Abstract
Exercise training has been shown not only to influence physical fitness positively but also cognition in healthy and impaired populations. However, some particular exercise types, even though comparable based on physical efforts, have distinct cognitive and sensorimotor features. In this study, the effects of different types of exercise, such as fast ball sports and dance training, on brain electrical activity were investigated. Electroencephalography (EEG) scans were recorded in professional dancer, professional fast ball sports athlete (FBSA) and healthy control volunteer groups consisting of twelve subjects each. In FBSA, power of delta and theta frequency activities of EEG was significantly higher than those of the dancers and the controls. Conversely, dancers had significantly higher amplitudes in alpha and beta bands compared to FBSA and significantly higher amplitudes in the alpha band in comparison with controls. The results suggest that cognitive features of physical training can be reflected in resting brain electrical oscillations. The differences in resting brain electrical oscillations between the dancers and the FBSA can be the result of innate network differences determining the talents and/or plastic changes induced by physical training.
Keywords: Exercise, Plasticity, EEG, Dancers, Fast ball sports athletes, Resting state networks
Introduction
Research suggests that both genetic and environmental factors affect the development and physical structure of the brain (Lenroot and Giedd 2008). Exercise training has also been shown not only to influence physical fitness positively, but also cognition (Kramer and Erickson 2007a, b). Its effects are positive for both normal and cognitively impaired adults, middle-aged adults, and children (Schneider et al. 2009; Gonzales et al. 2012).
The use of exercise and dance in the therapy of some medical conditions also supports the beneficial effects of exercise in different forms on brain functions. In Parkinson disease (PD), recent studies indicate that dance may be an effective alternative to traditional exercise for addressing areas of concern to individuals with PD such as gait and posture (Earhart 2009; Heiberger et al. 2011). Dance therapy is not only recommended for posture and balance rehabilitation, but also as an alternative to aerobic exercise (Belardinelli et al. 2008; Kattenstroth et al. 2010).
The underlying causes of the effects of exercise on brain function could be that exercise can increase levels of brain-derived neurotrophic factor (BDNF) and other growth factors that stimulate neurogenesis, increasing the resistance of the brain to insult and improve learning and mental performance. In addition to increasing levels of BDNF, exercise mobilizes gene expression profiles that would be predicted to benefit brain plasticity processes (Cotman and Berchtold 2002; Adlard et al. 2004).
There are strategies to examine the effects of external impacts like exercise and skills trainings on brain structures and functions. One method that analyzes dynamic changes in the brain with high time resolution incorporates electroencephalography (EEG) (Basar 2013).
Given the difficulties of recording EEG during movement, researchers have explored alternative ways to apply EEG methodologies in the sports sciences (Thompson et al. 2008). One approach is to record EEG outside of the execution of the sporting task itself to assess long-term outcomes or to understand pre-task, post-task short term cortical processes.
The EEG consists of the activity of oscillators generating rhythmic activity in several frequency ranges. Power spectral signal analysis of spontaneous EEG activity constitutes one of the most important and most commonly used analytical tools for evaluating neurophysiological signals in health and also in diseases (Lopes Da Silva 1978; Basar 1980, 2012; Basar et al. 2001; Basar and Guntekin 2013). A large body of evidence suggests that oscillatory activity in all frequency bands is linked to a broad variety of perceptual, sensorimotor, and cognitive operations (Basar et al. 2001; Aoki et al. 1999; Palva and Palva 2007).
In this study, the effects of different types of exercise, such as fast ball sports and dance training, on resting brain electrical activity were investigated. The plasticity induced changes of physical exercise and the training effects of repeated practice of skills over an extended period of time in fast ball sports and dancing may have additive effects on brain networks and functions. As dance and fast ball sports have specific differences related to cognition, sensorimotor execution, and functional neural networks, we hypothesize that those changes induced by training effect and physical exercise might differently reflect on slow and fast components of spontaneous brain electrical activity.
In ball sports, one has to adapt to the unexpected environmental changes related to the game, especially under severe time constraints. Fast ball sports athletes (FBSA) have to execute their actions in a very short time. FBSA anticipate future events by extracting advance sensory information from the opponent’s movements before the ball is released and have to inhibit the inappropriate actions that interfere with the correct ones (Williams and Grant 1999), which might be associated with delta activity. As they encounter continuously new information, FBSA might rely heavily on working memory-emotion based consciousness. In general, theta activity is associated with attentional processing, spatial navigation and with the processing of new (episodic) information and working memory (Basar-Eroglu et al. 1992; Sauseng et al. 2010).
