Abstract
Adsorbents comprising ferric hydroxide loaded on a variety of support materials are commonly used to remove arsenic from potable water. Although several studies have investigated the effects of support properties on arsenic adsorption, there have been no investigations of their effects on adsorbent regeneration. Furthermore, the effect of regenerant solution composition and the kinetics of regeneration have not been investigated. This research investigated the effects of adsorbent and regenerant solution properties on the kinetics and efficiency of regeneration of arsenate-loaded ferric hydroxide-based adsorbents. Solutions containing only 0.10–5.0 M NaOH or 0.10–1.0 M NaCl, as well as solutions containing both compounds, were used as regenerants. On all media, >99% of arsenate was adsorbed through complexation with ferric hydroxide. Arsenate recovery was controlled by both equilibrium and kinetic limitations. Adsorbents containing support material with weak base anion-exchange functionality or no anion-exchange functionality could be regenerated with NaOH solutions alone. Regeneration of media containing strong base anion (SBA)-exchange functionality was greatly enhanced by addition of 0.10 M NaCl to the NaOH regenerant solutions. Adsorbed silica had a significant effect on NaOH regeneration of media containing type I SBA-exchange functionality, but on other media, adsorbed silica had little impact on regeneration. On all media, 5–25% of arsenate was resistant to desorption in 1.0 M NaOH solutions. However, the use of 2.5–5.0 M NaOH solutions significantly reduced the desorption-resistant fraction.
Key words: : adsorbents, anion exchange, arsenate, ferric hydroxide, regeneration
Introduction
Drinking water contaminated with arsenic is a worldwide problem, especially in developing nations. Consumption of drinking water with high arsenic concentrations can lead to acute gastrointestinal illness, cardiac damage, vascular disorders, and skin and lung cancer (Stone, 2008). Chronic exposure to low concentrations of arsenic in drinking water has been suspected to cause bladder cancers, diabetes, high blood pressure, and embryonic heart defects (Samba and Wilson, 2008). More than 30 million people are regularly exposed to high (up to 5,000 μg/L) arsenic concentrations in Bangladesh, Vietnam, and other regions of the Indian subcontinent (Mukherjee et al., 2006). In other parts of the world, arsenic concentrations in potable water supplies are much lower. However, in many cases, the concentrations are above the 10 μg/L maximum recommendation of the World Health Organization.
In recent years, there has been substantial research in developing treatment technologies for removing arsenic from potable water (Mohan and Pittman, 2007). Most of the treatment technologies are based on ion exchange (Williams et al., 2009) or adsorption to granular ferric hydroxide (Mahler and Persson, 2013), activated alumina (Sarkar et al., 2008), or other metal oxide compounds (Bang et al., 2005; Hristovski et al., 2008; Awual et al., 2011). The most common types of adsorbents are based on ferric hydroxide, due to its strong binding of arsenate and arsenite anions. These adsorbents come in the form of porous ferric hydroxide granules or polymeric anion-exchange resins containing imbedded ferric hydroxide nanoparticles (Mohan and Pittman, 2007; Sylvester et al., 2007). Recently, ion-exchange fibers have been developed for removing arsenic, including fiberglass coated with conventional polystyrene–divinylbenzene copolymers (Dominguez et al., 2003), cellulosic fibers doped with iron nanoparticles (Guo and Chen, 2005), and derivatized polyacrylonitrile fibers loaded with ferric hydroxide (Vatutsina et al., 2007; Zhang et al., 2008).
Arsenic removal by ferric hydroxide-based media involves physical adsorption, chemical adsorption, and ion exchange (Lin and SenGupta, 2009). Physical adsorption, also known as outer-sphere complexation, results from dispersion and electrostatic interactions between the arsenate species (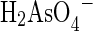 or
or  ) and ferric hydroxide. Chemical adsorption, also known as inner-sphere complexation, involves replacing an OH− or H2O ligand with an arsenate species (Manceau, 1995; Manning et al., 1998; Manning et al., 2002). This involves a chemical bond between one or more Fe atoms with one or more O atoms in arsenate. Investigators have used extended X-ray adsorption fine structure spectroscopy results along with structures derived from quantum chemistry modeling to determine the type of binding between arsenic and ferric hydroxides (Kubicki, 2005). Arsenate may form mono-, bi-, and tridentate surface complexes with one or more Fe atoms (Waychunas et al., 1993, 1995, 2004; Fendorf et al., 1997). Other oxyanions, such as orthosilicic acid (H4SiO4), also form a variety of ligand-exchange complexes with ferric hydroxide (Swedlund et al., 2010). Thus, dissolved silica will compete with arsenate for ligand-exchange binding sites on ferric hydroxide.
) and ferric hydroxide. Chemical adsorption, also known as inner-sphere complexation, involves replacing an OH− or H2O ligand with an arsenate species (Manceau, 1995; Manning et al., 1998; Manning et al., 2002). This involves a chemical bond between one or more Fe atoms with one or more O atoms in arsenate. Investigators have used extended X-ray adsorption fine structure spectroscopy results along with structures derived from quantum chemistry modeling to determine the type of binding between arsenic and ferric hydroxides (Kubicki, 2005). Arsenate may form mono-, bi-, and tridentate surface complexes with one or more Fe atoms (Waychunas et al., 1993, 1995, 2004; Fendorf et al., 1997). Other oxyanions, such as orthosilicic acid (H4SiO4), also form a variety of ligand-exchange complexes with ferric hydroxide (Swedlund et al., 2010). Thus, dissolved silica will compete with arsenate for ligand-exchange binding sites on ferric hydroxide.
