Abstract
We established a model of immune-mediated bone marrow (BM) failure in C57BL/6 (B6) mice with 6.5 Gy total body irradiation (TBI) followed by the infusion of 4–10 × 106 lymph node (LN) cells/recipient from FVB/N (FVB) donors. Forty-three percent animals succumbed, with surviving animals showing marked declines in blood neutrophils, red blood cells, platelets and total BM cells at 8 to 14 days following LN cell infusion. Lowering the TBI dose to 5 Gys or altering the LN source from FVB to BALB/cBy donors failed to produce BM destruction. Affected animals showed significant expansion and activation of CD8 T lymphocytes in both the blood and BM; cytotoxic T cells had elevated Fas ligand expression and were oligoclonal mainly displaying Vβ7 and Vβ17 T cell receptors. There were significant increases in blood plasma interferon gamma and tissue necrosis factor alpha in affected animals. Chemokine ligands CCL3, CCL4, CCL5, CCL20, CXCL2, CXCL5 and hematopoietic growth factors G-CSF, M-CSF, GM-CSF, VEGF were also elevated. In B6 mice carrying Fas gene knockout, BM failure was attenuated when they were infused with FVB LN cells. Our model establishes a useful platform to define the roles of individual genes and their products in immune-mediated BM failure.
Aplastic anemia (AA), the paradigm of bone marrow (BM) failure syndromes, anemia, neutropenia, and thrombocytopenia occur with a hypocellular and a regenerative BM [1]. While the etiology is unclear in most cases, most AA patients respond to immunosuppressive therapy [2–5], implicating the destruction of hematopoietic stem cells (HSCs) and progenitors by the immune system [6]. The immune mechanism was also supported by laboratory observations in which Th1 immune responses cytokine gamma interferon (IFN-γ) suppressed hematopoiesis [7,8], while immunosuppressive agents modulated effector to regulatory T cell conversion and Fas/FasL interaction to affect immune-mediated cell destruction [9–11].
BM failure had been successfully modeled in rodent animals by the infusion of allogeneic lymph node (LN) cells from donors mismatched at major histocompatibility complex (MHC) or minor-histocompatibility (minor-H) antigens [12,13]. Barnes and Mole produced the first mouse model of immune-mediated AA by infusing 1–10 × 106 LN cells from C3H donors into CBA/H recipients pre-irradiated at 450 – 600 rads of total body irradiation (TBI). Fatal AA developed in recipient animals, with reduced blood cell counts and an empty BM. Allogeneic LN cells were responsible for the pathology, since TBI alone or TBI plus infusion of irradiation-inactivated LN cells from the same source were ineffective in producing BM damage [12]. This pioneer work was extended to other strain combinations in different experimental settings to successfully recapitulate the major pathophysiological features of BM failure and to enable the study of disease mechanisms testing of therapeutic interventions [14–18].
We produced two mouse models using TBI plus allogeneic LN cell infusion approaches [19–21]. First, MHC heterozygous hybrid B6D2F1 and CByB6F1 mice carrying H2b/d were given 5 Gy TBI and an infusion of 5 × 106 LN cells from parental C57BL/6 (B6) donors (H2b/b). Pancytopenia and marrow hypoplasia developed within two to three weeks with pathological features mimicking human AA [19]. We then tested TBI plus B6 LN cell infusion into MHC-matched (H2b/b), minor-H mismatched, C.B10 recipients, and this specific strain combination also produced fatal BM failure [21]. In these models, BM destruction was mediated by expanded and activated donor T lymphocytes that targeted host BM cells [20]. Fas and Fas ligand (FasL)-associated cell death was the major pathway responsible for elimination of HSCs, hematopoietic progenitors, and other BM cellular components [22]; the perforin-granzyme B pathway played a minor role [23]. While a Th17 response was active early [24], Th1 cells were most important in mediating massive BM destruction [25,26]. Recent reports from others have provided new evidence of modulation of T-bet expression by Notch 1 and Ezh2 expression and the functional role of regulatory Th1 immune responses [27,28].
In the current study, we sought to model immune-mediated BM failure in B6 mice, as B6 are widely used in biomedical research especially for the development of transgenic and “knockout” animals. Our goal was to establish an experimental platform to test the roles of individual genes and molecules in immune-mediated marrow destruction. We successfully induced BM failure in B6 mice with 6.5 – 7.0 Gy TBI plus the infusion of 4–10 × 106 LN cells from FVB/N (FVB) donors. Recipient B6 mice developed severe pancytopenia and marrow hypocellularity. Oligoclonal expansion and activation of donor lymphocytes was characteristic. Affected animals also showed elevations in plasma inflammatory cytokines, chemokine ligands, and hematopoietic growth factors typical to marrow failure. We tested the utility of the model in mice deficient in Fas gene expression and found that BM failure was significantly attenuated, consistent with current hypothesis underling the pathophysiology of BM failure.
Materials and methods
Animals and induction of BM failure
Inbred B6, BALB/cBy (BALB) and FVB/NJ (FVB) mice, as well as induced mutants C57BL/6-Prf1tm1Sdz/J (Pfr−/−) and B6.MRL-Faslpr/J (Fas−/−) mice, were all obtained from the Jackson Laboratory (Bar Harbor, ME, USA), and were bred and maintained in NIH animal facilities under standard care and nutrition. Young adult male and female mice were used at two to ten months of age. All animal studies were approved by the Institutional Animal Care and Use Committee at the National Heart, Lung, and Blood Institute.
Inguinal, axillary, and lateral axillary LN were collected from FVB or BALB donors, homogenized with a mini-tissue grinder (A. Daigger & Company, Vernon Hills, IL, USA) in Iscove’s Modified Dullbecco’s Medium (IMDM, Life Technologies Corporation, Grand Island, NY, USA), washed, centrifuged, filtered through 90µM nylon mesh (Small Parts, Miami Lake, FL, USA), and counted by a Vi-Cell counter (Counter Cooperation, Hialeah, FL, USA). Diluted LN cells were injected through lateral tail vein to B6, Fas−/− or Prf−/− recipient mice at 4–10 × 106 cells/recipient in 400–500 µL IMDM. Recipients were pre-irradiated with 5, 6.5 or 7 Gy TBI using a 137cesium gamma source (J. L. Shepherd & Associates, Glandale, CA, USA). Recipients were bled and euthanized 8–14 days after LN cell infusion to obtain tissues for histological and cytological analyses.
Blood counts and flow cytometry
Blood was collected from the retro-orbital sinus into EDTA-added Eppendorf tubes. Complete blood counts (CBC) were performed in a HemaVet 950 analyzer (Drew Scientific, Inc. Waterbury, CT, USA). Plasma was separated by centrifugation at 8000 g for 5 minutes, and was stored at −30°C. After mouse euthanasia by CO2 inhalation, BM cells were extracted from bilateral tibiae and femurs, filtered through 90µM nylon mesh, and counted in a Vi-Cell counter. Peripheral blood leukocytes and BM cells were first incubated with ACK buffer twice for 10 minutes in order to lyse red blood cells (RBCs). Residual leukocytes were stained with various antibodies and analyzed on a LSR II or Canto II flow cytometer using the FACSDiva software (Becton Dickson, San Diego CA). To measure cell apoptosis, cells were first stained with an antibody mixture along with Annexin V in specific high calcium buffer using reagents from an Annexin V apoptosis detection kit from BD Biosciences, and were then added with 7AAD 10 minutes before cell acquisition.
