Abstract
The Müllerian duct forms the female reproductive tract consisting of the oviducts, uterus, cervix and upper vagina. Female reproductive tract function is vital to fertility, providing the site of fertilization, embryo implantation and fetal development. Developmental defects in the formation, and diseases of the female reproductive tract, including cancer and endometriosis, are prevalent in humans and can result in infertility and death. Further, because the Müllerian ducts are initially formed regardless of genotypic sex, mesenchymal-to-epithelial signaling is required in males to mediate Müllerian duct regression and prevents the development of Müllerian-derived organs. In males, defects in Müllerian duct regression result in the retention of female reproductive tract organs and have been described in several human syndromes. Although to date not reported in humans, ectopic activation of Müllerian duct regression signaling components in females can result in aplasia of the female reproduction tract. Clearly, Müllerian duct development is important to human health, however the molecular mechanisms remain largely undetermined. Molecular genetics studies of human disease and mouse models have provided new insights into molecular signaling during Müllerian duct development, regression and differentiation. This review will provide an overview of Müllerian duct development and important genes and signaling mechanisms involved.
Introduction
The Müllerian duct (MD) is the embryonic structure that develops into the female reproductive tract (FRT), including the oviduct, uterus, cervix and upper vagina. The FRT has essential functions in mammals, providing the site of fertilization, embryo implantation and fetal development. Defects in human FRT formation, thought to arise from abnormal embryonic development, are estimated to occur in up to 3% of births and often result in fertility problems. Diseases of the FRT are also prevalent in adult women and include uterine and cervical cancers, and endometriosis. Further, the reproductive tract of males and females initially contain identical pairs of fully formed Wolffian ducts (WDs) and MDs. During male sex differentiation, signaling between MD mesenchyme and epithelium mediate MD regression and prevent its development into a FRT. In males, defects in MD regression result in the retention of MD-derived organs and have been described in human persistent Müllerian duct, Urioste and Denys-Drash syndromes. Although to date not reported in humans, activation of the signaling pathways responsible for MD regression in females results in aplasia of the FRT (Kobayashi and Behringer, 2003). While an understanding of MD development is clearly important to human health, the cellular and molecular mechanisms of these processes remain largely unknown. Recent molecular genetics studies of human disease and mouse models have identified multiple genes important for MD development (Table 1). This review will provide an overview of MD formation, regression and differentiation and important genes and signaling mechanisms involved.
TABLE 1. Mouse and human genes involved in Müllerian duct development.
This table lists known genes involved in the development and differentiation of the Müllerian duct as demonstrated by mouse models and disease causing gene mutations in humans.
| Gene | Molecule Encoded | Expression (mouse) |
Reproductive Tract Phenotype (References) | |
|---|---|---|---|---|
| Mouse | Human | |||
| Amh | TGFβ Superfamily secreted protein |
Sertoli Cells | Ectopic FRT in males (N) (Behringer et al., 1994) | PMDS type I, Ectopic FRT in males (AR) (Belville et al., 1999; Belville et al., 2009; di Clemente and Belville, 2006; Salehi et al., 2012) |
| Amhr2 | TGFβ Superfamily type II Ser/Thr Transmembrane receptor |
MM | Ectopic FRT in males (N) (Mishina et al., 1996) | PMDS type I, Ectopic FRT in males (AR) (Belville et al., 1999; Belville et al., 2009; di Clemente and Belville, 2006; Salehi et al., 2012) |
|
beta catenin (Ctnnb1) |
Signaling Protein Adhesion |
MM, ME WE, WM |
Males - Ectopic FRT (C) (Kobayashi et al., 2011) Females- Hypotrophic uterine horns & defective oviduct coiling Myogenesis to adipogenesis switch (C) (Arango et al., 2005; Deutscher and Hung-Chang Yao, 2007; Jeong et al., 2009) |
NA |
| Digh1 | Scaffolding protein |
WE | Cervix and vagina aplasia (Iizuka-Kogo et al., 2007) | NA |
| Emx2 | Homeodomain Transcription Factor |
ME, WE | No FRT (N) (Miyamoto et al., 1997) | NA |
| Hoxa10 | Homeodomain Transcription Factor |
MM, WM | Homeotic transformation of anterior uterus to oviduct (N) (Benson et al., 1996) | Defects in MD fusion (AD) (Cheng et al., 2011; Ekici et al., 2013) |
| Hoxa11 | Homeodomain Transcription Factor |
MM, WM | Partial homeotic transformation of uterus to oviduct (N) (Gendron et al., 1997) | NA |
| Hoxa13 | Homeodomain Transcription Factor |
MM, WM | Agenesis of the caudal MD (N) (Warot et al., 1997) Homeotic transformation of cervix to uterus (Hd- Dominant negative allele of Hoxa13) (Post et al., 2000) Homeotic transformation of anterior cervix to uterus (compound Hoxa13+/−; Hoxd13−/−) (Warot et al., 1997) |
HFG syndrome (AD) (Goodman et al., 2000; Mortlock and Innis, 1997) |
| Lhx1 | Homeodomain Transcription Factor |
ME, WE | No FRT (N) (Kobayashi et al., 2004) | MRKH syndrome (AD) (Ledig et al., 2012) |
| Pax2 | Homeodomain Transcription Factor |
ME, WE | No FRT (N) (Torres et al., 1995) | NA |
| RAR α,β,γ | Retinoic acid receptors |
NA | Compound mutants; varying degrees of MD defect (malformation -> absence) (Mendelsohn et al., 1994) | NA |
| Tcf2 | Homeodomain Transcription Factor |
NA | NA | MODY5 with vaginal aplasia and rudimentary uterus (AD) (Bingham et al., 2002; Lindner et al., 1999). |
| Wnt4 | Wnt secreted protein |
MM | No FRT (N) (Vainio et al., 1999) Stratified luminal epithelial layer and reduced uterine gland numbers (C) (Franco et al., 2011) |
MRKH syndrome (AD) (Biason-Lauber et al., 2007) |
| Wnt5a | Wnt secreted protein |
MM, ME (<E13.5) MM (>E13.5) |
Posterior MD growth defects; absence of uterine glands (N) (Mericskay et al., 2004) |
NA |
| Wnt7a | Wnt secreted protein |
ME | Males - Ectopic FRT (N) (Parr and McMahon, 1998) Female - Homeotic transformation of oviduct to uterus and uterus to vagina no uterine glands abnormal mesenchyme differentiation (N) (Miller and Sassoon, 1998; Parr and McMahon, 1998) |
NA |
| Wnt9B | Wnt secreted protein |
WE | Absence of uterus and upper vagina (N) (Carroll et al., 2005) | NA |
| Wt1 | Zinc Finger Transcription Factor |
MM | NA | DDS, Ectopic FRT in males in some cases (Barakat et al., 1974; Denys et al., 1967; Manivel et al., 1987) |
AD, autosomal dominant; AR, autosomal recessive; C, conditional; DDS, Denys-Drash syndrome; FRT, female reproductive tract; HFG, hand-foot-genital; ME, Müllerian epithelium; MM, Müllerian mesenchyme; MODY5, maturity-onset diabetes of the young type 5; MRKH, Mayer-Rokitansky-Küster-Hauser; N, null; NA, not available; PMDS, persistent Müllerian duct syndrome; WE, Wolffian epithelium; WM, Wolffian mesenchyme.
Embryology of the Urogenital System
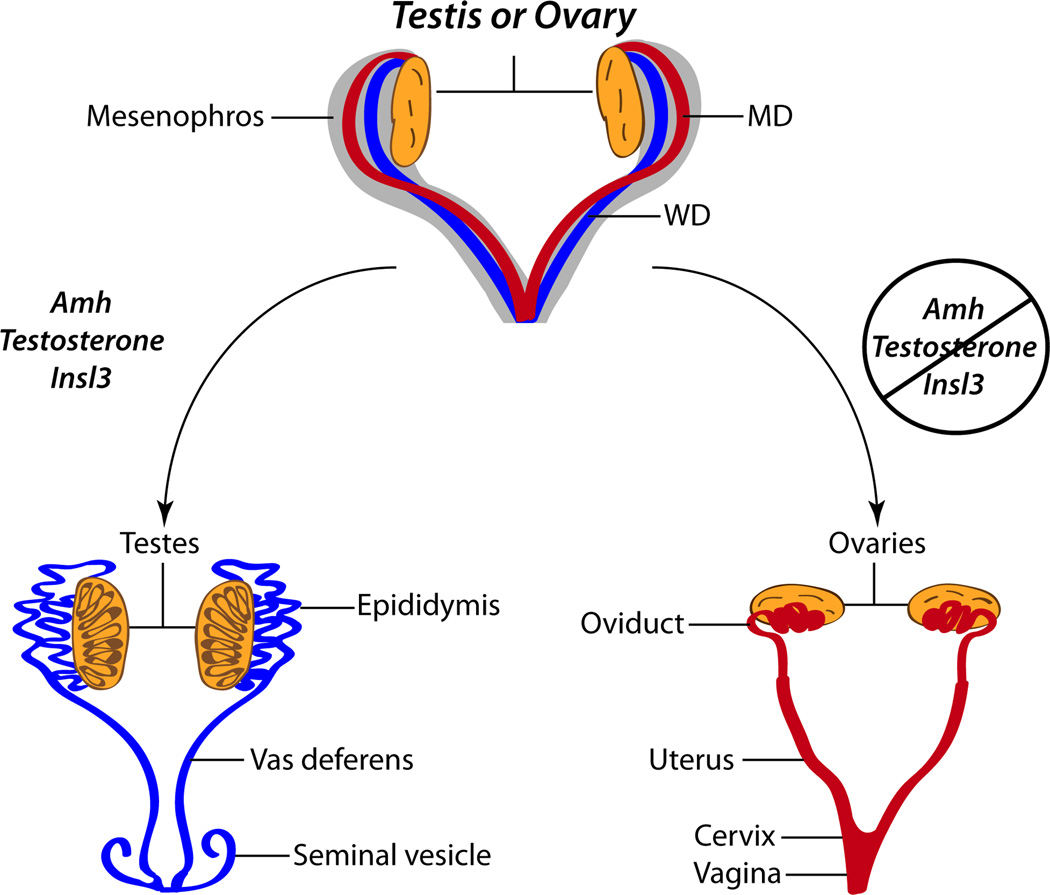
In vertebrates, the urogenital system originates from the intermediate mesoderm and consists of the kidneys, gonads, and urinary and reproductive tracts. Differentiation of the intermediate mesoderm into the urogenital tract begins shortly after gastrulation. First, signaling from the somite and surface ectoderm transduces mesenchymal-to-epithelial conversions in the intermediate mesoderm and the anterior to posterior formation of the nephric ducts, a pair of epithelial tubes joined at the cloaca (Mauch et al., 2000; Obara-Ishihara et al., 1999). Next, the primary kidney or pronephros transiently forms in the posterior region of the nephric ducts and subsequently degenerates (Bouchard et al., 2002; Saxen and Sariola, 1987). Then, posterior to the degenerating pronephros, the mesonephric duct (WD) develops and extends in an anteroposterior direction. The metanephros arises from inductive interactions between the ureteric bud that branches from the caudal WD and mesenchyme (reviewed in (Little et al., 2010)). Soon after formation of the WD, the paramesonephric duct (MD) appears and grows rostral to caudal adjacent to the WD until the duct joins at the urogenital sinus. Initially, the reproductive tracts of males and females are identical, containing two pairs of fully formed WDs and MDs. After sex determination, hormones produced in the fetal testis, anti-Müllerian hormone (AMH), testosterone, and insulin-like 3 (Insl3) trigger regression of the MD, differentiation of the WD into the male genital tract, consisting of the vas deferentia, epididymides, and seminal vesicles, and testicular descent, respectively. In females, lack of AMH, testosterone, and Insl3 in this developmental window permits differentiation of the MD into the female reproductive tract, consisting of the oviducts, uterus and upper vagina, passive degeneration of the WD, and maintenance of the ovaries in an abdominal position, respectively (Fig. 1) (reviewed in (Kobayashi and Behringer, 2003).
