMed] [Google Scholar]
- 20.Peppas NA. Pharm Acta Helv. 1985;60:110. [PubMed] [Google Scholar]
- 21.Antipov AA, Sukhorukov G, Donath E, Moehwald H. J Phys Chem B. 2001;105:2281. [Google Scholar]
- 22.Belova V, Gorin D, Shchukin D, Moehwald H. Angewandte Chemie. 2010;122:7129. doi: 10.1002/anie.201002069. [DOI] [PubMed] [Google Scholar]