Monoclonal antibodies for murine CD3 (clone 145-2C11), CD4 (clone GK 1.5), CD8 (clone 53-6.72), CD11a (clone 2D7), CD11b (clone M1/70), CD95 (Fas, clone Jo2), CD117 (c-Kit, clone 2B8), CD178.1 (FasL, clone MFL3), erythroid cells (clone Ter119), granulocytes (Gr1/Ly6-G, clone RB6-8C5), and stem cell antigen 1 (Sca-1, clone E13-161) were from BD-Biosciences (San Diego, CA). The anti-mouse T cell receptor β variable region antibody panel was also obtained from BD Biosciences. Anti-mouse CD45R (B220, clone RA3-6B2) was from Biolegend (San Diego, CA). Antibodies were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-cyanin 5 (PE-Cy5), phycoerythrin-cyanin 7 (PE-Cy7), allophycocyanin (APC) or allophycocyanin 7 (APC-Cy7).
Pathology and histology
Mice treated with 6.5 Gy TBI + 4–10 × 106 FVB LN cells or with 6.5 Gy TBI only were euthanized at days 12–14. Lung, liver, kidney, intestine, spleen and sternum tissues were fixed in 10% neutral buffered formalin, sectioned at 5µ thickness, and stained with H&E (VivoVitro Biotechnology Inc., Rockville, MD, USA). Slides were examined under a Zeiss Axioskop2 plus microscope and images were captured at 20 × magnification using a Zeiss AxioCam HRC camera (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Luminex assays for plasma cytokines
A premixed 39-plex kit was obtained from R & D Systems, Inc (Minneapolis, MN, USA). Plasma samples were filtered and loaded onto 96-well plates, and were incubated and washed according to the protocol from manufacturer. A minimum of 50 beads per analyte was acquired. Median fluorescence intensities were collected on a Luminex-200 instrument using Bio-Plex Manager software version 6.2 (Bio-Rad Laboratories Inc., Hercules, CA, USA). Standard curves for each cytokine were generated using the premixed lyophilized standards provided in the kit. Cytokine concentrations in samples were determined from the standard curve using a 5-point-regression to transform mean fluorescence intensities into concentrations. Each sample was run in duplicate and the average of the duplicates was used as the measured concentration.
Statistical analysis
JMP statistical discovery software (SAS Institute Inc, Cary, NC) was used to analyze CBC and BM cellular composition data through variance analysis with the compare all mean option for multiple comparisons [29]. Plasma cytokines were compared between TBI+FVB LN treated and TBI only animals using the Mann-Whitney test with Prism 6, as described earlier [30]. For both types of analyses, statistical significance was declared at P<0.05 and P<0.01 respectively.
Results
Induction of BM failure in B6 mice
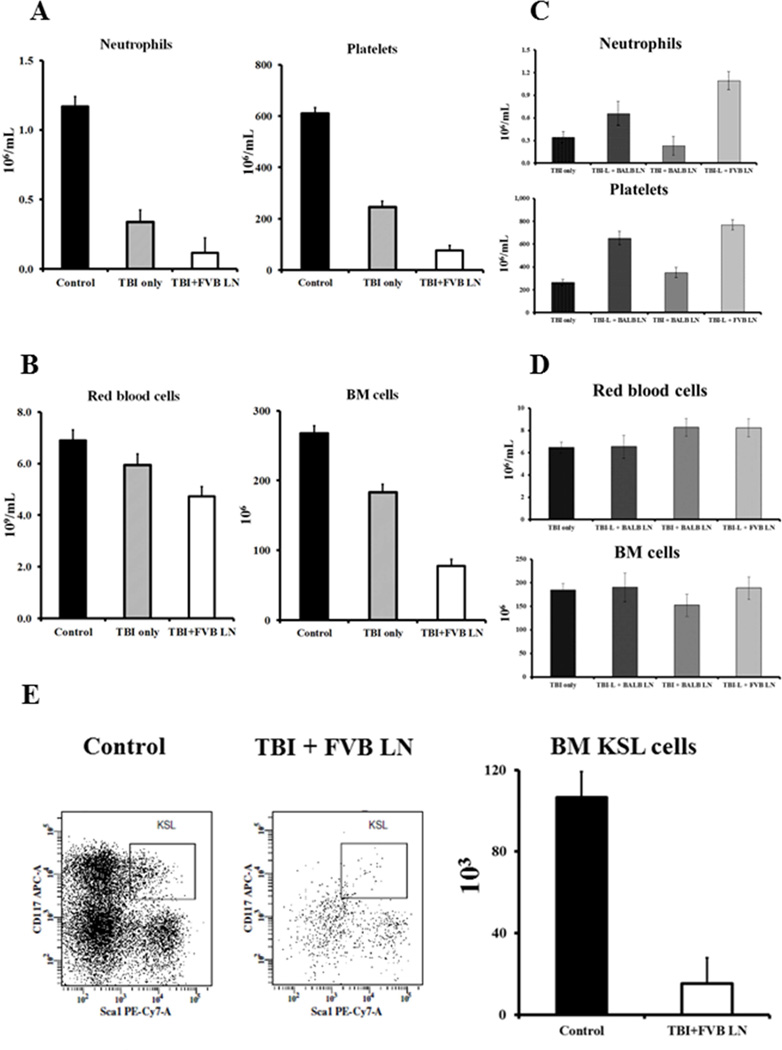
Infusion of 4–10 × 106 LN cells from FVB donors into normal B6 mice pre-irradiated with 6.5 Gy TBI (TBI + FVB LN) produced severe BM failure in recipients. We found 43% (19/44) animals succumbed between 8 and 14 days following LN cell infusion, and surviving animals had severe pancytopenia and marrow hypoplasia with significant declines in neutrophils (P>0.05) and platelets (P<0.01) (Fig 1A), as well as in red blood cells (RBCs, P<0.01) and total BM cells (P<0.01) (Fig 1B), when compared to TBI only animals or untreated controls. There were also declines in lymphocytes (P>0.05), white blood cells (WBCs, P>0.05), hemoglobin (Hb, P<0.01), hematocrit (HCT, P<0.01), and mean corpuscular volume (MCV, P<0.01) in TBI + FVB LN treated animals (data not shown). We were unable to induce marrow failure when we reduced the TBI dose to 5 Gys (TBI-L) or when we replaced FVB with BALB mice as LN cell donors: the TBI-L + BALB LN, TBI + BALB LN, and TBI-L + FVB LN treatment groups showed no cytopenia (Fig 1C) and no change in total BM cells (Fig 1D). FVB LN cells were tested at 4, 5, 8 and 10 × 106 cells per mouse respectively in combination with 6.5 Gy TBI. All cell doses were effective in producing marrow failure in B6 mice. Thus, we used 6.5 Gy TBI + 5 × 106 cells (TBI + FVB LN) as the standard regiment for induction of BM failure in B6 mice.
Figure 1.
Development of pancytopenia and BM hypoplasia. Normal B6 mice that received 6.5 Gy TBI + 4–10 × 106 FVB LN (TBI + FVB LN, N=25) treatment developed neutropenia and thrombocytopenia with significant declines in neutrophils (P<0.01) and platelets (P<0.01) (A). Affected mice also displayed anemia and BM hypoplasia with significant declines in RBCs (P<0.01) and total BM cells (P<0.01) (B) relative to mice that received no treatment (Control, N=21) or mice that received 6.5 Gys TBI without LN cell (TBI only, N=19). In parallel experiments, mice treated with 5.0 Gys TBI + 5 × 106 BALB LN (TBI-L + BALB LN, N=3), 6.5 Gy TBI + 5 × 106 BALB LN (TBI + BALB LN, N=5), or 5.0 Gys TBI + 5 × 106 FVB LN (TBI-L + FVB LN, N=5) showed no significant change in neutrophils and platelets (C), or RBCs and total BM cells (D). TBI + FVB LN treated mice (N=3) showed a significant reduction in the proportion (P<0.05) and total number (P<0.01) of BM KSL cell relative to untreated (N=3) controls (E).