FIG. 1. Sexual differentiation of the reproductive tracts.
The reproductive tracts prior to sexual differentiation are equivalent and contain a fully formed Wolffian duct (WD; represented in blue) and Müllerian duct (MD; represented in red). Hormones produced in the fetal testis, anti-Müllerian hormone (Amh), testosterone, and insulin-like 3 (Insl3), enable regression of the MD, differentiation of the WD into the male genital tract, and testicular descent, respectively. In females, lack of AMH, testosterone, and Insl3 at this developmental time permits differentiation of the MD into the female reproductive tract, passive degeneration of the WD, and maintenance of the ovaries in an abdominal position, respectively. The WD differentiates into the male reproductive tract consisting of the vas deferentia, epididymides, and seminal vesicles. The MD develops into the female reproductive tract consisting of the oviducts, uterus and upper vagina. Adapted from (Kobayashi and Behringer, 2003).
The elongating MDs reach and fuse with the urogenital sinus, to form the utero-vaginal duct that will give rise to the caudal uterus, cervix and upper vagina (Orvis and Behringer, 2007). The rostral region of the MD develops into the oviducts and rostral uterus. Uterine morphology between different mammalian species is highly diverse and varies in part because of differences in the extent of rostral MD fusion. For example, fusion in rodents is minimal, resulting in a duplex uterus (consisting of two individual uterine horns connected at the cervix) while in primates fusion extends more rostrally, resulting in a simplex uterus (consisting of a single uterine cavity) (Kobayashi and Behringer, 2003).
Müllerian Duct Formation
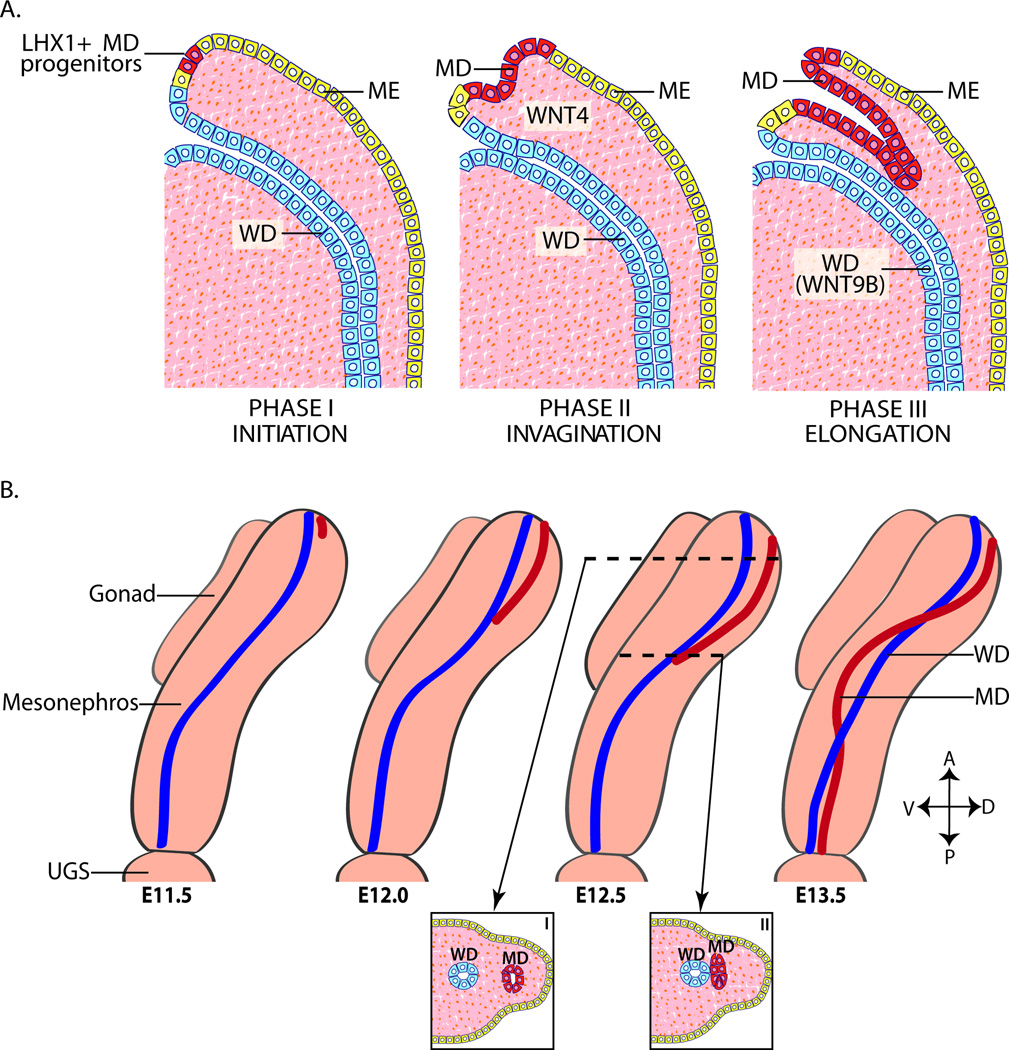
The MD forms in three distinct phases: initiation, invagination and elongation (Fig. 2A). The first phase, initiation, begins with the formation of a placode-like thickening and expression of the LIM (lin-11, Isl1 and mec-3) class homeodomain transcription factor, LHX1, in the rostral mesonephric epithelial cells fated to become MD epithelial cells (Orvis and Behringer, 2007). Lhx1 has important function in reproductive tract development of both sexes. One Lhx1 null male neonate had normal testes but lacked WD-derived organs (Kobayashi et al., 2004). Lhx1 null female mice form normal gonads but lack all MD-derived reproductive tract structures, including the oviducts, uterus and upper vagina (Kobayashi et al., 2004). Transcriptional co-factors, DACH1 and DACH2, function redundantly in and are required for the formation of the MD. The WD forms normally, however double Dach1/2 mutant mice have severe defects in MD formation and differentiation and reduced MD expression of Lhx1 and Wnt7a. This suggests that DACH proteins act upstream of Lhx1 and Wnt7a and regulate expression, either directly or indirectly, of these and possibly other factors important for MD formation (Davis et al., 2008).
FIG. 2. Müllerian duct formation.
(A) MD (represented in red) formation occurs in three phases: initiation, invagination, and elongation. Phase I (Initiation); MD progenitor cells in the mesonephric epithelium (represented in yellow) are specified and begin to express LHX1. Phase II (invagination): In response to Wnt4 signaling from the mesenchyme, LHX1+ MD progenitor cells invaginate caudally into the mesenophros towards the WD (represented in blue). Phase III (Elongation): The tip of the MD contacts the WD and elongates caudally in close proximity to the WD requiring structure and WNT9B signaling from the WD. (B) Beginning at ~ E11.5 in mice, the MD invaginates and extends posteriorly guided by the WD. During elongation, mesenchymal cells separate the WD and MD anterior to the growing tip (I). However at the MD tip, the MD and WD are in contact (II). At ~E12.5, the MD crosses over the WD to be located medially. Elongation is complete by ~E13.5 with the MDs reaching the UGS. Adapted from (Kobayashi and Behringer, 2003). A, anterior (dorsal); E, embryonic day in mouse; D, dorsal; ME, mesonephric epithelium; MD, Müllerian duct; P, posterior (caudal); urogenital sinus, UGS; V, ventral; WD, Wolffian duct.
In the second phase of MD formation, invagination, MD-specified cells from the mesonephric epithelium extend caudally towards the WD. Expression of Wnt4 in the mesonephric mesenchyme is necessary to signal the MD progenitor cells to begin invagination (Kobayashi et al., 2004; Vainio et al., 1999). The MD is absent in male and female Wnt4 null mice at E11.5 and E12.5 (Vainio et al., 1999). Loss of Wnt4 does not alter Lhx1 expression in the MD precursor cells however these cells fail to invaginate, indicating that Wnt4 is necessary for invagination but not specification (Kobayashi et al., 2004).
The final phase of MD formation, elongation, begins when the invaginating tip of the MD contacts the WD. MD elongation continues in close proximity to the WD until the MD fuses at the urogenital sinus (Masse et al., 2009; Orvis and Behringer, 2007). By E12.5 in the mouse, the MD has reached approximately the halfway point of its elongation path and crosses over the WD to be located medially. Elongation is complete by ~E13.5 with the MD reaching the urogenital sinus (Fig. 2B) (Gruenwald, 1941; Orvis and Behringer, 2007). While controversial in the past it is now believed that the origin of the MD epithelium cells is a cell population of the mesonephric epithelium likely found in the transition area between the pronephros and mesonephros (Guioli et al., 2007; Orvis and Behringer, 2007). There are cellular markers that are distinct between the WD, MD and mesonephric epithelium during MD formation. In mouse at E12.5, the WD expresses the epithelial markers cytokeratin 8 (CK8), pan cytokeratins and E-cadherin (CDH1) and lacks expression of the mesenchymal marker vimentin (Vim). Initially, the newly formed caudal portion of the MD is mesenchymal in nature and expresses Vim but is CDH1 negative. Later, the MD differentiates and expresses the standard epithelial cell markers with expression of CDH1 first evident in the most rostral region of the MD. At mouse E12.5 and E13.5, the mesonephric epithelium expresses both epithelial and mesenchymal cell markers (Orvis and Behringer, 2007).
