Abstract
Primitive expression (PE) is a form of dance therapy (DT) that involves an interaction of ethologically and socially based forms which are supplied for re-enactment. There exist very few studies of DT applications including in their protocol the measurement of neurophysiological parameters. The present pilot study investigates the use of the correlation coefficient (ρ) and mutual information (MI), and of novel measures extracted from ρ and MI, on electroencephalographic (EEG) data recorded in patients with schizophrenia while they undergo PE DT, in order to expand the set of neurophysiology-based approaches for quantifying possible DT effects, using parameters that might provide insights about any potential brain connectivity changes in these patients during the PE DT process. Indication is provided for an acute potentiation effect, apparent at late-stage PE DT, on the inter-hemispheric connectivity in frontal areas, as well as for attenuation of the inter-hemispheric connectivity of left frontal and right central areas and for potentiation of the intra-hemispheric connectivity of frontal and central areas, bilaterally, in the transition from early to late-stage PE DT. This pilot study indicates that by using EEG connectivity measures based on ρ and MI, the set of useful neurophysiology-based approaches for quantifying possible DT effects is expanded. In the framework of the present study, the causes of the observed connectivity changes cannot be attributed with certainty to PE DT, but indications are provided that these measures may contribute to a detailed assessment of neurophysiological mechanisms possibly being affected by this therapeutic process.
Keywords: Primitive expression, Dance therapy, Electroencephalography (EEG), Brain connectivity, Correlation coefficient, Mutual information
Introduction
Dance therapy
Dancing is one of the earliest forms of therapeutic experience and practice known. Dance therapy is the psychotherapeutic use of movement and dance through which the individual participates creatively in a process that furthers his cognitive, emotional, physical and social integration (American Dance Therapy Association 2009).
A variety of dance therapy and related methodology applications, with several groups of participants, has been presented in the literature, suggesting that dance therapy produces improvements such as redefining and enhancing the body image; clarifying ego boundaries; increasing self esteem; contributing to the relief of physical tension, anxiety and aggression; strengthening cognitive and kinaesthetic orientation; as well as increasing happiness and the capacity for communication (Dosamantes 1990; Berrol et al. 1997; Jeong et al. 2005; Lee et al. 2008; Röhricht et al. 2011; Margariti et al. 2012). In particular, Primitive Expression (PE), a form of dance therapy which this paper relates to, involves an interaction of ethologically and socially based forms that are supplied for re-enactment, and includes an incentive for successful performance as well as a challenge to “transcend” (Schott-Billmann 1985, 1997). In PE dance therapy, with the use of percussion, roles are played and figures of myth are enacted. Opportunities are provided for satisfying hidden desires, expressing a wide range of feelings, exploring new behaviours and trying out unfamiliar stances, thus leading to a therapeutic experience (Margariti 2012; Margariti et al. 2012).
The evaluation of the possible benefits of dance therapy in patients undergoing treatment can be furthered when the dance therapy protocol assesses not only psychological/behavioral changes possibly related to the dance therapy process, but also neurophysiological changes. In this perspective, studies exist in the literature indicating an emerging interest for including in the protocol of dance therapy the measurement of neurophysiological parameters. In Bojner-Horwitz et al. (2003), a study concerning fibromyalgia patients, stress hormones were measured in relation to the effect of dance/movement therapy. Increased cortisol levels were found in the patient group that underwent therapy vs the control group of patients that did not participate in dance therapy. This increase was related to significant changes in the self-reported movement pain, mobility, and life energy of the treatment group. In another study (Jeong et al. 2005), dance therapy was applied to depressed non-medicated adolescents and was found to modulate hormonal and neurotransmitter release, which may be involved in therapeutic processes. In a previously published study by our group (Margariti et al. 2012), a PE dance therapy-based protocol was applied to a small group of medicated psychiatric patients with psychotic, obsessive compulsive and depressive disorders. In an effort to assess neurophysiological changes as a result of the PE process, the electroencephalographic (EEG) activity was recorded in a subset of the patients suffering from psychotic disorders. A relatively short duration of PE dance therapy treatment led to quantifiable modifications in psychological state, behavior and, to some extent, brain physiology as expressed by EEG recordings. Specifically, it was found that the patients experienced an increase in their happiness level, expressed a positive attitude to the PE dance therapy process by utilizing appropriate word associations, and, for the group of patients that had EEG recordings, there was an increase in the alpha EEG frequency activity (8–12 Hz) which may relate to the above psychological and behavioural changes.
EEG similarity measures
The investigation of the “similarity”, “association” or “relation” existing between voltage waveforms recorded in pairs of scalp-recorded or intracranial EEG electrodes has attracted research interest concerning both healthy subjects and psychiatric patients. The general assumption underpinning such research is that if there is similarity between two simultaneous voltage recordings, each corresponding to an electrode in the electrode pair, then, presumably, there is increased probability that the brain areas underlying the locations of the corresponding electrodes are involved in similar or related neurophysiological processes. This investigation approach can be extended to MEG measurements (Stam et al. 2007), as well as to quantities derived from the voltage measurements, e.g., intracranial current source densities (Cao and Slobounov 2010).
A basic parameter quantifying the relation between two signals/variables (or, more generally, two sets of measurements) is the correlation coefficient ρ (called also the Pearson product moment correlation), which is defined as the covariance of the two signals, normalized by the square root of the product of the individual variances. It ranges between −1 and +1 and measures the degree of linearity between the two signals (Le 2003). A value approaching +1 (−1) indicates “positive” (“negative”) correlation. For example, an augmentation (decrease) in one of the signals corresponds to an augmentation (decrease) in the other signal. A value approaching 0 indicates that there is no linear relation between the two signals.
A parameter closely related to the correlation coefficient is the coherence of two signals γ2 which has values between 0 and 1, and which has attracted a lot of interest in EEG studies. It is defined as the squared magnitude of the cross spectral density function of the two signals, normalized by the product of the individual auto spectral density functions. A relatively high coherence value in a given frequency range between two EEG recordings indicates a high degree of linear relation between the two recordings in that frequency range, while a low coherence value indicates a low degree of linear relation. A significant amount of work on EEG coherence in patients suffering from schizophrenia has been reported in the last decades, in an effort to locate pairs of brain regions whose coherence is different from that in patients with other psychiatric illnesses or in normal controls (Shaw et al. 1983; Morrison-Stewart et al. 1996; Tauscher et al. 1998; Wada et al. 1998; Winterer et al. 2001; Ford et al. 2002; Sritharan et al. 2005).
High values of EEG correlation coefficient or coherence may be caused by anatomical connections or functional coupling between the brain regions generating the recorded EEG signals (Thatcher et al. 1986; Gray and Singer 1989). Values approaching 0 do not imply that there is no association whatsoever between the signals, but that there is no linear association between them. It must be kept in mind that high values of correlation coefficient or coherence can occur independently of the level of the amplitude of the two signals whose correlation coefficient or coherence are computed. For example, a high value of EEG correlation coefficient or coherence may correspond to either concurrent excitation or inhibition of the two brain areas presumably producing the recorded EEG signals (Manganotti et al. 1998).
The interest of investigators has been extended to the quantification of non-linear relations that may exist between two signals. A variety of parameters have been proposed for measuring such relations, for example the mean phase coherence and the general synchronization index (Wilmer et al. 2010), the synchronization likelihood (Stam et al. 2006) and the mutual information (MI), originating in information theory (Shannon 1948; Kolmogorov 1968). MI detects linear and nonlinear statistical dependencies between two recorded signals and can be used as a measure of the level of coupling or of information transmission between these signals, quantifying the information gained about one of the signals from the measurement of the other. MI is maximized when the two signals are identical, while it is minimized to zero when the signals are statistically independent. MI has been applied to EEG signal analysis. It has been used for automatic sleep stage scoring (Gersch et al. 1977), for investigating information transmission among different cortical areas in waking and sleep states of healthy subjects (Xu et al. 1997), in epilepsy (Albano et al. 2000; Chen et al. 2000) and for classification of behavioural states (Bonita et al. 2014). Parameters based on MI have also been used in the investigation of the interdependency existing between pairs of EEG voltage waveforms, in Altzheimer’s disease patients versus normal controls (Jeong et al. 2001), in schizophrenic patients versus normal controls (Na et al. 2002) and in the study of sleep deprivation effects in healthy subjects (Na et al. 2006). The estimation of MI has been shown to be sensitive to the algorithms used (see also section “Computation of correlation coefficient and mutual information” below) (Quian Quiroga et al. 2002; Duckrow and Albano 2003). A recent comparison of four time-domain measures for quantifying correlations in scalp-recorded EEG data, including the Pearson product moment correlation, two non-parametric correlation measures and MI, has indicated that linear measures should be used in combination with non-linear measures (Bonita et al. 2014). The metric used for comparing the measures was their ability to discriminate between behavioral states. MI tended to outperform the other measures concerning the between-state discrimination, even when short-time (1 s) data segments were available. Additionally, MI discrimination was more robust to noise than the other measures.
Aim of the study
It appears that there is contradictory evidence as to the presence of intra and inter-hemispheric EEG relationships in patients suffering from schizophrenia. For example, in Na et al. (2002) high intra and inter-hemispheric EEG connectivity levels were reported, and in Merrin et al. (1989) an increased intra-hemispheric EEG coherence in the theta frequency band was found. On the other hand, in Gordon et al. (2010) a frontal inter-hemispheric asymmetry in the alpha EEG frequency band was reported, in Morrison-Stewart et al. (1996) a reduced inter-hemispheric EEG coherence in anterior brain regions was shown, and in Winterer et al. (2001) it was suggested that a decreased inter-hemispheric EEG coherence in temporal cortices may be a trait marker for schizophrenia.
Given the positive effects of PE dance therapy on psychiatric patients, including patients with schizophrenia (Margariti et al. 2012), and given the interest in utilizing brain connectivity measures for assessing patients with schizophrenia, the present pilot study presents methodology investigating the use of the correlation coefficient and MI on EEG data recorded in patients with schizophrenia while they undergo a dynamic therapeutic process, as exemplified by a PE dance therapy protocol. The presented work expands the set of neurophysiology-based approaches for quantifying possible therapeutic effects of dance therapy, using EEG-based measures that might provide insights about any potential brain connectivity changes in these patients during PE dance therapy.