To verify damage to HSCs and progenitors, we analyzed BM c-Kit+Sca-1+Lin− (KSL) cells. TBI + FVB LN treatment caused significant decline (P<0.05) in the proportion of BM KSL cells (0.018 ± 0.006%) relative to those in untreated B6 (0.047 ± 0.006%) controls (Fig 1E). This change, along with a significant decline in total BM cells, resulted in a seven-fold reduction in total BM KSL cells in BM failure mice (Fig 1E).
Clonal T cell expansion
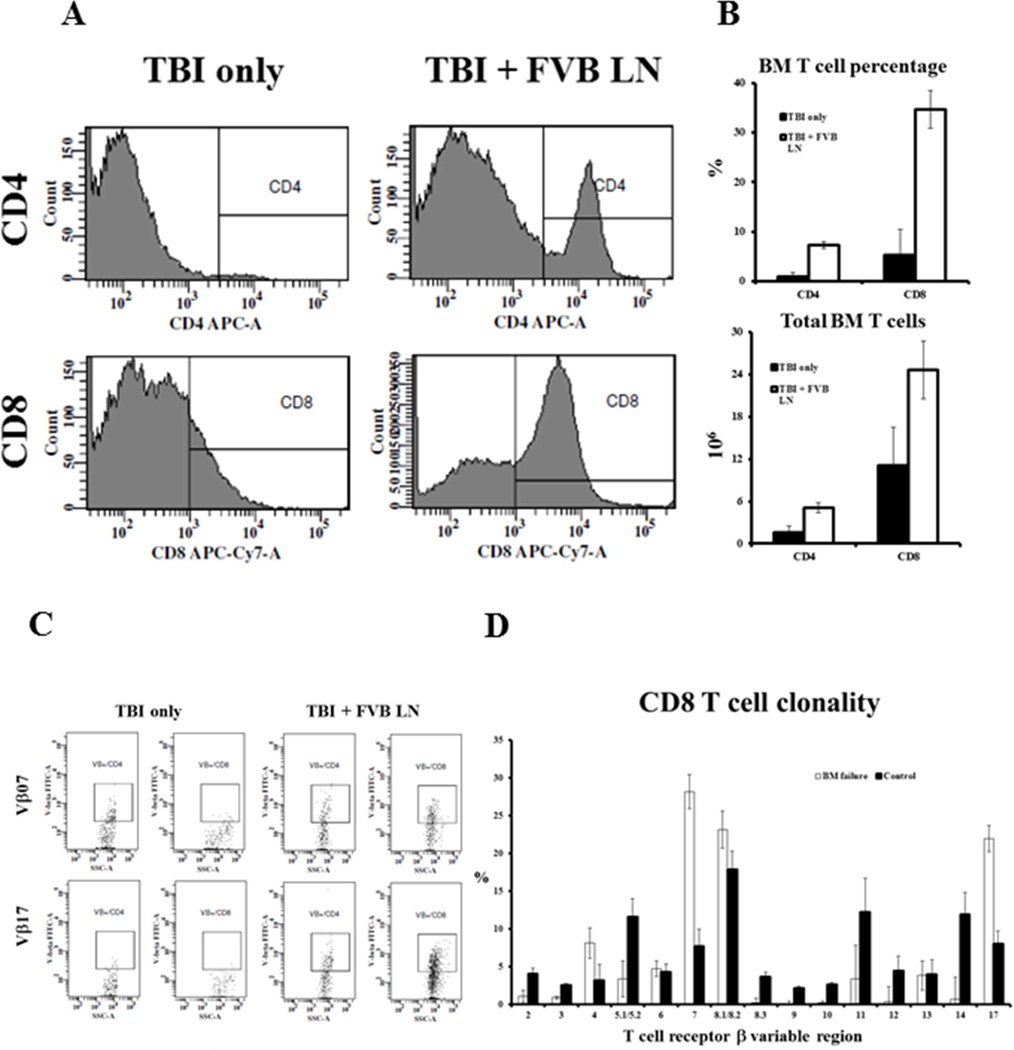
A characteristic feature of immune-mediated BM failure is T cell-mediated destruction of BM hematopoietic cells. In this new model, we found greatly-increased proportions of BM CD4 and CD8 T cells in TBI + FVB LN treated mice (Fig 2A). On average, CD4 T cells increased 8-fold (P<0.01) while CD8 T cells increased 7-fold (P<0.01) in the BM of TBI + FVB LN treated animals (Fig 2B). Even considering the decline in total BM cells, there was a 3-fold (P<0.05) increase in total CD4 cells and a 2-fold increase in total CD8 cells (P<0.05) in TBI + FVB LN-treated animals relative to TBI-only controls (Fig 2B). We further examined BM CD4 and CD8 T cell β variable region (Vβ) representation. Among the 15 Vβ groups, Vβ 7 and Vβ 17a were consistently up-regulated in TBI + FVB LN treated animals (Fig 2C). In CD4 T cells, Vβ 7 increased from 16.5 ± 4.7% to 23.4 ± 4.7% (P>0.05) while Vβ 17a increased from 12 ± 1.9% to 21 ± 1.9% (P<0.05) in TBI + FVB LN cell treated animals (data not shown). In CD8 T cells, the Vβ 7 proportion increased from 7.7 ± 2.3 % to 28 ± 2.3% (P<0.01), and the Vβ 17a proportion increased from 8.0 ± 1.8% to 22 ± 1.8% (P<0.01), in TBI + FVB LN cell infused animals relative to TBI-only controls (Fig 2D). Over representation of Vβ 7 and Vβ 17a CD4 and CD8 T cells indicated oligoclonal T cell expansion in this immune-mediated BM failure model.
Figure 2.
Oligoclonal T cell expansion. Proportions (A) and total numbers (B) of CD4 (P<0.01 and P<0.05) and CD8 (P<0.01 and P<0.05) T cells were significantly increased in the BM of mice that received TBI + FVB LN (N=21) treatment than those that received TBI only (N=12). The expanded T cells in the BM of TBI + FVB LN treated mice (N=3) had distinctive Vβ 7 and Vβ 17a over representation relative to TBI only controls (N=3) (C). Percentages of Vβ 7 (P<0.01) and Vβ 17a (P<0.01) CD8 T cells were significantly higher in the BM of TBI + FVB LN treated animals than in TBI only controls (D).