Development of the MD is independent of sex genotype and occurs rostral to caudal. Only MD cells at the most caudal tip are in physical contact with WD cells during elongation (Fig. 2B) (Orvis and Behringer, 2007). Mesenchymal cells are present between the MD and WD and mesonephric epithelium in regions rostral to the caudal tip of the MD (Gruenwald, 1941; Orvis and Behringer, 2007). Specification of MD precursor cells and the initial invagination of the mesonephric epithelium occur independently of the WD. However elongation requires signaling and structure from the WD.
Organ culture studies demonstrated that physical disruption of the WD causes MD truncation, highlighting the link between WD and MD formation (Gruenwald, 1941). The dependence of MD elongation on the WD has also been shown in several mutant mouse models in which the WD either fails to form (Lhx1 and Pax2 mutants), degenerates shortly after formation (Emx2 mutants) or lacks key signaling molecules (Wnt9b mutants). In Lhx1 null mice, the WD is completely absent and the MD fails to form (Kobayashi et al., 2004). Paired-box gene 2 (Pax2) is expressed in the WD and MD and is necessary for MD formation. Pax2 null mice die shortly after birth, failing to form kidneys and reproductive tracts. In Pax2 homozygous null mice, the rostral portion of the WD forms at E9.5 but does not elongate. By E12.5 the truncated WD has begun to degenerate. MD initiation and invagination occur normally but MD elongation occurs only along the truncated WD. By E16.5, the truncated WD and MD are absent (Torres et al., 1995). Homeobox gene Emx2 knockout mice lack kidneys, reproductive tracts and gonads in males and females and die shortly after birth due to renal dysfunction. The WD forms normally at E10.5, but by E11.5 the WD is degenerating. The MD fails to form in mutants and is absent at E13.5 (Miyamoto et al., 1997). Although the structure of the WD is unaffected in Wnt9b mutant mice, MD elongation is blocked. This suggests that the WD guides elongation through the secreted WNT9B signal. As in studies with a disruption of WD structure, loss of WNT9B signaling did not affect MD specification and initial invagination, only caudal elongation (Carroll et al., 2005).
While the primary cause of MD loss in Lhx1, Pax2 and Emx2 null mice is likely a result of WD defects, these homeodomain transcription factors are suggested to have later functions in MD development. Chimera studies suggest that Lhx1 is required cell-autonomously for the formation of the MD epithelium (Kobayashi et al., 2004). It is also probable Pax2 functions cell autonomously during MD formation and/or maintenance. PAX2 protein is expressed in both the MD and WD epithelium at E13.5. Further Pax2 is thought to be required for the mesenchyme-to-epithelium transitions in the intermediate mesoderm necessary for both WD and MD formation (Torres et al., 1995). Similarly, Emx2 is expressed in both the WD and MD epithelium at E13.5 suggesting additional roles in MD development (Miyamoto et al., 1997).
Caudal growth of the MD epithelium is thought to occur primarily as a result of proliferation of the MD epithelium. Cells from the WD or from the mesonephric epithelium following MD specification do not contribute significantly to the growing MD (Guioli et al., 2007; Orvis and Behringer, 2007). During elongation, studies in mouse and chick have shown proliferation is occurring along the length of the MD (Guioli et al., 2007; Jacob et al., 1999; Orvis and Behringer, 2007). Organ culture of mouse urogenital ridges in which the rostral MD has been removed leaving the caudal MD tip region showed completion of MD elongation. Therefore, cells contained in the caudal MD tip are capable of completing MD elongation in the absence of the rostral MD (Orvis and Behringer, 2007). In organ culture studies of rat urogenital ridges, migration of MD epithelial cells has been shown to be occurring in the rostral to caudal direction during MD elongation. Further, following extended culture with BrdU, both dividing and non-dividing cells are found in the MD tip suggesting migration may contribute to MD elongation (Fujino et al., 2009).
The PI3K/ AKT pathway also has a role in MD elongation. Treatment with PI3K inhibitors in rat urogenital organ culture blocks MD elongation. PI3K inhibition also deterred lateral migration of the mesenchymal cells that separate the WD and MD. However PI3K inhibition did not affect rostral to caudal migration of the MD epithelial cells in the already formed portions of duct. Slight increases in apoptosis were observed in the MD after PI3K inhibition, but likely do not explain the MD elongation defect. The authors hypothesize that PI3K may be required to activate enzymes that break down the extra-cellular matrix that would otherwise block invasion by the caudal tip (Fujino et al., 2009).
Retinoic acid (RA) signaling is also required for the formation and/or maintenance of the MD. RA, a morphogen derived from vitamin A, has important functions in antero-posterior patterning of the body axis and during limb development (Dreyer and Ellinger-Ziegelbauer, 1996; Robert and Lallemand, 2006). RA receptor (RAR) genes have redundant function in MD formation. In mouse, single gene mutants of RARα1, RARα2, RARβ2 or RARγ, have no defects in female reproductive tract development. However the MD is completely absent at E12.5 in RARα/RARβ2 compound mutants not attributable to any defects in WD formation. Additionally other combinations of RAR mutations resulted in partial MD loss caudally. Thus suggesting RA signaling has important functions in MD but not WD formation (Kastner et al., 1997; Mendelsohn et al., 1994). Caudal defects in MD elongation are also observed in Discs large homolog 1 (Dlgh1) null mice that cause MD fusion failure and obstruction which results in aplasia of the cervix and vagina (Iizuka-Kogo et al., 2007).
Sex Differentiation; Müllerian Duct Regression in Males
Anti-Müllerian hormone signaling pathway
During male development mesenchyme-epithelia interactions mediate MD regression to prevent its development into a uterus and oviduct (Fig. 1). MD regression requires binding and signal transduction from the transforming growth factor-β (TGF-β) family member anti-Müllerian hormone (AMH) secreted from the Sertoli cells of the fetal testis and its type 1 and 2 receptors expressed in MD mesenchyme (reviewed in (Josso et al., 1993; Kobayashi and Behringer, 2003). The transcription of the Amh gene is directly regulated by multiple factors in the testis-determining pathway including SRY-box containing gene 9 (Sox9), steroidogenic factor 1 (Sf1), Wilms tumour homologue (Wt1), and DSS-AHC critical region on the X-chromosome gene 1 (Dax1) (Arango et al., 1999; De Santa Barbara et al., 1998; Nachtigal et al., 1998; Shen et al., 1994). Females do not express AMH during fetal development thus allowing differentiation of the MD.
The first observable histological change during regression in males is the appearance of the “sworl” pattern of the mesenchymal cells surrounding the MD in the most rostral region (Dyche, 1979; Orvis and Behringer, 2007). At the onset of regression, differences in the MD also appear at the cellular level between the sexes. Initially the forming MD is mesoepithelial in nature with cell markers consistent with a mesenchymal cell tube however the morphology is consistent with a true epithelial cell tube (Dyche, 1979; Orvis and Behringer, 2007). Beginning at E13.5, the female MD begins to express the epithelial cell marker E-cadherin (CDH1) apically and show evidence of apicobasal polarity while the MD in males remains unchanged (Orvis and Behringer, 2007). This has also been observed in several other species including rat (Dohr et al., 1987; Paranko and Virtanen, 1986), human (Magro and Grasso, 1995), chick (Jacob et al., 1999), but not in golden hamster (Viebahn et al., 1987). AMH-induced MD regression occurs in a specific window in time during development and after this time the MD is no longer sensitive to AMH-induced regression (Josso et al., 1976). This window corresponds with the time frame in which the MD is not yet expressing epithelial-specific markers (Orvis and Behringer, 2007). One hypothesis is that the mesoepithial nature of the MD may facilitate regression in males.
Genetic experiments in mouse and naturally occurring mutations in the human have demonstrated that AMH is necessary and sufficient for Müllerian duct regression. Amh null male mice have normal development of the testis and male reproductive tract, however Müllerian-derived tissues develop, causing infertility by physically blocking sperm release (Behringer et al., 1994). Further, female transgenic mice ectopically expressing human AMH lack MD-derived tissues (Behringer et al., 1990). Additionally, mutations in the human AMH gene are causative of ~45% of Persistent Müllerian Duct Syndrome (PMDS) cases, a rare autosomal recessive disorder. Like Amh null mice, male patients with PMDS are normally virilized, but have female reproductive organs including a uterus and fallopian tubes. PMDS is most often diagnosed because of cryptorchidism, a failure of the testis to descend, and/or inguinal hernia (Belville et al., 1999; Belville et al., 2009; di Clemente and Belville, 2006; Salehi et al., 2012).
Amhr2 positive cells in the MD mesenchyme transduce the AMH hormone signal secreted from the fetal testis (Mishina et al., 1999). AMH signaling occurs in a paracrine manner and begins with AMH binding to its type 2 receptor (AMHR2). AMHR2 then forms a heteromeric complex with and then phosphorylates and activates a type 1 receptor. This activation results in the phosphorylation of an R-Smad and supposedly formation of an R-SMAD/SMAD-4 complex that translocates into the nucleus to transcriptionally activate AMH signaling pathway target genes. AMH type 1 receptors ALK2 (AVCR2) and ALK3 (BMPR1A), and AMH R-Smad effectors (SMAD1, SMAD5 and SMAD8) function redundantly in MD regression and are shared with the bone morphogenetic protein (BMP) pathway. ALK3 is considered the primary type 1 receptor required for regression however ALK2 is capable of transducing the AMH signal in the absence of ALK3. Conditional knockout of Alk2 in the MD mesenchyme does not block MD regression. However approximately half of all Alk3 conditional mutant males and 100% of mutants with conditional knockout of both Alk2 and Alk3 failed to regress the MD (Jamin et al., 2002; Orvis et al., 2008).
