Abstract
Visual objects presented around the time of saccadic eye movements are strongly mislocalized towards the saccadic target, a phenomenon known as “saccadic compression.” Here we show that perisaccadic compression is modulated by the presence of a visual saccadic target. When subjects saccaded to the center of the screen with no visible target, perisaccadic localization was more veridical than when tested with a target. Presenting a saccadic target sometime before saccade initiation was sufficient to induce mislocalization. When we systematically varied the onset of the saccade target, we found that it had to be presented around 100 ms before saccade execution to cause strong mislocalization: saccadic targets presented after this time caused progressively less mislocalization. When subjects made a saccade to screen center with a reference object placed at various positions, mislocalization was focused towards the position of the reference object. The results suggest that saccadic compression is a signature of a mechanism attempting to match objects seen before the saccade with those seen after.
Keywords: perisaccadic, mislocalization, visual space
Introduction
We frequently shift our gaze with rapid saccadic eye movements, each shifting the images on our retinae. It is still unclear how the visual system perceives a smooth and continuous world from the series of snapshots gleaned during each fixation. However, much evidence suggests that saccades impact dramatically on visual processing, in many ways (Ross, Morrone, Goldberg, & Burr, 2001). One of the most robust effects is that saccades cause briefly presented perisaccadic stimuli to be grossly mislocalized, seen compressed towards the saccadic target (Morrone, Ross, & Burr, 1997; Ross, Morrone, & Burr, 1997). Although the phenomenon of saccadic compression has now been replicated in many laboratories (Lappe, Awater, & Krekelberg, 2000; Matsumiya & Uchikawa, 2001; Ostendorf, Fischer, Finke, & Ploner, 2007; Pola, 2011; Richard, Churan, Guitton, & Pack, 2009), its exact functional role remains obscure. Whereas a shift in the direction of the saccade may be interpreted as a predictive compensation, the compression, which involves shifts in both directions, is harder to explain.
Earlier studies yielded mixed evidence regarding the question whether visual information (e.g., the visual saccade target) drives perisaccadic compression: In an antisaccade task, compression focuses on the saccade landing, not on the visual target (Awater & Lappe, 2005). Compression also correlates with saccade peak velocity (Ostendorf et al., 2007). These results suggest a motor influence on compression. The role of visual factors has been suggested by the finding that in darkness only a uniform shift in saccade direction is observed (e.g., Honda, 1989; Dassonville, Schlag, & Schlag-Rey, 1992; Schlag & Schlag-Rey, 2002). Lappe et al. (2000) reported that compression depended on the presence of postsaccadic visual references, while in their absence only the uniform shift occurred. However, the presence of references only before the saccadic onset is also sufficient to induce compression (Morrone, Ma-Wyatt, & Ross, 2005), leaving open the exact mechanism and timing of the effect of visual references. Other studies, however, show that mislocalization can also be produced by visual references alone, with the eye stationary, when retinal motion is artificially simulated. Again, the actual amount of shift and compression observed is different for the different studies, so the issue is still under debate (Morrone et al., 1997; Ostendorf, Fischer, Gaymard, & Ploner, 2006; Zimmermann et al., 2013). Recently, Cicchini, Binda, Burr, and Morrone (2013) studied mislocalization of pairs of bar-stimuli (very similar in shape and dynamics); one presented perisaccadically, the other before or after. Over a wide range of space and time, the perisaccadic stimulus was mislocalized towards the stimulus presented during pre- or post-saccadic fixation. From these experiments, the authors concluded that saccadic compression may be related to the mechanisms attempting to match objects seen before saccades with those seen after, as originally hypothesized by Deubel, Schneider, and Bridgeman (1996). When there is only one stimulus, flashed briefly just before saccade initiation, the system attempts to pair it with a visual salient stimulus seen after fixation, with similar shape and dynamics (abruptly appearing).
On this view, having no presaccadic visual input should abolish, or at least reduce perisaccadic compression: Stimuli flashed briefly at the time of saccades to an empty screen should not be compressed. We tested this hypothesis by measuring saccadic compression when observers make saccades without a saccadic target. However, unlike earlier studies that tested mislocalization in complete darkness, our experiments were carried out in normal light, thus making them more comparable to natural viewing conditions. As predicted, saccadic compression is greatly reduced. We also show that flashing visual references at the time of saccades causes mislocalization towards them, even though they are not near the saccadic landing point.