Materials and methods
Subjects
The subjects that participated in the present study were undergoing PE dance therapy. The methodology of the PE dance therapy technique has been described in a previous study (Margariti et al. 2012). The present study took place in the 1st Psychiatric Clinic of the University of Athens Medical School at the Eginition Hospital, Athens, Greece. The participants were 9 inpatients suffering from schizophrenia (age range 19–51; 4 women and 5 men). The data of 5 of these participants were used also in a previous study (Margariti et al. 2012). Patients were under appropriate pharmacotherapy, mostly atypical antipsychotics. EEG recordings were obtained from the patients, but the recordings of one of the patients were not used due to artifacts. There were 12 PE dance therapy sessions, 2 times per week, for 6 weeks. The study conformed to the Helsinki declaration on human experimentation and was approved by the ethics committee of the Eginition Hospital. Written informed consent was obtained from all participants.
EEG recording procedure and EEG pre-processing
EEG recordings took place before and after the 5th and the 11th PE dance therapy sessions. An EEG recording session, in the awake resting state with eyes closed, lasting for about 3 min, took place before the 5th dance therapy session (denoted in the following as s1), after the 5th dance therapy session (s2), as well as before (s3) and after (s4) the 11th dance therapy session. In the following, by “session” it will be meant “EEG recording session”. When referring to dance therapy sessions, this will be made explicit 5 in the text. At each session, EEG was recorded with six electrodes, at F3, F4, C3, C4, O1 and O2, using as reference the right ear and as ground an electrode placed at the left ear.
Data from 8 patients were used in the analysis. For each patient and each session, a data segment as near as possible to the end of the session and lasting 32 s was selected by visual inspection for further analysis. The selection of the segment was based on the segment being as clear as possible from artifacts, in as many electrodes as possible. If there were electrode recordings with unacceptably high level of artifacts in the duration of the selected segment, those electrodes were excluded from further analysis. Artifacts that led to exclusion were amplitude excursions exceeding by at least 50 % the average amplitude of EEG waveform peaks in the rectified recording, as well as parts of EEG that possessed unequivocally EMG motion artifacts and EOG artifacts. If there did not exist a 32-s segment with more than four electrodes being sufficiently artifact-free, then a shorter segment was sought. This resulted in having 17 segments where all 6 electrodes were kept for analysis, 11 segments where one electrode was excluded and three segments where two electrodes were excluded. For one patient, for session s2, a segment lasting 20 s was selected, since longer-lasting segments did not have more than three acceptable electrodes. For that 20-s segment, all six electrodes were included in the study. For another patient, for session s3, all recordings were corrupted with unacceptably high level of artifacts and consequently the whole session was excluded from further analysis. The use of automated methods for detecting and removing artifacts was not applied in this work due to the following considerations: Such methods have the benefit of reducing the time needed for detecting artifacts and are, therefore, especially suitable when the process has to be repeated for long recordings. However, when the recordings of interest are short (a few minutes long) and include a few electrodes, as was the case in the present study, careful visual inspection by experts, with predefined criteria, might be considered an effective way to detect artifacts and safe as well for avoiding artifact misses which can occur even with automated methods (Lawhern et al. 2012). As a matter of fact, visual inspection is usually the “gold-standard” method against which automated detection methods are compared (Shao et al. 2009; Bhattacharyya et al. 2013). Finally, it is of interest to note that automatic artifact removal, in addition to removing artifacts, in many cases might distort the original signal in the time windows where no artifacts were initially present (Shao et al. 2009; Bhattacharyya et al. 2013).
The recorded data, hence referred in the following as “unfiltered”, were subsequently filtered in order to extract specific EEG rhythms. Band-pass 128th-order FIR filters were used, with low-frequency and high-frequency cut-offs at 0.4 and 3.5 Hz, respectively, for delta rhythm, at 4 and 7 Hz for theta rhythm, at 8 and 12 Hz for alpha rhythm, at 13 and 20 Hz for “low-frequency” beta rhythm (referred to in the following as “low beta” rhythm) and, finally, at 21 and 30 Hz for “high-frequency” beta rhythm (referred to in the following as “high beta” rhythm). The voltage waveform for subject i (i = 1, …, 8), for electrode j (j = F4, C4, O2, F3, C3, O1), for session sk (k = 1, …, 4) and for EEG rhythm rm (m = U, D, T, A, Bl, Bh, corresponding to the unfiltered data, and the delta, theta, alpha, low beta and high beta rhythm data, respectively) is denoted as , where are the recorded samples, for the selected time segment, per subject and session. Hence, when it is stated that a computation was done “per EEG rhythm”, it should be kept in mind that the same processing or computation was done also for the unfiltered data.
Computation of correlation coefficient and mutual information
For two stochastic variables (stochastic processes) X and Y, the zero-time lag correlation coefficient function (or correlation coefficient ρ) is defined as (Bendat and Piersol 1986):
| 1 |
where CXY is the zero-time lag covariance function of X and Y. If the two variables are exactly linearly related, then ρ = ±1. If ρ = 0, they are uncorrelated. As stated in the Introduction, the correlation coefficient can be used for quantifying signal similarity. If x(t) represents stochastic process X and y(t) stochastic process Y, and if x(t) and y(t) are identical to each other, i.e., x(t) = y(t) for every t, then ρXY = 1. If x(t) and y(t) are uncorrelated with each other, then it might be inferred that no “similarity” exists between them. When an intermediate situation exists, where x(t) and y(t) have some level of similarity without being identical, it will be 0 < |ρXY| < 1.
The mutual information (MI) between two stochastic variables X and Y is given by (Shannon 1948; Kolmogorov 1968; Fraser and Swinney 1986; Vastano and Swinney 1988):
| 2 |
where fX(x) and fY(y) are the marginal probability density functions of variables X and Y, respectively, and fXY(x,y) is their joint probability density function. MI(X,Y) can be considered as a measure of the amount of information about a random vector contained in another random vector. MI is equal to zero if the two variables are statistically independent, and greater than zero if the variables are interdependent. MI has also been termed “average amount of mutual information (AAMI)” (Mars and van Arragon 1982), or “cross-mutual information (CMI)” (Jeong et al. 2001; Na et al. 2002, 2006). MI has been proposed as an extension of the correlation coefficient for investigating the connectivity of pairs of variables/systems that presents both linear and non-linear structure (Alonso et al. 2010; Cruz et al. 2011; Bonita et al. 2014). Since brain electrophysiology, as recorded by the EEG, may involve, at least in part, non-linear processes (Pritchard et al. 1995; Jeong et al. 1998; Meyer-Lindenberg et al. 1998; Andrzejak et al. 2001), it is of interest to investigate both the correlation coefficient and MI when analysing voltage recordings of pairs of electrodes.
In contradistinction to the correlation coefficient and MI, which operate in the time domain, the coherence function operates directly in the frequency domain. This characteristic of coherence might present advantages in providing insight into the similarity of the EEG of pairs of electrodes for specific brain rhythms (Gardner 1992; Nunez et al. 1997). Since the present work is a pilot study on the application of similarity measures in EEG signals extracted from schizophrenic patients undergoing dance therapy, we decided to use only the correlation coefficient and MI, and not coherence, in order to reduce the complexity of the analysis by using two measures that both operate in the time domain. However, since the focus of interest is on the relationships existing per pair of electrodes and per EEG rhythm, in both the case of correlation coefficient and of MI, the signal was first band-pass filtered in the bands of the specific EEG rhythms, as explained in the previous section, and then the correlation coefficient and MI were computed per rhythm.
In real-life measurements, the realization of a stochastic variable implies the existence of a finite number of values that the variable takes. Therefore, assuming that the realizations of X and Y are represented by the finite sets {xi}, i = 1,…,Mx and {yj}, j = 1,…, My, respectively, the elements xi and yj have a probability of occurrence pX(xi) = Prob(X = xi) ≥ 0 and pY(yj) = Prob(Y = yj) ≥ 0, respectively, and the joint probability of occurrence is given by pXY(xi,yj) = Prob(X = xi, Y = yj) ≥ 0. Then MI is defined as:
| 3 |
The estimation of MI in Eq. (3) is based on the estimation of the marginal and joint probability density functions (pdf). Following the histogram-based estimation of pdf (Moddemeijer 1989), assume that the range spanned by {xi} is divided into si, i = 1,…,SI, segments of length Δsi, and the range spanned by {yi} is divided into sj, j = 1,…,SJ, segments, of length Δsj. If the “cell” of size ΔsiΔsj that is produced in the (x,y) plane by any two segments si and sj includes data points (observed joint events), then pXY(x, y) is estimated as having a uniform value across the cell and the histogram-based estimator of MI is given by
| 4 |
where N is the data sample size of the observed joint events, and and are the marginal “counts”. Various approaches have been proposed concerning the topic of segment size and whether it might vary within the range spanned by the variables (Huseman 1986; Fraser and Swinney 1986; Steuer et al. 2002; Cellucci et al. 2005; Fernandes and Gloor 2010). Based on the extensive comparative evaluation of non-equidistant and equidistant segmentation schemes presented by Huang (2001), and taking into account that there were always more than 4,096 samples available (Huang 2001), an equidistant segmentation scheme proposed by van Bergen (1986) was used in this study.
The values of were computed using Eq. (1), with and , per session sk, per EEG rhythm rm and per subject i, for every pair j, j′ (j ≠ j′) of electrodes, for which artifact-free voltage data existed for both electrodes of the pair. For further analysis, only ρ values for which the significance value (p value) was p ≤ 0.05 were kept. p values were computed during testing the hypothesis of no correlation. Each p value was the probability of getting a correlation as large as the calculated value of ρ by random chance, when the true correlation between the time-series was zero. Since one of the aims of the present study was the comparison of several ρ and MI values, and since MI is never negative, the squared values (ρ2) of the significant ρ values were used in subsequent analysis, instead of the ρ values. ρ2 represents the proportion of the variability of Y, accounted for by X (Le 2003). This approach also leads to a unified handling of both “positive” and “negative” correlations, as expressed by positive and negative values of ρ, respectively, in contrast to the non-existence of any correlated activity, and, presumably, of no underlying connectivity between the brain regions under the respective electrode pair, as expressed by ρ values tending to 0.