High pH solutions are most commonly used for regeneration of ferric hydroxide-based arsenate adsorption media (Sarkar et al., 2007). Many experimental studies have shown that arsenate binding to ferric hydroxides decreases with increasing pH values (Waychunas et al., 1995; Fendorf et al., 1997; Dixit and Hering, 2003). This has been attributed to electrostatic repulsion between negatively charged arsenate species, such as H2AsO4− and HAsO42−, and the negative surface charge that develops on ferric hydroxides at pH values above their point of zero net proton charge (pHpznpc).
There have been several studies where arsenate-loaded ferric hydroxide-based adsorbent media have been regenerated in column experiments (DeMarco et al., 2003; Cumbal and SenGupta, 2005; Lin and SenGupta, 2009; Tresintsi et al., 2014). These studies have used NaOH solutions with concentrations ranging from 0.8% to 10% and elution times ranging from 60 to 140 min. In two of these studies, NaCl was added to the regenerant solutions at concentrations of 3% or 10% (Cumbal and SenGupta, 2005; Lin and SenGupta, 2009). Each study used only one eluent composition and the effects of the eluent concentration on the kinetics and extent of arsenate release were not investigated. Furthermore, the amount of adsorbed arsenic remaining on the adsorbents after regeneration was not quantified. One commonality between these studies is that the arsenic adsorption capacity was reduced after the first adsorption/regeneration cycle (Moller et al., 2009; Boldaji et al., 2010). This may be due to the incomplete recovery of arsenate from the media or to changes in properties of the media upon exposure to the high pH regenerant solutions.
When regenerating arsenate-loaded adsorbent media, it is desirable to minimize both the eluent volume and chemical additions. Therefore, it is important to understand the effects of eluent composition on the equilibrium and kinetics of arsenic desorption. The goal of this research was to investigate the effects of eluent composition and time on regeneration of arsenate-loaded ferric hydroxide adsorbent media. Toward that end, the effects of 0.10–5.0 M NaOH and 0.10–1.0 M NaCl regenerant solutions on arsenic release from one granular and three fibrous ferric hydroxide-based media were investigated. The media were selected to span a range in support properties that included weak base anion (WBA) and types I and II strong base anion (SBA)-exchange sites. The type II SBA-exchange sites are weaker bases than type I sites, and are more easily regenerated with NaOH.
Experimental Protocol
Reagents
All solutions were prepared from ultrapure water (UPW) (18.2 MΩ-cm). All chemicals were analytical grade and were obtained from Sigma-Aldrich (Saint Louis, MO). The test solutions in the column breakthrough experiments consisted of 600 μg/L As(V), prepared from sodium arsenate dibasic heptahydrate (Na2HAsO4·7H2O), >99.7% purity; 15 mM NaCl, >99.0% purity; and 0 or 30 mg/L SiO2, prepared from sodium metasilicate nonahydrate (Na2SiO3·9H2O), >98.0% purity. The 600 μg/L influent arsenate concentration was selected to be comparable with concentrations often encountered in drinking water supplies in developing nations (Mukherjee et al., 2006). High influent arsenic concentrations lead to more rapid media exhaustion, thereby increasing the need for media regeneration (Sarkar et al., 2007). The pH of feed solutions was adjusted to 8.0 using 0.10 M NaOH or HCl solutions. The NaCl was included in the breakthrough solutions as an inert background electrolyte since it does not chemically adsorb to ferric hydroxides, but will compete with arsenate for anion-exchange sites on the adsorbent media. Silica was included in the breakthrough solutions because it has been found to compete strongly with arsenic for complexation sites on ferric hydroxide media (Meng et al., 2000; Moller and Sylvester, 2008), adsorbs to SBA, but not WBA-exchange sites (Bornak, 2003), and is often present in groundwater at concentrations of 1–3 orders of magnitude greater than arsenic. The inclusion of silica in the breakthrough experiments at a concentration that is 50 times greater than that for arsenate will lead to significant competitive inhibition of arsenate complexation. High surface coverage of chemically adsorbed silica may also affect the ratio of mono- versus bidentate complex formation by preventing arsenate from forming bonds with adjacent ligand-exchange sites (He et al., 2011).
Materials
Four ferric hydroxide-based adsorbent media spanning a range in support properties were selected for the study. Fiban As5 and Fiban A1 media were obtained from the National Academy of Science (Minsk, Belarus), and ArsenXnp was obtained from the Purolite Company (Bala Cynwyd, PA). An adsorbent prepared in our laboratory from homopolymer polyacrylonitrile (PAN) obtained from Heading Filter Material Co., Ltd., (Zhejiang, China) was also investigated (Chaudhary and Farrell, 2014). The Fiban As5 material consists of a nonwoven PAN felt that has been treated with hydrazine and N-N′-dimethyl-1,3-propanediamine and contains 4.2 meq/g WBA-exchange groups consisting of ternary amines (Soldatov, 2008; Nesteronok and Soldatov, 2012). The Fiban A1 medium consists of a polystyrene–divinylbenzene copolymer that has been treated with trimethylamine and contains 2.4 meq/g of quaternary amines with type I SBA-exchange functionality (Soldatov, 2008). The ArsenXnp medium consists of a polystyrene–divinylbenzene copolymer that has been treated with dimethylethanolamine and contains type II SBA-exchange functionality (Zaganiaris, 2011). The ArsenXnp and Fiban As5 media come doped with ferric hydroxide from the manufacturer. The Fiban A1 medium was loaded with ferric hydroxide by soaking in 6% ferric chloride solution for 60 min and then raising the pH to 8.0 by NaOH addition (Lin and SenGupta, 2009). The PAN-Fe medium was prepared from homopolymer PAN needle-punched felt fabric with a weight of 500 g/m2 and a fiber diameter of 15 μm (Chaudhary and Farrell, 2014). The PAN-Fe adsorbent was loaded with ferric hydroxide without any pretreatment of the fiber and thereby contains no ion-exchange functionality. The same iron loading method used for the Fiban A1 was used for the PAN-Fe medium. Before use, the adsorbents were soaked in UPW overnight at room temperature, and then thoroughly rinsed with UPW before drying at 50°C. The SBA-exchange capacity for each medium after loading with ferric hydroxide was determined by measuring sulfate uptake at a pH value of 12.0. Properties of the adsorbents are listed in Table 1.