Methods
The subject was seated 57 cm from a 22-inch CRT color monitor (Barco Calibrator, Duluth, GA) with head stabilized by chin- and head-rest. The visible visual field was 40° × 30°. Stimuli were presented on the monitor with a vertical frequency of 120 Hz at a resolution of 800 × 600 pixels. Eye movements were monitored by the EyeLink 2000 system (SR Research, Ltd., Ottawa, Ontario, Canada), which samples gaze positions with a frequency of 2000 Hz. Viewing was binocular but only the dominant eye was recorded. The system detected start and end of a saccade when eye velocity exceeded or fell below 22°/sec and acceleration was above or below 4000°/sec2. In all experiments the background was red (7 cd/m2) and the fixation points and saccade targets were black (0.5 cd/m2), to minimize retinal afterimages. In all experiments the edges of the monitor were clearly visible. In the first experiment, we collected 8,721 trials in total, in the second experiment, 10,699, and in the third experiment, 5,423.
Participants
At total of 13 subjects participated in the study, some in several experiments. Seven subjects (one author, six naive subjects, mean age 33 years) participated in Experiment 1. Six subjects (one author, five naive subjects, mean age 34 years, two of them participated also in Experiment 1) participated in Experiment 2. Four subjects participated in Experiment 3 (one author, three naive subjects, mean age 31 years, two of them participated in Experiments 1 and 2). All subjects had normal or corrected-to-normal vision. Subjects gave informed consent. The experiments were carried out along the principles laid down in the declaration of Helsinki.
Procedures
First, we measured mislocalization of brief stimuli at the time of saccades made to a visual saccadic target (“target on” condition). After 1000 ms plus a random duration between 300–500 ms fixation, the fixation point was extinguished and a saccade target appeared 15° to the right of fixation. Within all experiments saccade direction as well as saccade starting and end point positions were held constant. Subjects saccaded immediately to the saccade target. At various times around saccade onset a probe dot (0.75° × 0.75°, luminance 18.6 cd/m2) was flashed for 8 ms in one of six possible positions. After saccade execution a mouse cursor appeared, with which the subject indicated the perceived position of the probe dot (see Figure 1A, B).
Figure 1.
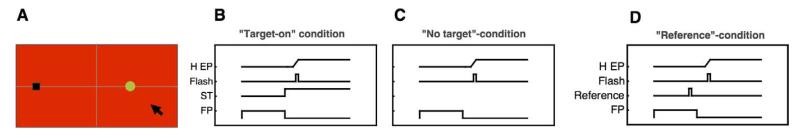
(A) Setup used in all experiments. Stimuli were presented on a red background. The fixation point (black rectangle) was displayed 19° to the left of the screen center, and the probe dot (green disk) in one of four different positions. Subjects saccaded to the center of the screen, either following the presentation of a visually saccade target or with no saccade target. After saccade execution a mouse cursor appeared with which subjects localized the perceived probe-dot position. (B) Timecourse of events in the “target-on” saccade task. The trial started with a fixation point 19° to the left of screen center. After a variable time between 1300 and 1500 ms, the saccade target was presented for the rest of the trial and the fixation point disappeared. Subjects then saccaded to the saccade target. A probe dot was flashed for 8 ms at various times around saccade onset. (C) Timecourse of events in the “no-target” condition. The fixation point is shown for a variable time between 1300 and 1500 ms. Subjects made a saccade into the middle of the screen when the fixation point was switched off. A probe dot was flashed for 8 ms at various times around saccade onset. (D) Timecourse of events in the “no-target” condition. The fixation point is shown for a variable time between 1300 and 1500 ms. Subjects made a saccade into the first quarter of the screen (−10°) when the fixation point was switched off. One hundred milliseconds before offset of the fixation point, a reference object was presented in one of three possible locations and remained on screen. A probe dot was flashed for 8 ms at various times around saccade onset.
We next tested mislocalization during saccades performed to a location where no saccade target had ever been presented (“no target” condition). Subjects were instructed to perform a saccade to the center of the screen as soon as the fixation point was switched off. When the fixation point disappeared the screen remained blank. At no time during these sessions was any saccade target shown (see Figure 1C). In this condition we tested six different probe dot positions.
In order to test the full timecourse of the attraction of a visual reference on the flashed probe bar, we presented the reference at various times before, during and after saccade execution and measured compression for each of these times separately. In these trials a fixation point was shown for 1000 ms. The subject saccaded to screen center as soon as the fixation point disappeared. The saccade target could appear at any time around saccade onset, and was presented always in the center of the screen for 60 ms.
We also tested mislocalization with irrelevant landmarks presented at the time of saccades. Subjects were instructed to make saccades into the first left quarter of the screen, which was located 10° to the left of the screen center, without a saccade target, and 100 ms before offset of the fixation point (which served as to go-signal to perform the saccade) a landmark was presented at one of three possible positions on the screen. The landmarks remained visible until the end of the trial. The landmark had the same color (black) and shape (rectangle) as the fixation point used for the first series of experiments.