In dancing, movement and gestural representations with the rhythm of the music are practiced repeatedly in interpersonal coordination in space and time. Figures are practiced to perfection to minimize unexpected events, which might involve beta band activity. Dancers heavily use motor control in terms of postural control, equilibrium maintenance, and stabilization, as well as sequence learning (Bläsing et al. 2012). Motor experience, imagination and memory are also important components in practicing dance where alpha band activity might be involved (Isoglu-Alkac and Strüber 2006; Klimesch et al. 2007; Klimesch 2012).
In the current study, EEGs of modern dancers and FBSA were compared. Our hypotheses were (1) acquired and/or innate features of modern dance and fast ball sports can reflect spontaneous brain electrical activity; (2) dancers may have higher fast activity whereas FBSA may have higher slow activity.
Materials and methods
Participants
Twelve physically active, modern dancers (6 males; age: 30.58 ± 1.09; education year: 15.91 ± 0.69; activity year: 9.91 ± 0.65; daily practicing hour: 2.5 ± 0.72), 12 professional FBSA (6 males; age: 24.33 ± 0.43; education year: 14.75 ± 0.13; activity year: 9.83 ± 0.51; daily practicing hour: 3.08 ± 0.90), and 12 healthy control volunteers (6 males; age: 23.08 ± 0.94; education year: 14.33 ± 0.47), all right-handed, were included in the study. All participants had neither neurological or psychiatric disorders, nor sensory deficiencies. The study was performed in accordance with the Declaration of Helsinki. The study was approved by the ethical committee of Istanbul University, Istanbul Faculty of Medicine, Approval No: 2010/450-123.
Electrophysiological recordings
The EEGs were recorded from 19 scalp electrode sites of the 10–20 system (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2) unipolarly, using an electro-cap system referenced to the earlobes and the grounding electrode being at Fpz. The MITSAR (WinEEG) recording system and analysis software were used. The recording of bipolar electrooculographic (EOG) data monitored blinking and eye movements. Electrode impedance were kept under 5 kΩ. The raw data were digitized at a sampling rate of 500 Hz with an online bandpass filter of 0.3–30 Hz in continuous recording mode. EEGs for 5 min were recorded while the participants kept their eyes closed. Each subject was given instructions to stay relaxed and awake without eye movements to avoid motion artifacts. Epochs that had EEG amplitudes higher than ±100 µV was rejected. Epochs having higher than ±50 µV waves in 0.5–1 and 20–30 Hz bands were also rejected. After automatically artifact rejection the remaining epochs were visually inspected for eye movement or blink artifact in the EOG channel.
Power spectrum
The spectral power in background EEG has been used to investigate neural activity during the eyes-closed conditions. After the artifacts elimination, the relative EEG power spectra were calculated at 0.5–30 Hz band in all channels and epochs of 4 s with Fast Fourier Transformation algorithm. Mean relative power was obtained by averaging the relative power per frequency band in fifty epochs. Subsequently, delta (0.5–4 Hz), theta (over 4–8 Hz), alpha (8–12 Hz) and beta (over 12–30 Hz) powers were extracted from the total spectrum.
Statistical analysis
Group differences in mean relative power were assessed by two-way analysis of variance (ANOVA) for frequency bands of delta, theta, alpha, and beta. The first group factor included three levels: dancers, FBSA, and control volunteers; the second factor was the location of the electrodes (channel) (19 levels: Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2). Post hoc Bonferroni test was used where necessary. Statistical analyses were carried out with SPSS version 20.0, with alpha set at 0.05.
Results
Demographic data
Demographic data of the groups were compared by ANOVA and post hoc Bonferroni tests. The ages of dancers were significantly higher than the other groups (F(2, 33) = 21.33; p = 0.001). There was no significant difference for the education, activity and daily practicing hours between the groups.