Table 1.
Physical Properties of Adsorbent Media
| Structure | ArsenXnpa | Fiban As5b | Fiban A1c | PAN-Fe |
|---|---|---|---|---|
| Iron content (mg/g) | 250 | 60 | 110 | 75 |
| Diameter (μm) | 300–1,200 | 44–54 | 5–50 | 15–20 |
| Form of ferric iron | Poorly crystalline | Amorphous | Amorphous | Amorphous |
| Shape | Spherical porous grain | Cylindrical fiber | Cylindrical fiber | Cylindrical fiber |
| Ion-exchange groups | Type II 4° amine | 3° amine | Type I 4° amine | None |
| SBA capacity post Fe loading (meq/g) | 1.7 | 0 | 2.4 | 0 |
Column experiments
Arsenic loading onto the media was performed in packed columns containing ∼1 g of adsorbent. The test solutions were passed through each column using a liquid chromatography pump, and the arsenic loading was controlled by varying the empty bed contact time (EBCT) from 1.0 to 4.0 min. The columns were regenerated using NaOH alone, NaCl alone, and NaOH plus NaCl at concentrations ranging from 0.10 to 5.0 M. The regeneration experiments were all conducted with an EBCT of 2.0 min. This short EBCT was selected to allow the kinetics of arsenate desorption to be investigated and to minimize the solution-phase mass transfer resistance for arsenic release from the media. These regeneration conditions were selected to investigate the arsenate release mechanisms and are not intended to represent practical regeneration conditions. Samples were collected using a Gilson FC-204 fraction collector. Properties of the packed columns and operating conditions are summarized in Table 2. Arsenic loading on the media after the regeneration experiments was determined by dissolving the ferric hydroxide using 1.0 M HCl solutions with an EBCT of 4.0 min, as illustrated in Supplementary Fig. S1. To ensure complete dissolution of the ferric hydroxide, the acid solutions were passed through the columns until the concentration of iron in the effluent approached the analytical detection limit of <1 μg/L. Effluent samples were filtered with 0.45-μm syringe filters and analyzed for both arsenic and iron using a PerkinElmer Optima 2100 DV inductively coupled plasma optical emission spectrometer.
Table 2.
Summary of Experimental Conditions for Column Experiments
| Parameters | ArsenXnp | Fiban As5 | Fiban A1 | PAN-Fe |
|---|---|---|---|---|
| Hydraulic loading rate (m/h) | ||||
| Breakthrough | 0.74 | 0.82 | 0.89 | 1.03 |
| Hydraulic loading rate (m/h) | ||||
| Regeneration | 1.49 | 1.65 | 1.78 | 2.06 |
| Flow rate (mL/min) | ||||
| Breakthrough | 0.62 | 0.69 | 0.75 | 0.86 |
| Flow rate (mL/min) | ||||
| Regeneration | 1.25 | 1.38 | 1.49 | 1.73 |
| EBCT (min) | ||||
| Breakthrough | 4.0 | 4.0 | 4.0 | 4.0 |
| EBCT (min) | ||||
| Regeneration | 2.0 | 2.0 | 2.0 | 2.0 |
| Bulk density (g/mL) | 0.40 | 0.36 | 0.33 | 0.29 |
| Bed volume (mL) | 2.5 | 2.8 | 3.0 | 3.5 |
| Mass (g) | 1.0 | 1.0 | 1.0 | 1.0 |
EBCT, empty bed contact time.
Results and Discussion
Arsenate adsorption
Column breakthrough experiments measuring effluent concentrations of arsenate and silica were performed for all media (Supplementary Fig. S2). The adsorbed arsenic and silica concentrations per gram of dry media were (mg-As, mg-SiO2): Fiban A1 (2.1, 11.9), Fiban As5 (0.9, 20.0), ArsenXnp (3.6, 12.8), and PAN-Fe (0.6, 5.2). When silica was absent in the feed solution, adsorbed arsenate concentrations increased by factors of 2.3–10.8. This significant reduction in arsenate adsorption by dissolved silica is similar to that reported by others (Meng et al., 2000; Su and Puls, 2001; Liu et al., 2007).
Regeneration of ferric hydroxide-based adsorbents
Figure 1 illustrates the effect of 0.10 M NaOH solutions with an EBCT of 2.0 min on arsenate recovery from the four media. The recovery profiles were generated by integrating the elution concentration profiles according to:
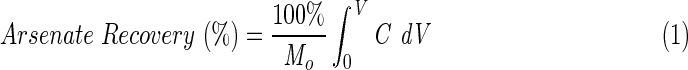 |
FIG. 1.
Fractional arsenate recovery from adsorbent media using 0.10 M NaOH with an empty bed contact time (EBCT) of 2.0 min. PAN-Fe (◯), Fiban As5 (Δ), ArsenXnp ( ), Fiban A1 (■).
), Fiban A1 (■).
where C is the mass concentration of arsenate in the eluent (g/cm3) solution, Mo is the arsenate mass (g) on the medium at the start of the elution experiment, and V is the volume of eluent (cm3). The number of empty bed volumes (EBVs) is calculated as V÷EBV. For the Fiban A1 and ArsenXnp media, less than 3% of the adsorbed arsenic was released after 125 EBVs. In contrast, 85% of the arsenic was released from the Fiban As5 medium and 78% of the arsenic was recovered from the PAN-Fe medium. This difference in behavior can be attributed to differences in ion-exchange functionality of the four media.