The values of were computed using Eq. (4) per session sk, per EEG rhythm rm and per subject i, for every pair j,j′ (j ≠ j′) of electrodes, for which artifact-free voltage data existed for both electrodes of the pair. In Huang (2001) it was shown that when ρ ≤ 0.1, for a subject i and a pair j, j′ of electrodes, then the bias error that is present in MIest(X,Y) is not negligible. MIest values for such cases were excluded from further analysis, in a conservative approach to the use of MIest values in the present study.
Statistical analysis
Statistical analysis was done, per EEG rhythm rm, in order to find whether there were significant differences among the sets of values, for k = 1,…,4. The 4 sets did not have independent values, since, for each pair of electrodes j, j′, there existed up to four values of that corresponded to the same subject i. Therefore, a repeated-measures approach had to be followed. The same analysis was also implemented for the values, for k = 1, …, 4. The distribution of each set, for both the ρ2 and the MI values, was checked for normality using the Kolmogorov–Smirnov test and, additionally, by inspecting the distribution histogram of the set. Both methods indicated clearly that the distributions were non-normal, so the Friedman non-parametric test was used. When the Friedman test indicated significant differentiation among the four sets, then post hoc tests were performed, in order to investigate the existence of significant differences between pairs of sessions, using the Wilcoxon signed-rank test, with Bonferroni multiple measures adjustment. The analysis was performed for the whole set of electrode pairs, as well as for three sub-sets of the electrode pairs: the 1st sub-set included the left hemisphere electrode pairs (F3–C3, F3–O1, C3–O1), the 2nd the right hemisphere electrode pairs (F4–C4, F4–O2, C4–O2) and the 3rd all other electrode pairs, i.e., those related to inter-hemispheric connectivity.
The time separation between EEG recording sessions s2 and s1 and between EEG recording sessions s4 and s3 was much smaller compared to the time separation between s3 and s1 and between s4 and s2. Therefore, it could be tentatively conjectured that the difference values
might reflect an “acute” effect on brain connectivity occurring at the (“middle-stage”) 5th dance therapy session, and that the difference values
might reflect an “acute” effect on brain connectivity occurring at the (“late-stage”) 11th dance therapy session. For example, if a specific value of was positive, this might indicate that for that specific subject, electrode pair and EEG rhythm, the brain connectivity, as far as it might be related to correlation coefficient values, increased “just” after the completion of the 5th dance therapy session, compared to the situation “just” before the 5th dance therapy session. In accordance with what was stated above, concerning the use of ρ2 values, a positive value of might indicate (1) an increase in positively correlated activity, (2) an increase in negatively correlated activity, (3) a transition from a low positively correlated activity to a higher (in absolute terms) negatively correlated activity, or (4) a transition from a low (in absolute terms) negatively correlated activity to a higher positively correlated activity. At this point it should be stressed that what caused these changes could not be ascertained in the framework of the present study (see “Discussion”).
Furthermore, it could be tentatively conjectured that the difference values
might reflect a “long-term” effect on brain connectivity, that took place between just before the 5th and just before the 11th dance therapy sessions, while the difference values
might reflect (1) a “long-term” effect on brain connectivity, that took place between just after the 5th and just before the 11th dance therapy sessions, and (2) the “acute” effect on brain connectivity as a result of the (“late-stage”) 11th dance therapy session. Again, as was the case for the “acute” changes, the cause of these “long-term” changes could not be ascertained in the framework of the present study (see “Discussion”). Comparable statements can be made for the corresponding MI difference values , , and .
Statistical analysis was done, per EEG rhythm rm, in order to find whether there were significant differences among the sets of , , and values. For brevity, these sets will be denoted in the following as sets , , and , respectively. The distribution of each set was checked for normality using the Kolmogorov–Smirnov test and by inspecting the distribution histogram of the set. When one or both methods indicated that at least one of the 4 sets had a non-normal distribution, the Friedman test was used. When the Friedman test indicated a significant differentiation among the 4 sets, then post-hoc Wilcoxon signed-rank tests with Bonferroni multiple measures adjustment were performed, in order to investigate the existence of significant differences among “session pairs”, i.e., {2,1}, {4,3}, {3,1} and {4,2}. The analysis was performed for the whole set of electrode pairs but not for the 3 sub-sets described above. There were cases when the distribution of all 4 sets was normal, i.e., the Kolomogorov-Smirnov test did not reach a significant value and the visual inspection of the histograms did not indicate non-normality. In such cases, a repeated-measures ANOVA test was applied, with one within-subjects factor, the “session pair”, i.e., {2,1}, {4,3}, {3,1} and {4,2}. When the repeated-measures ANOVA test indicated significant differentiation among the 4 sets, then post-hoc tests with Bonferroni multiple measures adjustment were performed, in order to investigate the existence of significant differences between “session pairs”. The same analysis was repeated for the sets of , , and values, denoted in the following as sets , , and , respectively.
Preliminary inspection of difference values showed that the pooling of both negative and positive values in each set resulted in a “cancellation” effect, whereby the absolute mean value of the samples of each set was much smaller than both the mean value of the sub-set composed of samples with positive values and the absolute mean value of the sub-set composed of samples with negative values. This, in turn, resulted in the fact that, even when statistically significant differences could emerge between two sets, the extraction of conclusions would be meaningless concerning the difference values present in the sets. To give a typical example, post hoc comparisons showed a significant differentiation between set (which had a mean value of −0.084) and set (which had a mean value of 0.020) and between set (which had a mean value of −0.096) and set (which had a mean value of 0.009), while the mean value of the sub-set of composed of samples with positive values was 0.215 and the absolute mean value of the sub-set composed of samples with negative values was 0.308. In order to overcome this, we also analyzed, per EEG rhythm rm, the absolute difference values , , and , as well as the absolute difference values , , and . A Kolmogorov–Smirnov test and inspection of the distribution histogram of each absolute difference values set, for both squared correlation coefficient (denoted in the following as sets , , and ) and MI (denoted in the following as sets , , and ), indicated that the distributions were non-normal, so the Friedman test was used. When the test indicated significant differentiation among the 4 “session pair” sets, then post hoc Wilcoxon signed-rank tests, as described above, were performed, in order to investigate the existence of significant differences between “session pair” sets. The analysis was performed for the whole set of electrode pairs. It should be kept in mind that by using the absolute difference values, i.e., we ignore the information concerning the sign of i.e., if it was or . Instead, we get indications only on the absolute value “level” or “strength” of the mechanisms modifying brain connectivity, as reflected by higher or lower values of . For example, if the set is significantly different from the set and it happens that the mean value of the set is higher than the mean value of the set , this might indicate that the acute effect, for the alpha rhythm, in the 11th dance therapy session is “stronger” than the acute effect in the 5th dance therapy session, but we cannot have knowledge on whether the acute effect of each session corresponded to a reduction or an increase of ρ2 values. The same remarks apply also for MI.
An additional path of analysis was also pursued, concerning the differences of ρ2 values, per electrode pair and rhythm, between sessions s2 and s1, s4 and s3, s3 and s1 and s4 and s2. For example, concerning the set of differences of ρ2 values , for each electrode pair j,j′(j ≠ j′), per EEG rhythm rm, the sign (positive or negative) of was noted, for each subject i. Since the minimum numerical computation resolution that was kept in the present study, for both ρ2 and MI values, was 0.01, only values | were kept, in order to exclude from the analysis values that were in the range of the minimum resolution. If, for a specific electrode pair, taking into account all subjects, there was a majority of positive values of , then it was concluded that a “majority indication” (mI) existed for , i.e. a “majority indication” (mI) existed for an increase of compared to . Inversely, if, for the same electrode pair, taking into account all subjects, there was a majority of negative values of , then it was concluded that a “majority indication” (mI) existed for i.e. a “majorsity indication” (mI) existed for a decrease of compared to . The same analysis was performed for the 3 other session pairs, i.e., for , and values, as well as for , , and values. The existence of mI was investigated only for those electrode pairs for which ρ2 or MI difference values were available for analysis for all the 8 subjects, for session pairs {2,1} and {4,2}, or for 7 subjects, for session pairs {3,1} and {4,3}. It is reminded that for one patient, EEG recording session s3 was excluded from analysis due to extensive artifacts, so for session pairs {3,1} and {4,3} at most 7 subjects possessed data available for analysis. It should be stressed that the reason for investigating mI was to detect trends of either strengthening or weakening of the “coupling” between the voltage waveforms at pairs of electrodes, in the transition from a session to another. On the other hand, the existence of a mI did not give any indication on the specific (i.e., per subject) values of those differences and, consequently, on the degree of the strengthening or weakening of the “coupling” between the voltage waveforms at pairs of electrodes, in the transition from a session to another.
Summing up the methodological approach proposed in the present study, its novelty concerns (1) the investigation of statistically significant differentiation among the sets of difference values , , and , as well as among the sets , , and , (2) the investigation of statistically significant differentiation among the sets of the absolute difference values , , and , as well as among the sets , , and , and (3) the introduction of the “majority indication” (mI) parameter. Steps (1), (2) and (3) are used in addition to the conventional investigation of the existence of significant differences among the sets of values, and among the sets of values, for k = 1,…,4 (the four EEG recording sessions). To the knowledge of the authors, this additional 3-step approach has not been used in the literature concerning the application of ρ and MI to the study of brain connectivity using EEG measures. The motivation for proposing this methodology is the presence of four sets of data (EEG recordings) that are temporally defined. As explained above, there are two pairs of sets, each pair corresponding to one PE DT session and containing one pre-DT and one post-DT EEG recording set. The pre-DT and post-DT sets of each pair were recorded within a short time distance (approximately an hour), before and after the respective PE DT session. The two dance sessions were separated by a much longer period, approximately two and one half weeks. Therefore, the proposed approach might help in clearly differentiating between “acute” and “long-term” changes of brain connectivity. Specifically step (3) enabled the detection of overall trends in either strengthening or weakening of the “coupling” between the voltage waveforms at pairs of electrodes, in the transition from a recording set to another, even when such trends were not indicated by conventional statistical evaluation.