Topographic mapping of the groups
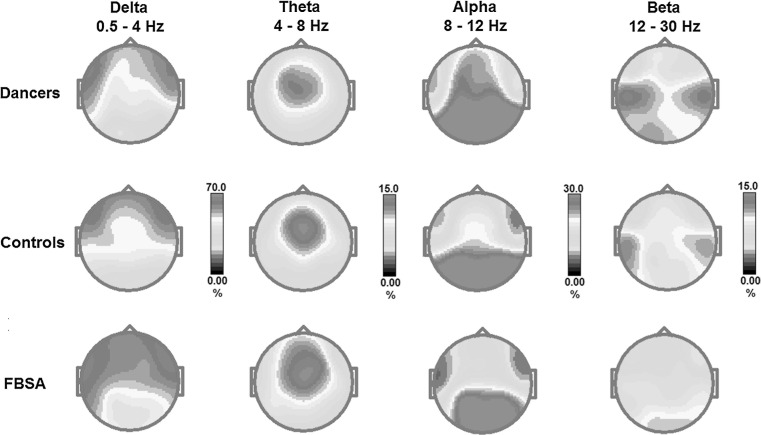
Figure 1 displays maps showing topographical details of the each frequency during the eyes-closed condition in the dancers, controls and FBSA for the 19 channels. The maps of FBSA were characterized by evident slow (delta and theta) frequency bands. Compared with the FBSA, the dancers showed higher amplitudes of the alpha and beta frequencies.
Fig. 1.
Maps show topographical details of the delta, theta, alpha and beta frequencies during eyes-closed condition for the dancers, controls and fast ball sports athletes (FBSA). The maps of FBSA were characterized by an evident slow (delta and theta) frequency bands. Compared with the FBSA, the dancers showed higher amplitude of the alpha and beta frequencies. The controls were in between the dancers and the FBSA
Comparison of EEG power of dancers, FBSA and controls
Delta (0.5–4 Hz) frequency
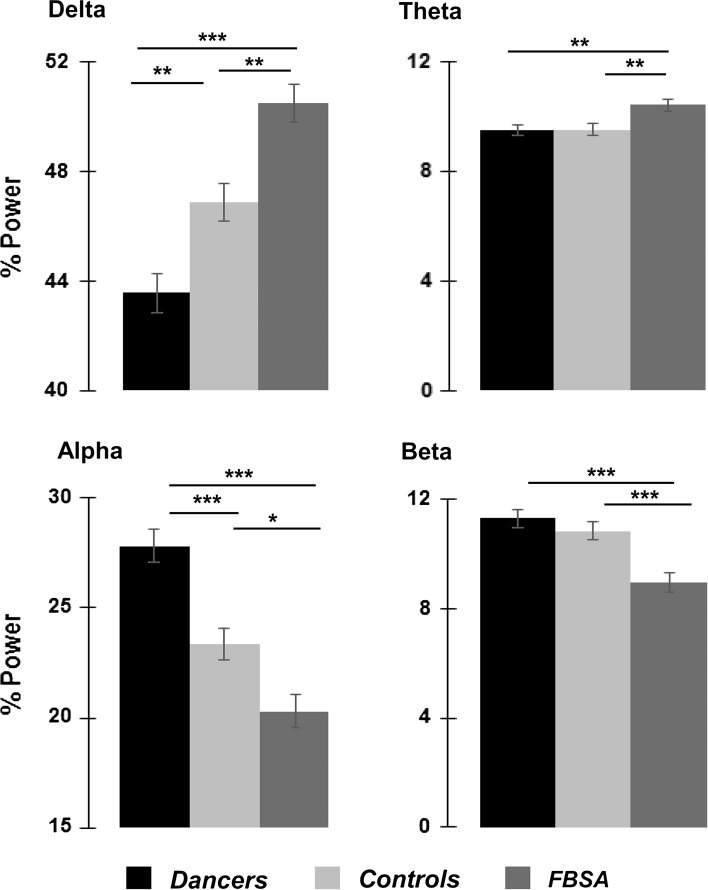
The delta band power was statistically significant for group (F(2, 627) = 24.76; p = 0.0001; see Figs. 1, 2) and channel (F(18, 627) = 16.76; p = 0.0001). In post hoc tests (Bonferroni), delta power of the FBSA was significantly higher than the dancers (p = 0.0001) and controls (p = 0.001). Also, delta power of the dancers was significantly lower than the controls (p = 0.003).
Fig. 2.