T cell activation and Fas-Fas ligand expression
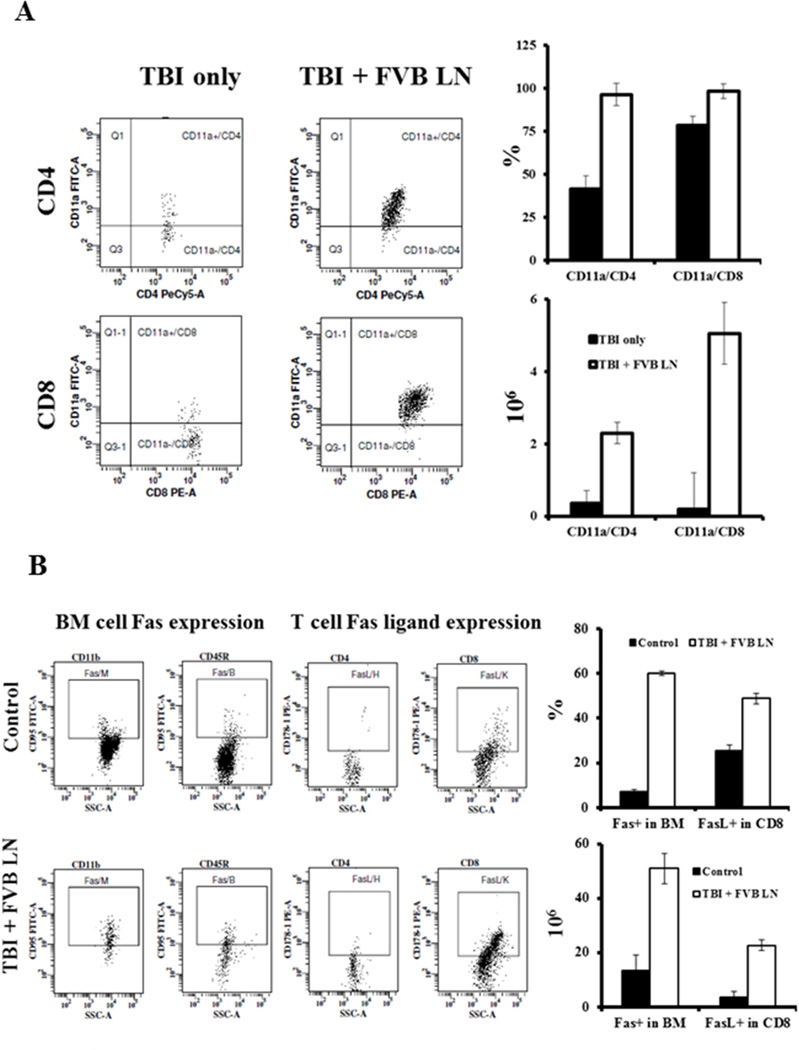
In addition to CD4 and CD8 T cell expansion, there was also marked up-regulation of T cell activation, as infiltrates showed high proportions of CD4 and CD8 T cells expressing the T cell activation marker CD11a (Fig 3A). In the BM of TBI + FVB LN-treated animals, 96 ± 6.4% CD4 T cells and 98 ± 4.3% CD8 T cells were CD11a+, significantly higher (P<0.01 and P<0.05, respectively) than those in TBI only controls. In absolute terms, total number of CD4+CD11a+ T cells was 6.5-fold higher (P<0.01), while total number of CD8+CD11a+ T cells was 27-fold higher (P<0.01), in the BM of TBI + FVB LN-treated mice (Fig 3A). T cell CD11a expression was also up-regulated in the peripheral blood of TBI + FVB LN treated animals (data not shown), although to a lesser degree than seen in BM.
Figure 3.
T cell activation and enhanced Fas/Fas ligand expression. In addition to a significant T cell expansion in the BM, B6 mice that received TBI + FVB LN treatment (N=10) also had significantly higher proportions of CD11a+ cells, a marker of T cell activation, in both CD4 (P<0.01) and CD8 (P<0.05) subsets, causing net gains of CD11a+CD4+ (P<0.01) and CD11a+CD8+ (P<0.01) T cells in the BM relative to TBI only controls (A). Residual BM cells from TBI + FVB LN treated mice (N=3) showed elevated Fas expression (P<0.01), while BM T cells, especially CD8 T cells, showed higher level of Fas ligand expression (P<0.01) relative to BM cells from untreated control animals (B).
To define changes relevant to BM destruction, we examined the expression of Fas and FasL. Fas expression was up-regulated on all BM cell fractions in TBI + FVB LN treated animals, in which Fas+ total BM cells was at 60 ± 0.9%, 8.3-fold higher (P<0.01) than that in TBI only (7.2 ± 0.9%) controls (Fig 4B). In the fraction of expanded BM T cells from TBI + FVB LN-treated mice, 49 ± 2.5% CD8 T cell expressed FasL, 2-fold higher (P<0.01) than the 25 ± 2.5% FasL-expressing BM CD8 T cells in TBI-only animals (Fig 3B). FasL expression on expanded BM CD4 T cells, surprisingly, was not up-regulated in BM failure animals (Fig 3B). Overall, there were 3.8-times more (P<0.01) Fas+ BM cells and 6.2-times more (P<0.01) FasL+ CD8 T cells, in the BM of TBI + FVB LN cell-infused animals relative to TBI only controls (Fig 3B).
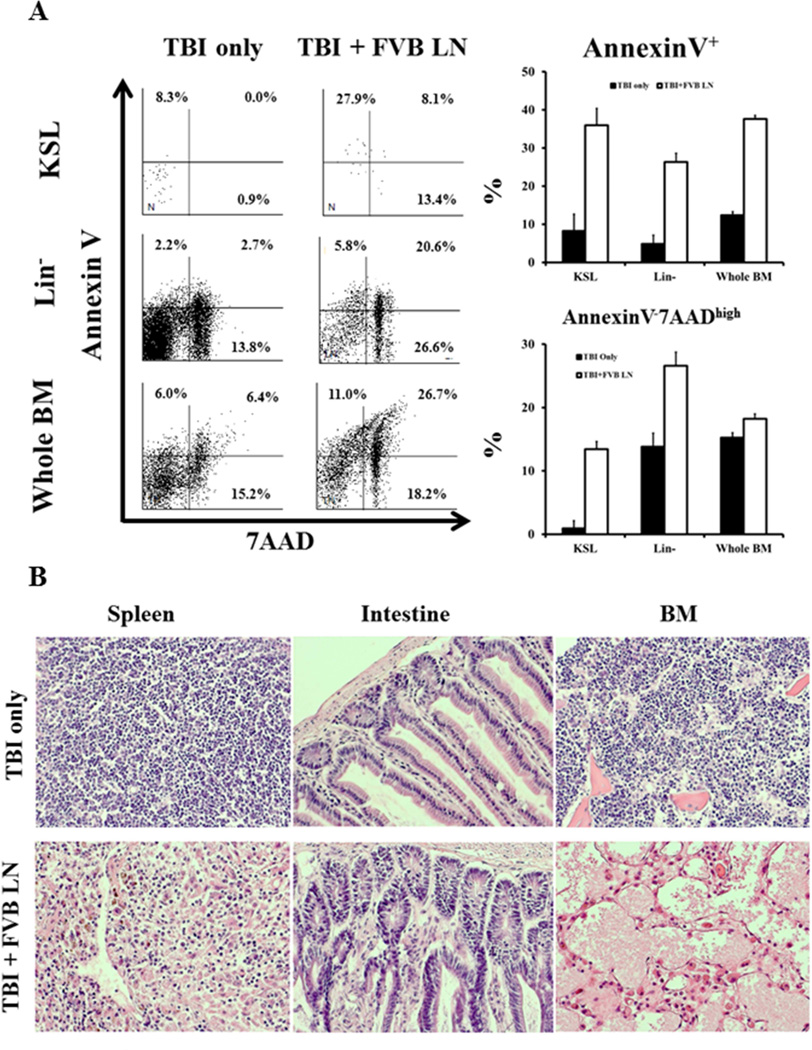
Figure 4.