The two media with SBA-exchange sites released very little arsenate when challenged with 0.10 M NaOH. The data in this article show that complex formation with ferric hydroxide was responsible for more than 99% of the arsenate uptake by the ArsenXnp and Fiban A1 media. Additionally, the data show that 0.10 M NaOH does promote desorption of the arsenate from the ferric hydroxide sites on the media. However, the SBA-exchange sites can retain arsenate released by the ferric hydroxide through ion exchange. In contrast, the PAN-Fe and Fiban As5 media have zero SBA-exchange functionality, and arsenate released from the ferric hydroxide cannot be retained elsewhere on the media in 0.10 M NaOH.
The desorption behavior for Fiban As5 and PAN-Fe media in Fig. 1 cannot be attributed to release from WBA-exchange sites since very little of the arsenate was retained through ion exchange on any of the media. Figure 2 shows the effect of 0.10–1.0 M NaCl eluent solutions on arsenate release from all four media. The arsenic concentrations in the effluent solutions for the data in Fig. 2 are shown in Supplementary Fig. S3. Eluents with NaCl concentrations of 0.10, 0.20, 0.50, 0.75, and 1.0 M were used in succession, each for 120 EBVs. After eluting with 600 EBVs of NaCl solutions, only 3.0% of the arsenate was released from the Fiban As5 and only 1.3% was released from the PAN-Fe. This further confirms that the mechanism for arsenic retention by these two media was not ion exchange, but rather chemisorption to ferric hydroxide. The effect of 0.10–1.0 M NaOH solutions on arsenate release from the Fiban As5 and PAN-Fe media, shown in Fig. 2, confirms this conclusion. Changing the eluent from 1.0 M NaCl to 0.10 M NaOH increased the rate of arsenate release from the Fiban As5 medium by a factor of 737 and by a factor of 1,757 for the PAN-Fe medium. Further increases in NaOH concentration resulted in spikes in the effluent arsenate concentrations (Supplementary Fig. S3a, b). For example, changing the eluent from 0.10 to 0.20 M NaOH immediately increased the arsenate concentration in the column effluent from 178 to 227 μg/L, but this increased effluent concentration lasted less than 50 EBVs, and the arsenate release rates reverted back to the trend established in the 0.10 M NaOH solution. Increasing the NaOH concentration from 0.20 to 0.50 M resulted in a factor of 2.4 increase in the effluent arsenate concentration. These immediate increases in effluent arsenate concentrations with increasing NaOH concentration indicate that equilibrium partitioning between the liquid and solid phases was limiting effluent concentrations. Interruption of the 0.50 M NaOH eluent flow for 12 h, followed by eluting with 0.75 M NaOH, resulted in a factor of 18 increase in the effluent arsenate concentration (Supplementary Fig. S3b). This indicates that mass transfer or desorption rate limitations were also limiting the arsenate release rates from the PAN-Fe medium. Similar behavior was observed for the Fiban As5 medium (Supplementary Fig. S3a).
FIG. 2.
Fractional arsenate recovery from adsorbent media with increasing concentrations of NaCl or NaOH or using an EBCT of 2.0 min. The concentrations of both eluents were 0.10, 0.20, 0.50, 0.75, and 1.0 M, with each concentration covering 120 bed volumes. After 600 empty bed volumes (EBVs), the eluent was changed from 1.0 M NaCl to 0.10 M NaOH.
Figure 2 shows that the NaCl eluents had a greater effect on arsenate release for the Fiban A1 and ArsenXnp media, where 6.5% and 4.1%, respectively, of the arsenate was released by the series of 0.10–1.0 M NaCl eluents. This release could result from arsenate adsorbed to SBA-exchange sites or could result from Cl− displacement of adsorbed silicate anions, which then displace arsenate bound to ferric hydroxide. Silica concentrations in the column effluent during elution with NaCl indicate that the latter explanation is more likely. For example, during elution with NaCl solutions, effluent silica concentrations in the column effluents averaged 100 times greater than effluent arsenate concentrations for the Fiban A1 medium. Thus, although arsenate adsorbs to ferric hydroxide more strongly than silica (Gao et al., 2013), the much higher concentrations of dissolved silica resulted in arsenate displacement from the ferric hydroxide, especially at high pH.
Lack of arsenate retention through ion exchange by the Fiban A1 is also supported by the data in Fig. 3 comparing the effects of 0.10–1.0 M NaCl solutions on arsenate release in systems with and without adsorbed silica. The absence of adsorbed silica decreased the fraction of arsenate released by the 0.10–1.0 M NaCl solutions by a factor of 10 for the Fiban A1 medium. This indicates that arsenate release from the silica-loaded Fiban A1 medium in NaCl solutions resulted from silica released from ion-exchange sites displacing arsenate from complexation with ferric hydroxide. This also confirms that >99% of the arsenate that was retained by the Fiban A1 medium during the breakthrough experiments was adsorbed through complexation with ferric hydroxide. Similar behavior was observed for the ArsenXnp medium, where the NaCl-promoted arsenate release in the silica-containing column was a factor of 16 greater than that in the column without silica (Supplementary Fig. S4). Thus, the two media with SBA-exchange sites retain significant silica through ion exchange. During the elution experiments with NaCl, 67% and 57%, respectively, of the retained silica was released from the Fiban A1 and ArsenXnp media.
FIG. 3.
Fractional arsenate recovery from Fiban A1 medium with increasing concentrations of NaCl or NaOH using an EBCT of 2.0 min. The concentrations of both eluents were 0.10, 0.20, 0.50, 0.75, and 1.0 M, with each concentration covering 120 bed volumes. After 600 EBVs, the eluent was changed from 1.0 M NaCl to 0.10 M NaOH. Media were loaded with feed solutions containing 0 or 30 mg/L of SiO2. Initially adsorbed arsenate in the column without silica (3.8 mg/g) was a factor of 1.9 times greater than that in the silica-containing column (2.0 mg/g).