Statistical analysis of the relative spectral power in three groups for the delta, theta, alpha and beta frequencies during eyes closed condition. The significance between the groups shows the results of the Bonferroni post hoc testing at the 19 channels. *p < 0.05; **p = 0.001; ***p = 0.0001
Theta (4–8 Hz) frequency
The theta band power was statistically significant for group (F(2, 627) = 6.46; p = 0.002; see Figs. 1, 2) and channel (F(18, 627) = 15.93; p = 0.0001). Bonferroni post hoc testing indicated that the theta power was significantly higher in the FBSA compared with dancers (p = 0.005) and controls (p = 0.006).
Alpha (8–12 Hz) frequency
The alpha band power was statistically significant for group (F(2, 627) = 25.32; p = 0.0001; see Figs. 1, 2) and channel (F(18, 627) = 24.42; p = 0.0001). The alpha power of dancers was higher than the FBSA (p = 0.0001) and also controls (p = 0.0001) with Bonferroni post hoc testing. Also, the FBSA had smaller alpha power than controls (p = 0.01).
Beta (12–30 Hz) frequency
The beta band power was statistically significant for group (F(2, 627) = 13.87; p = 0.0001; see Figs. 1, 2) and channel (F(18, 627) = 4.38; p = 0.0001). The beta power of the dancers was higher than the FBSA (p = 0.0001) and also the controls (p = 0.0001) with Bonferroni post hoc testing.
Discussion
The results of the study showed different EEG band power patterns for the FBSA, dancers and controls. Among FBSA, powers of slow frequencies were significantly higher than the dancers and controls. Conversely, dancers had significantly higher amplitudes in alpha and beta bands compared with FBSA. Also dancers had significantly higher amplitudes in alpha than controls.
EEG data in professional dancers and FBSA are relatively scarce. In one study, expert dancers produced more alpha during mental imagination of creative dance than novices (Fink et al. 2009), whereas there were no significant differences in the EEG during imagination of waltz dancing between the groups (Belardinelli et al. 2008). In previous studies, increased alpha power was found for high-fit relative to low-fit subjects (Dustman et al. 1990). Lardon and Polich reported that compared with controls young adult exercise subjects produced less baseline delta but greater spectral power in alpha, theta, and beta bands (Lardon and Polich 1996). The underlying causes of these findings were explained at least in part as being related to improved neurotransmitter functioning and increased vascularization.
In healthy controls that learned to dance tango, a shift in cortical activation, with increased activity in the premotor and supplementary motor areas during imagined walking following a series of tango lessons was reported (Sacco et al. 2006).
Cognitive features that are apparently different in dancers compared with FBSA include the use of self-reflection and self-awareness in the emotional, motor, and kinesthetic domains. The movements, gestures, and figures are practiced repeatedly to perfection and unexpected events are suppressed. There is synchronization of one’s movements to those of another dance partner or to the rhythm of the music, where timing and attention have important effects (Minvielle-Moncla et al. 2008).
Imagery is also a crucial part of dance and dance training. Dancers use mental imagery in creating new material and artistic expression which might associate with alpha activity (Fink et al. 2009); kinesthetic imagery is used to heighten the imagery of kinesthetic sensations. Motor imagery is also used in dance training to help memorize complex movements. It is accompanied with beta band activity observed in a broad range of cortical areas (Bläsing et al. 2012). In the motor system, beta band activity apparently relates to maintenance of the current motor set and the dominance of endogenous top-down influences that override the effect of potentially novel, or unexpected, external events. To bring the gestures and movements to perfection in dance seems to relate closely to beta band activity (Engel and Fries 2001).
However, the features that distinguish expertise in fast ball sports are a better adaptation of the perceptual motor system to unexpected environmental changes under time constraints (Runigo et al. 2010). This may be the result of shorter visuomotor delay and efficient reprogramming of motor responses within limited time periods (Nakamoto and Mori 2012). According to Knyazev, functional delta oscillations appear to be implicated in the synchronization of brain activity with autonomic functions and in cognitive processes related to attention and the detection of motivationally salient stimuli in the environment (Knyazev 2012).