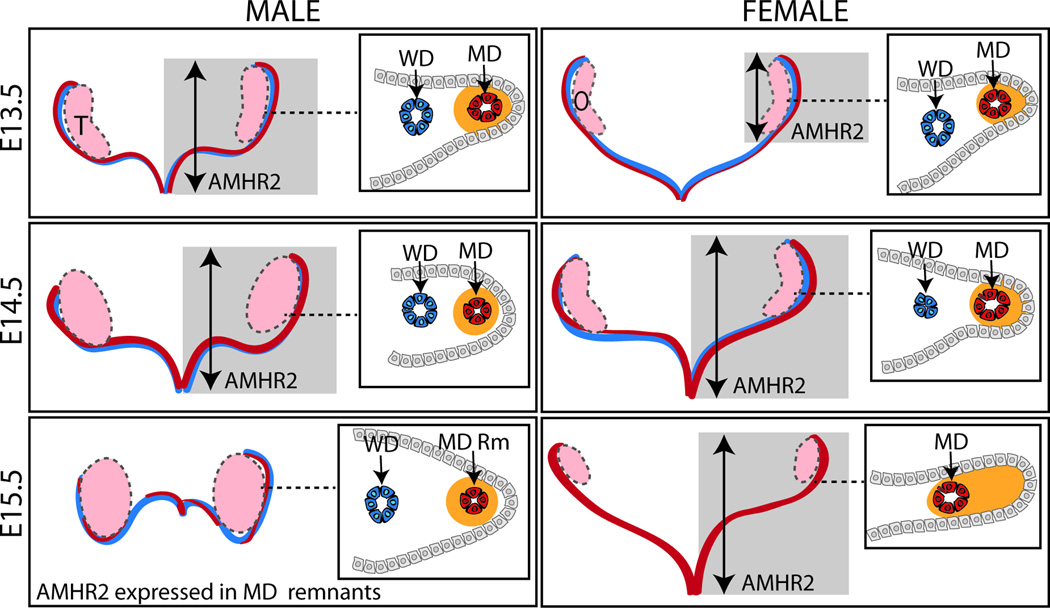
ALK2 and ALK3 are believed to function in a distinct temporal and spatial manner during regression. Initially, Amhr2 is expressed in the mesonephric epithelium. In the mesonephric epithelium, ALK2 appears to mediate AMH signaling which directs Amhr2 positive cells to undergo an epithelial to mesenchymal transition and migrate to surround the MD epithelium forming the distinct “sworl” pattern observed at E15.5 (Zhan et al., 2006). This is followed by the breakdown of the basement membrane and subsequent loss of the epithelium (Dyche, 1979; Orvis and Behringer, 2007; Trelstad et al., 1982). In females, Amhr2 expressing cells are found in the mesonephric epithelium on the antimesometrial side of the MD, but without AMH signaling these cells do not undergo migration to surround the MD epithelium or cellular changes up to at least E15.5 (Fig. 3) (Arango et al., 2008; Orvis et al., 2008; Zhan et al., 2006).
FIG. 3. Müllerian duct regression.
At E13.5 in mice, male and female WD (represented in blue) and MD (represented in red) are fully formed. In males, AMHR2 is expressed throughout the length (represented by gray box and arrow) and in the MD mesenchyme (represented in orange) in a tight ring around the MD epithelium (cross-section inset). In females, AMHR2 expression is caudally expressed and found in the MD mesenchyme on the antimesometrial side of the MD epithelium. By E14.5, expression of AMHR2 is found along the length of the MD in both sexes. At E14.5 in females, the WD is beginning to degenerate and is absent by E15.5. Portions of the MD have regressed in males by E15.5 in an apparently random pattern and AMHR2 expression is limited to the remaining MD remnants. E, embryonic day in mouse; MD, Müllerian duct; O, ovary; Rm, remnant; T, testis; WD, Wolffian duct.
Mechanisms known to be involved in regression include epithelial cell migration, epithelial to mesenchymal transformations and apoptosis (Allard et al., 2000; Austin, 1995; Hutson et al., 1984). The pattern of regression of the MD is hypothesized to occur in a rostral to caudal wave corresponding to Amhr2 expression. A statically imaged time course of ex vivo cultured rat urogenital ridges (males and AMH-treated females) during regression showed rostrally a reduction in MD diameter (Picon, 1969; Tsuji et al., 1992). Further Allard et al. showed a positive correlation between the rostral-caudal wave of Amhr2 expression and the pattern of increased apoptosis of the MD epithelium during regression in rat (Allard et al., 2000). In contrast, studies from the Amhr2-lacZ knock-in mouse model indicate that although Amhr2 expression initiates in a rostral to caudal wave, it is expressed along the entire length of the MD at E13.5 well before any overt changes in the male MD (Fig. 3) (Arango et al., 2008). Further studies will be needed to clarify the spatial-temporal patterning and cell behaviors, including migration and apoptosis, during MD regression.
Genetic studies in mice and human indicate that AMHR2 is the sole type 2 receptor required for AMH signaling and is likely dedicated to the AMH-signaling pathway. Male Amhr2 and Amh mutant mice have identical phenotypes; normally virilized with persistent MD-derived organs (Behringer et al., 1994; Mishina et al., 1996; Mishina et al., 1999). Additionally, the phenotype of hAMH expressing transgenic female mice is rescued by Amhr2 mutation (Behringer et al., 1990; Mishina et al., 1999). Further, mutation of the AMHR2 gene accounts for about half of the cases of PMDS with known molecular etiology (Belville et al., 2009; di Clemente and Belville, 2006; Salehi et al., 2012).
The Wilms’ tumor 1 (Wt1) transcription factor gene is a direct activator of Amhr2 transcription. Amhr2 transcript levels are reduced in Wt1 null mice and both transcripts are co-expressed in the developing MD. Additionally, Wt1 expression mirrors the sexually dimorphic pattern observed for Amhr2 expression during MD regression in the urogenital ridge. Further, in vitro assays show WT1 activates transcription of Amhr2 and binds to elements in the Amhr2 proximal promoter (Klattig et al., 2007). Two alternate splice variants of Amhr2, Amhr2-Δ exon 2 and Amhr2-Δ exon 9 and 10, are found in adult rat and mouse. The Amhr2 splice variants act in a dominant negative manner when co-transfected with full length AMHR2 and AMH in in vitro luciferase reporter assays. The dominant negative effect of the splice variants was reduced at higher levels of AMH concentration corresponding to local levels of AMH in the gonads. The authors hypothesize that splice variants may be expressed at high levels only in a particular subset of gonadal cells and regulate AMH signaling in these cells. Alternatively, splice variants may have site specific effects independent of the presence of AMH ligand or may have a role in the transport of ligand into the cell and might be important in trafficking across the blood brain barrier (Imhoff et al., 2013). Expression of these splice variants during MD regression has not been determined and it is currently unknown what if any role they have during reproductive tract development.
Wnt signaling plays multiple roles in MD development and is needed for formation, regression and differentiation. Prior to the onset of MD regression in males, WNT7A signaling from the epithelium to mesenchyme of the MD activates Amhr2 expression in both sexes and is also required for appropriate differentiation of the MD. Wnt7a mutant males retain MD-derived organs because Amhr2 expression is lost in the MD mesenchyme, thus blocking the AMH-signaling pathway. Consistent with the differentiation defects observed in the Wnt7a mutant female reproductive tract, in mutant male, the ectopic female reproductive tract shows no evidence of oviduct coiling and is a simple epithelial tube (Parr and McMahon, 1998). This is in contrast to Amh and Amhr2 null mouse models where the differentiation of the mutant female and male MD-derived organs is relatively normal (Behringer, 1994; Behringer et al., 1994; Mishina et al., 1996). The Frizzled (Fzd) genes encode the seven trans-membrane protein receptors for the WNT ligand which are required for both canonical and non-canonical WNT signaling pathways. A dedicated FZD receptor has not been identified for WNT7A in the MD. Previous studies showed interactions between WNT7A and FZD10 activated the WNT pathway (Kawakami et al., 2000). Additionally, in mouse, Wnt7a and Fzd10 have overlapping expression patterns in the MD (Nunnally and Parr, 2004). Although this identified FZD10 as a potential candidate receptor for WNT7A, Fzd10 knockout mice have no reproductive tract phenotype (Fzd 10: MGI Direct Data Submission MGI:3604450). Similar to Fzd10, Fzd1 expression is found in the MD mesenchyme and epithelium at E14.5, but Fzd1 knockout males have no MD regression defects (Deutscher and Hung-Chang Yao, 2007; Lapointe et al., 2012). This suggests multiple FZD receptors are capable of interaction with WNT7A and function redundantly during MD regression.
AMH signaling; downstream molecular mechanisms
Several studies suggest WNT signaling is also important to the downstream molecular signaling cascade required for MD regression during male reproductive tract differentiation. However, the exact role of WNT signaling following activation of the AMH signaling pathway remains unclear. Either inactivation or constitutive activation of β-CATENIN (CTNNB1) in the MD mesenchyme causes retention of MD-derived tissues in mutant males independent of AMH expression suggesting tight control of CTNNB1 activation is necessary for MD regression (Kobayashi et al., 2011; Tanwar et al., 2010). In the canonical WNT signaling pathway, nuclear localized CTNNB1 in a complex with T-cell factor/ lymphoid enhancer factor (TCF/LEF) transcription factors regulate expression of target genes. CTNNB1 is also known to have roles in cell tight junction formation and adhesion and may therefore be functioning independent of WNT signaling during regression (Brembeck et al., 2006). CTNNB1 activates Lef1 transcription and upregulates Lef1 promoter activity in vitro (Filali et al., 2002; Vadlamudi et al., 2005). Additionally, Ctnnb1 inactivation results in the loss of LEF1 up-regulation normally observed in the MD mesenchyme of males during regression (Kobayashi et al., 2011). This suggested LEF1 may be required downstream of WNT/β-CATENIN signaling to induce MD regression during male reproductive tract differentiation. However, Lef1 null male mice have normal MD regression (Mullen and Behringer, unpublished observations) (van Genderen et al., 1994). Several additional WNT pathway factors have also been identified that are expressed in a sex-specific pattern in the mesenchyme during AMH-induced MD regression including Wnt4, Wnt5a, and Frizzled-related Wnt pathway genes Sfrp1, Sfrp2, and Sfrp5. Knockout of Wnt4 in the MD mesenchyme does not interfere with MD regression (Kobayashi et al., 2011). No defects in MD regression have been reported in Wnt5a−/− mice (Mericskay et al., 2004). Likewise, loss of function of Sfrp2 and Sfrp5 caused no defects in MD regression (Cox et al., 2006). Double knockout Sfrp1−/−/Sfrp2−/− mice appear to have a slight delay, but MD regression is complete at later embryonic stages (Warr et al., 2009). These results suggest that WNT pathway factors have redundant function during MD regression or alternatively are not required for regression. Further studies will be needed to clarify the roles of WNT signaling during later stages of MD regression.