Results
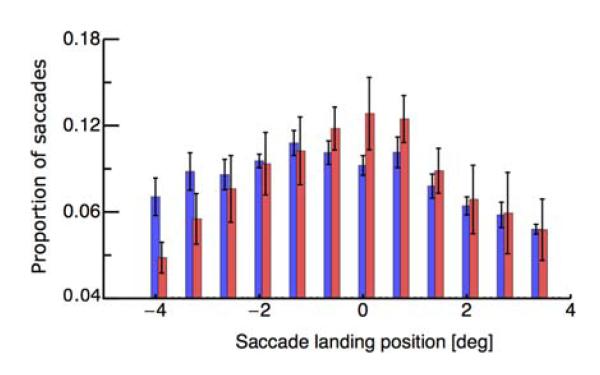
In Experiment 1, subjects fixated a fixation point and performed a saccade in one of two conditions: Either a visual target appeared and subjects saccaded to it, or subjects saccaded to the center of the screen without any visual target. All trials in which saccades landed within a region of ±3° around the saccade target position went into analysis. We first checked whether saccade landing positions differed in the two conditions. Figure 2 shows the landing distribution of saccades to a target at 0° (shown in red) and the distribution of saccades to screen center (shown in blue). Data are averaged across all subjects. The average landing position is at −0.2°, indicating the typical saccade undershoot. A paired t-test did not reveal significant differences between the landing positions in the “target on” and the “no target” condition (p = 0.38). As can be seen in Figure 2 most saccades landed close to screen center (0°).
Figure 2.
Distributions of saccade-landing in the “target-on” (blue), and the “no-target” condition (red). Saccade landing positions were binned into 0.5° bins. The required landing position was at 0°. Negative values correspond to hypometric saccades and positive values to hypermetric saccades. Error bars represent SEM across subjects.
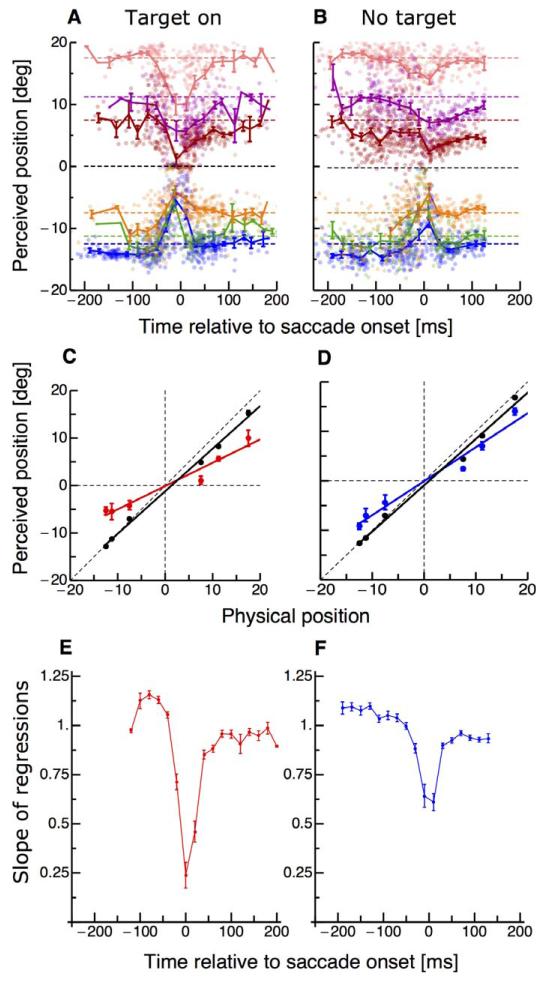
Figure 3A shows the results for localization of a brief probe dot, which was presented at various times around onset of saccades to a target. In each trial the probe dot appeared in one out of six possible locations. Consistent with previous studies with saccades in the “target-on” condition (Ross et al., 2001), there is clear compression at saccadic onset, with the probe dot perceived localized towards the saccadic target. We then repeated the experiment with subjects making saccades without visual saccade targets. In these trials, subjects fixated the fixation point and as soon as it was turned off saccaded to the middle of the screen. Figure 3B shows the localization results when no saccade target was presented. In this condition, with no visual saccade target, the probe dots were localized more veridically. Figure 3C and D summarizes the perisaccadic effects, plotting averaged perceived position across the seven subjects as a function of physical position, for perisaccadic trials (stimuli presented between −25 to +25 ms from saccadic onset for the target-on and the no-target conditions. If perception is veridical, the points should fall on the equality line, with a slope of 1. If compression is total, all stimuli will be seen at the same position, so the slope of the best-fitting regression will be zero. To obtain a descriptor of the degree of compression, we calculated the linear regression of these plots, giving us a “compression index”: the smaller the index, the greater the compression.