The increased spontaneous alpha and beta EEG band powers in dancers is a parallel finding to increased alpha and beta power, which correlates positively with activity in the default mode network (DMN) and self-referential networks that were identified in concomitant fMRI and EEG studies (Laufs et al. 2003; Mantini et al. 2007). The activity during the eyes-closed resting state is linked to cognitive processes like random episodic memory (Buckner and Carroll 2006) and related imagery, conceptual processing (Greene et al. 2001), stimulus-independent thought (Calhoun et al. 2004), and self-reflection (Mason et al. 2007). Most of the cognitive processes that signify self-referential network also might be involved in dance performance. Spontaneous resting activity, measured by blood oxygen level-dependent (BOLD) in fMRI in the resting awake or anesthetized brain, is organized in multiple highly specific functional anatomical networks (resting state networks, RSNs), only one of which corresponds to the DMN (RSA 1) (De Luca et al. 2006; Damoiseaux et al. 2006). Those RSNs strongly overlap with sensorimotor, visual, auditory, attention, language, and default networks that are commonly modulated during active behavioral tasks (Fox et al. 2006; Mantini et al. 2007; Harrison et al. 2008).
As alpha and beta powers correlate with self-referential cognitive processes, theta and delta powers were found to be correlated with sensory networks as visual, auditory, and sensorimotor systems respectively (Jann et al. 2010). Increased delta and theta powers in the FBSA compared with dancers and the controls also might suggest decreased arousal in the FBSA. Relatively short recording time and instructions given before the recordings make this possibility unlikely, but not impossible. In this study, the mean age of dancers was 6 years more than the FBSA and the controls. However, in this decade age group (3rd), significant differences between EEG power bands seem unlikely.
This is the first study comparing the resting brain electrical activity of two different exercise types from cognitive and sensorimotor perspectives, namely, professional dancers and FBSA. EEG band power differences of FBSA and dancers from the control subjects support the previous studies that showed exercise induces changes in the brain. The differences that were shown also may indicate innate network differences of the individuals or reflect their talents that made them choose or excel at a particular exercise type. However, long and time consuming exercise for years also may lead to plastic changes on the disposed neural networks. The findings confirm association of the functional significance of the EEG bands and the cognitive and sensorimotor features of the particular exercise. However, to delineate the differences between the FBSA and dancers whether innate or induced, findings from our cross-sectional study should be replicated with longitudinal studies using different paradigms delineating the more specific aspects of the EEG band differences.
Conclusion
Being an elite dancer or a FBSA requires long-term, regular exercise and skill training that can induce plastic changes involving the brain. Two professional groups apparently have different cognitive and sensorimotor features that might be innate or induced by the particular training or both. The different distribution of the EEG bands between the two groups confirms the association of features of the exercise type and functional significance of the EEG bands.
Acknowledgments
Authors would like to thank Edanz Group Ltd. for English editing of this paper and to thank Prof. Dr. Sacit Karamürsel for his critical comments.
Contributor Information
Numan Ermutlu, Email: numan.ermutlu@istanbulbilim.edu.tr.
Ilker Yücesir, Email: iyucesir@yahoo.com.
Gökçer Eskikurt, Email: gokcereskikurt@gmail.com.
Tan Temel, Email: tantemel@torkdance.com.
Ümmühan İşoğlu-Alkaç, Phone: +90 212 4142000, Email: alkac@istanbul.edu.tr, Email: ummalkac@gmail.com.
References
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The time course of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA. Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor tasks. Clin Neurophysiol. 1999;110:524–537. doi: 10.1016/S1388-2457(98)00064-9. [DOI] [PubMed] [Google Scholar]
- Basar E. A review of alpha activity in integrative brain function: fundamental physiology, sensory coding, cognition and pathology. Int J Psychophysiol. 2012;86:1–24. doi: 10.1016/j.ijpsycho.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Başar E. EEG-brain dynamics. Relation between EEG and brain evoked potentials. Amsterdam: Elsevier; 1980. [Google Scholar]
- Basar E. Brain–Body–Mind in the nebulous Cartesian system: a holistic approach by oscillations. New York: Springer; 2013. [Google Scholar]
- Basar E, Guntekin B. Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. In: Basar E, Basar-Eroglu C, Ozerdem A, Rossini PM, Yener GG, editors. Application of brain oscillations in neuropsychiatric diseases (supplements to clinical neurophysiology, vol 62) 1. Amsterdam: Elsevier BV; 2013. pp. 303–341. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Başar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-G. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Lacalaprice F, Ventrella C, Volpe L, Faccenda E. Waltz dancing in patients with chronic heart failure new form of exercise training. Circ Heart Fail. 2008;1:107–114. doi: 10.1161/CIRCHEARTFAILURE.108.765727. [DOI] [PubMed] [Google Scholar]