Sexually dimorphic expression patterns during regression have also been identified for Matrix metalloproteinase 2 (Mmp2). Mmp2 is upregulated in the male MD mesenchyme during regression and this up regulation is lost in Amh null males. Morpholino knockdown of Mmp2 in organ culture blocks regression and decreases MD epithelium apoptosis (Roberts et al., 2002). Null Mmp2-mutant mice however have no defects in MD regression. This may suggest redundant function with other genes (Itoh et al., 1997; Roberts et al., 2002). The PI3K/ AKT pathway may also have a role in AMH signal transduction during regression. Activated phospho-AKT (p-AKT) is present in equal amounts in the WD and MD in both sexes prior to regression. In rats, synchronous with the initiation of MD regression, p-AKT is decreased in males at E15.5 and is undetectable at E16.5. Females maintain p-AKT expression. This pattern is also observed in mice (Fujino et al., 2009). Although PI3K signaling has been shown to prevent apoptosis and the epithelial to mesenchymal transitions that take place during MD regression, it is not clear if this reduction in p-AKT is a cause or effect of regression (Allard et al., 2000; Dyche, 1979; Fujino et al., 2009; Trelstad et al., 1982; Zhan et al., 2006). AMH signaling has been shown to inactivate the PI3K pathway by blocking autophosphorylation of the EGF receptor in the MD epithelium by inhibiting tyrosine kinase (Hurst et al., 2002; Hutson et al., 1984). Although multiple genes have been identified using candidate approaches, the role of many of these signaling pathways and molecules remains unclear due to the possibility of functional redundancy.
Müllerian duct differentiation in females
Once the MD is formed it differentiates into a functional oviduct, uterus, cervix and upper vagina. Correct patterning and differentiation of the MD is dependent on a complex network of Hox and Wnt genes. Further it is known that steroid hormones also regulate many of the genes necessary for proper MD differentiation during organogenesis and adulthood (Masse et al., 2009). Abdominal B (AbdB) homeobox genes (Hoxa9, Hoxa10, Hoxa11 and Hoxa13) of the mammalian Hoxa cluster are required for differentiation and segmental patterning of the MD. AbdB genes are expressed along the anterior-posterior axis of the MD according to their 3’ to 5’ order in the Hoxa cluster. Hoxa9 is expressed in the oviduct. Hoxa10 is expressed in the mesenchyme of the uterus. Hoxa11 is expressed in the posterior uterus and cervix. Hoxa13 is expressed in the cervix and upper vagina (Taylor et al., 1997; Warot et al., 1997). Hox10 expression is necessary for correct specification of tissue boundaries in the male and female reproductive tract. In Hoxa10 null mice, male and female reproductive tracts display posterior to anterior homeotic transformation. At E17.5, Hoxa10 is present only in the portion of the MD that will differentiate into the uterus. Mutations in Hoxa10 result in homeotic transformation of 25% of the proximal uterus into oviduct (Benson et al., 1996). Three heterozygous mutations in the HOXA10 gene with predicted loss of function have been associated with uterine malformations. Patients with HOXA10 mutations had uterine defects ranging from septate uterus and vagina with a duplex cervix to a didelphic uterus indicative of MD fusion defects (Cheng et al., 2011; Ekici et al., 2013). In adult uterus, Hoxa10 represses Emx2 and is found in an inverse expression pattern suggesting a further role for Emx2 in MD patterning and differentiation (Troy et al., 2003). Overlapping expression of patterns of Hoxa10 and Hoxa11 suggests they have partially redundant function during MD differentiation. Further, exposure to the non-steroidal estrogen diethylstilbestrol (DES) in mice caused a posterior shift in Hoxa9 expression likely a result of down regulation of Hoxa10 and Hoxa11 (Block et al., 2000). Sex steroids mediate Hoxa10 and Hoxa11 expression levels. Women exposed to DES during development have malformations of the reproductive tract consistent with anterior transformation (Cermik et al., 2003; Taylor et al., 1999). Hoxa11 null mice have a thinner and shorter uterus lacking glands consistent with a partial homeotic transformation (Gendron et al., 1997). Mutant mice in which the Hoxa11 homeodomain was replaced with the Hoxa13 homeodomain displayed posterior homeotic transformation of the female reproductive tract with the posterior uterus becoming cervix/vagina. This demonstrates that Hoxa11 and Hoxa13 have unique functions in MD differentiation and that Hoxa13 is upstream of factors required for differentiation of the MD into cervix and vagina (Zhao and Potter, 2001). Although Hoxa13 null mutants die between E13.5 to E14.5, mutant female embryos are missing the caudal portion of the MD suggesting Hoxa13 has function during MD formation in addition to MD differentiation. Defects in caudal MD formation were also observed in a Hoxa13 paralogue, Hoxd13, mutant females at birth (Warot et al., 1997).
The WNT pathway is required for MD patterning and differentiation. Wnt7a has important function in both MD regression in males and MD differentiation in females. Wnt7a is expressed throughout the MD epithelium prior to birth. After birth expression is maintained in the oviductal and uterine epithelium but is down-regulated in the vaginal epithelium (Miller et al., 1998). In adult and neonate Wnt7a mutant females, the uterus is smaller in length and diameter and the uterine wall is thinner with less smooth muscle. Oviduct differentiation occurs but coiling and elongation are absent and uterine glands are not present. Posteriorly, a homeotic transformation occurs of oviduct to uterus and uterus to vagina in the Wnt7a null females (Miller and Sassoon, 1998; Parr and McMahon, 1998). Wnt7a also appears to be necessary for the maintenance of Hoxa10 and Hoxa11 expression with the Wnt7a null females showing reduced expression of the two genes. Additionally, the similarity between the Hoxa11 null and Wnt7a null mutant phenotype (thin, small uterus lacking glands) is consistent with upstream regulation of Hoxa11 by Wnt7a (Miller and Sassoon, 1998; Parr and McMahon, 1998).
Recent studies using conditional knockout and tissues explanted under the kidney capsule have also clarified the role for WNT signaling at later stages of MD differentiation. These WNT signaling molecules include Wnt4, Wnt5a and CTNNB1, which were not previously described because of early embryonic lethality (Wnt5a and CTNNB1) or early roles in MD formation (Wnt4). The role of Wnt4 in MD formation is well established. A recent conditional knockout study also demonstrates Wnt4 is important for MD differentiation. Conditional inactivation of Wnt4 in the uterine luminal and glandular epithelium, stroma and myometrium using Progesterone receptor (PR)-Cre resulted in reduced uterine gland number and a stratified luminal epithelial layer instead of a simple columnar epithelial cell layer (Franco et al., 2011). The receptor for Wnt4 during MD development has not been identified. However, Frizzled1 (Fzd1) expression has been found in the developing mesenophros in both the MD mesenchyme and epithelium (Deutscher and Hung-Chang Yao, 2007). Further, 3 of 17 Fzd1−/− null females had a uterine phenotype similar to the Wnt4 conditional mutant females suggesting FZD1 may be acting as the WNT4 receptor (Lapointe et al., 2012). However because of the low penetrance of uterine defects in the Fzd1−/− mutant females it is likely other FZD receptors are also able to transduce the WNT4 signal.
The Wnt5a gene is required for development of the posterior (caudal) region of the MD and glandular genesis. Wnt5a null mice have short, coiled uterine horns, but lack a cervix and vagina. In kidney capsule explant studies of mutant uterine horns, both Wnt7a in the luminal epithelium and Wnt5a in the uterine stroma were required for gland formation independent of canonical pathway member Lef1 (Mericskay et al., 2004). Conditional deletion of Ctnnb1 in the MD mesenchyme using Amhr2-Cre causes a hypoplastic uterus with uterine hypotrophy, reduced uterine glands and uncoiled oviducts. In Ctnnb1 conditional mutants, reduced proliferation but not apoptosis contributes to the hypoplasia of the uterus. No differences were found in the expression patterns of Wnt4 and Wnt5a in the mesenchyme or Wnt7a in the epithelium (Deutscher and Hung-Chang Yao, 2007). The mutant phenotype including lack of coiling mimics the Wnt7a null phenotype (Parr and McMahon 1998). Distinct from the Wnt7a null mice, deletion of Ctnnb1 in the MD mesenchyme resulted in the differentiation of smooth muscle cells into adipose tissue postnatally causing the uterus to become fat filled. This suggests other WNTs may be needed for uterine differentiation or alternatively the role of Ctnnb1 in the mesenchyme is independent of WNTs and is functioning instead to control cell adhesion or the formation of cell tight junctions. These studies suggest that the initial differentiation of the myometrium does not require Ctnnb1 but in its absence there is a progressive shift from smooth muscle tissue to adipose tissue (Arango et al., 2005; Deutscher and Hung-Chang Yao, 2007). Ctnnb1 conditional ablation using PR-Cre resulted in a thinner uterus of normal length with reduced gland numbers at sexual maturity. Constitutive activation of CTNNB1 using PR-Cre reduced uterus length and caused hyperplasia of uterine glands. Reductions in the size of the uterus as a result of ablation or activation of Ctnnb1 suggest tight control of WNT signaling is required for proper MD differentiation and development (Jeong et al., 2009).
The Wnt 4 and β-catenin (Ctnnb1) conditional mutant females and Wnt7a−/− and Wnt5a−/− null females have similar defects in MD differentiation. These are also similar to the uterine phenotype caused by DES exposure suggesting a link between WNT signaling and estrogen signaling. WNT signaling ligands in the luminal epithelium may be required to prevent the formation of a stratified epithelial layer in the uterus in response to estrogen signaling (Franco et al., 2011).