Results
Statistical evaluation showed that there existed significant differentiation among the four sets of values (one set of values for each session) of ρ2 for the delta (x2(3) = 13.116, p = 0.004) and low beta (x2(3) = 15.979, p = 0.001) EEG rhythms, when all possible electrode pair combinations were included in the test. Post-hoc tests indicated that there existed a statistically significant reduction in the ρ2 values from s1 (mean () = 0.38, standard deviation (SD) = 0.29) to s2 ( = 0.31, SD = 0.27) (p = 0.001) for the delta rhythm, and a statistically significant augmentation in the ρ2 values from s2 ( = 0.23, SD = 0.24) to s4 ( = 0.29, SD = 0.23) (p = 0.006) and from s3 ( = 0.19, SD = 0.22) to s4 (p = 0.001) for the low beta rhythm. When only the electrode pairs related to inter-hemispheric connectivity were included in the analysis, again there existed significant differentiation among the 4 sets of ρ2 values for the delta (x2(3) = 9.8, p = 0.02) and low beta (x2(3) = 9.205, p = 0.027) rhythms. Post-hoc tests indicated that there existed a statistically significant augmentation in the ρ2 values from s3 ( = 0.20, SD = 0.25) to s4 ( = 0.26, SD = 0.22) (p = 0.004) for the low beta rhythm. When only the left hemisphere electrode pairs (F3–C3, F3–O1, C3–O1) were included in the analysis, there existed significant differentiation among the four sets of ρ2 values for the alpha (x2(3) = 14.294, p = 0.003), low beta (x2(3) = 10.781, p = 0.013) and high beta (x2(3) = 11.582, p = 0.009) rhythms, but this finding was not accompanied by significant post hoc differentiations between specific sets.
The statistical evaluation results for the four sets of MI values were similar to those for the four sets of ρ2 values, concerning the existence of significant differentiation among the four sets, when the analysis was performed for the whole set of electrode pairs, as well as for the set of electrodes pairs related to inter-hemispheric connectivity. For the analysis for the whole set of electrode pairs, the similarity of ρ2 and MI values was evident also at the level of post hoc comparisons. There existed significant differentiation among the four sets of MI values for the delta (x2(3) = 13.5, p = 0.004) and low beta (x2(3) = 11.011, p = 0.012) rhythms, when all electrode pairs were included in the test. Post-hoc tests indicated that there existed a statistically significant reduction in the MI values from s1 ( = 0.61, SD = 0.47) to s2 ( = 0.47, SD = 0.39) (p < 0.001) for the delta rhythm, and a statistically significant augmentation in the MI values from s3 ( = 0.26, SD = 0.26) to s4 ( = 0.32, SD = 0.29) (p = 0.01) for the low beta rhythm. When only the electrode pairs related to inter-hemispheric connectivity were included in the analysis, again there existed significant differentiation among the four sets of MI values for the delta (x2(3) = 10.035, p = 0.018) and low beta (x2(3) = 10.864, p = 0.012) rhythms. Post-hoc tests indicated that there existed a statistically significant reduction in the MI values from s1 ( = 0.62, SD = 0.50) to s2 ( = 0.48, SD = 0.42) (p = 0.003) for the delta rhythm. When only the left hemisphere electrode pairs (F3–C3, F3–O1, C3–O1) were included in the analysis, there existed significant differentiation among the four sets of MI values only for the alpha rhythm (x2(3) = 7.851, p = 0.049), but this finding was not accompanied by significant post hoc differentiations between specific sets.
Concerning the absolute difference of squared correlation coefficient value sets (e.g., , , and ), the mean value of the sets and was less than the mean value of the sets and , for the unfiltered data and for all EEG rhythms. Statistical evaluation showed that there existed significant differentiation among the four sets for every EEG rhythm, as well as for the unfiltered EEG data. Specifically, for unfiltered data, delta, theta, alpha, low beta and high beta rhythms, the Friedman test results were x2(3) = 43.09 (p < 0.001), x2(3) = 26.66 (p < 0.001), x2(3) = 15.324 (p = 0.002), x2(3) = 37.037 (p < 0.001), x2(3) = 54.760 (p < 0.001) and x2(3) = 42.443 (p < 0.001), respectively. Post-hoc tests showed that for the unfiltered data and for all EEG rhythms, except for the delta rhythm, there existed statistically significant differentiation between sets and , and , and , and . For the delta rhythm there existed statistically significant differentiation between sets and , and , and . The mean value and standard deviation of each set, per rhythm, is given in Table 1, and the p value, for every pair-wise comparison that reached statistical significance, is given in Table 2.
Table 1.
Mean value (standard deviation) of the sets of absolute difference of squared correlation coefficient values , , and , for the unfiltered data and EEG rhythms
| Unfiltered data | EEG rhythm | |||||
|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Low beta | High beta | ||
| 0.0913 (0.0915) | 0.1559 (0.1231) | 0.09 (0.1) | 0.0651 (0.1002) | 0.06 (0.088) | 0.07 (0.087) | |
| 0.0927 (0.0986) | 0.1152 (0.1301) | 0.0938 (0.0906) | 0.1061 (0.1287) | 0.0864 (0.1122) | 0.084 (0.0802) | |
| 0.2234 (0.2104) | 0.264 (0.2251) | 0.23 (0.226) | 0.20 (0.215) | 0.1928 (0.1966) | 0.1872 (0.1751) | |
| 0.2088 (0.1820) | 0.2614 (0.1993) | 0.2075 (0.2068) | 0.1963 (0.1948) | 0.1901 (0.1817) | 0.1624 (0.14) | |
The four sets corresponded to the difference between sessions s2 and s1, s4 and s3, s3 and s1, s4 and s2
Table 2.
p value of pair-wise comparisons between the sets of absolute difference of squared correlation coefficient values that reached statistical significance
| Pair-wise comparison | Unfiltered data | EEG rhythm | ||||
|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Low beta | High beta | ||
| and | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 |
| and | <0.001 | – | 0.001 | <0.001 | <0.001 | <0.001 |
| and | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| and | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Concerning the absolute difference of MI value sets, (e.g., , , and ) the mean value of the sets and was less than the mean value of the sets and , for the unfiltered data and for all EEG rhythms. Statistical evaluation results coincided almost exactly with those concerning the corresponding sets for the absolute squared correlation coefficient difference values. There existed significant differentiation among the 4 sets for every EEG rhythm, as well as for the unfiltered EEG data. Specifically, for unfiltered data, delta, theta, alpha, low beta and high beta rhythms, the Friedman test results gave (x2(3) = 37.355, p < 0.001), (x2(3) = 17.937, p < 0.001), (x2(3) = 17.156, p = 0.001), (x2(3) = 23.257, p < 0.001), (x2(3) = 38.633, p < 0.001), and (x2(3) = 31.723, p < 0.001), respectively. Post-hoc tests showed that for the unfiltered data and for all rhythms, except for the delta rhythm, there existed statistically significant differentiation between sets and , and , and , and . For the delta rhythm there existed statistically significant differentiation between sets and , and , and . The mean value and standard deviation of each set, per rhythm, is given in Table 3, and the p-value, for every pair-wise comparison that reached statistical significance, is given in Table 4.
Table 3.
Mean value (standard deviation) of the sets of absolute difference of MI values , , and , for the unfiltered data and EEG rhythms
| Unfiltered data | EEG rhythm | |||||
|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Low beta | High beta | ||
| 0.1353 (0.1375) | 0.2447 (0.1768) | 0.1446 (0.1374) | 0.1085 (0.1372) | 0.0750 (0.0938) | 0.0927 (0.1125) | |
| 0.1270 (0.1509) | 0.1584 (0.1898) | 0.1250 (0.1388) | 0.1225 (0.1502) | 0.0926 (0.1176) | 0.0901 (0.0968) | |
| 0.3137 (0.2954) | 0.371 (0.3415) | 0.315 (0.3076) | 0.2709 (0.2649) | 0.228 (0.2238) | 0.2712 (0.3371) | |
| 0.26 (0.2466) | 0.3443 (0.3002) | 0.2606 (0.2826) | 0.2597 (0.2711) | 0.2073 (0.2285) | 0.1850 (0.1893) | |
The four sets corresponded to the difference between sessions s2 and s1, s4 and s3, s3 and s1, s4 and s2
Table 4.
p value of pair-wise comparisons between the sets of absolute difference of MI values that reached statistical significance
| Pair-wise comparison | Unfiltered data | EEG rhythm | ||||
|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Low beta | High beta | ||
| and | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 |
| and | 0.001 | – | 0.007 | <0.001 | <0.001 | 0.002 |
| and | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| and | <0.001 | <0.001 | 0.002 | 0.001 | <0.001 | <0.001 |
Figure 1a presents, in a pictorial form, the “majority indications” (mI) existing for , corresponding to either an increase (solid line) or a decrease (dashed line), respectively, of values compared to values. Figure 1b–d presents the mI existing for , and , respectively. Figure 2a–d presents the mI existing for , , and , respectively. By inspecting Fig. 1 and focusing on electrode pairs with electrodes from opposite hemispheres, it is observed that there exists a majority of positive values for , for m = D, T, A, Bl and Bh. In other words, there exists a mI for , for m = D, T, A, Bl and Bh, i.e., there exists a mI for the increase of ρ2 values in the frontal electrode pair F4–F3, for all rhythms, in the transition from session s3 to s4. mI for the increase of ρ2 values for the pair F4–F3 exist also for the transitions from session s1 to s3 and from session s2 to s4, for the low and high beta rhythms. By inspecting Fig. 2, it is observed that for MI values there exists a mI for , for m = A, Bl and Bh. Concerning other inter-hemispheric electrode pairs with common trends of mI across sessions and/or rhythms, it is observed in Fig. 1 that there exist a mI for < , for m = D, T, A, Bl, Bh and U, i.e., there is a mI for the decrease of ρ2 values for the electrode pair C4–F3, for all rhythms, as well as for the unfiltered data, in the transition from session s1 to s3. By inspecting Fig. 2, it is observed that for MI values there exists exactly the same mI pattern as for ρ2 values.