Elevation in hematopoietic cell apoptosis and BM destruction. BM cells from TBI + FVB LN treated mice (N=5) had significantly higher proportions of Annexin V+ (including both 7AADhigh and 7AADlow) apoptotic cells in KSL (P<0.01), Lin− (P<0.01) and whole BM (P<0.01) cell fractions relative to TBI only (N=5) controls. TBI + FVB LN treated mice also had significantly higher proportions of Annexin V−7AADhigh dead cells in KSL (P<0.01), Lin− (P<0.01) and whole BM (P<0.05) cells (A). Spleen, intestine, and sternum tissues from TBI only (N = 5) and TBI + FVB LN (N = 6) treated B6 mice were sectioned and hematoxylin & eosin stained for histological observations. In comparison to TBI only controls, TBI + FVB LN cell treated mice had mild to moderate inflammation with lymphocyte infiltration in the spleen and intestines, along with severe BM damage showing empty marrow space (B).
Increased apoptosis and BM cell destruction
Up-regulation of Fas expression on BM cells and expansion of FasL+CD8 T cells suggested that the Fas/FasL pathway mediated BM destruction by increasing cell apoptosis. Indeed, BM cells from TBI + FVB LN cell-treated mice had significantly higher proportions of KSL (P<0.01), Lin− (P<0.01) and whole BM (P<0.01) cells that entered apoptosis showing membrane binding to Annexin V (including both 7AADlow and 7AADhigh cells) relative to the same BM cell fractions from TBI only animals (Fig 4A). In addition, proportion of Annexin V−7AADhigh dead cells was also significantly higher in KSL (P<0.01), Lin−(P<0.01) and whole BM (P<0.05) cells from TBI+FVB LN cell-infused animals than from TBI only controls (Fig 4A).
TBI + FVB LN cell infusion caused mild to moderate inflammation in the spleen and intestines, with disappearance of the germinal centers and mild infiltration of lymphocytes (Fig 4B). However, the major pathology was the elimination of KSL cells, Lin- cells and other cellular elements in the BM (Fig 4B).
Alterations in plasma cytokines
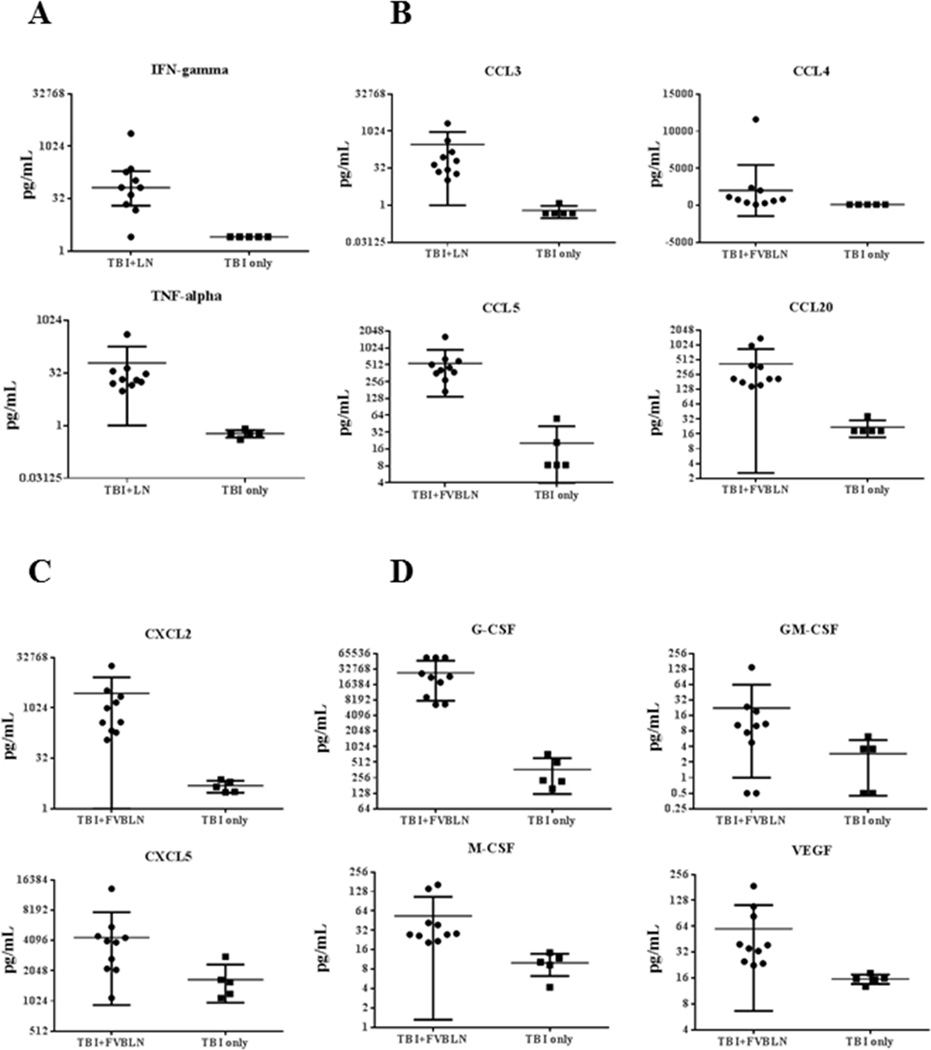
Development of BM failure was associated with changes in blood plasma cytokine levels as measured in a 39-plex Luminex assay. Most notable were a 26-fold increase in IFN-γ (P<0.01) and a 34-fold increase in TNF-α (P<0.01) in BM failure animals (Fig 5A). Also significantly increased in the plasma of BM failure mice were the chemokine (C-C motif) ligands CCL3 at 112-fold (P<0.01), CCL4 at 6-fold (P<0.01), CCL5 at 52-fold (P<0.01), and CCL20 at 11-fold (P<0.01), relative to TBI-only controls (Fig 5B). Two chemokine (C-X-C motif) ligands, CXCL2 and CXCL5, increased 154-fold (P<0.01) and 2.5-fold (P<0.05), respectively, in FVB LN cell-infused animals (Fig 5C). BM failure mice also had significant elevations in hematopoietic growth factors: G-CSF (100-fold, P<0.01), GM-CSF (2.8-fold, P<0.05), M-CSF (2.7-fold, P<0.01), and VEGF (2.4-fold, P<0.01) (Fig 5D). There were also significant increases in 12 other plasma cytokine concentrations in BM failure mice relative to TBI-only controls (Table 1). Three cytokines, CCL21, IGF-I and IL-13, were significantly down-regulated (Table 1), while 12 other cytokines, including IL-17a, showed no significant change during the development of BM failure (Table 2).
Figure 5.
Up-regulation in inflammatory cytokines, chemokines and hematopoietic growth factors. Luminex measurements of blood plasma cytokine concentrations revealed significantly increased inflammatory cytokines IFN-γ (P<0.05) and TNF-α (P<0.01) (A); chemokine (C-C motif) ligands CCL3 (P<0.01), CCL4 (P<0.01), CCL5 (P<0.01) and CCL20 (P<0.01) (B); chemokine (C-X-C motif) ligand CXCL2 (P<0.01) and CXCL5 (P<0.05) (C); and hematopoietic growth factors G-CSF (P<0.01), GM-CSF (P<0.05), M-CSF (P<0.01), and VEGF (P<0.01) (D) in BM failure mice that received FVB LN cell infusion (N = 10) in comparison to animals that received TBI only (N = 5).