Human Müllerian duct formation and differentiation defects
Aberrant development of the MD is a relatively frequent cause of human birth defects. Defects include MD aplasia, MD persistence, and MD fusion and patterning defects. Multiple medical syndromes are associated with female reproductive tract abnormalities. Molecular genetic studies of these patients have identified candidate factors involved in some cases. However the molecular genetic cause of these syndromes remains unknown in the majority of cases (Kobayashi and Behringer, 2003). Several well-characterized syndromes are described; Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome (OMIM 277000), maturity-onset diabetes of the young type 5 (MODY5) (OMIM 604284), persistent Müllerian duct (PMDS) syndrome (OMIM 261550), Urioste syndrome (OMIM 235255), Denys-Drash syndrome (DDS) (OMIM 194080) and Hand-foot-genital (HFG) syndrome (OMIM 140000).
Müllerian duct aplasia syndromes
MRKH occurs in about 1 in 4500 female births and is characterized by the absence of the uterus and upper vagina in genetic females (46XX) (Folch et al., 2000). In the majority of affected patients, ovarian development is normal and the lower third of the vagina is present. The molecular etiology of most cases of MRKH is unknown and occurs most often due to sporadic mutations but familial cases have been described with autosomal dominant inheritance with incomplete penetrance and variable expressivity. MRKH syndrome is further classified as type I (typical; isolated to the reproductive tract) or type II (atypical; associated with additional developmental defects) (Ledig et al., 2011). Müllerian duct aplasia, unilateral renal agenesis, and cervicothoracic somite anomalies (MURCS) association is a severe form of MRKH type II. Patients with MURCS association have renal and skeletal defects in addition to uterine and vagina aplasia or agenesis (Oppelt et al., 2006). Plausible causes of MRKH are mutations increasing either AMH or AMHR2 activity and/or expression that could result in MD regression in females. However, no defects in either the AMH or AMHR2 genes have been discovered to date (Oppelt et al., 2005).
Multiple genes have been associated with MRKH including WNT4, TCF2 (also known as HNF1β or v-HNF1), LHX1 and short stature homeobox (SHOX). Heterozygous loss of function mutations in the human WNT4 gene causes a complete absence of a uterus and upper vagina. In addition, female patients with WNT4 mutations also have excess androgens and symptoms of virilization (Biason-Lauber et al., 2007; Biason-Lauber et al., 2004). This is consistent with the phenotype of Wnt4 null mice in which the MD does not development and testosterone biosynthesis is ectopically activated in the ovary (Vainio et al., 1999). The most common mutations associated with MRKH are deletions of chromosomal region 17q12 which contains both TCF2 and LHX1 genes with approximately 6% of examined cases of MRKH carrying this deletion. TCF2 is a POU domain containing transcription factor widely expressed during development with function in epithelial differentiation (Coffinier et al., 1999a; Kolatsi-Joannou et al., 2001). Heterozygote mutations of TCF2 were first associated with MODY5 with additional malformations in renal development and function. A subset of female patients with heterozygote mutations in TCF2 have malformations of the reproductive tract including bicornuate uterus, uterus didelphys, and Müllerian aplasia and renal defects in the absence of diabetes (Bingham et al., 2002; Lindner et al., 1999). This suggests an important role for TCF2 in urogenital tract formation and maintenance. In mouse, Tcf2 is expressed in the reproductive tract epithelium during development and persists in the adult (Coffinier et al., 1999a; Reber and Cereghini, 2001). However the function of Tcf2 in mouse urogenital development remains undetermined due to the early embryonic lethality of Tcf2 null mutant mice and the normal phenotype of the heterozygous mutants (Barbacci et al., 1999; Coffinier et al., 1999b). To date, MKRH syndrome has also been associated with five heterozygous mutations in the human LHX1 gene; four missense mutations and a frame shift mutation leading to a stop codon (Ledig et al., 2012; Ledig et al., 2011; Sandbacka et al., 2013). Additionally, Lhx1 has been shown in mice to be essential for MD formation (Kobayashi et al., 2004). Partial duplication of the SHOX gene was found in two daughters with MRKH type I and their unaffected father (Gervasini et al., 2010).
Müllerian duct persistence syndromes
The development of a uterus and oviduct in human males has been noted in three syndromes; PMDS, Urioste syndrome and DDS. PMDS patients have normal testis development and the presence of MD-derived female reproductive organs. The syndrome is usually diagnosed while correcting undescended testes in pediatric patients. Reduced fertility is common in PMDS patients and potential causes include structural abnormalities caused by MD remnants, cryptorchidism past the age of 2 years, and damage to the vas deferens during orchidopexy. There is also an increased risk of malignancy in the ectopic MD-derived organs if not surgically removed and in the testes due to cryptorchidism. Eleven cases of malignancy in the retained MD organs have been reported and laparoscopic removal of the MD structures in PMDS patients is recommended (Farikullah et al., 2012). PMDS is further classified with type I males having undetectable levels of AMH and type II males with normal AMH levels. The majority of PMDS cases are caused by mutations in the AMH (type I) or AMHR2 (type II) genes with each representing about half of the cases with known molecular etiology (Belville et al., 2009; di Clemente and Belville, 2006; Salehi et al., 2012).
Urioste syndrome is an autosomal recessive disorder associated with the retention of MD-derived tissues in males. In addition to a persistent MD phenotype patients also have lymphangiectasia and postaxial polydactyly. The molecular basis of this syndrome is currently unknown (Urioste et al., 1993).
Denys-Drash syndrome (DDS) is characterized by partial gonad dysgeneis, congenital or infantile nephropathy, and Wilms’ tumor. The molecular cause of DDS in almost all cases is dominant loss of function mutations in the zinc finger DNA binding domain of WT1. In multiple cases of DDS, patients have MD-derived uterus and vagina remnants in addition to developed vas deferens and epididymis (Barakat et al., 1974; Denys et al., 1967; Manivel et al., 1987). Amh and Amhr2 are regulated by WT1 therefore reductions in AMH ligand and its receptor are postulated to cause the defects in MD regression seen in DDS patients (Hossain and Saunders, 2003; Klattig et al., 2007; Nachtigal et al., 1998). The most common WT1 gene mutation in DDS is a missense mutation in exon 9, 1180C>T (R394W). The presence of retained MD structures is found in some but not all patients including those from the same family (Coppes et al., 1992; Zhu et al., 2013). Mouse models heterozygous either for the Wt1 null allele or the R394W mutation have no evidence of MD regression defects (Gao et al., 2004). Together this suggests that genetic background and/or environmental factors may play an important role in determining the penetrance of MD regression defects in DDS patients.
Müllerian duct fusion and patterning defect syndrome
HFG is an autosomal dominant syndrome that results in shortened thumbs and great toes and genital defects including hypospadias in males and a range of female reproductive tract defects from a longitudinal vagina or double vagina to double uterus and cervix. Incomplete MD fusion during embryogenesis gives rise to these defects in females with HFG (Goodman and Scambler, 2001). The similarity of the Hypodactly (Hd) mutant mouse phenotype with a spontaneous dominant negative mutation in the first exon of the Hoxa13 gene to the limb and genital defects in humans first identified Hoxa13 as a potential candidate gene (Post et al., 2000). Although, MD fusion defects are not present in Hd and Hoxa13−/− mice, mild hypospadias of the vagina is observed in a portion of the mutant females (Post et al., 2000; Warot et al., 1997). Additionally, one in six female compound mutants of Hoxa13 and its paralogue, Hoxd13, had MD fusion defects (Warot et al., 1997). Further, six heterozygous mutations in the human HOXA13 gene have been reported in families with HFG to date (Goodman et al., 2000; Mortlock and Innis, 1997).
Conclusions
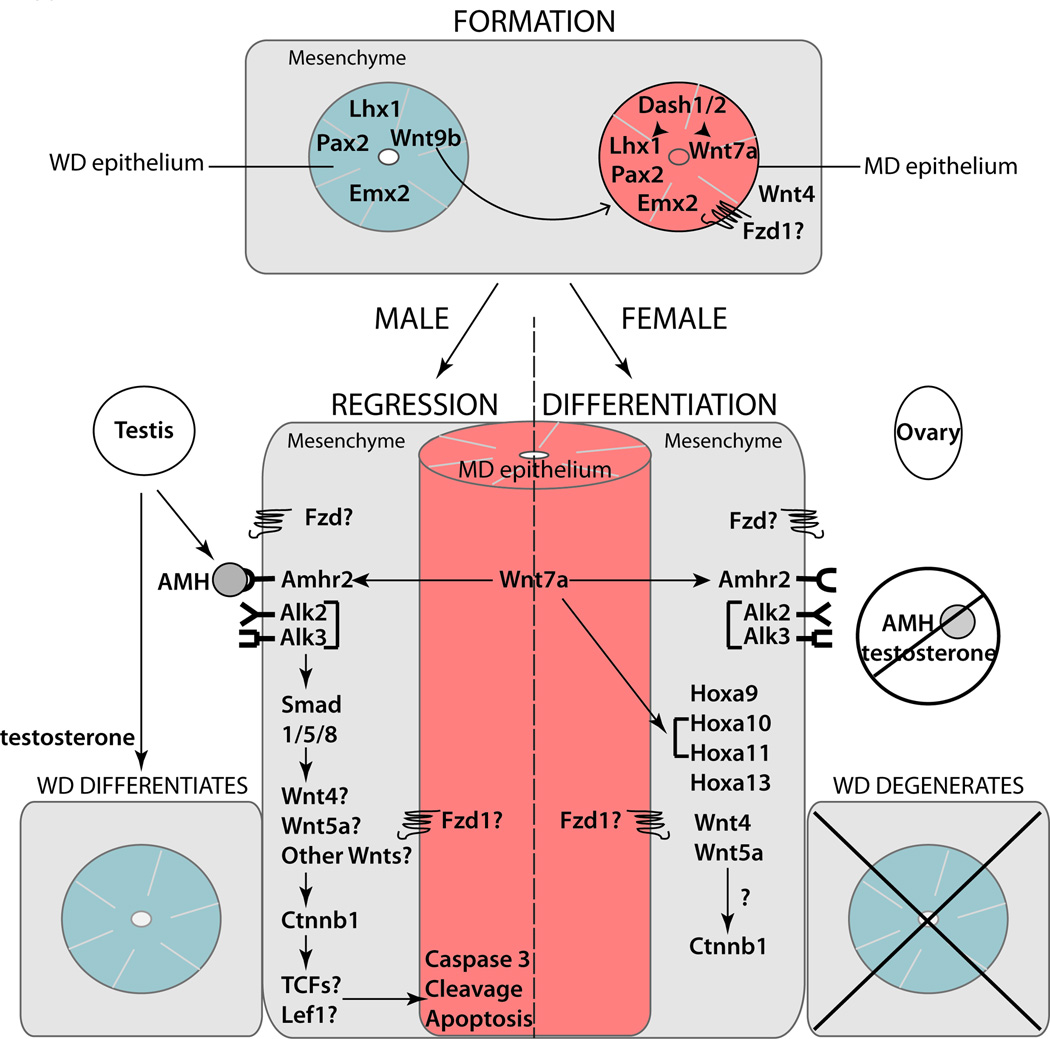
Much progress has been made in understanding the molecular genetics of MD development and several important signaling pathways have been identified (Fig. 4.). Study of knockout and conditional knockout mouse model phenotypes and molecular genetic studies of human diseases of FRT development have provided key insights into the complex signaling cascade involved. However potential functional redundancy of many of these factors including WNT signaling pathway members, MMPS and HOX genes has made it difficult to assess their in vivo function. For example, MD regression requires tight regulation of CTNNB1 activation but thus far a single WNT required for MD regression has not been identified. Further it is likely other factors yet to be identified are involved in MD development. Expression profiling using next generation sequencing technologies should identify genes that are differentially expressed during MD formation and differentiation. By using Cre recombinase lines expressing in the MD epithelium (Wnt7a–Cre) and mesenchyme (Amhr2-Cre) it will be possible to globally uncover the transcriptome of these juxtaposed tissue types. Mesenchyme-epithelia interactions are important regulators of development and many defects in FRT development are the result of aberrant cell-cell communication and signaling. Understanding the molecular and cellular mechanisms of MD formation and differentiation will give key insights into FRT development and disease.