Figure 3.
(A–B) Localization of the probe dot, as a function of time relative to saccadic onset. Different colors refer to the six different positions of the probe dot. Dashed color-coded lines indicate the physical position of the flashed probe dot. Data were averaged across subjects and trials. The dots report single trials from all subjects. (C–D) Average perceived position, as a function of physical position, for probe dots displayed perisaccadically (during the interval −25 to +25 ms, relative to saccadic onset). Data are taken for the “target-on” and the “no-target” condition. Lines show best fitting regressions. Data shown in black are from trials where the probe appeared long before saccade onset and data in colors from trials in which the probe appeared ±25 around saccade onset. The slopes of the regression lines estimate the “compression index,” 0.49 for the target-on condition and 0.68 for no-target. Data are averaged across subjects and error bars represent SEM. (E–F) Timecourse of compression (given by the slope of the regression of apparent against physical positions), in time bins of 25 ms width, for the two conditions: “target-on” (red color) and “no-target” (blue color). An index of 1 indicates veridical perception, and 0, maximal compression. Error bars are derived by bootstrapping.
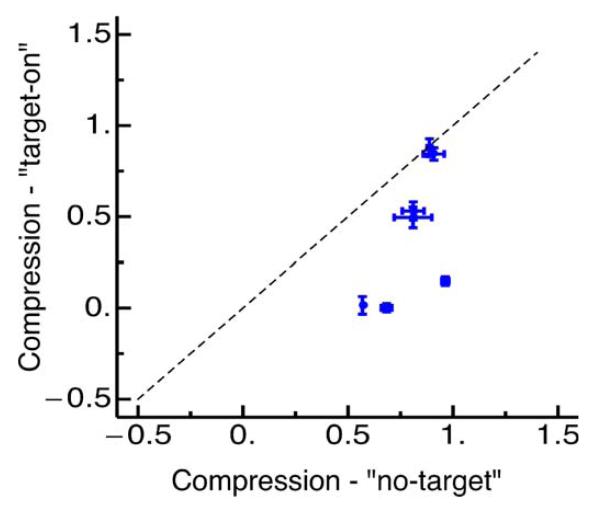
Figure 3E and F show how the compression indices vary with time (relative to saccade onset) for all three conditions. Long before and after the saccade the slope is near 1, implying no-compression. Close to saccade onset the slope reduces strongly in the “target-on” condition, as the probe dots appear compressed towards the saccade target. In the “no-target” condition the compression is less, never falling below 0.6. Figure 4 compares compression strength in the target-on condition against compression strength in the no-target condition for all subjects. Five out of seven subjects show stronger compression in the target-on than in the no-target condition: The other two do not show much compression in either condition. In general compression was significantly stronger in the target-on condition (paired t-test, p = 0.03).
Figure 4.
Compression indices from all seven subjects in the target-on against the no-target condition. Error bars are SEM across subjects.
The magnitude of perisaccadic compression has been shown to correlate with saccade peak velocity (Ostendorf et al., 2007). Although the correlation is between individuals rather than trials within the same individual, it is conceivable that saccades to a blank screen might have lower peak velocities than visually-guided saccades, and this may drive the lower compression. To exclude this possibility, we split our trials into two groups of low and high velocities (above or below the median for each subject) and recalculated the compression indices for fast and slow trials of each subject. Compression magnitude was statistically indistinguishable between these two groups in the “target-on” (paired t-test, p = 0.35) and in the “no target” (paired t-test, p = 0.42) condition. We can therefore exclude this possible artifact.
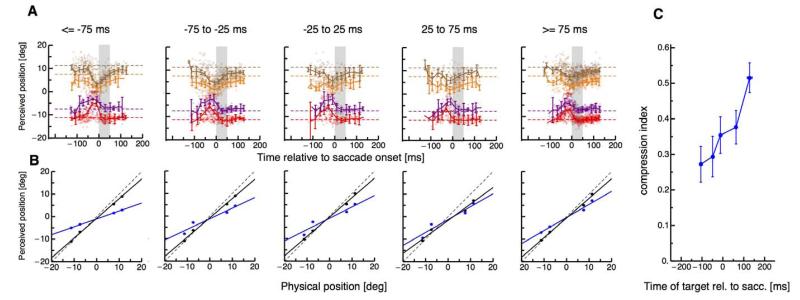
To study the timecourse of the interaction between a visual reference and mislocalization of the probe dot we presented the reference at various times relative to saccade initiation. Subjects were instructed to saccade to the screen center as soon as the fixation point was extinguished. They were explicitly told not to wait for the presence of a target but to aim for the center of the screen as in the no-target condition in Experiment 1. We binned the data into five bins of saccade target onset (aligned to saccade onset). Figure 5A shows the perceived positions of the probe dots for each of the five bins, with data pooled across the six subjects. The first two panels of Figure 5A show data from trials in which the reference was presented before saccade execution. Compression is strong in these bins, consistent with previous data using the “target-on” saccade task (Ross et al., 2001). However, when the reference was presented close to the onset of the saccade (third panel of Figure 5A), compression decreased. Compression was low for both conditions when the saccade target was presented after saccade execution. Figure 5B shows the average perceived against the physical position of the probe dots. As before, the compression indices were defined as the slope of the linear regression of the perceived position in the perisaccadic trials against physical position. These are plotted as a function of presentation time of the reference in Figure 5C. References presented at least 200 ms before saccade execution caused larger compression than references that appeared after saccade onset. A one-way ANOVA confirmed significant differences in compression strength depending on the relative timing of the reference (df = 4, F = 3.69, p = 0.01). As before, we checked whether saccade landing positions were affected by the different presentation times of the visual reference. A one-way ANOVA did not reveal any significant differences in saccade landing between the five time bins (df = 4, F = 0.13, p = 0.96).