Table 1.
Plasma cytokines significantly up or down regulated during BM failure
| Cytokines | TBI+FVB LN | TBI only | Statistics |
|---|---|---|---|
| N | 10 | 5 | |
| IL-6 (pg/mL) | 416.6 (38.2 – 74301.9) | 32.6 (31.4 – 71.2) | P<0.01 |
| IL-10 (pg/mL) | 285 (48 – 9805) | 3.2 (3.2 - 3.2) | P<0.01 |
| IL-12P70 (pg/mL) | 82.6 (28.0 – 386.2) | 39.5 (21.7 – 69.5) | P<0.05 |
| IL-23P19 (pg/mL) | 471.5 (74.1 – 10011.7) | 8.8 (8.8 – 10.0) | P<0.01 |
| CD32 (pg/mL) | 950 (504 – 2624) | 34.6 (22.5 – 50.0) | P<0.01 |
| CD257 (pg/mL) | 79633 (35395 – 118554) | 24486 (21582 – 26213) | P<0.01 |
| FGF21 (pg/mL) | 2223 (134 – 54176) | 231.9 (15.2 – 379.6) | P<0.01 |
| JE (pg/mL) | 4105 (899 – 90710) | 92.8 (92.8 - 92.8) | P<0.01 |
| KC (pg/mL) | 5292 (7 – 21884) | 682.7 (162.7 – 1203.0) | P<0.05 |
| Lipocalin-2 (pg/mL) | 43253 (43253 – 43253) | 37596 (23086 – 43253) | P<0.01 |
| PC-9 (pg/mL) | 194214 (120027 – 289800) | 49992 (37583 – 54746) | P<0.01 |
| CCL21 (pg /mL) | 62114 (52973 – 111122) | 118165 (81457 – 131254) | P<0.05 |
| IGF-1 (pg/mL) | 250.6 (23.4 – 2824.2) | 2607 (1474.9 – 6344.5) | P<0.01 |
| IL-13 (pg/mL) | 143.2 (12.8 – 315.1) | 279.5 (188.7 – 354.0) | P<0.05 |
Data shown as Median (range) with statistical significance based on Mann-Whitney test
Table 2.
Plasma cytokines not significantly affected during BM failure
| Cytokines | TBI+FVB LN | TBI only |
|---|---|---|
| N | 10 | 5 |
| CXCL12 (pg/mL) | 948.6 (658.6 – 2008.5) | 712.7 (544.9 – 1499.8) |
| Epo (pg/mL) | 1939 (457 – 25137) | 1384 (660 – 2736) |
| FGF-b (pg/mL) | 504.3 (108.3 – 1200.9) | 196.4 (125.0 – 289.7) |
| IL-1a (pg/mL) | 151.6 (68.8 – 2107.1) | 108.2 (76.5 – 137.8) |
| IL-1b (pg/mL) | 37.5 (37.5 – 3335.6) | 37.5 (37.5 - 37.5) |
| IL-2 (pg/mL) | 4.6 (0.3 – 16.2) | 3.4 (0.3 – 17.4) |
| IL-4 (pg/mL) | 233.8 (152.2 – 258.1) | 215.2 (193.2 – 236.9) |
| IL-5 (pg/mL) | 9.5 (2.0 – 43.6) | 10.9 (10.3 – 18.3) |
| IL-17a (pg/mL) | 21 (21.0 – 82.2) | 21 (21.0 - 21.0) |
| IL-33 (pg/mL) | 9.5 (9.5 – 141.2) | 9.5 (9.5 - 9.5) |
| MMP-9 (pg/mL) | 1571 (710 – 3590) | 3446 (2501 – 3972) |
| Resistin (pg/mL) | 11271 (1684 – 25720) | 18752 (13258 – 23863) |
Data shown as Median (range) values. There was no statistical significance between TBI+FVB LN and TBI only animals in any of the cytokines listed in this table based on Mann-Whitney test
Attenuated BM failure in Fas−/− mice
To illustrate the usefulness of the B6 BM failure model, we infused FVB LN cells into sublethally irradiated (6.5 Gy TBI) Fas−/− and Prf−/− mice. As anticipated, Prf−/− mice developed severe BM failure, in which all mice were dead at day 10 following FVB LN cell infusion (No CBC data was collected and no cellular analysis was performed for these animals; data not shown).
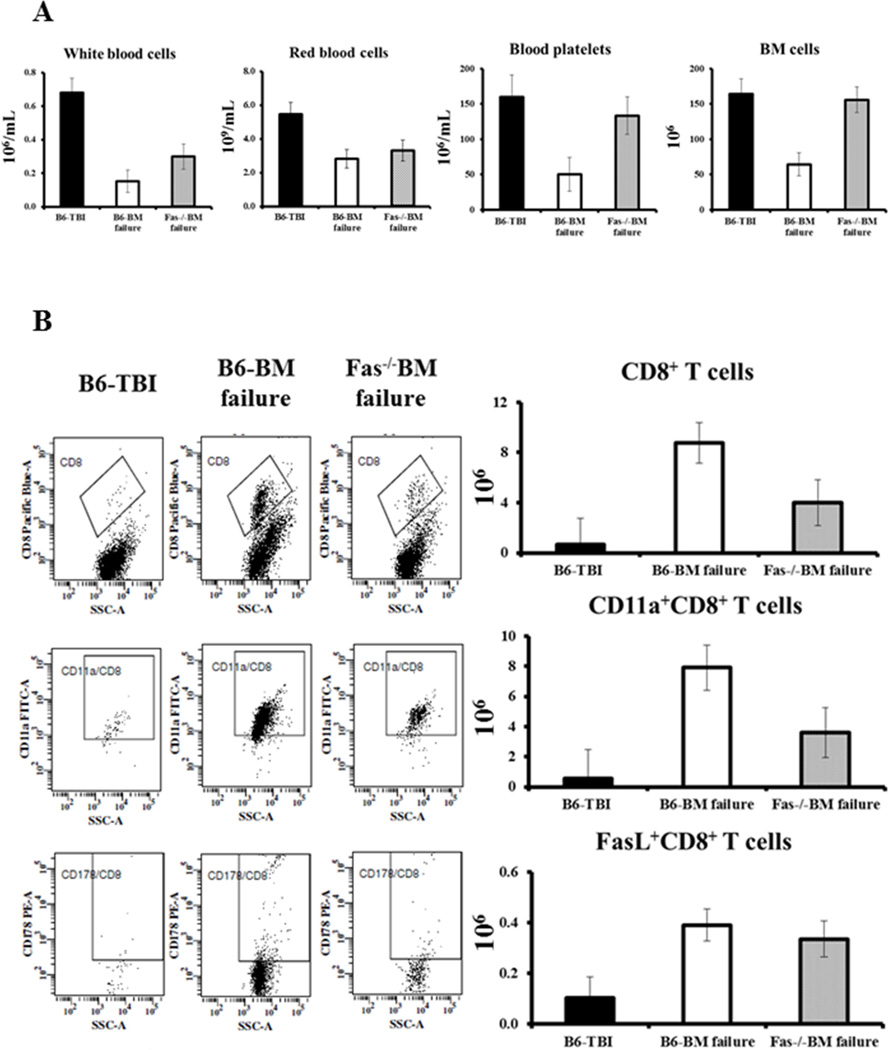
After 6.5 Gy TBI, the infusion of 5 × 106 FVB LN cells caused different levels of pancytopenia and BM failure in B6 and Fas−/− mice. In B6 mice, LN cell infusion caused a 78% decline in WBCs (P<0.01), 48% decline in RBCs (P<0.05), 69% decline in platelets (P<0.05), and 61% decline in total BM cells (P<0.01), relative to TBI-only controls (Fig 6A). The declines in these cellular components were much less severe in Fas−/− mice, at 56%, 40%, 17% and 5% respectively (Fig 6A). The reduced BM damage was associated with fewer CD8 T cells (P<0.05) and fewer CD11a+CD8+ T cell (P<0.05) in the BM of Fas−/− mice (Fig 6B). Of interest, BM FasL+ CD8 T cell numbers were relatively similar in B6 BM failure and Fas−/−BM failure mice (Fig 6B).