FIG. 4. Genes involved in Müllerian duct development.
Multiple factors and signaling pathways in the mesenchyme (represented in gray) and epithelium of both ducts (WD epithelium represented in blue; MD epithelium represented in red) act in concert to direct MD development. Hormones and key genes required for MD formation, regression in males and differentiation in females are indicated in this figure. MD, Müllerian duct; WD, Wolffian duct.
References
- Allard S, Adin P, Gouedard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development. 2000;127:3349–3360. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Austin HB. DiI analysis of cell migration during mullerian duct regression. Dev Biol. 1995;169:29–36. doi: 10.1006/dbio.1995.1123. [DOI] [PubMed] [Google Scholar]
- Barakat AY, Papadopoulou ZL, Chandra RS, Hollerman CE, Calcagno PL. Pseudohermaphroditism, nephron disorder and wilms' tumor: a unifying concept. Pediatrics. 1974;54:366–369. [PubMed] [Google Scholar]
- Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- Behringer RR. The in vivo roles of mullerian-inhibiting substance. Curr Top Dev Biol. 1994;29:171–187. doi: 10.1016/s0070-2153(08)60550-5. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature. 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Belville C, Josso N, Picard JY. Persistence of Mullerian derivatives in males. Am J Med Genet. 1999;89:218–223. doi: 10.1002/(sici)1096-8628(19991229)89:4<218::aid-ajmg6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Belville C, Marechal JD, Pennetier S, Carmillo P, Masgrau L, Messika-Zeitoun L, Galey J, Machado G, Treton D, Gonzales J, et al. Natural mutations of the anti-Mullerian hormone type II receptor found in persistent Mullerian duct syndrome affect ligand binding, signal transduction and cellular transport. Hum Mol Genet. 2009;18:3002–3013. doi: 10.1093/hmg/ddp238. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, De Filippo G, Konrad D, Scarano G, Nazzaro A, Schoenle EJ. WNT4 deficiency--a clinical phenotype distinct from the classic Mayer-Rokitansky-Kuster-Hauser syndrome: a case report. Hum Reprod. 2007;22:224–229. doi: 10.1093/humrep/del360. [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with Mullerian-duct regression and virilization in a 46, XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- Bingham C, Ellard S, Cole TR, Jones KE, Allen LI, Goodship JA, Goodship TH, Bakalinova-Pugh D, Russell GI, Woolf AS, et al. Solitary functioning kidney and diverse genital tract malformations associated with hepatocyte nuclear factor-1beta mutations. Kidney Int. 2002;61:1243–1251. doi: 10.1046/j.1523-1755.2002.00272.x. [DOI] [PubMed] [Google Scholar]
- Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:238–243. doi: 10.1210/jc.2002-021072. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Zhu Y, Su D, Wang J, Cheng L, Chen B, Wei Z, Zhou P, Wang B, Ma X, et al. A novel mutation of HOXA10 in a Chinese woman with a Mullerian duct anomaly. Hum Reprod. 2011;26:3197–3201. doi: 10.1093/humrep/der290. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Barra J, Babinet C, Yaniv M. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev. 1999a;89:211–213. doi: 10.1016/s0925-4773(99)00221-x. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999b;126:4785–4794. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- Coppes MJ, Liefers GJ, Higuchi M, Zinn AB, Balfe JW, Williams BR. Inherited WT1 mutation in Denys-Drash syndrome. Cancer Res. 1992;52:6125–6128. [PubMed] [Google Scholar]
- Cox S, Smith L, Bogani D, Cheeseman M, Siggers P, Greenfield A. Sexually dimorphic expression of secreted frizzled-related (SFRP) genes in the developing mouse Mullerian duct. Mol Reprod Dev. 2006;73:1008–1016. doi: 10.1002/mrd.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Harding M, Moayedi Y, Mardon G. Mouse Dach1 and Dach2 are redundantly required for Mullerian duct development. Genesis. 2008;46:205–213. doi: 10.1002/dvg.20385. [DOI] [PubMed] [Google Scholar]
- De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol. 1998;18:6653–6665. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys P, Malvaux P, Van Den Berghe H, Tanghe W, Proesmans W. [Association of an anatomo-pathological syndrome of male pseudohermaphroditism, Wilms' tumor, parenchymatous nephropathy and XX/XY mosaicism] Arch Fr Pediatr. 1967;24:729–739. [PubMed] [Google Scholar]
- Deutscher E, Hung-Chang Yao H. Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol. 2007;307:227–236. doi: 10.1016/j.ydbio.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Clemente N, Belville C. Anti-Mullerian hormone receptor defect. Best Pract Res Clin Endocrinol Metab. 2006;20:599–610. doi: 10.1016/j.beem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Dohr G, Tarmann T, Schiechl H. Different antigen expression on Wolffian and Mullerian cells in rat embryos as detected by monoclonal antibodies. Anat Embryol (Berl) 1987;176:239–242. doi: 10.1007/BF00310057. [DOI] [PubMed] [Google Scholar]
- Dreyer C, Ellinger-Ziegelbauer H. Retinoic acid receptors and nuclear orphan receptors in the development of Xenopus laevis. Int J Dev Biol. 1996;40:255–262. [PubMed] [Google Scholar]
- Dyche WJ. A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- Ekici AB, Strissel PL, Oppelt PG, Renner SP, Brucker S, Beckmann MW, Strick R. HOXA10 and HOXA13 sequence variations in human female genital malformations including congenital absence of the uterus and vagina. Gene. 2013;518:267–272. doi: 10.1016/j.gene.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Farikullah J, Ehtisham S, Nappo S, Patel L, Hennayake S. Persistent Mullerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Mullerian remnants. BJU Int. 2012;110:E1084–E1089. doi: 10.1111/j.1464-410X.2012.11184.x. [DOI] [PubMed] [Google Scholar]
- Filali M, Cheng N, Abbott D, Leontiev V, Engelhardt JF. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- Folch M, Pigem I, Konje JC. Mullerian agenesis: etiology, diagnosis, and management. Obstet Gynecol Surv. 2000;55:644–649. doi: 10.1097/00006254-200010000-00023. [DOI] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25:1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino A, Arango NA, Zhan Y, Manganaro TF, Li X, MacLaughlin DT, Donahoe PK. Cell migration and activated PI3K/AKT-directed elongation in the developing rat Mullerian duct. Dev Biol. 2009;325:351–362. doi: 10.1016/j.ydbio.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Maiti S, Sun G, Ordonez NG, Udtha M, Deng JM, Behringer RR, Huff V. The Wt1+/R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome. Mol Cell Biol. 2004;24:9899–9910. doi: 10.1128/MCB.24.22.9899-9910.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- Gervasini C, Grati FR, Lalatta F, Tabano S, Gentilin B, Colapietro P, De Toffol S, Frontino G, Motta F, Maitz S, et al. SHOX duplications found in some cases with type I Mayer-Rokitansky-Kuster-Hauser syndrome. Genet Med. 2010;12:634–640. doi: 10.1097/GIM.0b013e3181ed6185. [DOI] [PubMed] [Google Scholar]
- Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, Holmes LB, Donnenfeld AE, Feingold M, et al. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. 2000;67:197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman FR, Scambler PJ. Human HOX gene mutations. Clin Genet. 2001;59:1–11. doi: 10.1034/j.1399-0004.2001.590101.x. [DOI] [PubMed] [Google Scholar]
- Gruenwald P. The relation of the growing müllerian duct to the wolffian duct and its importance for the genesis of malformations. The Anatomical Record. 1941;81:1–19. [Google Scholar]
- Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Hossain A, Saunders GF. Role of Wilms tumor 1 (WT1) in the transcriptional regulation of the Mullerian-inhibiting substance promoter. Biol Reprod. 2003;69:1808–1814. doi: 10.1095/biolreprod.103.015826. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Abbott B, Schmid JE, Birnbaum LS. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts early morphogenetic events that form the lower reproductive tract in female rat fetuses. Toxicol Sci. 2002;65:87–98. doi: 10.1093/toxsci/65.1.87. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Fallat ME, Kamagata S, Donahoe PK, Budzik GP. Phosphorylation events during Mullerian duct regression. Science. 1984;223:586–589. doi: 10.1126/science.6607531. [DOI] [PubMed] [Google Scholar]
- Iizuka-Kogo A, Ishidao T, Akiyama T, Senda T. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development. 2007;134:1799–1807. doi: 10.1242/dev.02830. [DOI] [PubMed] [Google Scholar]
- Imhoff FM, Yang D, Mathew SF, Clarkson AN, Kawagishi Y, Tate WP, Koishi K, McLennan IS. The type 2 anti-Mullerian hormone receptor has splice variants that are dominant-negative inhibitors. FEBS Lett. 2013;587:1749–1753. doi: 10.1016/j.febslet.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Jacob M, Konrad K, Jacob HJ. Early development of the mullerian duct in avian embryos with reference to the human. An ultrastructural and immunohistochemical study. Cells Tissues Organs. 1999;164:63–81. doi: 10.1159/000016644. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josso N, Cate RL, Picard JY, Vigier B, di Clemente N, Wilson C, Imbeaud S, Pepinsky RB, Guerrier D, Boussin L, et al. Anti-mullerian hormone: the Jost factor. Recent Prog Horm Res. 1993;48:1–59. doi: 10.1016/b978-0-12-571148-7.50005-1. [DOI] [PubMed] [Google Scholar]
