Summary
During vertebrate egg maturation, cytokinesis initiates after one pole of the bipolar metaphase I spindle attaches to the oocyte cortex, resulting in the formation of a polar body and the mature egg. It is not known what signal couples the spindle pole positioning to polar body formation. We approached this question by drawing an analogy to mitotic exit in budding yeast, as asymmetric spindle attachment to the appropriate cortical region is the common regulatory cue. In budding yeast, the small G protein Cdc42 plays an important role in mitotic exit following the spindle pole attachment [1]. We show here that inhibition of Cdc42 activation blocks polar body formation. The oocytes initiate anaphase but fail to properly form and direct a contractile ring. Endogenous Cdc42 is activated at the spindle pole-cortical contact site immediately prior to polar body formation. The cortical Cdc42 activity zone, which directly overlays the spindle pole, is circumscribed by a cortical RhoA activity zone; the latter defines the cytokinetic contractile furrow [2]. As the RhoA ring contracts during cytokinesis, the Cdc42 zone expands, maintaining its complementary relationship with the RhoA ring. Cdc42 signaling may thus be an evolutionarily conserved mechanism that couples spindle positioning to asymmetric cytokinesis.
Results and Discussion
To investigate whether Cdc42 has a role in first polar body formation during oocyte maturation, we employed highly specific inhibitors to block individual members of the Rho family GTPases: RhoA, Rac1, and Cdc42. Injection of dominant-negative Cdc42 (HA-Cdc42T17N) or dominant-negative Rac1 (HA-Rac1T17N) mRNA caused no visible changes in oocyte morphology nor affected progesterone-induced GVBD (Figure 1A), in spite of the fact that both were expressed at high levels (Figure 1D). Contrary to a previous report [3], we did not observe consistent acceleration of progesterone-induced GVBD in oocytes injected with HA-Cdc42T17N compared to uninjected oocytes (data not shown). Injection of C3 toxin mRNA, on the other hand, caused depigmentation of the animal hemisphere, similar to treatment of oocytes with cytochalasin B. The depigmentation interfered with assessment of GVBD in intact oocytes (Figure 1A). Nonetheless, it was evident that C3-injected oocytes, as well as oocytes treated with cytochalasin B, also responded to progesterone by undergoing GVBD, as determined upon fixing and bisecting the treated oocytes (data not shown).
Figure 1.

Cdc42T17N Inhibited First Polar Body Formation
(A) Typical images of oocytes after different treatment. All except for control (GV) were treated with progesterone overnight. When cytochalasin B (CB, 5 μg/ml) was used, it was added 2 hr before the addition of progesterone. Oocytes injected with Cdc42T17N or Rac1T17N mRNA were incubated for at least 6 hr before the addition of progesterone. Oocytes injected with C3 mRNA were immediately placed in medium containing progesterone.
(B) Oocytes treated as described in (A) were fixed, stained with Sytox green, and viewed from the animal pole under a dissecting fluorescence microscope. First polar body (PB) is indicated.
(C) Control oocytes and oocytes injected with Cdc42T17N or Rac1T17N mRNA were coinjected with tubulin-Oregon green 514 conjugate (Molecular Probes, 5 nl per oocyte) before the addition of progesterone. Following overnight incubation, the oocytes were fixed, stained with propidium iodide (red), and viewed following lateral bisection of the oocytes. Pictures are representative confocal images viewed from the cutting surface. C3 mRNA-injected oocytes and CB-treated oocytes were fixed, bisected, and costained with anti-tubulin antibodies (red) and Sytox green. A dashed line indicates the oocyte cortex in each image.
(D) Uninjected (control) oocytes and oocytes injected with mRNA for HA-Cdc42T17N or HA-Rac1T17N were incubated overnight. Extracts were prepared and analyzed by SDS-PAGE followed by immunoblotting with antibodies against HA.