Figure 6.
Attenuation of FVB LN cell-induced BM failure in Fas−/− mice. We infused FVB LN cells into sublethally-irradiated (6.5 Gy TBI) normal B6 mice (B6-BM failure, N=5) or Fasdeficient B6 mice (Fas−/−BM failure, N=4). Relative to TBI only controls (B6-TBI, N=3), B6-BM failure mice showed significant declines in WBCs, RBCs, platelets and total BM cells (A). In contrast, pancytopenia was significantly buffered in Fas−/−BM failure mice in which blood platelets and total BM cells were essentially not different from B6-TBI controls (A). There were significant attenuation in the expansion of CD8 T cells (P<0.05) and CD11a+ CD8 T cells (P<0.05) in Fas−/−BM failure animals, but total FasL+ CD8 T cell number was relatively similar in B6-BM failure and Fas−/−BM failure mice (B).
Discussion
Immune-mediated BM failure was successfully created in inbred B6 mice with 6.5–7.0 Gy TBI and the infusion of 4–10 × 106 LN cells from inbred FVB donors. Marrow failure took an acute course, as 43% of recipients were found dead at days 8–14 following LN infusion. Surviving animals developed severe pancytopenia and marrow hypoplasia, as seen in CByBF1 hybrid and C.B10 congenic recipients, as described previously [19–21]. Necropsy of dead animals found no lesion in the brain, heart, lung and pancreas. The specific cause of death could not be determined; liver and kidney appeared pale in some animals while some animals had suspected intestinal hemorrhage. Together with findings of pancytopenia from moribund animals analyzed at the same time range, we speculate that the cause of early animal death was due to severe pancytopenia, especially severe thrombocytopenia.
An appropriate donor-recipient strain combination (FVB ® B6), titrated amount of TBI (6.5–7.0 Gys), and sufficient LN cells (4–10 × 106) are all critical to ensure successful induction of BM failure, as has been reported [31]. Lowering the TBI dose or altering the LN cell source from FVB to BALB mice failed to result in BM damage in our study, consistent with early observations of Barnes and Mole (in their immune-mediated AA model, reversing donor and recipient strains by infusing CBA/H LN cells to C3H recipients did not induce aplastic anemia [12]). Thus, not all MHC and minor-H mismatched LN cell infusion pairs result in BM failure. A key element in our current model is the use of FVB mice as LN cell donors. The FVB strain originated from outbred Swiss mice, conferring sensitivity to the Friend leukemia virus B. FVB mice carry H2q/q [32], which is a complete mismatch with B6 mice that carry H2b/b at MHC.
Limited Vβ display of both CD4 and CD8 T cells in the BM of FVB LN cell-infused animals in our current study represents clonally restricted effector T cell expansion, as previously described in immune-mediated BM failure animal models [20], and consistent with observations from patients [33,34]. In AA patients at disease presentation, both Th1 and Th2 cells are significantly increased, while immunosuppressive regulatory T cells are decreased [6,25,35]. These immune system features have been replicated in previous mouse models, in which the Th1 immune response was the major contributor to BM damage [26–28]. In the current model, in comparison of FVB LN cell-infused animals and TBI-only controls, we found significant elevations in IFN-γ and TNF-α, again consistent with observations in human AA [6]. Increased IL-10 could also be the result of an increased Th2 immune response, or contributory to hematopoietic stem cell proliferation (IL-10 can stimulate HSC self-renewal)[36]. In our current model, 7 out of 10 BM failure mice did not have detectable circulating IL-17a and the other 3 BM failure mice had only moderate increases in plasma IL-17a. As Th17 up-regulation was detected only in severe AA patients [6], and only at early stage disease in previous mouse experiments [24], the role of the Th17 immune response in BM failure maybe limited.
Previously we reported different cytokine signature profiles for AA and myelodysplastic patients in which increases in thrombopoietin and G-CSF were characteristic to AA [30]. We observed a consistent increase in plasma G-CSF level in our B6 mouse model in the current study. In contrast, plasma levels of CCL5 and CXCL5 were low in AA patients [30] but increased in our B6 model. Perhaps chemokine ligand levels are stage specific in BM failure, and CCL5 and CXCL5 are present during massive T cell expansion and localization in the BM, as in the B6 mouse model. Future studies are needed to clearly define the roles of chemokine ligands in the development of BM failure, especially their role in T cell activation and homing of effector cells to inflammatory sites [37].
The roles of Fas/FasL and perforin/granzyme B pathways in BM failure had been defined in previous studies [9,22,23,38]. That Prf−/− mice were found dead at day 10 following TBI and FVB LN cell infusion is expected, since perforin deficiency might augment granzyme B expression in Prf−/− animals, making them more sensitive toward FVB LN cells that have normal perforin expression. Our observation of BM failure attenuation in Fas−/− mice is consistent with previous reports, confirming Fas/FasL as the major cell death pathway responsible for immune-mediated BM destruction [9,22,38]. Reduced BM damage in Fas−/− mice following FVB LN cell infusion also provided evidence showing that immune-mediated BM failure can be extended to B6 mice carrying gene mutations, deletions, insertions and other genetic manipulations. Consideration should be given to ensure sufficient backcrosses of any mutant to the B6 background to reduce unwanted donor genome attachment. When that is achieved, experiments could be performed using specific gene knockout and transgenic animals on the B6 background to receive FVB LN cell infusion to test various genes as potential sources of inciting antigens or as key molecules for T cell homing and lodging, such as Stat3, CCL3 and CCL5 conditional knockout mice. Deficiency or over-expression of a particular gene should significantly reduce or enhance sensitivity toward FVB LN cell-mediated BM damage, as was observed here in Fas−/− mice.
Highlights.