- Josso N, Picard JY, Trah D. The antimullerian hormone. Recent Prog Horm Res. 1976;33:117–167. doi: 10.1016/b978-0-12-571133-3.50011-8. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Wada N, Nishimatsu S, Nohno T. Involvement of frizzled-10 in Wnt-7a signaling during chick limb development. Dev Growth Differ. 2000;42:561–569. doi: 10.1046/j.1440-169x.2000.00545.x. [DOI] [PubMed] [Google Scholar]
- Klattig J, Sierig R, Kruspe D, Besenbeck B, Englert C. Wilms' tumor protein Wt1 is an activator of the anti-Mullerian hormone receptor gene Amhr2. Mol Cell Biol. 2007;27:4355–4364. doi: 10.1128/MCB.01780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, Behringer RR. beta-Catenin is essential for Mullerian duct regression during male sexual differentiation. Development. 2011;138:1967–1975. doi: 10.1242/dev.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatsi-Joannou M, Bingham C, Ellard S, Bulman MP, Allen LI, Hattersley AT, Woolf AS. Hepatocyte nuclear factor-1beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol. 2001;12:2175–2180. doi: 10.1681/ASN.V12102175. [DOI] [PubMed] [Google Scholar]
- Lapointe E, Boyer A, Rico C, Paquet M, Franco HL, Gossen J, DeMayo FJ, Richards JS, Boerboom D. FZD1 regulates cumulus expansion genes and is required for normal female fertility in mice. Biol Reprod. 2012;87:104. doi: 10.1095/biolreprod.112.102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig S, Brucker S, Barresi G, Schomburg J, Rall K, Wieacker P. Frame shift mutation of LHX1 is associated with Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome. Hum Reprod. 2012;27:2872–2875. doi: 10.1093/humrep/des206. [DOI] [PubMed] [Google Scholar]
- Ledig S, Schippert C, Strick R, Beckmann MW, Oppelt PG, Wieacker P. Recurrent aberrations identified by array-CGH in patients with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil Steril. 2011;95:1589–1594. doi: 10.1016/j.fertnstert.2010.07.1062. [DOI] [PubMed] [Google Scholar]
- Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet. 1999;8:2001–2008. doi: 10.1093/hmg/8.11.2001. [DOI] [PubMed] [Google Scholar]
- Little M, Georgas K, Pennisi D, Wilkinson L. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol. 2010;90:193–229. doi: 10.1016/S0070-2153(10)90005-7. [DOI] [PubMed] [Google Scholar]
- Magro G, Grasso S. Expression of cytokeratins, vimentin and basement membrane components in human fetal male mullerian duct and perimullerian mesenchyme. Acta Histochem. 1995;97:13–18. doi: 10.1016/S0065-1281(11)80202-3. [DOI] [PubMed] [Google Scholar]
- Manivel JC, Sibley RK, Dehner LP. Complete and incomplete Drash syndrome: a clinicopathologic study of five cases of a dysontogenetic-neoplastic complex. Hum Pathol. 1987;18:80–89. doi: 10.1016/s0046-8177(87)80199-5. [DOI] [PubMed] [Google Scholar]
- Masse J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol. 2009;53:411–424. doi: 10.1387/ijdb.082680jm. [DOI] [PubMed] [Google Scholar]
- Mauch TJ, Yang G, Wright M, Smith D, Schoenwolf GC. Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev Biol. 2000;220:62–75. doi: 10.1006/dbio.2000.9623. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- Miller C, Pavlova A, Sassoon DA. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev. 1998;76:91–99. doi: 10.1016/s0925-4773(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Whitworth DJ, Racine C, Behringer RR. High specificity of Mullerian-inhibiting substance signaling in vivo. Endocrinology. 1999;140:2084–2088. doi: 10.1210/endo.140.5.6705. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998;93:445–454. doi: 10.1016/s0092-8674(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Nunnally AP, Parr BA. Analysis of Fz10 expression in mouse embryos. Dev Genes Evol. 2004;214:144–148. doi: 10.1007/s00427-004-0386-4. [DOI] [PubMed] [Google Scholar]
- Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development. 1999;126:1103–1108. doi: 10.1242/dev.126.6.1103. [DOI] [PubMed] [Google Scholar]
- Oppelt P, Renner SP, Kellermann A, Brucker S, Hauser GA, Ludwig KS, Strissel PL, Strick R, Wallwiener D, Beckmann MW. Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: recommendations for clinical diagnosis and staging. Hum Reprod. 2006;21:792–797. doi: 10.1093/humrep/dei381. [DOI] [PubMed] [Google Scholar]
- Oppelt P, Strissel PL, Kellermann A, Seeber S, Humeny A, Beckmann MW, Strick R. DNA sequence variations of the entire anti-Mullerian hormone (AMH) gene promoter and AMH protein expression in patients with the Mayer-Rokitanski-Kuster-Hauser syndrome. Hum Reprod. 2005;20:149–157. doi: 10.1093/humrep/deh547. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, Roberts AB, Umans L, Huylebroeck D, Zwijsen A, et al. Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Mullerian hormone-induced Mullerian duct regression in the mouse. Biol Reprod. 2008;78:994–1001. doi: 10.1095/biolreprod.107.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranko J, Virtanen I. Epithelial and mesenchymal cell differentiation in the fetal rat genital ducts: changes in the expression of cytokeratin and vimentin type of intermediate filaments and desmosomal plaque proteins. Dev Biol. 1986;117:135–145. doi: 10.1016/0012-1606(86)90356-8. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Picon R. [Action of the fetal testis on the development in vitro of the Mullerian ducts in the rat] Arch Anat Microsc Morphol Exp. 1969;58:1–19. [PubMed] [Google Scholar]
- Post LC, Margulies EH, Kuo A, Innis JW. Severe limb defects in Hypodactyly mice result from the expression of a novel, mutant HOXA13 protein. Dev Biol. 2000;217:290–300. doi: 10.1006/dbio.1999.9550. [DOI] [PubMed] [Google Scholar]
- Reber M, Cereghini S. Variant hepatocyte nuclear factor 1 expression in the mouse genital tract. Mech Dev. 2001;100:75–78. doi: 10.1016/s0925-4773(00)00493-7. [DOI] [PubMed] [Google Scholar]
- Robert B, Lallemand Y. Anteroposterior patterning in the limb and digit specification: contribution of mouse genetics. Dev Dyn. 2006;235:2337–2352. doi: 10.1002/dvdy.20890. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Visser JA, Ingraham HA. Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development. 2002;129:1487–1496. doi: 10.1242/dev.129.6.1487. [DOI] [PubMed] [Google Scholar]
- Salehi P, Koh CJ, Pitukcheewanont P, Trinh L, Daniels M, Geffner M. Persistent Mullerian duct syndrome: 8 new cases in Southern California and a review of the literature. Pediatr Endocrinol Rev. 2012;10:227–233. [PubMed] [Google Scholar]
- Sandbacka M, Laivuori H, Freitas E, Halttunen M, Jokimaa V, Morin-Papunen L, Rosenberg C, Aittomaki K. TBX6, LHX1 and copy number variations in the complex genetics of Mullerian aplasia. Orphanet J Rare Dis. 2013;8:125. doi: 10.1186/1750-1172-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Tanwar PS, Zhang L, Tanaka Y, Taketo MM, Donahoe PK, Teixeira JM. Focal Mullerian duct retention in male mice with constitutively activated beta-catenin expression in the Mullerian duct mesenchyme. Proc Natl Acad Sci U S A. 2010;107:16142–16147. doi: 10.1073/pnas.1011606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Igarashi P, Olive DL, Arici A. Sex steroids mediate HOXA11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab. 1999;84:1129–1135. doi: 10.1210/jcem.84.3.5573. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. The epithelial-mesenchymal interface of the male rate Mullerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Troy PJ, Daftary GS, Bagot CN, Taylor HS. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol. 2003;23:1–13. doi: 10.1128/MCB.23.1.1-13.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Shima H, Yonemura CY, Brody J, Donahoe PK, Cunha GR. Effect of human recombinant mullerian inhibiting substance on isolated epithelial and mesenchymal cells during mullerian duct regression in the rat. Endocrinology. 1992;131:1481–1488. doi: 10.1210/endo.131.3.1505479. [DOI] [PubMed] [Google Scholar]
- Urioste M, Rodriguez JI, Barcia JM, Martin M, Escriba R, Pardo M, Camino J, Martinez-Frias ML. Persistence of mullerian derivatives, lymphangiectasis, hepatic failure, postaxial polydactyly, renal and craniofacial anomalies. Am J Med Genet. 1993;47:494–503. doi: 10.1002/ajmg.1320470413. [DOI] [PubMed] [Google Scholar]
- Vadlamudi U, Espinoza HM, Ganga M, Martin DM, Liu X, Engelhardt JF, Amendt BA. PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J Cell Sci. 2005;118:1129–1137. doi: 10.1242/jcs.01706. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Lane EB, Ramaekers FC. The mesonephric (wolffian) and paramesonephric (mullerian) ducts of golden hamsters express different intermediate-filament proteins during development. Differentiation. 1987;34:175–188. doi: 10.1111/j.1432-0436.1987.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Warr N, Siggers P, Bogani D, Brixey R, Pastorelli L, Yates L, Dean CH, Wells S, Satoh W, Shimono A, et al. Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol. 2009;326:273–284. doi: 10.1016/j.ydbio.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, Teixeira J, Donahoe PK. Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development. 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Potter SS. Functional specificity of the Hoxa13 homeobox. Development. 2001;128:3197–3207. doi: 10.1242/dev.128.16.3197. [DOI] [PubMed] [Google Scholar]
- Zhu C, Zhao F, Zhang W, Wu H, Chen Y, Ding G, Zhang A, Huang S. A familial WT1 mutation associated with incomplete Denys-Drash syndrome. Eur J Pediatr. 2013 doi: 10.1007/s00431-013-2004-9. [DOI] [PubMed] [Google Scholar]