The effects of NaOH solutions on arsenate release by the Fiban A1 and ArsenXnp media are also shown in Fig. 2. Replacing the 1.0 M NaCl eluent by 0.10 M NaOH increased the arsenate release rate by a factor of 95 for the Fiban A1 and by a factor of 19 for the ArsenXnp. This increase was due to the release of arsenate chemically adsorbed to ferric hydroxide. Further increases in the NaOH concentration increased the rates of arsenate release, as shown by the inflection points in the data in Fig. 2 and by the effluent arsenate concentration profiles in Supplementary Fig. S3c and d in the Supplementary Data The fact that increasing the NaOH concentration and interrupting the eluent flow for 12 h increased the arsenic concentrations in the eluent indicates that both equilibrium and kinetic factors were controlling arsenate release.
For the Fiban A1 medium, silica also had a significant effect on arsenate release by NaOH, as shown in Fig. 3. Eluents containing 0.10 and 0.20 M NaOH released only 6% of the arsenate in the column without silica and 42% in the column with silica. The factor of 7 greater arsenate release in the silica-containing column can be attributed to silicate anions preventing arsenate released from ferric hydroxide from being retained by the SBA-exchange sites. In the column without silica, the only anion in the eluent solutions that could bind to the SBA-exchange sites was arsenate. Therefore, in solutions containing only NaOH, a significant fraction of the arsenate could be retained by the type I SBA sites on the Fiban A1 medium.
In the Fiban A1 column without silica, only 45% of the arsenate could be recovered by 0.10–1.0 M NaOH eluents, whereas in the column with silica, Fig. 3 shows that 82% could be recovered. The arsenate remaining on the Fiban A1 medium after elution with 1.0 M NaOH represents only 3% of the SBA-exchange capacity of the medium. Therefore, the 1.0 M NaOH was able to regenerate most, but not all, of the SBA-exchange sites and indicates that some sites show stronger arsenate binding than others. Although the chemical compositions of the SBA sites on the Fiban A1 medium are all the same, not all the sites have the same relative affinity for AsO43− versus OH−. Previous investigators have found that the spacing of the ion-exchange sites can affect the selectivity of SBA-exchange media (Clifford and Weber, 1983). The positioning of the SBA sites within the media depends on the tacticity and orientation of the polymer chains. In locations where two or three SBA sites are in close proximity, an AsO43− anion can interact with more than one amine site and there may be a much stronger preference for a multivalent over a monovalent anion. In contrast, in locations where SBA sites are spaced so that an AsO43− can interact with only a single site, monovalent ions may be preferred over multivalent ions (Clifford and Weber, 1983).
The effect of silica on arsenate release by NaOH solutions was much smaller on media without type I SBA sites. As shown in Supplementary Fig. S4, for the ArsenXnp medium, the arsenate release rate in the 0.10 M NaOH eluent was 50% greater in the silica-containing column compared with the column without silica. This difference can likely be attributed to silicate anions preventing arsenate adsorption to SBA sites in 0.10 M NaOH. This effect of silica on the ArsenXnp medium is much smaller than for the Fiban A1 due to differences in the type of SBA-exchange sites. The type II sites on the ArsenXnp medium are weaker bases than the type I sites on Fiban A1 and are more easily regenerated by NaOH (Bornak, 2003; SenGupta et al., 2004), and thus there will be less arsenate retained through ion exchange. The difference in regenerability of the SBA sites by NaOH can be seen by comparing the NaOH concentration that resulted in a dramatic increase in the arsenate release rate in columns without silica. For the type II media, this concentration is 0.20 M (Supplementary Fig. S4), while for the type I media, 0.50 M (Fig. 3) is required. Thus, the main effect of silica on arsenate release is that it competes with arsenate for type II SBA sites in 0.10 M NaOH solutions and with type I sites in 0.10 and 0.20 M NaOH solutions.
In the presence of other anions, such as Cl−, silica does not have a significant effect on arsenate release from media containing SBA-exchange sites. Figure 4 and Supplementary Figure S5 show that arsenate release from SBA media with and without adsorbed silica is similar. For media without SBA-exchange sites, silica does not significantly affect arsenate release, even without other anions present in the NaOH eluent, as illustrated in Supplementary Fig. S6 in the Supplementary Data for the PAN-Fe medium.
FIG. 4.
Fractional arsenate recovery from Fiban A1 with 0.10 M NaOH and 0.10 M NaOH plus 0.10 M NaCl using an EBCT of 2.0 min. Media were loaded with feed solutions containing 0 or 30 mg/L of SiO2. Initially adsorbed arsenate in the column without silica (1.9 mg/g) was a factor of 1.1 times greater than that in the silica-containing column (1.8 mg/g).
Chloride ions have an effect on arsenate release only for media that contain SBA-exchange sites. Figure 5 shows the effect of adding 0.10 M NaCl to 0.10 M NaOH eluent solutions after 120 EBVs. For the two media containing SBA-exchange sites, rates of arsenate release increased by factors of 374 and 93, respectively, for Fiban A1 and ArsenXnp media. Conversely, Cl− anions had no effect on arsenate release from the media lacking SBA-exchange sites. These dramatic increases in arsenic release rates can be attributed to the ability of Cl− ions to displace AsO43− ions from the SBA-exchange sites. The weak ability of 0.10 M NaOH to displace ion-exchanged AsO43− can be attributed to the weak affinity of OH− ions for SBA media (Alexandratos, 2009). Although the SBA sites may have a greater affinity for the trivalent AsO43− over monovalent Cl−, the concentration of Cl− was a factor of 2.4×105 greater than the arsenate concentrations when NaCl was added to the eluent for the Fiban A1 medium. When all media are regenerated by a combination of 0.10 M NaOH plus 0.10 M NaCl from the start, all media behave qualitatively similar, as shown in Supplementary Fig. S7 in the Supplementary Data.