Fig. 1.
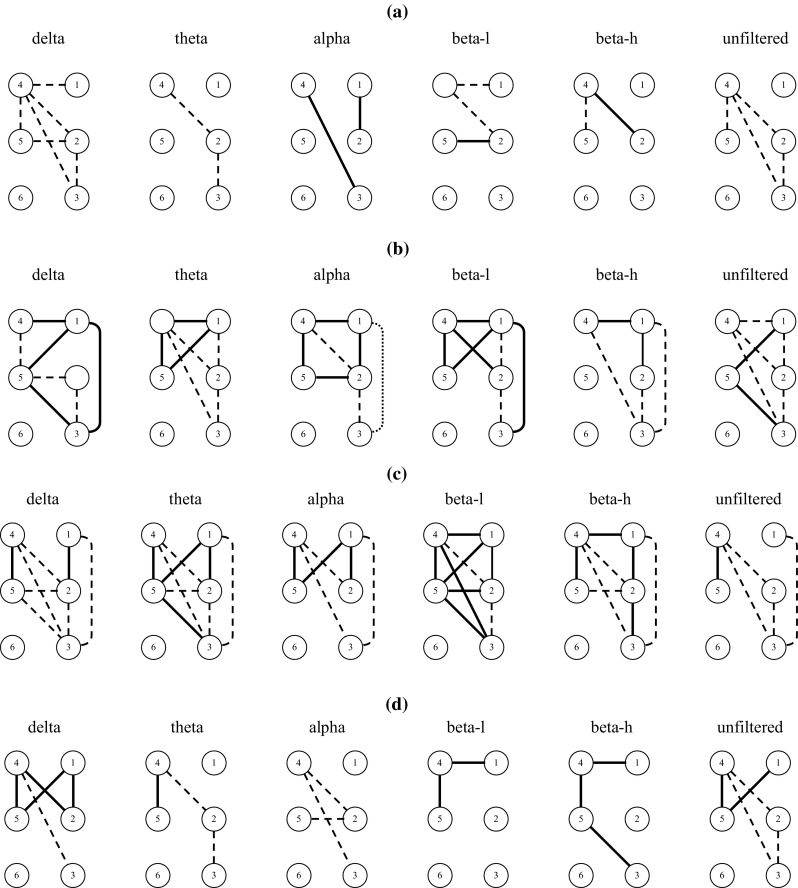
“Majority indications” (mI) existing for a positive (solid line) or a negative (dashed line) value, respectively, of (a), (b), (c) and (d), for delta, theta, alpha, low-frequency beta (beta-l), high-frequency beta (beta-h) rhythms and for the unfiltered data. Numbers 1, 2, 3, 4, 5, 6 denote electrodes F4, C4, O2, F3, C3, O1, respectively
Fig. 2.
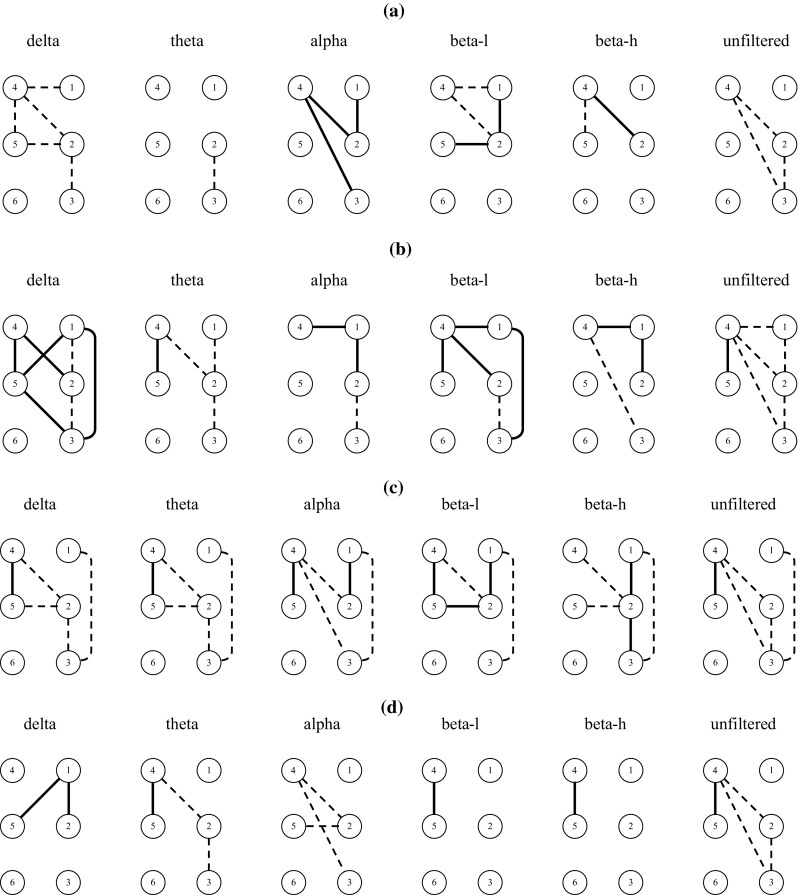
“Majority indications” (mI) existing for a positive (solid line) or a negative (dashed line) value, respectively, of (a), (b), (c) and (d), for delta, theta, alpha, low-frequency beta (beta-l), high-frequency beta (beta-h) rhythms and for the unfiltered data. Numbers 1, 2, 3, 4, 5, 6 denote electrodes F4, C4, O2, F3, C3, O1, respectively
Next, by inspecting Fig. 1, and focusing on electrode pairs with electrodes belonging to the same hemisphere, it is observed that there exist mI for > and for > , for m = D, T, A, Bl and Bh, i.e., there exists a mI for the increase of ρ2 values for the fronto-central electrode pairs, bilaterally, for all rhythms, in the transition from session s1 to s3. This increase of ρ2 values for the fronto-central electrode pairs presents itself bilaterally for all rhythms only in this transition. A bilateral increase of ρ2 values for the fronto-central electrode pairs is also observed for the alpha rhythm in the transition from session s3 to s4. It is also observed for the delta rhythm in the transition from session s2 to s4. The mI for MI values, for electrode pairs with electrodes belonging to the same hemisphere, tend to follow the patterns that are present for ρ2 values, with some exceptions. Accordingly, mI for the increase of MI values, in the transition from session s1 to s3, are present in the left-hemisphere fronto-central electrode pair (F3–C3) for the delta, theta, alpha and low beta rhythms, while for the right-hemisphere pair (F4–C4) the same exists for the alpha, low and high beta rhythms. Therefore, the bilateral presence of mI for the increase of MI values in fronto-central electrode pairs exists only for alpha and low beta rhythms and only in the transition from session s1 to s3.
As far as trends in mI indicating a decrease of ρ2 values for electrode pairs in electrodes belonging to the same hemisphere, Fig. 1 shows that there exist mI for < , for < and for < , for m = D, T and U, i.e., there exist mI for the decrease of ρ2 values for the electrode pair C4–O2, for the delta and theta rhythms, as well as for the unfiltered data, in the transitions from session s1 to s2, from session s3 to s4 and from session s1 to s3. Especially in the transition from session s3 to s4, the mI for a decrease in ρ2 values for the electrode pair C4–O2 are extended to all rhythms. In the transition from session s2 to s4, there exist a mI for < and for < . By inspecting Fig. 2, it is observed that for the MI values there exist exactly the same mI pattern as for the ρ2 values, presented above, with the exception that there are no mI for . Additionally, Fig. 1 shows that there exist mI for < , for m = D, T, A, Bh and U, i.e., there exist mI for the decrease of ρ2 values for the electrode pair F4–O2, for the delta, theta, alpha and high beta rhythms, as well as for the unfiltered data, in the transition from session s1 to s3. By inspecting Fig. 2, it is observed that for the MI values there exist exactly the same mI pattern as for the ρ2 values, with the addition (and this is a rare case when the trend for mI for the MI values is “richer” than that for the ρ2 values) that there are mI for the decrease of values as compared to values. Therefore, there exist mI for the decrease of MI values for the electrode pair F4–O2, for all EEG rhythms, as well as for the unfiltered data, in the transition from session s1 to s3.
Summarizing the above results concerning mI, there was mI for the increase of ρ2 values for all individual EEG rhythms, and for the increase of MI values for the alpha and beta rhythms, in the frontal electrode pair F4–F3 in the transition from session s3 to s4. There was also mI for the decrease of ρ2 and MI values at the electrode pair C4–F3, for all EEG rhythms, for the transition from session s1 to s3. For electrode pairs with electrodes belonging to the same hemisphere, there was mI for the increase of ρ2 values between frontal and central electrodes bilaterally, for all individual EEG rhythms, for the transition from session s1 to s3. There was also mI for the decrease of both ρ2 and MI values at the right fronto-occipital electrode pair, for all rhythms except beta low for ρ2, in the transition from session s1 to s3. Additionally, there was mI for the decrease of ρ2 and MI values at the right centro-occipital electrode pair, for all EEG rhythms, except high beta for MI, in the transition from session s3 to s4.
Discussion
This is a pilot study presenting methodology for the investigation of the use of the correlation coefficient and MI on EEG data recorded in patients with schizophrenia while they undergo a dynamic therapeutic process, as exemplified by a PE dance therapy protocol. The present work expands the set of neurophysiology-based approaches for quantifying possible therapeutic effects of dance therapy, using EEG-based measures that might provide insights about any potential brain connectivity changes in these patients during PE dance therapy. The presented results are intriguing and point to the possibility that such a therapy might involve brain connectivity changes with a potential benefit to the patients (see below). However, the framework of this study does not allow the elucidation of the specific causes for the observed changes (see below). Nevertheless, the presented work provides an important methodological background for future studies which could provide such elucidation.