Figure 5.
(A) Localization of the probe dot relative to onset of a horizontal 15° single saccade. Different colors refer to the four different positions of the probe dot. Dashed lines indicate the physical position of the flashed probe dot. Data were averaged across subjects and trials. The dots report single trials from all subjects. Data are binned with respect to the onset of the saccade target relative to saccade onset. Each panel shows data from one of the five bins. Error bars represent SEM. (B) Average perceived against physical probe dot position for probes presented around saccade onset (magenta) and for probes presented long before saccade onset (black). The averages were calculated from the data shown in Figure 4A correspondingly for each data bin. (C) Compression magnitude as a function of the timing of the second saccade target. Vertical bars are SD of the compression magnitude derived by bootstrapping.
That there is less compression for saccades without transient visual references suggests that it is the saccades target itself that contributes strongly to compression. To investigate this further, we had subjects make saccades without a visual reference (as before) to the first quarter of the screen, but presented clear and stable visual landmarks at one of three possible positions on the screen. They appeared abruptly 100 ms before the signal to saccade, and then remained until next trial.
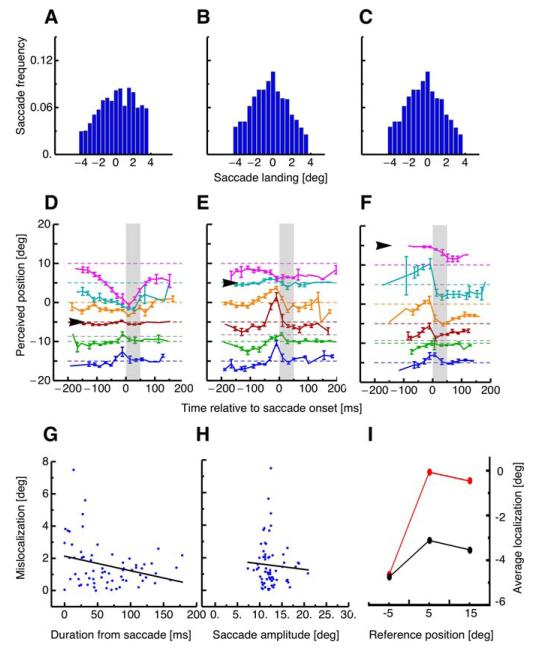
Figure 6A–C shows the distributions of saccade landing for each of the three different reference positions, pooled across the four subjects. When the reference was shown at −5° (Figure 6A), saccades overshot the required landing position (always at 10°) by 0.38°. When the reference was presented at 5°, saccades overshot the target by 0.17°. When the reference was at 15°, saccades landed close to the required landing position, at −0.09°. Kolmogorov–Smirnov tests revealed that all three distributions were statistically indistinguishable.
Figure 6.
(A–C) Distribution of saccade landing when the reference was shown at −5° (A), +5° (B) or +15° (C). Saccade landing positions were binned into 0.5° bins. The arrows show the mean of the distributions. (D–F) Localization of the probe dot relative to onset of a horizontal 15° saccade performed into the first quarter of the screen (at 10°) with no saccade target. The black arrows near the ordinates show the position of the irrelevant references at −5, +5, and +5°. Different colors refer to the six different positions of the probe dot. Dashed lines indicate the physical position of the flashed probe dot. The gray dashed line (third from bottom) represents the average saccade landing. Data were averaged across subjects and trials. The dots report single trial from all subjects. Data are binned with respect to the onset of the saccade target relative to saccade onset. Error bars represent SEM. (G) Absolute mislocalization, calculated as perceived minus real position, against absolute (positive and negative) from saccade onset. Data were fit with the function y = 2.13 − 0.01x. (H) Absolute mislocalization against saccade amplitude. Data were fit with the function y = 1.98 − 0.03x. (I) Mean mislocalization (averaged over all probe positions) as a function of position of the reference stimulus. Trials from the perisaccadic range (−25 to 25 ms from onset) are shown in red and those from the presaccadic range (Y to Y ms) are shown in black. Perisaccadic stimuli are clear biased in the direction of the reference stimulus.