FIG. 5.
Fractional arsenate recovery from adsorbent media with 0.10 M NaOH and 0.10 M NaOH plus 0.10 M NaCl using an EBCT of 2.0 min. PAN-Fe (◯), Fiban As5 (Δ), ArsenXnp ( ), Fiban A1 (■).
), Fiban A1 (■).
Arsenate release rates were likely impacted by a boundary layer and intraparticle diffusion limitations and by equilibrium partitioning and desorption reaction kinetics. The relative importance of each of these mechanisms changed over the course of the regeneration process. Eluting the Fiban As5 and PAN-Fe with 0.10 M NaOH resulted in release rates as high as 5.1% recovery per EBV as shown by the data first data points for each medium in Fig. 5. This fast initial rate likely represents a boundary layer mass transfer limitation on arsenate desorption. The same conclusion can be made for the Fiban A1 and ArsenXnp after commencement of elution with 0.10 M NaOH plus 0.10 M NaCl, as illustrated in Fig. 5. After the first 10–20 EBVs of regenerant, the arsenate release rates became limited by other factors. For example, the data in Fig. 6 show that increasing the regenerant concentration for the PAN-Fe medium from 1.0 to 2.5 M NaOH did not affect arsenate release rates over the first 10 EBVs, but did impact the release rates after that point. The greater release rates in the 2.5 M eluent indicate that equilibrium partitioning between the solution and solid phases was a factor in controlling the rate of arsenate release. However, further increasing the NaOH concentration to 5.0 M at 100 EBVs did not further increase the rate of arsenate release. This indicates that the kinetics of the desorption reaction and/or slow intraparticle diffusional mass transfer limited arsenate release rates during this time. Further evidence of equilibrium control of arsenate release can be seen by the immediate increases in the arsenic desorption rates after increasing the eluent concentrations, as shown in Supplementary Fig. S3.
FIG. 6.
Fractional arsenate recovery from PAN-Fe using 0.10 M NaOH, followed by 0.10 M NaOH plus 0.10 M NaCl, or 2.5 M, followed by 5.0 M NaOH.
Effect of time on arsenate release can be seen in the experiments where the elution was paused for 12 h, as shown in Supplementary Fig. S3. This 12 h pause resulted in arsenate concentrations in the columns increasing by factors of 10–20 after recommencing the elution experiments. These increases are 5–10 times greater than the increases associated with increasing the eluent concentrations without pausing the experiment. Thus, either the desorption reactions were kinetically slow or intragranular mass transfer limited the arsenic release rates. At this point, boundary layer mass transfer limitations were not a factor in controlling desorption rates. As shown in the Supplementary Data, the desorption rates were more than two orders of magnitude slower than those controlled by boundary layer mass transfer. The small diffusional distances associated with the fibrous materials suggest that the kinetics of the desorption reactions may be the most important factor. This hypothesis has been previously proposed and attributed to high activation barriers for breaking mono- and bidentate surface complexes (He et al., 2011; Farrell and Chaudhary, 2013).
Leaching of iron from the media was small (<0.5%), and in most cases, the cumulative amount of iron released was less than 0.2% of the iron on the media. Greater amounts of iron were released with increasing NaOH concentrations as illustrated in Supplementary Fig. S8 for the PAN-Fe medium in solutions with NaOH concentrations as high as 5.0 M. Iron leaching can likely be attributed to the formation of 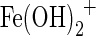 . Comparison of arsenic concentrations in filtered and unfiltered samples indicated that there were no filterable particulates in the effluent solutions, as illustrated in Supplementary Fig. S9.
. Comparison of arsenic concentrations in filtered and unfiltered samples indicated that there were no filterable particulates in the effluent solutions, as illustrated in Supplementary Fig. S9.
The short EBCT used in the regeneration experiments resulted in very low efficiencies from the standpoint of moles of arsenic recovered per moles of NaCl or NaOH passed through the columns. Supplementary Table S1 and Supplementary Figures S10 and S11 in the Supplementary Data show the moles of eluent required to recover 1 mol of arsenate in the experiments shown in Fig. 2. For the NaCl eluents that were each used for 120 EBVs, the moles of NaCl required to recover 1 mol of arsenate ranged from 3.2×104 for the ArsenXnp in 0.10 M NaCl to 3.2×107 for the PAN-Fe in 0.75 M NaCl. For the NaOH eluents that were each used for 120 EBVs, the moles of NaOH required to recover 1 mol of arsenate ranged from 5.8×103 in 0.10 M NaOH to 5.8×106 in 1.0 M NaOH for the Fiban As5 medium. The factors of 10–20 increases in effluent arsenate concentrations after pausing the elution experiments for 12 h indicate that the moles of NaOH required per mole of arsenic recovered could be greatly reduced using a discontinuous regeneration process.
Summary
This study showed that media without SBA-exchange sites could be regenerated using solutions containing only NaOH. For media with SBA-exchange sites, the addition of NaCl to the eluent solution was required to prevent arsenate (AsO43−) that was released from complexation with the ferric hydroxide from binding with SBA-exchange sites that can retain arsenate even in 1.0 M NaOH solutions. Increasing NaOH concentrations from 0.10 to 2.5 M resulted in increasing arsenate release rates, indicating that equilibrium constraints limited regeneration rates in solutions with NaOH concentrations less than 2.5 M. The majority of the arsenate could be recovered on a time scale of less than 1 h in solutions with NaOH concentrations of 0.10 M. However, 5–25% of the arsenate was desorption resistant and could not be recovered in more than 20 h (600 EBVs) using solutions with concentrations as high as 1.0 M. The data here indicate that slow flow rates and high NaOH concentrations will help to minimize the volume of waste produced during the regeneration process.