It has been suggested that the selection of the methodology for computing MI might influence the computational results and this has been an active topic of research (Fernandes and Gloor 2010). The results for ρ2 and MI values in this study, for all the various measures used, i.e., the values themselves, the absolute differences of the values and the mI, were similar. Differentiation among the four sets of ρ2 values (one set per each session), for the sets including all electrode pairs and for the sets including only the electrode pairs related to inter-hemispheric connectivity, reached statistical significance for the same rhythms (alpha and low beta) for which statistical significance was reached for the differentiation among the corresponding four sets of MI values. The results for the existence of statistical significance of differentiation among the sets , , and were identical to the results for sets , , and , up to the level of post-hoc pair-wise tests. The similarity in statistical trends between most of the results of MI and those of ρ2 might indirectly give assurance that the methodology used in the present study for computing MI is not producing spurious results.
Concerning similarity of mI results for MI and ρ2, the mI for the differences , Q-W ∈ {(2-1), (4-3), (3-1), (4-2)}, were more than those for . A vast majority of mI for had a corresponding mI for . As stated in Methodology, ρ2 is a measure of the degree of linearity between two variables or systems, while MI has been proposed as an extension of the correlation coefficient for investigating pairs of variables or systems that may present both linear and non-linear connectivity, resulting in both linear and nonlinear dependence between time series emanating from those variables/systems. Therefore, one might expect that the set of electrode pairs jj′ (j ≠ j′) which possesses mI for would be a sub-set of the set of electrode pairs which possesses mI for , which is contrary to what was observed in the present study. The reason for this apparent discrepancy might be the fact that we investigated the existence of mI only for those electrode pairs for which ρ2 or MI difference values were available for analysis for all the eight subjects, for session pairs {2,1} and {4,2}, or for 7 subjects, for session pairs {3,1} and {4,3}. Therefore, the bigger number of mI for might be the result of the existence of more pairs of electrodes that had non-significant MI values and significant ρ2 values, than pairs of electrodes with the inverse situation, i.e., significant MI values and non-significant ρ2 values.
Concerning the sets , , , , the mean values of the sets and were significantly less than the mean value of the set , for the unfiltered data and for all specific EEG rhythms. In Methodology, it was suggested that might reflect an “acute” effect on brain connectivity occurring at the 5th dance therapy session, that might reflect an “acute” effect on brain connectivity occurring at the 11th dance therapy session and that might reflect a “long-term” effect on brain connectivity, that took place between just before the 5th and just before the 11th dance therapy sessions. The absolute difference values, i.e., , sG-H ∊ {s2-1, s4-2, s3-1, s4-2}, provide indications only on the absolute value “level” or “strength” of the mechanisms modifying brain connectivity, as reflected by higher or lower values of . With the above in mind, the finding that the mean values of the sets and were significantly less than the mean value of the set might indicate that the “acute” effects on brain connectivity at the 5th dance therapy session and at the 11th dance therapy session were weaker than the “long-term” effects on brain connectivity that took place between just before the 5th and just before the 11th dance therapy sessions. The same held also for the sets , , and .
Concerning mI for the increase or decrease of ρ2 values, for electrode pairs with electrodes in opposite hemispheres, the mI for the increase of ρ2 values for the frontal electrode pair F4–F3 that existed in all EEG rhythms in the transition from session s3 to s4 might indicate an “acute” effect, specific for the 11th dance therapy session. For the mI for the increase of MI values, there is a comparable result for alpha, low beta and high beta EEG rhythms. The above findings might provide an indication for an acute potentiation effect occurring at the “late-stage” 11th dance therapy session on the inter-hemispheric connectivity in frontal areas, influencing all EEG rhythms. Concerning the other trend that was detected for inter-hemispheric electrode pairs, i.e., that there was a mI for the decrease of ρ2 and MI values for the electrode pair C4–F3 for all EEG rhythms in the transition from session s1 to s3, it might be conjectured that there was an attenuation of the inter-hemispheric connectivity of left frontal to right central areas, influencing all EEG rhythms, taking place between the 5th and 11th dance therapy sessions.
Concerning intra-hemispheric connectivity, the mI for the increase in ρ2 values between frontal and central electrodes bilaterally, for all rhythms, existed only for the transition from session s1 to s3, probably suggesting a “long-term” potentiation effect on the intra-hemispheric connectivity of frontal and central areas, bilaterally, influencing all EEG rhythms, that took place between the 5th and 11th dance therapy sessions. On the other hand, the mI for the decrease of ρ2 and MI values that existed in the right centro-occipital electrode pair for the unfiltered data and all EEG rhythms, except high beta for MI, in the transition from session s3 to s4, might suggest an “acute” attenuation effect on the intra-hemispheric connectivity of right central and occipital regions, occurring at the “late-stage” 11th dance therapy session. Nevertheless, since for the delta and theta rhythms and the unfiltered data this trend is not restricted to the transition from s3 to s4, the specificity of this “acute” late-stage effect is reduced. Finally, the mI for the decrease of both ρ2 and MI values that existed in the right fronto-occipital electrode pair for the unfiltered data and all EEG rhythms except low beta for ρ2, in the transition from session s1 to s3, might suggest a “long-term” attenuation effect on the intra-hemispheric connectivity of right frontal to occipital areas that took place between the 5th and 11th dance therapy sessions. A note of caution concerning the above conclusions involving right-hemisphere occipital region connectivity is that, due to the fact that the recording from electrode O1 was often corrupted by noise and not included in the processing, results concerning mI for electrode pairs including occipital leads might have been partly biased in favor of detecting trends in the right instead of the left hemisphere.
Although no previous studies exist, to the knowledge of the authors, investigating the possible effects of dance therapy on brain connectivity of psychotic patients by using electrophysiological parameters, it is interesting to examine the results of the present study in the context of previous studies investigating the use of brain connectivity measures, such as coherence or MI, extracted from the EEG of schizophrenic patients. It should be noted that the focus of our study was on detecting “acute” or “long-term” effects, as attested by the comparative evaluation of ρ2 and MI values among the different EEG recording sessions, and that, as mentioned in Methodology, the reasons causing these effects cannot be ascertained in the framework of the present study (see below). Suppose that these effects indicate, for example, an attenuation (potentiation) of the connectivity between two brain regions, as attested by the existence of mI for the decrease (increase) of ρ2 and/or MI values between two EEG recording sessions. If previous studies, using coherence or MI, had indicated that the connectivity of the same two brain regions is stronger (weaker) in schizophrenic patients versus normal controls, then it might be conjectured that an attenuation (potentiation) effect might be an indication of a positive influence, implying an improvement in patient state. Although such positive influences cannot be exclusively attributed to PE dance therapy in the framework of the present study, they would be in accordance to the results of the authors in Margariti et al. (2012) and Margariti and Ventouras (2012), who showed positive observable changes in the psychological state and behavior of the patients (increased level of happiness and word associations commensurate with the PE therapeutic process).
The investigators in Na et al. (2002), using A-CMI, which is a parameter related to but not identical to the MI parameter used in the present study, found an increase of inter-hemispheric A-CMI in schizophrenic patients versus normal controls, between left anterior and right posterior (“posterior” in that study included central region electrodes), between right anterior and left posterior, between left and right posterior regions, as well as between right frontal and right occipital regions, among others. In that study, the recorded EEG was not filtered for extracting individual rhythm information, so comparisons should be limited only to results of the present study concerning the unfiltered data. As stated before, our results showed a “long-term” attenuation effect on the inter-hemispheric connectivity of left frontal and right central areas, influencing all EEG rhythms and unfiltered data, taking place between the 5th and 11th dance therapy sessions. Concerning intra-hemispheric connectivity, the results of the present study suggest a “long-term” attenuation effect on the connectivity of right frontal and right occipital areas, taking place between the 5th and 11th dance therapy sessions. Our results indicate positive influences, since in Na et al. (2002) an increase in connectivity between left anterior and right posterior as well as between right frontal and right occipital areas was found in schizophrenic patients. Additionally, concerning the theta rhythm, the existence of mI for a decrease of ρ2 and MI values in electrode pair C4–O2 for the transition from session s1 to s2, session s3 to s4 and session s1 to s3, and in electrode pair F4–O2 for the transition from session s1 to s3, seems to indicate positive influences, since in Merrin et al. (1989) it was found that intra-hemispheric theta coherence was increased in schizophrenic patients versus normal controls.
A number of studies have indicated a decrease of inter-hemispheric coherence in schizophrenic patients versus normal controls. In Shaw et al. (1983) it was shown that, during a visual imagery task, inter-hemispheric coherence increases in healthy right-handers and neurotic patients, and decreases in healthy left-handers and schizophrenic patients. In Morrison-Stewart et al. (1996), during the performance of a frontal activation task, normal subjects showed an increased inter-hemispheric coherence between anterior brain regions compared to schizophrenic patients. In Winterer et al. (2001) it was suggested that a decreased inter-hemispheric coherence between temporal cortices may be a trait marker for schizophrenia. In Higashima et al. (2006), increases in the beta-band coherence of resting EEG in frontal electrode pairs, during the treatment of patients hospitalized for acute exacerbations of schizophrenia, were associated with improvement in the total score and the score on the positive subscale of the Brief Psychiatric Rating Scale, suggesting that functional disconnection between the left and right frontal lobes may be related to the generation of psychotic symptoms. This functional disconnection can be normalized following antipsychotic treatment. If viewed in the context of those studies, the acute potentiation effect on the inter-hemispheric connectivity of frontal areas (as attested by the mI for an increase of ρ2 and MI values between the left and right frontal electrodes), occurring at the late-stage 11th PE dance therapy session, might indicate a positive influence, since it tends to alleviate the reduced inter-hemispheric connectivity of schizophrenic patients.