(E) Uninjected oocytes (control, lanes 1 to 4) and oocytes injected with HA-Cdc42T17N mRNA (lanes 5 to 8) were incubated overnight before the addition of progesterone. Individual oocytes were lysed at GVBD or at the indicated times following GVBD. GV oocytes (without progesterone) were lysed at the same time as the GVBD 3 hr oocytes. Extracts were subjected to MPF assays (top panel) or immunoblotting with antibodies against cyclin B2 (middle panel) or xFZY (lower panel). Shown is a representative of three independent experiments.
We wished to determine whether Cdc42T17N affected the transient inactivation of maturation-promoting factor (MPF) following GVBD; this transient inactivation of MPF is thought to be important for the completion of meiosis I [4]. To analyze MPF dynamics in control oocytes and oocytes injected with Cdc42T17N, we withdrew individual oocytes at GVBD, 1 hr or 3 hr following GVBD. Extracts were prepared and analyzed for MPF activity. As shown in the top panel of Figure 1E, the transient inactivation of MPF was evident in both control oocytes (lane 3) and in oocytes injected with Cdc42T17N (lane 7). Similarly, both groups of oocytes exhibited similar degradation and resynthesis of cyclin B2 (middle panel). Accumulation of the APC/C activator xFzy [5] at GVBD was also normal in oocytes injected with Cdc42T17N (bottom panel). These results indicated that inhibition of Cdc42 did not affect APC/C activation or the biphasic pattern of MPF activity.
Although inhibition of Cdc42 had no apparent effect on GVBD or the biphasic pattern of MPF activity, analysis of chromosome morphology revealed that while control oocytes (97% or 261/268 in seven experiments) and HA-Rac1T17N-injected oocytes (98% or 122/125 in three experiments) had completed meiosis, with a “flower” pattern of metaphase II chromosome array in the presence of the first polar body, oocytes injected with HA-Cdc42T17N (97% or 178/184 in six experiments) had not emitted the first polar body, but had a similar “flower” pattern of chromosome arrays that were bigger and contained approximately twice as many distinguishable chromosomes (Figure 1B). Significantly, the single metaphase spindle in Cdc42T17N oocytes was bipolar and could be seen asymmetrically attached to the oocyte cortex, similar to metaphase II spindles found in control oocytes or oocytes injected with HA-Rac1T17N (Figure 1C). On the other hand, no chromosome arrays could be seen from the animal pole (or anywhere else on the oocyte surface) in C3-injected oocytes, nor could we detect the presence of the first polar body (data not shown). These results were similar to those obtained earlier by others in oocytes treated with cytochalasin B [6]. These similarities strongly suggested that the metaphase spindle in C3-injected oocytes failed to translocate/anchor to the oocyte cortex. We examined the oocytes after bisecting them and found bipolar spindles deeply imbedded in oocytes injected with C3 or treated with cytochalasin B (Figure 1C for example). However, we could detect spindles in less than 10% of the examined oocytes. We attributed the rare occurrence of spindle detection in these experiments to the intrinsically “hit-or-miss” nature of the technique (the spindle would be detectable only if it happened to be within 50 μm or so of the cutting surface due to the limitation of the confocal imaging), rather than indicating the lack of such spindles.
To precisely determine at which point in the process of oocyte maturation Cdc42 activity was required, we monitored chromosome changes in live oocytes incubated with Hoechst dye, as previously done in mouse oocytes [7]. As shown in Figure 2A, control oocytes exhibited a single chromosome array (metaphase I) before chromosome separation (anaphase I). Within a few minutes, individual chromosomes became unidentifiable, indicating that oocytes had entered telophase or cytokinesis. Well-formed metaphase II chromosome array could be seen less than 3 hr after GVBD, in the presence of the first polar body. Cdc42T17N-injected oocytes exhibited similar metaphase I spindles and also clearly underwent chromosome separation (anaphase I). Remarkably, following a period of separation of the two chromatin masses, a single metaphase spindle formed and became stable (175 min). No polar body was emitted, and the single metaphase spindle contained approximately twice as many identifiable chromosomes as the metaphase II spindle in control oocytes. It should be pointed out that the time-lapse experiments were performed with the use of an epifluorescence microscope; as a result, one of the two separating chromosome arrays was often out of focus. We have analyzed several dozen oocytes (about one-third were control oocytes and two-thirds were oocytes injected with Cdc42T17N) from four donor frogs. Although anaphase initiation could be seen as early as 110 min and as late as 140 min following GVBD, we observed no significant difference in the timing of anaphase in control oocytes versus oocytes injected with Cdc42T17N (data not shown). The other striking feature was that anaphase was very brief—lasting no more than 1–2 min (Figure 2A and data not shown). These results suggested that Cdc42T17N-injected oocytes failed to initiate or complete cytokinesis and that the oocytes had achieved a “metaphase II” arrest with a tetraploid chromosome complement. To eliminate the possibility that the Hoechst dye and/or the UV altered chromosome behavior in these time-lapse experiments, we also analyzed a large number of control and Cdc42T17N-injected oocytes fixed at different times following GVBD. These analyses confirmed that Cdc42T17N did not affect anaphase initiation but blocked the first polar body emission (see Figure S1 in the Supplemental Data available with this article online).