Supplementary Material
Acknowledgment
Grant number P42 ES004940 from the National Institutes of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), supported this work. The views of the authors do not necessarily represent those of the NIEHS, NIH.
Author Disclosure Statement
No competing financial interests exist.
Author's Note
The ArsenXnp medium is no longer available from Purolite. It has been replaced by FerrIX A34E, which is a ferric hydroxide-loaded type I SBA-exchange resin.
References
- Alexandratos S.D. (2009). Ion-exchange resins: A retrospective from industrial and engineering chemistry research. Ind. Eng. Chem. Res. 48, 388 [Google Scholar]
- Awual R., El-Safty S.A., and Jyo A. (2011). Removal of trace arsenic(V) and phosphate from water by a highly selective ligand exchange adsorbent. J. Environ. Sci. 23, 1947. [DOI] [PubMed] [Google Scholar]
- Bang S., Patel M., Lippincott L., and Meng X. (2005). Removal of arsenic from groundwater by granular titanium dioxide adsorbent. Chemosphere 60, 389. [DOI] [PubMed] [Google Scholar]
- Boldaji M.R., Nabizadeh R., Dehghani M.H., Nadafi K., and Mahvi A.H. (2010). Evaluating the performance of iron nanoparticle resin in removing arsenate from water. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 45, 946. [DOI] [PubMed] [Google Scholar]
- Bornak W.E. (2003). Ion Exchange Deionization. Littleton, CO: Tall Oaks Publishing [Google Scholar]
- Chaudhary B.K., and Farrell J. (2014). Preparation and characterization of homopolymer polyacrylonitrile (PAN) based fibrous sorbents for arsenic removal. Environ. Eng. Sci. 31, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford D., and Weber W.J. (1983). The determinants of divalent/monovalent selectivity in anion exchangers. React. Polym. 1, 77 [Google Scholar]
- Cumbal L., and SenGupta A.K. (2005). Arsenic removal using polymer-supported hydrated iron(III) oxide nanoparticles: Role of donnan membrane effect. Environ. Sci. Technol. 39, 6508. [DOI] [PubMed] [Google Scholar]
- DeMarco M.J., SenGupta A.K., and Greenleaf J.E. (2003). Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res. 37, 164. [DOI] [PubMed] [Google Scholar]
- Dixit S., and Hering J.G. (2003). Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 37, 4182. [DOI] [PubMed] [Google Scholar]
- Dominguez L., Economy J., Benak K., and Mangun C.L. (2003). Anion exchange fibers for arsenate removal derived from a vinylbenzyl chloride precursor. Polym. Adv. Technol. 14, 632 [Google Scholar]
- Farrell J., and Chaudhary B.K. (2013). Understanding arsenate reaction kinetics with ferric hydroxides. Environ. Sci. Technol. 47, 8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendorf S., Eick M.J., Grossl P., and Sparks D.L. (1997). Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environ. Sci. Technol. 31, 315 [Google Scholar]
- Gao X., Root R., Farrell J., Ela W. P., and Chorover J. (2013). Effect of silicic acid on arsenate and arsenite retention mechanisms on 6-L ferrihydrite: A spectroscopic and batch adsorption approach. Appl. Geochem. 38, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., and Chen F. (2005). Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ. Sci. Technol. 39, 6808. [DOI] [PubMed] [Google Scholar]
- He G., Pan G., and Zhang M. (2011). Studies on the reaction pathway of arsenate adsorption at water-TiO2 interfaces using density functional theory. J. Colloid Interface Sci. 364, 476. [DOI] [PubMed] [Google Scholar]
- Hristovski K., Westerhoff P., Moller T., Sylvester P., Condit W., and Mash H. (2008). Simultaneous removal of perchlorate and arsenate by ion-exchange media modified with nanostructured iron(hydr)oxide. J. Hazard. Mater. 152, 397. [DOI] [PubMed] [Google Scholar]
- Kubicki J.D. (2005). Comparison of As(III) and As(V) complexation onto Al- and Fe-hydroxides. In Advances in Arsenic Research: Integration of Experimental and Observational Studies and Implications for Mitigation. ACS Symposium Series 915, p. 104 [Google Scholar]
- Lin J.C., and SenGupta A.K. (2009). Hybrid anion exchange fibers with dual binding sites: Simultaneous and reversible sorption of perchlorate and arsenate. Environ. Eng. Sci. 26, 1673 [Google Scholar]
- Liu R., Qu J., Xia S., Zhang G., and Li G. (2007). Silicate hindering in situ formed ferric hydroxide precipitation: Inhibiting arsenic removal from water. Environ. Eng. Sci. 24, 707 [Google Scholar]
- Mahler J., and Persson I. (2013). Rapid adsorption of arsenic from aqueous solution by ferrihydrite-coated sand and granular ferric hydroxide. Appl. Geochem. 37, 179 [Google Scholar]
- Manceau A. (1995). The mechanism of anion adsorption on iron oxides: Evidence for the bonding of arsenate tetrahedral on free Fe(O, OH)6 edges. Geochim. Cosmochim. Acta 59, 3647 [Google Scholar]
- Manning B.A., Fendorf S. E., and Goldberg S. (1998). Surface structures and stability of arsenic(III) on goethite: Spectroscopic evidence for inner-sphere complexes. Environ. Sci. Technol. 32, 2383 [Google Scholar]