The arrows of Figure 6D–F indicate the positions of the landmarks, with the corresponding color-coded data points showing the perceived position of the probe dots flashed at the time of saccade onset. Around saccade onset localization clearly shifts toward the position of the reference. Figure 6G shows mean mislocalization, averaged over all stimulus positions, given by the reported minus the physical position, plotted against absolute time difference (positive or negative) from saccade onset. A significant correlation (r = −0.29, p = 0.008) confirmed that mislocalization increased the closer in time to saccade start probe stimuli were presented. On the other hand, there was no significant correlation (r = −0.06, p = 0.311) between mislocalization and saccade amplitude, indicating that differences in saccade landing do not explain the results.
Figure 6I plots mean mislocalization, again averaged across all probe locations, as a function of the position of the reference. Mislocalization from the perisaccadic range (shown in red) is dragged in the direction of the reference objects, far more than when for presaccadic latencies (shown in black). A one-way ANOVA confirmed that the reference position modulated mislocalization significantly (df = 2, F = 5.67, p = 0.02).
Discussion
The main finding of this study is that saccadic compression was reduced when subjects made saccades to a region with no visible target. When irrelevant objects were abruptly presented during these saccades into open space, compression occurred towards them, rather than to the landing point. These results suggest that perisaccadic compression does not center on the saccade landing position but on an attempt to match the transient stimulus to objects with similar properties seen before the saccade. If a reference is presented, it is maximally effective if presented some 200 ms before the eyes move. It can be switched off before saccade execution, so subjects make a visually-guided saccade, and will still cause compression. When the reference is presented after saccade execution compression is weaker, consistent with an earlier report that references after saccadic onset are less powerful in driving compression (Morrone et al., 2005). These results suggest that the focus of compression is driven by the most salient event around saccade planning or execution. The saccade landing position can act as reference for compression since it still occurs when there is no visual saccade target. However, the strongest reference for compression is a visual stimulus which is presented shortly before saccade initiation. A visual stimulus can even attract the compression focus when it is not the saccade target, thereby driving compression away from the saccade landing position.
We must however also point out that compression was not completely reduced by the absence of visual references. This might suggest that other factors are also involved, such as a corollary discharge signal. Alternatively, remote visual references such as the monitor edges may have contributed to the compression.
Much previous research has suggested that visual references may be important for saccadic compression. Many studies performed in the dark report little or no compression, but only a shift in the direction of the saccade (e.g., Honda, 1989; Dassonville et al., 1992; Schlag & Schlag-Rey, 2002). Lappe et al. (2000) used a memory-guided paradigm to measure compression, and reported that in conditions of complete darkness (other than fixation and saccadic targets), saccades caused a uniform shift in localization, with no compression. Although these (and also our) results are complicated by the fact that the saccade target itself is mislocalized when there are no other visual references present (Morrone et al., 2005; Awater & Lappe, 2006), they do suggest that compression depends to some extent on visual information. In more natural conditions of visible background illumination it seems that it is not so much the visual references after the saccade that are important, but the abrupt visual presentation of the saccade target or other transient visual signals before saccade initiation, to allow it to be encoded in visual memory. Other studies have shown that the contrast (Michels & Lappe, 2004) and also the visibility (Georg et al. 2008) of the perisaccadic stimulus modulate the compression strength.
We believe that these results reflect the action of mechanisms attempting to match pre- with post-saccadic images (Cicchini et al., 2013; Deubel et al., 1996). Under normal viewing, objects exist both before and after the saccade. The task for the visual system is to identify what goes with what, and integrate appropriate objects with each other. Much work suggests that this process is aided by a corollary discharge signal (Wurtz, Joiner, & Berman, 2011) accompanying each saccade, informing the system of the intended saccade so that it can compensate for it. However, several authors (e.g., Bridgeman et al. 1975; Deubel et al. 1996; Irwin, 1991) point out that “cancellation” theories cannot explain the subjective impression of stability across saccades, for any small mismatch between the extra-retinal signal and the actual eye movement would result in an apparent displacement of visual images. One explicit theory along these lines is Deubel et al.’s (1996) “reference object theory of visual stability,” which argues that the first object acquired after the saccade serves as an anchor point for the localization of the visual scene (Deubel, Koch, & Bridgeman, 2010), so that stability does not need to rely on precise eye-position information (Deubel, Schneider, & Bridgeman, 2002).