We developed a new model of immune-mediated bone marrow failure in C57BL/6 mice
Irradiation dose and specific donor-recipient combination are critical to model development
Bone marrow of model animals had clonal T cell expansion and T cell Fas ligand up-regulation
Inflammatory cytokine gamma interferon and tissue necrosis factor alpha were increased
Mice carrying germline Fas deletion had bone marrow destruction significantly attenuated
Acknowledgment
This research was supported by funds from National Heart, Lung, and Blood Institute Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh JC, Bacigalupo A, Schrezenmeier H, Tichelli A, Risitano AM, Passweg JR, Killick SB, Warren AJ, Foukaneli T, Aljurf M, Al-Zahrani HA, Hochsmann B, Schafhausen P, Roth A, Franzke A, Brummendorf TH, Dufour C, Oneto R, Sedgwick P, Barrois A, Kordasti S, Elebute MO, Mufti GJ, Socie G. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119:5391–5396. doi: 10.1182/blood-2012-02-407684. [DOI] [PubMed] [Google Scholar]
- 3.Scheinberg P, Townsley D, Dumitriu B, Scheinberg P, Weinstein B, Rios O, Wu CO, Young NS. Horse antithymocyte globulin as salvage therapy after rabbit antithymocyte globulin for severe aplastic anemia. Am J Hematol. 2014;89:467–469. doi: 10.1002/ajh.23669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and ciclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133:622–627. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
- 6.Kordasti S, Marsh J, Al-Khan S, Jiang J, Smith A, Mohamedali A, Abellan PP, Veen C, Costantini B, Kulasekararaj AG, Benson-Quarm N, Seidl T, Mian SA, Farzaneh F, Mufti GJ. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119:2033–2043. doi: 10.1182/blood-2011-08-368308. [DOI] [PubMed] [Google Scholar]
- 7.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–4157. [PubMed] [Google Scholar]
- 8.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165:538–546. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 9.Killick SB, Cox CV, Marsh JC, Gordon-Smith EC, Gibson FM. Mechanisms of bone marrow progenitor cell apoptosis in aplastic anaemia and the effect of anti-thymocyte globulin: examination of the role of the Fas-Fas-L interaction. Br J Haematol. 2000;111:1164–1169. doi: 10.1046/j.1365-2141.2000.02485.x. [DOI] [PubMed] [Google Scholar]
- 10.Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;2013:76–81. doi: 10.1182/asheducation-2013.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, Munson PJ, Herndon TM, Chen J, Young NS. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes DW, Mole RH. Aplastic anaemia in sublethally irradiated mice given allogeneic lymph node cells. Br J Haematol. 1967;13:482–491. doi: 10.1111/j.1365-2141.1967.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen J. Animal models for acquired bone marrow failure syndromes. Clin Med Res. 2005;3:102–108. doi: 10.3121/cmr.3.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knospe WH, Steinberg D, Speck B. Experimental immunologically mediated aplastic anemia (AA) in H-2k identical, Mls (M) locus different mice. Exp Hematol. 1983;11:542–552. [PubMed] [Google Scholar]
- 15.Knospe WH, Steinberg D, Gratwohl A, Speck B. Experimental immunologically mediated aplastic anemia (AA) in mice: cyclosporin A fails to protect against AA. Int J Cell Cloning. 1984;2:263–271. doi: 10.1002/stem.5530020406. [DOI] [PubMed] [Google Scholar]
- 16.Knospe WH, Husseini SG, Chiu KM, Fried W. Immunologically mediated aplastic anemia in mice: evidence of hematopoietic stromal injury and injury to hematopoietic stem cells. Exp Hematol. 1994;22:573–581. [PubMed] [Google Scholar]
- 17.Kubota K, Mizoguchi H, Miura Y, Kano S, Takaku F. Experimental hypoplastic marrow failure in the mouse. Exp Hematol. 1978;6:791–800. [PubMed] [Google Scholar]
- 18.Nemoto K, Hayashi M, Abe F, Takita T, Nakamura T, Takeuchi T, Umezawa H. Therapy of experimental immunologically mediated aplastic anemia in mice by various immunosuppressive and antitumor agents. Transplant Proc. 1988;20:545–548. [PubMed] [Google Scholar]
- 19.Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp Hematol. 2004;32:1163–1172. doi: 10.1016/j.exphem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Lipovsky K, Ellison FM, Calado RT, Young NS. Bystander destruction of hematopoietic progenitor and stem cells in a mouse model of infusion-induced bone marrow failure. Blood. 2004;104:1671–1678. doi: 10.1182/blood-2004-03-1115. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, Young NS. Minor antigen h60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J Immunol. 2007;178:4159–4168. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- 22.Omokaro SO, Desierto MJ, Eckhaus MA, Ellison FM, Chen J, Young NS. Lymphocytes with aberrant expression of Fas or Fas ligand attenuate immune bone marrow failure in a mouse model. J Immunol. 2009;182:3414–3422. doi: 10.4049/jimmunol.0801430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarcon AK, Desierto MJ, Zhou W, Visconte V, Gibellini F, Chen J, Young NS. Role of perforin-mediated cell apoptosis in murine models of infusion-induced bone marrow failure. Exp Hematol. 2009;37:477–486. doi: 10.1016/j.exphem.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Latour RP, Visconte V, Takaku T, Wu C, Erie AJ, Sarcon AK, Desierto MJ, Scheinberg P, Keyvanfar K, Nunez O, Chen J, Young NS. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116:4175–4184. doi: 10.1182/blood-2010-01-266098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomou EE, Keyvanfar K, Young NS. T-bet, a Th1 transcription factor, is up-regulated in T cells from patients with aplastic anemia. Blood. 2006;107:3983–3991. doi: 10.1182/blood-2005-10-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y, Desierto MJ, Chen J, Young NS. The role of the Th1 transcription factor T-bet in a mouse model of immune-mediated bone-marrow failure. Blood. 2010;115:541–548. doi: 10.1182/blood-2009-03-211383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roderick JE, Gonzalez-Perez G, Kuksin CA, Dongre A, Roberts ER, Srinivasan J, Andrzejewski C, Jr, Fauq AH, Golde TE, Miele L, Minter LM. Therapeutic targeting of NOTCH signaling ameliorates immune-mediated bone marrow failure of aplastic anemia. J Exp Med. 2013;210:1311–1329. doi: 10.1084/jem.20112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong Q, He S, Xie F, Mochizuki K, Liu Y, Mochizuki I, Meng L, Sun H, Zhang Y, Guo Y, Hexner E, Zhang Y. Ezh2 Regulates Transcriptional and Posttranslational Expression of T-bet and Promotes Th1 Cell Responses Mediating Aplastic Anemia in Mice. J Immunol. 2014;192:5012–5022. doi: 10.4049/jimmunol.1302943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. JMP Statistics and Graphics Guide, Version 3. Cary, NC: SAS Institute; 1998. [Google Scholar]
- 30.Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, Ganetzky RD, McCoy JP, Jr, Maciejewski JP, Young NS. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96:602–606. doi: 10.3324/haematol.2010.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu KM, Knospe WH. Immunologically mediated aplastic anemia in mice: effects of varying the source and composition of donor cells. Exp Hematol. 1987;15:269–275. [PubMed] [Google Scholar]
- 32.Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risitano AM, Kook H, Zeng W, Chen G, Young NS, Maciejewski JP. Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by V beta CDR3 spectratyping and flow cytometry. Blood. 2002;100:178–183. doi: 10.1182/blood-2002-01-0236. [DOI] [PubMed] [Google Scholar]
- 34.Zeng W, Maciejewski JP, Chen G, Young NS. Limited heterogeneity of T cell receptor BV usage in aplastic anemia. J Clin Invest. 2001;108:765–773. doi: 10.1172/JCI12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, Kajigaya S, Barrett AJ, Young NS. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110:1603–1606. doi: 10.1182/blood-2007-01-066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang YJ, Yang SJ, Park G, Cho B, Min CK, Kim TY, Lee JS, Oh IH. A novel function of interleukin-10 promoting self-renewal of hematopoietic stem cells. Stem Cells. 2007;25:1814–1822. doi: 10.1634/stemcells.2007-0002. [DOI] [PubMed] [Google Scholar]
- 37.Comerford I, Kara EE, McKenzie DR, McColl SR. Advances in understanding the pathogenesis of autoimmune disorders: focus on chemokines and lymphocyte trafficking. Br J Haematol. 2014;164:329–341. doi: 10.1111/bjh.12616. [DOI] [PubMed] [Google Scholar]
- 38.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]