- Manning B.A., Hunt M.L., Amrhein C., and Yarmoff J.A. (2002). Arsenic(III) and arsenic(V) reactions with zerovalent iron corrosion products. Environ. Sci. Technol. 36, 5455. [DOI] [PubMed] [Google Scholar]
- Meng X., Bang S., and Korfiatis G.P. (2000). Effects of silicate sulfate and carbonate on arsenic removal by ferric chloride. Water Res. 34, 1255 [Google Scholar]
- Mohan D., and Pittman C.U. (2007). Arsenic removal from water/wastewater using adsorbents: A critical review. J. Hazard. Mater. 142, 1. [DOI] [PubMed] [Google Scholar]
- Moller T., and Sylvester P. (2008). Effect of silica and pH on arsenic uptake by resin/iron oxide hybrid media. Water Res. 42, 1760. [DOI] [PubMed] [Google Scholar]
- Moller T., Sylvester P., Shepard D., and Morassi E. (2009). Arsenic in groundwater in New England—point-of-entry and point-of-use treatment of private wells. Desalination 243, 293 [Google Scholar]
- Mukherjee A., Sengupta M.K., Hossain M.A., Ahamed S., Das B., Nayak B., Lodh D., Rahman M.M., and Chakraboti D. (2006). Arsenic contamination in groundwater: A global perspective with emphasis on the Asian scenario. J. Health Popul. Nutr. 24, 142. [PubMed] [Google Scholar]
- Nesteronok P.V., and Soldatov V.S. (2012). Acid-base properties of ion exchangers. IV. Synthesis and potentiometric analysis of polyampholytes on the base of polyacrylonitrile fibers. Solvent Extr. Ion Exch. 30, 414 [Google Scholar]
- Samba S., and Wilson R. (2008). Arsenic in food and water—A brief history. Toxicol. Ind. Health 217. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Blaney L.M., Gupta A., Ghosh D., and SenGupta A.K. (2007). Use of ArsenXnp, a hybrid anion exchanger, for arsenic removal in remote villages in the Indian subcontinent. React. Funct. Polym. 67, 1599 [Google Scholar]
- Sarkar S., Blaney L.M., Gupta A., Ghosh D., and SenGupta A.K. (2008). Arsenic removal from groundwater and its safe containment in a rural environment: Validation of sustainable approach. Environ. Sci. Technol. 42, 4268. [DOI] [PubMed] [Google Scholar]
- SenGupta A.K., Marcus Y., and Marinsky J.A. (2004). Ion Exchange and Solvent Extractions: A Series of Advances. New York: Marcel Dekker, Inc [Google Scholar]
- Soldatov V.S. (2008). Synthesis and the main properties of fiban fibrous ion exchangers. Solvent Extr. Ion Exch. 26, 457 [Google Scholar]
- Stone R. (2008). Arsenic and paddy rice: A neglected cancer risk? Science 321, 184. [DOI] [PubMed] [Google Scholar]
- Su C., and Puls R.W. (2001). Arsenate and arsenite removal by zerovalent iron: Effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ. Sci. Technol. 35, 4562. [DOI] [PubMed] [Google Scholar]
- Swedlund P.J., Miskelley G.M. and McQuillan A.J. (2010). Silicic acid adsorption and oligomerization at the ferrihydrite-water interface: ATR-IR spectra based on a model surface structure. Langmuir 26, 3394. [DOI] [PubMed] [Google Scholar]
- Sylvester P., Westerhoff P., Moller T., Badruzzaman M., and Boyd O. (2007). A hybrid sorbent utilizing nanoparticles of hydrous iron oxide for arsenic removal from drinking water. Environ. Eng. Sci. 24, 104 [Google Scholar]
- Tresintsi S., Simeonidis K., Katsikini M., Paloura E.C., Bantsis G., and Mitrakas M. (2014). A novel approach for arsenic adsorbents regeneration using MgO. J. Hazard. Mater. 265, 217. [DOI] [PubMed] [Google Scholar]
- Vatutsina O.M., Soldatov V.S., Sokolova V.I., Johann J., Bissen M., and Weissenbacher A. (2007). A new hybrid (polymer/inorganic) fibrous sorbent for arsenic removal from drinking water. React. Funct. Polym. 67, 184 [Google Scholar]
- Waychunas G., Trainor T.P., Eng P., Newville M., Catalano J.G., and Brown G.E. (2004). Surface EXAFS and diffraction study of arsenate sorption on hematite single crystals. In Presented at the 227th National Meeting of the American Chemical Society Anaheim, CA, March 28–April 1 [Google Scholar]
- Waychunas G.A., Davis J. A., and Fuller C.C. (1995). Geometry of sorbed arsenate on ferrihydrite and crystalline FeOOH: Re-evaluation of EXAFS results and topological factors in predicting sorbate geometry, evidence for monodentate complexes. Geochim. Cosmochim. Acta 59, 3655 [Google Scholar]
- Waychunas G.A., Rea B.A., Fuller C.C., and Davis J.A. (1993) Surface chemistry of ferrihydrite: Part 1: EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochim. Cosmochim. Acta 57, 2251 [Google Scholar]
- Williams T.S., Chen A.S.C., Wang L., and Paolucci A.M. (2009). Arsenic removal from drinking water by adsorptive media U.S. EPA demonstration project at Wellman, TX. Final Performance Evaluation Report, EPA/600/R-09/145 [Google Scholar]
- Zaganiaris E.J. (2011). Ion Exchange Resins and Synthetic Adsorbents in Food Processing. Paris, France: Books on Demand GmbH [Google Scholar]
- Zhang X., Jiang K., Tian Z., Huang W., and Zhao L. (2008). Removal of arsenic in water by an ion-exchange fiber with amino groups. J. Appl. Polym. Sci. 110, 3934 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