The present study has several limitations which mainly pertain to the relatively few electrodes used, the small number of subjects, and the fact that no control groups were used. Specifically, there was no matched group of medicated patients not undergoing the PE dance therapy treatment, in order to control for possible long-term medication effects. In addition, there was no matched group of medicated patients undergoing a physical activity protocol, in order to assess any effects of physical activity inherent in the PE dance therapy process. Unfortunately, patient admission and stay in the psychiatric clinic where the study took place precluded the use of a larger group of patients as well as the use of control groups. The clinic admits a limited number of psychiatric patients for a short period of time (a few weeks), with an unavoidable patient “dropout”. Consequently, enrollment of a larger group of schizophrenic patients and of groups of matched controls for the present work was not possible. Therefore, it was decided to study only a relatively small group of patients undergoing PE dance therapy, with the expectation that the proposed methodology could lead to preliminary encouraging results, without aiming at obtaining “scientific evidence” concerning effects attributed specifically to PE dance therapy. In addition, it was decided to use the minimum number of electrodes needed in order to infer any “gross” inter and intra-hemispheric brain connectivity patterns. This decision was taken in order to increase patient cooperation (patients would engage in dance therapy while wearing the electrodes) and reduce the time between the end of the dance therapy session and the EEG recording following the session. Consequently, the small number of electrodes drastically limited the detail of spatial information that could be extracted about connectivity changes, resulting in connectivity information concerning bilateral or unilateral pairs of only broad regions such as frontal, central and occipital.
A point of caution is that in the recording montage the right earlobe was used as a reference, while the ground was represented by an electrode placed at the left ear. In general, since no appreciable EEG activity is expected to be present in the earlobes (except possibly in the case of epileptogenic spikes and sharp waves originating in the temporal lobe, which was not the case in our work, since our subjects did not suffer from epilepsy), their use as reference is common (Reilly 1999). Nevertheless, the possibility existed for non-EEG electrical activity (artifact signal) to be present in the earlobe where the reference electrode was located. In that case, due to a possible uneven propagation of the artifact signal on the scalp, there might have been inter-hemispheric asymmetry issues, due to the non-EEG artifact.
An important point that should be taken into account is that there exists great variability in the results of the various studies concerning brain connectivity measures in schizophrenic patients. As stated in French and Beaumont (1984), the different electrode montages, the presence or absence of psychotropic medications, the imprecision of diagnostic categories, the degree of severity of the disorder, as well as age and sex differences, all likely affect the coherence values, possibly explaining the numerous contradicting results among studies estimating coherence for discriminating between schizophrenia and “normal” subjects. The above problems probably also exist for MI computations, where the field is less investigated. Therefore, the inferences that can be made from the results of the present study concerning the connectivity of specific brain regions underlying the recording electrodes should take into account the inherent difficulties of brain connectivity studies that are based on scalp-recorded electrophysiological measures in schizophrenic patients. Nevertheless, future investigations, in addition to utilizing appropriate control groups and more electrodes, could also include the coherence measure, since it has been used extensively in brain connectivity studies of schizophrenic populations. Furthermore, techniques addressing the issue of causal interaction among electrode recordings, taking into account linear and non-linear interactions, such as the Transfer Entropy measure, should be undertaken (Madulara et al. 2012; Ma et al. 2013).
The present work should be viewed as a pilot study primarily describing a methodological path for applying correlation and mutual information-based metrics, derived from scalp EEG electrodes, to assess inter and intra-hemispheric brain connectivity in a group of schizophrenic patients studied during a dynamic therapeutic process, as exemplified by a PE dance therapy protocol. As a matter of fact, this is the first time that such an analysis is used in the investigation of schizophrenic patients undergoing such a therapeutic process. The study indicated that by using EEG similarity measures, i.e., ρ2 and MI, in addition to detecting the presence or absence of individual EEG rhythms (Margariti et al. 2012), the set of neurophysiology-based approaches for quantifying possible dance therapy effects is expanded and may contribute to a detailed assessment of neurophysiological mechanisms possibly being affected by PE dance therapy. It should be stressed that until studies are implemented that surpass the main limitations of the present work (i.e., a limited number of electrodes and subjects, as well as the lack of control cohorts), any claims of “scientific evidence” for brain connectivity modifications incurring solely as a result of the dance therapy protocol used here are extremely tentative. As a matter of fact, excluding any possible influence of the dance therapy protocol, such modifications could be attributed to medication, to physical exercise, or even to the interaction of the subjects with the experimenters, or to some combination of these factors. Having the above limitations in mind, it would be of interest to investigate in future studies whether the preliminary results of the present work, concerning potentiation (attenuation) of brain connectivity in the aforementioned areas, might be replicated and attributed specifically to the applied dance therapy protocol.
Acknowledgments
We would like to thank the 1st Psychiatric Clinic of the University of Athens Medical School, Eginition Hospital, for providing the patients and overall encouragement for this study. In particular, we appreciate the contribution of Dr. E. Daskalopoulos and of Profs. E. Aggelopoulos, D. Dikeos, A. Pehlivanidis, N. Stefanis, and G. Vaslamatzis. The support of Profs. G. Papadimitriou and C. Soldatos is especially appreciated.
References
- Albano AM, Cellucci CJ, Harner RJ, Rapp PE. Optimization of embedding parameters for prediction of seizure onset with mutual information. In: Mees AI, editor. Nonlinear dynamics and statistics. Boston: Birkhaeuser; 2000. pp. 435–451. [Google Scholar]
- Alonso JF, Mananas MA, Romero S, Hoyer D, Riba J, Barbanoj MJ. Drug effect on EEG connectivity assessed by linear and nonlinear couplings. Hum Brain Mapp. 2010;31:487–497. doi: 10.1002/hbm.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Dance Therapy Association (2009) About dance/movement therapy. American Dance Therapy Association. http://www.adta.org/. Accessed 20 October 2014
- Andrzejak RG, Lehnertz K, Mormann F, Rieke C, David P, Elger CE. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: dependence on recording region and brain state. Phys Rev E. 2001;64:061907. doi: 10.1103/PhysRevE.64.061907. [DOI] [PubMed] [Google Scholar]
- Bendat J, Piersol A (1986) Random data. Analysis and measurement procedures, 2nd edn. Wiley, New York
- Berrol CF, Ooi WL, Katz SS. Dance/movement therapy with older adults who have sustained neurological insult: a demonstration project. Am J Dance Th. 1997;19:135–160. doi: 10.1023/A:1022316102961. [DOI] [Google Scholar]
- Bhattacharyya S, Biswas A, Mukherjee J, Majumdar AK, Majumdar B, Mukherjee S, Singh AK. Detection of artifacts from high energy bursts in neonatal EEG. Comput Biol Med. 2013;43:1804–1814. doi: 10.1016/j.compbiomed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Bojner-Horwitz E, Theorell T, Anderberg UM. Dance/movement therapy and changes in stress-related hormones: a study of fibromyalgia patients with video-interpretation. Art Psychother. 2003;30:255–264. doi: 10.1016/j.aip.2003.07.001. [DOI] [Google Scholar]
- Bonita JD, Ambolode LCC, II, Rosenberg BM, Cellucci CJ, Watanabe TA, Rapp PE, Albano AM. Time domain measures of inter-channel EEG correlations: a comparison of linear, nonparametric and nonlinear measures. Cogn Neurodyn. 2014;8:1–15. doi: 10.1007/s11571-013-9267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Slobounov S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA and sLORETA analyses of EEG signals. IEEE T Neur Sys Reh. 2010;18:11–19. doi: 10.1109/TNSRE.2009.2027704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellucci CJ, Albano AM, Rapp PE. Statistical validation of mutual information calculations: comparison of alternative numerical algorithms. Phys Rev E. 2005;71:066208. doi: 10.1103/PhysRevE.71.066208. [DOI] [PubMed] [Google Scholar]
- Chen F, Xu J, Gu F, Yu X, Meng X, Qiu Z. Dynamic processes of information transmission complexity in human brains. Biol Cybern. 2000;83:355–366. doi: 10.1007/s004220000158. [DOI] [PubMed] [Google Scholar]
- Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J Neurophysiol. 2011;106:2012–2023. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosamantes E. Movement and psychodynamic pattern changes in long-term dance/movement therapy groups. Am J Dance Th. 1990;12:27–44. doi: 10.1007/BF00844313. [DOI] [Google Scholar]
- Duckrow RB, Albano AM. Comment on ‘‘Performance of different synchronization measures in real data: a case study on electroencephalographic signals’’. Phys Rev E. 2003;67:063901–1–063901-3. doi: 10.1103/PhysRevE.67.063901. [DOI] [PubMed] [Google Scholar]
- Fernandes AD, Gloor GB. Mutual information is critically dependent on prior assumptions: would the correct estimate of mutual information please identify itself? Bioinformatics. 2010;26:1135–1139. doi: 10.1093/bioinformatics/btq111. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/S0006-3223(01)01335-X. [DOI] [PubMed] [Google Scholar]
- Fraser AM, Swinney HL. Independent coordinates for strange attractors from mutual information. Phys Rev A. 1986;33:1134–1140. doi: 10.1103/PhysRevA.33.1134. [DOI] [PubMed] [Google Scholar]