Figure 2.
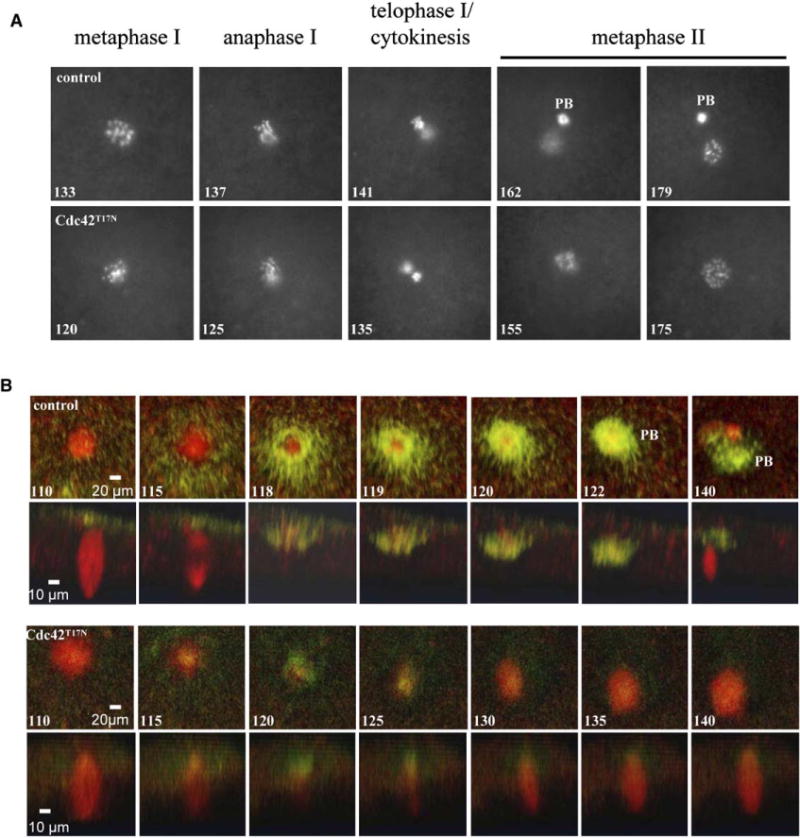
Cdc42T17N Inhibits Contractile Ring Formation
(A) En face series from time-lapse experiments showing the typical chromosomal changes in control oocytes (top) and oocytes injected with Cdc42T17N (bottom). Time lapses (in minutes) are from the first appearance of a maturation spot (i.e, GVBD = 0). PB, first polar body.
(B) Time-lapse experiments showing the F-actin (green) contractile ring in relationship with the spindle (red) in control oocytes (first row, en face series; second row, Z series). Similar experiments (third row, en face series; fourth row, Z series) reveal the lack of contractile ring in oocytes injected with Cdc42T17N, despite the correct spindle positioning and anaphase initiation. Time lapses (minutes) are from the first appearance of a maturation spot (i.e., GVBD = 0).