- Bläsing B, Calvo-Merino B, Cross ES, Jola C, Honisch J, Stevens CJ. Neurocognitive control in dance perception and performance. Acta Psychol. 2012;139:300–308. doi: 10.1016/j.actpsy.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2006;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magn Reson Imaging. 2004;22:1181–1191. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:291–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckman CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of longdistance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Emmerson WY, Ruhling R, Shearer DE, Steinhaus LA, Johnson S, Bonekat H, Shigeoka J. Age and fitness effects on EEG, ERPs, visual sensitivity, and cognition. Neurobiol Aging. 1990;11:193–200. doi: 10.1016/0197-4580(90)90545-B. [DOI] [PubMed] [Google Scholar]
- Earhart GM. Dance as therapy for individuals with Parkinson disease. Eur J Phys Rehabil Med. 2009;45:231–238. [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 2001;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fink A, Graif B, Neubauer AC. Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. Neuroimage. 2009;46:854–862. doi: 10.1016/j.neuroimage.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales MM, Tarumi T, Kaur S, Nualnim N, Fallow BA, Pyron M, Tanaka H, Haley AP. Aerobic fitness and the brain: ıncreased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr. 2012;25:126–134. doi: 10.1007/s10548-012-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yücel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. PNAS. 2008;115:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiberger L, Maurer C, Amtage F, Mendez-Balbuena I, Schulte-Mönting J, Hepp-Reymond MC, Kristeva R. Impact of a weekly dance class on the functional mobility and on the quality of life of individuals with Parkinson’s disease. Front Aging Neurosc. 2011;3:1–15. doi: 10.3389/fnagi.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoglu-Alkac U, Strüber D. Necker cube reversals during long-term EEG recordings: sub-bands of alpha activity. Int J Psychophysiol. 2006;59:179–189. doi: 10.1016/j.ijpsycho.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Jann K, Kottlow M, Dierks T, Boesch C, Koenig T. Topographic electrophysiological signatures of fMRI resting state networks. PLoS ONE. 2010;5:12945. doi: 10.1371/journal.pone.0012945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattenstroth JC, Kolankowska I, Kalisch T, Dinse HR. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front Aging Neurosc. 2010;2:1–9. doi: 10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36:677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Effects of physical activity on cognition, well-being, and brain: human interventions. Alzheimer Dement. 2007;3:45–51. doi: 10.1016/j.jalz.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lardon MT, Polich J. EEG changes from long-term physical exercise. Biol Psychol. 1996;44:19–30. doi: 10.1016/S0301-0511(96)05198-8. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. PNAS. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: initial findings from a neuroimaging study of pediatric twins. Dev Psychopathol. 2008;20:1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes Da Silva FH. Analysis of EEG non-stationarities. Electroencephalogr Clin Neurophysiol. 1978;Suppl 34:163–179. [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Crafton ST, Macrae CN. Wandering minds: the default network and stimulus independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minvielle-Moncla J, Audiffren M, Macar F, Vallet C. Overproduction timing errors in expert dancers. J Mot Behav. 2008;40:291–300. doi: 10.3200/JMBR.40.4.291-300. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Mori S. Experts in fast-ball sports reduce anticipation timing cost by developing inhibitory control. Brain Cognit. 2012;80:23–32. doi: 10.1016/j.bandc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Runigo CL, Benguigui N, Bardy BG. Visuo-motor delay, information movement coupling, and expertise in ball sports. J Sports Sci. 2010;28:327–337. doi: 10.1080/02640410903502782. [DOI] [PubMed] [Google Scholar]
- Sacco K, Cauda F, Cerliani L, Mate D, Duca S, Geminiani GC. Motor imagery of walking following training in locomotor attention, the effect of ‘the tango lesson’. NeuroImage. 2006;32:1441–1445. doi: 10.1016/j.neuroimage.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34:1015–1022. doi: 10.1016/j.neubiorev.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Schneider S, Vogt T, Frysch J, Guardiera P, Strüder HK. School sport—a neurophysiological approach. Neurosci Lett. 2009;467:131–134. doi: 10.1016/j.neulet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Thompson T, Steffert T, Ros T, Leach J, Gruzelier J. EEG applications for sport and performance. Methods. 2008;45:279–288. doi: 10.1016/j.ymeth.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Williams AM, Grant A. Training perceptual skill in sport. Int J Sport Psychol. 1999;30:194–220. [Google Scholar]