Our recent work (Cicchini et al., 2013) also shows that both visual and nonvisual processes govern fusion of stimuli flashed around the time of saccades. We suggest that “remapping” neurons, of the type described by Goldberg and others, in the lateral intraparietal area and elsewhere (Duhamel, Colby, & Goldberg, 1992) have spatiotemporal receptive fields that integrate pre- and post-saccadic stimuli of similar properties, bridging the perceptual gap. At the time the eye movement is planned (well before it actually occurs), receptive fields of many units shift in the direction of the saccade to the position they will occupy after the eyes move. Then, as the eyes move, the receptive fields relax to their resting positions. During this entire period from the moment the remapping shift is completed until the next remapping, the receptive field is centered on the same region of space: In other words it is transiently spatiotopic. There is also evidence for neural corollaries of compression. Zirnsak et al. (2014) tested perisaccadic compression of visual space while recording neurons on the frontal eye fields (FEF) of the monkey. They found that FEF receptive fields did not shift parallel to the saccade direction but moved towards the saccade target.
However, it is possible that the corollary discharge signal is not extremely precise. Indeed it may define only the general range over which matching could occur, and the fine-scale matching could be achieved by visually guided mechanisms images, as suggested by many (Burr & Morrone, 2012; Cicchini et al., 2013; Deubel et al., 1996, 2002). That is to say, both extraretinal and retinal may combine to preserve pre- and post-saccadic continuity.
A single flashed stimulus, like the one used here and typically used to study saccadic mislocalization, is very rare in nature: Stimuli are usually present both before and after saccades. Under these unnatural conditions, perhaps the saccadic target acts as an attractor to the flashed stimulus, and causes it to be mislocalized at the position of the target. This is the natural consequence of the mechanism attempting to perceive continuity. When no saccadic target exists, compression is reduced. Interestingly, with the no-target condition, there was some mislocalization in the direction of the saccade for stimuli near the initial fixation (Figure 2B). Cicchini et al. (2013; see also Burr & Morrone, 2012) suggest that two mechanisms contribute to cross-saccade fusion, a corollary discharge signal, setting the general “road-map” of what should be integrated with what (well described by an acceptance region tilted in space-time), and a visual mechanism that fuses pre- with post-saccadic images.
Eye movements are intrinsically related to shifts in attention. One of us (Zimmermann, Fink, & Cavanagh, 2013) has shown that compression can also be induced during fixation if the effects of a saccade are mimicked by a visual mask. Together with the finding of some compression during simulated saccades (Ostendorf et al., 2006), the mechanism that produces perisaccadic compression might thus be active in situations where objects in the visual scene have to be matched across a visual gap. This process might share similar mechanisms to attention shifts triggered by the onset of a visual target signal. The stronger compression in the target-on compared to the no-target condition could arise because no attention shift is triggered in the latter condition. In Experiment 3 where irrelevant references were shown at various positions, attention could have been captured by the visual signal of the references, thereby modulating the focus of compression.
To conclude, this research shows that the visual signal of the saccade target plays an important role in determining the strength and focus of perisaccadic compression, probably activating mechanisms attempting to match pre- with post-saccadic fixations, ensuring visual stability.
Acknowledgments
This research was supported by the European Union, STANIB and ESCPLAIN (FP7-ERC).
Footnotes
Commercial relationships: none.
Contributor Information
Eckart Zimmermann, Cognitive Neuroscience (INM3), Institute of Neuroscience and Medicine, Research Centre Juelich, Juelich, Germany.
M. Concetta Morrone, Department of Translational Research on New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy Scientific Institute Stella Maris (IRCSS), Pisa, Italy concetta@in.cnr.it.