The apparent failure of Cdc42T17N-injected oocytes to initiate/complete cytokinesis prompted us to examine whether Cdc42T17N inhibited formation or contraction of the actomyosin-based cytokinetic contractile ring. We performed time-lapse experiments with oocytes coinjected with Alexa phalloidin and rhodamine-tubulin (Figure 2B). In control oocytes, F-actin did not accumulate in the spindle region until about 115 min after GVBD (Figure 2B). The F-actin was initially found in a broad region around and over the spindle and then narrowed as it contracted inward beneath the forming polar body (Figure 2B, top two series). From the spindle morphology, it was clear that the contractile ring formed at the end of anaphase, as indicated by the lack of microtubule signal in the central region of the spindle (Z series, 115 min). The metaphase II spindle became visible at 140 min, or about 20 min after the severing of the polar body (122 min). In Cdc42T17N-injected oocytes, although F-actin accumulation was evident at the proper time, it was far less abundant than in control cells. Further, rather than closing downward underneath the spindle and driving polar body emission, the actin ring closed over the top of the spindle (Figure 2B, bottom two series), indicating that Cdc42 activity is required not only for normal actin accumulation but also for proper direction of the force of the contractile ring.
To determine whether Cdc42 activation occurred at the time of polar body formation, we utilized the GFP-wGBD probe previously developed for visualization of Cdc42 activity in Xenopus oocytes and eggs [8]. Cdc42 activity was undetectable in GV oocytes (data not shown) or GVBD oocytes until several minutes before emission of the first polar body, at which point a faint patch of increased Cdc42 activity appeared at the animal pole overlaying the spindle microtubules (Figure 3A, 51:50). This patch increased in intensity and, within 2–4 min after the appearance of the patch, cytokinesis ensued, as the cap spread downward in concert with formation of the polar body and eventually surrounded the entire polar body as it was pinched off (Figure 3A, 63:00). This process is better appreciated in the Z series of another oocyte, taken at higher magnification (Figure 3B). Importantly, it is apparent that the point at which the Cdc42 activity starts to go up corresponds to the point where the spindle looks smallest (Figure 3A, 51:20). It looks smallest because at that point it is finally oriented and anchored more or less perpendicular to the oocyte cortex. In contrast, in the earlier time points, it is still tilted at various angles. Cdc42 activation was never observed to occur in association with spindles that were more than 15° off axis (7/7 experiments). Cdc42 activity remained high on the polar body for at least 10 min post-polar body emission but eventually subsided (data not shown). In oocytes injected with Cdc42T17N, Cdc42 activation was greatly diminished (Figure S2), consistent with our previous finding that Cdc42T17N partially inhibits Cdc42 activation during wound healing in Xenopus oocytes [9]. Furthermore, the remaining GFP-wGBD signals were more diffused and disappeared much sooner than those in control oocytes (Figure S2).
Figure 3.

Spindle-Associated Activation of Cdc42 during Meiosis I in Frog Oocytes
(A) En face series from 4D movie showing metaphase I spindle (red) and GFP-wGBD (green). An increase in wGBD signal is apparent (arrowheads, 51:20) coincident with metaphase I spindle assuming perpendicular orientation relative to the cortex.
(B) Double-label Z series of wGBD (green) and metaphase I spindle (red) during the formation of the first polar body. Active Cdc42 is initially apparent as a patch over the metaphase I spindle (00:00; equivalent to 51:20 in [A]) and then spreads downward from its concentration above the spindle (arrow, 02:00). Active Cdc42 ultimately surrounds first polar body (16:40). All times are in min:sec from the start of imaging.
We have recently demonstrated that a microtubule-dependent RhoA activity zone directs cytokinetic furrow formation in several models, including Xenopus polar body emission [2]. To determine the relationship between Cdc42 activity and RhoA activity during polar body formation, active Rho and Cdc42 were simultaneously imaged in living oocytes [9]. As previously reported [2], RhoA activity first appeared as a circular zone in the cortical region around the metaphase I spindle (Figure 4A, 3:40). Cdc42 activity, in contrast, was localized to the inside of the circular RhoA activity zone, with little or no overlap. (The small dot at 00:00, and in all en face pictures, represents nonspecific enrichment of both rGBD and wGBD probes associated with the cytoplasm in the region of the spindle, which serves as a convenient indicator of the spindle position; see [2]). The RhoA activity zone then contracted and eventually ended at midbody at the completion of cytokinesis (11:20) [2]. As the RhoA zone contracted, the Cdc42 activity patch spread down, trailing the RhoA activity zone, to eventually cover the whole polar body (Figure 4A, Z series).