- French CC, Beaumont JG. A critical review of EEG coherence studies of hemisphere function. Int J Psychophysiol. 1984;1:241–254. doi: 10.1016/0167-8760(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Gardner WA. A unifying view of coherence in signal processing. Signal Process. 1992;29:113–140. doi: 10.1016/0165-1684(92)90015-O. [DOI] [Google Scholar]
- Gersch W, Yonemoto J, Naitoh P. Automatic classification of multivariate EEGs using an amount of information measure and the eigenvalues of parametric time series model features. Comput Biomed Res. 1977;10:297–318. doi: 10.1016/0010-4809(77)90044-1. [DOI] [PubMed] [Google Scholar]
- Gordon E, Palmer DM, Cooper N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clin EEG Neurosci. 2010;41:178–183. doi: 10.1177/155005941004100404. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashima M, Takeda T, Kikuchi M, Nagasawa T, Koshino Y. Functional connectivity between hemispheres and schizophrenic symptoms: a longitudinal study of interhemispheric EEG coherence in patients with acute exacerbations of schizophrenia. Clin EEG Neurosci. 2006;37:10–15. doi: 10.1177/155005940603700104. [DOI] [PubMed] [Google Scholar]
- Huang X (2001) The Use of the Average Amount of Mutual Information (AAMI) as a Signal Similarity Index. Master’s Thesis, University of Houston
- Huseman JAS (1986) Histogram estimators of bivariate densities. Doctoral Dissertation, Rice University, Houston
- Jeong J, Kim DJ, Chae JH, Kim SY, Ko HJ, Paik IH. Nonlinear analysis of the EEG of schizophrenics with optimal embedding dimension. Med Eng Phys. 1998;20:669–676. doi: 10.1016/S1350-4533(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Jeong J, Gore JC, Peterson BS. Mutual information analysis of the EEG in patients with Alzheimer’s disease. Clin Neurophysiol. 2001;112:827–835. doi: 10.1016/S1388-2457(01)00513-2. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Hong SC, Myeong SL, Park MC, Kim YK, Suh CM. Dance movement therapy improves emotional responses and modulates neurohormones in adolescents with mild depression. Int J Neurosci. 2005;115:1711–1720. doi: 10.1080/00207450590958574. [DOI] [PubMed] [Google Scholar]
- Kolmogorov A. Logical basis for information theory and probability theory. IEEE T Inform Theory. 1968;14:662–664. doi: 10.1109/TIT.1968.1054210. [DOI] [Google Scholar]
- Lawhern V, Hairston WD, McDowell K, Westerfield M, Robbins K. Detection and classification of subject-generated artifacts in EEG signals using autoregressive models. J Neurosci Meth. 2012;208:181–189. doi: 10.1016/j.jneumeth.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Le C. Introductory biostatistics. Hoboken: Wiley; 2003. [Google Scholar]
- Lee JPS, Kim HS, Kim CY. Clinical application of dance therapy in psychiatric outpatients with schizophrenia. J Korean Neuropsychiatr Assoc. 2008;47:279–285. [Google Scholar]
- Ma C, Pan X, Wang R, Sakagami M. Estimating causal interaction between prefrontal cortex and striatum by transfer entropy. Cogn Neurodyn. 2013;7:253–261. doi: 10.1007/s11571-012-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madulara MD, Francisco PAD, Nawang S, Arogancia DC, Cellucci CJ, Rapp PE, Albano AM. EEG transfer entropy tracks changes of information transfer on the onset of vision. Int J Mod Phys. 2012;17:9–18. [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallett M. Task-related coherence and task-related spectral power changes during sequential finger movements. Electroen Clin Neuro. 1998;109:50–62. doi: 10.1016/s0924-980x(97)00074-x. [DOI] [PubMed] [Google Scholar]
- Margariti A (2012) Results of the primitive expression form of dance therapy in a psychiatric population. Doctoral Dissertation, University of Peloponnese, Nafplion, Greece
- Margariti A, Ventouras E (2012) Quantification of changes in behavior and in the inter and intra-hemispheric EEG. 4th European Congress of the International Neuropsychiatric Association, Athens, Greece
- Margariti A, Ktonas P, Hondraki P, Daskalopoulou E, Kyriakopoulos G, Economou NT, Tsekou H, Paparrigopoulos T, Barbousi V, Vaslamatzis G. An application of the primitive expression form of dance therapy in a psychiatric population. Art Psychother. 2012;39:95–101. doi: 10.1016/j.aip.2012.01.001. [DOI] [Google Scholar]
- Mars NJI, van Arragon GW. Time delay estimation in non-linear systems using average amount of mutual information analysis. Signal Process. 1982;4:139–153. doi: 10.1016/0165-1684(82)90017-2. [DOI] [Google Scholar]
- Merrin EL, Floyd TC, Fein G. EEG coherence in unmedicated schizophrenic patients. Biol Psychiatry. 1989;25:60–66. doi: 10.1016/0006-3223(89)90147-9. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Bauer U, Krieger S, Lis S, Vehmeyer K, Schuler G, Gallhofer B. The topography of non-linear cortical dynamics at rest, in mental calculation and moving shape perception. Brain Topogr. 1998;10:291–299. doi: 10.1023/A:1022227108139. [DOI] [PubMed] [Google Scholar]
- Moddemeijer R. On estimation of entropy and mutual information of continuous distributions. Signal Process. 1989;16:233–248. doi: 10.1016/0165-1684(89)90132-1. [DOI] [Google Scholar]
- Moddemeijer R (1997) The variance of the mutual information estimator. Technical Report CS-R9701, University of Groningen, The Netherlands
- Morrison-Stewart SL, Velikonja D, Corning WC, Williamson P. Aberrant interhemispheric alpha coherence on electroencephalography in schizophrenic patients during activation tasks. Psychol Med. 1996;26:605–612. doi: 10.1017/S0033291700035674. [DOI] [PubMed] [Google Scholar]
- Na SH, Jin SH, Kim SY, Ham BJ. EEG in schizophrenic patients: mutual information analysis. Clin Neurophysiol. 2002;113:1954–1960. doi: 10.1016/S1388-2457(02)00197-9. [DOI] [PubMed] [Google Scholar]
- Na SH, Jin SH, Kim SY. The effects of total sleep deprivation on brain functional organization: mutual information analysis of waking human EEG. Int J Psychophysiol. 2006;62:238–242. doi: 10.1016/j.ijpsycho.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ. EEG coherency. I: statistics, reference electrode, volume conduction, laplacians, cortical imaging, and interpretation at multiple scales. Electroen Clin Neuro. 1997;103:499–515. doi: 10.1016/S0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Duke DW, Krieble KK. Dimensional analysis of resting human EEG. II: surrogate-data testing indicates nonlinearity but not low-dimensional chaos. Psychophysiology. 1995;32:486–491. doi: 10.1111/j.1469-8986.1995.tb02100.x. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Kraskov A, Kreuz T, Grassberger P. Performance of different synchronization measures in real data: a case study on electroencephalographic signals. Phys Rev E. 2002;65:041903–1–041903-13. doi: 10.1103/PhysRevE.65.041903. [DOI] [PubMed] [Google Scholar]
- Reilly EL (1999) EEG recording and the operation of the apparatus. In: Niedermeyer E, Lopes Da Silva F (eds) Electroencephalography, basic principles, clinical applications, and related fields, (4th Ed.). Williams & Wilkins, Baltimore, pp. 142–143
- Röhricht F, Papadopoulos N, Holden S, Clarke T, Priebe S. Therapeutic processes and clinical outcomes of body psychotherapy in chronic schizophrenia—an open clinical trial. Art Psychother. 2011;38:196–203. doi: 10.1016/j.aip.2011.06.001. [DOI] [Google Scholar]
- Schott-Billmann F. Possession, Danse et Therapie. Paris: Sand; 1985. [Google Scholar]
- Schott-Billmann F. Quand la Danse Guerit. Paris: La Recherche en Danse; 1997. [Google Scholar]
- Shannon C. A mathematical theory of communication. Bell Systems Tech J. 1948;27(379–423):623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x. [DOI] [Google Scholar]
- Shao SY, Shen KQ, Ong CJ, Wilder-Smith E, Li XP. Automatic EEG artifact removal: a weighted support vector machine approach with error correction. IEEE Trans Biomed Eng. 2009;56:336–344. doi: 10.1109/TBME.2008.2005969. [DOI] [PubMed] [Google Scholar]
- Shaw JC, Colter N, Resek G. EEG coherence, lateral preference and schizophrenia. Psychol Med. 1983;13:299–306. doi: 10.1017/S0033291700050911. [DOI] [PubMed] [Google Scholar]
- Sritharan A, Line P, Sergejew A, Silberstein R, Egan G, Copolov D. EEG coherence measures during auditory hallucinations in schizophrenia. Psychiat Res. 2005;136:189–200. doi: 10.1016/j.psychres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P (2006) Magnetoencephalographic examination of resting-state functional connectivity in Alzheimer’s disease. Neuroimage 32:1335–1344 [DOI] [PubMed]
- Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multichannel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer R, Kurths J, Daub CO, Weise J, Selbig J. The mutual information: detecting and evaluating dependencies between variables. Bioinformatics. 2002;18:S231–S240. doi: 10.1093/bioinformatics/18.suppl_2.S231. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Fischer P, Neumeister A, Rappelsberger P, Kasper S. Low frontal electroencephalographic coherence in neuroleptic-free schizophrenic patients. Biol Psychiat. 1998;44:438–447. doi: 10.1016/S0006-3223(97)00428-9. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroen Clin Neuro. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- van Bergen R (1986) A discrete mutual information estimator of continuous signals. Master’s Thesis, Twente University, Enschede, The Netherlands
- Vastano JA, Swinney HL. Information transport in spatiotemporal systems. Phys Rev Lett. 1988;60:1773–1776. doi: 10.1103/PhysRevLett.60.1773. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nanbu Y, Kikuchi M, Koshino Y, Hashimoto T. Aberrant functional organization in schizophrenia: analysis of EEG coherence during rest and photic stimulation in drug-naive patients. Neuropsychobiology. 1998;38:63–69. doi: 10.1159/000026518. [DOI] [PubMed] [Google Scholar]
- Wilmer A, de Lussanet MHE, Lappe M. A method for the estimation of functional brain connectivity from time-series data. Cogn Neurodyn. 2010;4:133–149. doi: 10.1007/s11571-010-9107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Rädler T, Hyde T, Coppola R, Weinberger DR. An association between reduced interhemispheric EEG coherence in the temporal lobe and genetic risk for schizophrenia. Schizophr Res. 2001;49:129–143. doi: 10.1016/S0920-9964(00)00128-6. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu ZR, Liu R, Yang QF. Information transmission in human cerebral cortex. Physica D. 1997;106:363–374. doi: 10.1016/S0167-2789(97)00042-0. [DOI] [Google Scholar]