David C. Burr, Department of Neuroscience, Psychology, Pharmacology and Child Heath, University of Florence, Florence, Italy Institute of Neuroscience CNR, Pisa, Italy dave@in.cnr.it
References
- Awater H, Lappe M. Mislocalization of perceived saccade target position induced by perisaccadic visual stimulation. Journal of Neuroscience. 2006;426(1):12–20. doi: 10.1523/JNEUROSCI.2407-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman B, Hendry D, Stark L. Failure to detect displacement of the visual world during saccadic eye movements. Vision Research. 1975;15(6):719–722. doi: 10.1016/0042-6989(75)90290-4. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC. Constructing stable spatial maps of the world. Perception. 2012;41(11):1355–1372. doi: 10.1068/p7392. [DOI] [PubMed] [Google Scholar]
- Cicchini GM, Binda P, Burr DC, Morrone MC. Transient spatiotopic integration across saccadic eye movements mediates visual stability. Journal of Neurophysiology. 2013;109(4):1117–1125. doi: 10.1152/jn.00478.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Schlag J, Schlag-Rey M. Oculomotor localization relies on a damped representation of saccadic eye displacement in human and nonhuman primates. Visual Neuroscience. 1992;9:261–269. doi: 10.1017/s0952523800010671. [DOI] [PubMed] [Google Scholar]
- Deubel H, Koch C, Bridgeman B. Landmarks facilitate visual space constancy across saccades and during fixation. Vision Research. 2010;50(2):249–259. doi: 10.1016/j.visres.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX, Bridgeman B. Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Research. 1996;6(7):985–996. doi: 10.1016/0042-6989(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX, Bridgeman B. Transsaccadic memory of position and form. Progress in Brain Research. 2002;140:165–180. doi: 10.1016/S0079-6123(02)40049-0. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255(5040):90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Georg K, Hamker FH, Lappe M. Influence of adaptation state and stimulus luminance on peri-saccadic localization. Journal of Vision. 2008;8(1):1–11. doi: 10.1167/8.1.15. http://www.journalofvision.org/content/8/1/15, doi:10.1167/8.1.15. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Honda H. Perceptual localization of visual stimuli flashed during saccades. Perception & Psychophysics. 1989;45:162–174. doi: 10.3758/bf03208051. [DOI] [PubMed] [Google Scholar]
- Irwin DE. Information integration across saccadic eye movements. Cognitive Psychology. 1991;23(3):420–456. doi: 10.1016/0010-0285(91)90015-g. [DOI] [PubMed] [Google Scholar]
- Lappe M, Awater H, Krekelberg B. Postsaccadic visual references generate presaccadic compression of space. Nature. 2000;403(6772):892–895. doi: 10.1038/35002588. [DOI] [PubMed] [Google Scholar]
- Matsumiya K, Uchikawa K. Apparent size of an object remains uncompressed during presaccadic compression of visual space. Vision Research. 2001;41(23):3039–3050. doi: 10.1016/s0042-6989(01)00174-2. [DOI] [PubMed] [Google Scholar]
- Michels L, Lappe M. Contrast dependency of saccadic compression and suppression. Vision Research. 2004;44(20):2327–2336. doi: 10.1016/j.visres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Ma-Wyatt A, Ross J. Seeing and ballistic pointing at perisaccadic targets. Journal of Vision. 2005;5(9):7, 741–754. doi: 10.1167/5.9.7. http://www.journalofvision.org/content/5/9/7, doi:10.1167/5.9.7. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Morrone MC, Ross J, Burr DC. Apparent position of visual targets during real and simulated saccadic eye movements. Journal of Neuroscience. 1997;1517(20):7941–7953. doi: 10.1523/JNEUROSCI.17-20-07941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf F, Fischer C, Finke C, Ploner CJ. Perisaccadic compression correlates with saccadic peak velocity: Differential association of eye movement dynamics with perceptual mislocalization patterns. Journal of Neuroscience. 2007;27(28):7559–7563. doi: 10.1523/JNEUROSCI.2074-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf F, Fischer C, Gaymard B, Ploner CJ. Perisaccadic mislocalization without saccadic eye movements. Neuroscience. 2006;137(3):737–745. doi: 10.1016/j.neuroscience.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Pola J. An explanation of perisaccadic compression of visual space. Vision Research. 2011;51(4):424–434. doi: 10.1016/j.visres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Richard A, Churan J, Guitton DE, Pack CC. The geometry of perisaccadic visual compression. Journal of Neuroscience. 2009;29(32):10160–10170. doi: 10.1523/JNEUROSCI.0511-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Burr DC. Compression of visual space before saccades. Nature. 1997;386(6625):598–601. doi: 10.1038/386598a0. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends in Neuroscience. 2001;24(2):113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Through the eye, slowly: Delays and localization errors in the visual system. Nature Reviews Neuroscience. 2002;3:191–215. doi: 10.1038/nrn750. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Joiner WM, Berman RA. Neuronal mechanisms for visual stability: Progress and problems. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1564):492–503. doi: 10.1098/rstb.2010.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Fink G, Cavanagh P. Perifoveal spatial compression. Journal of Vision. 2013;13(5):21, 1–9. doi: 10.1167/13.5.21. http://www.journalofvision.org/content/13/5/21, doi:10.1167/13.5.21. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Zirnsak M, Steinmetz NA, Noudoost B, Xu KZ, Moore T. Visual space is compressed in prefrontal cortex before eye movements. Nature. 2014;507(7493):504–507. doi: 10.1038/nature13149. [DOI] [PMC free article] [PubMed] [Google Scholar]