Figure 4.

Coordination of RhoA and Cdc42 in Polar Body Formation
(A) Double-label 4D movie series showing eGFP-rGBD (indicating active RhoA, red) and GFP-wGBD (indicating active Cdc42, green). RhoA activity first appears as a zone around a patch of Cdc42 activity (3:40). As RhoA zone contracts to form the first polar body, Cdc42 activity spreads to cover the polar body (more evident in the Z series, 11:20). Note the lack of colocalization of rGBD and wGBD in any of the pictures. All times in min:sec from the start of imaging.
(B) The schematic (top, en face view; bottom, lateral view) depicts the relationship between RhoA activity zone (red) and Cdc42 activity zone (green) and the possible microtubule-mediated activation of the two small GTPases. Brown circles represent spindle poles (microtubule organizing centers). Black lines are microtubules, with their plus ends (+) and minus ends (−) indicated. Purple bars are separating chromosomes in late anaphase or telophase.
Thus, in the presence of a dominant-negative Cdc42T17N, oocytes are able to undergo GVBD, form a bipolar metaphase I spindle, translocate and anchor the spindle to the animal pole cortex, and initiate anaphase, but they fail to properly form and direct the actomyosin-based contractile ring and consequently fail to emit the first polar body. As active Cdc42 is first observed in the cortex in close association with one pole of the metaphase I spindle (Figure 3B), it is tempting to speculate that a protein associated with the spindle pole might be responsible for Cdc42 activation, analogous to the budding yeast mitotic exit signaling in which the spindle-pole-based Tem1 (a Ras-like GTPase) is activated by the cortex-based Lte1 (a putative Tem1 guanine nucleotide exchange factor) when the daughter’s spindle pole contacts the bud cortex. In this regard, it is worth noting that a Cdc42-specific activator, xGef [10], is found to be a binding partner for a known spindle-pole-associated protein, CPEB [11].
How does Cdc42 regulate contractile ring formation and contraction? Based on results from the wound-healing model [9, 12] we suspect that Cdc42 activity promotes formation of a region of high actin turnover, which could abet the function of a region of more stable actin templated by the RhoA zone. That is, a bordering region of rapid actin turnover could both provide more F-actin for incorporation into the contractile ring and relax the cortex on the polar body itself. This relaxation, in turn, could help ensure that the closing contractile array is directed downward rather than over the top of the spindle.
What are the signaling relationships between the RhoA and Cdc42 activity zones? The RhoA zone is also dependent on spindle microtubules [2]. Furthermore, RhoA and Cdc42 appear to be activated almost simultaneously and form nonoverlapping and complementary activity zones. We propose (Figure 4B) that RhoA is controlled by aster microtubules emanating from the spindle pole approaching the cortex, as previously suggested [2]. This notion is consistent with studies of polar body emission in clams [13] as well as demonstrations of microtubuleplus end-associated RhoA Gefs [14]. As the spindle pole makes tight contact with the overlaying cortex, Cdc42 is activated by an activator associated with the spindle pole (microtubule-minus ends). The differential activation of RhoA and Cdc42 by microtubules, together with a possible direct inhibition of RhoA by Cdc42 [15], could explain the complementary and mutually exclusive temporal relationship of RhoA and Cdc42 activity zones during polar body formation. The complementary relationship between Cdc42 and RhoA reported here in polar body emission is similar to that in single-cell wound healing in which Cdc42 activity and RhoA activity form concentric but nonoverlapping zones, driving wound closure [9]. Further, segregation of the RhoA and Cdc42 zones during wound healing is microtubule dependent, suggesting the existence of a mechanism for microtubule-dependent control of rho GTPase activity zones that is broadly conserved in asymmetric cell division as well as wound repair.
Conclusion
We conclude that Cdc42, together with RhoA, couples asymmetric spindle positioning to cytokinesis in first polar body formation during Xenopus oocyte maturation. Our previous study [2] has indicated that microtubule-dependent RhoA activity defines the cytokinetic contractile ring. In this study, we provide the first evidence that spindle pole-cortex contact activates cortical Cdc42. The Cdc42 activity zone directly overlays the spindle pole on the inside of the RhoA activity ring. The complementary nature of the two GTPases thus explains how spindle pole attachment to the cortex is mechanistically coupled to the formation, and the contraction, of the cytokinetic contractile ring during polar body formation. We believe that in contrast to the role of RhoA in cytokinesis, which is likely universal [2], the function of Cdc42 in cytokinesis discovered here is unique for polar body formation and perhaps some other forms of asymmetric cell division (such as budding yeast mitosis).
Supplementary Material
Acknowledgments
We thank the following colleagues for reagents: Dr. Nathalie Lamarche for Cdc42 and Rac cDNA, Dr. Alan Hall for C3 cDNA, Dr. Thierry Lorca for anti-xFZY, and Dr. James Maller for anti-cyclin B2. Dr. Andrew Ridsdale provided invaluable advice on confocal imaging at OHRI in Ottawa. This work was supported by operating grants from the Canadian Institute of Health Research (MT15381) and from National Cancer Institute of Canada (to X.J.L.) and from the National Institute of Health (to W.M.B.). X.J.L. holds a Premier’s Research Excellence Award from the province of Ontario.
Footnotes
The Supplemental Data for this article, including Supplemental Experimental Procedures and figures, can be found online at http://www.current-biology.com/cgi/content/full/16/2/214/DC1/.
References
- 1.Hofken T, Schiebel E. Novel regulation of mitotic exit by the Cdc42 effectors Gic1 and Gic2. J Cell Biol. 2004;164:219–231. doi: 10.1083/jcb.200309080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cau J, Faure S, Vigneron S, Labbe JC, Delsert C, Morin N. Regulation of Xenopus p21-activated kinase (X-PAK2) by Cdc42 and maturation-promoting factor controls Xenopus oocyte maturation. J Biol Chem. 2000;275:2367–2375. doi: 10.1074/jbc.275.4.2367. [DOI] [PubMed] [Google Scholar]
- 4.Gerhart J, Wu M, Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taieb FE, Gross SD, Lewellyn AL, Maller JL. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr Biol. 2001;11:508–513. doi: 10.1016/s0960-9822(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 6.Gard DL, Cha BJ, Roeder AD. F-actin is required for spindle anchoring and rotation in Xenopus oocytes: a re-examination of the effects of cytochalasin B on oocyte maturation. Zygote. 1995;3:17–26. doi: 10.1017/s0967199400002331. [DOI] [PubMed] [Google Scholar]
- 7.Maro B, Verlhac MH. Polar body formation: new rules for asymmetric divisions. Nat Cell Biol. 2002;4:E281–E283. doi: 10.1038/ncb1202-e281. [DOI] [PubMed] [Google Scholar]
- 8.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5:727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 9.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reverte CG, Yuan L, Keady BT, Lacza C, Attfield KR, Mahon GM, Freeman B, Whitehead IP, Hake LE. XGef is a CPEB-interacting protein involved in Xenopus oocyte maturation. Dev Biol. 2003;255:383–398. doi: 10.1016/s0012-1606(02)00089-1. [DOI] [PubMed] [Google Scholar]
- 11.Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell. 2000;103:435–447. doi: 10.1016/s0092-8674(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 12.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154:785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pielak RM, Gaysinskaya VA, Cohen WD. Formation and function of the polar body contractile ring in Spisula. Dev Biol. 2004;269:421–432. doi: 10.1016/j.ydbio.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Rogers SL, Wiedemann U, Hacker U, Turck C, Vale RD. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr Biol. 2004;14:1827–1833. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 15.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


