Abstract
Vaginal exfoliative cytology is commonly used in biomedical and toxicological research to classify the stages of the rodent estrous cycle. However, mouse vaginal exfoliative cytology is commonly used as a stand-alone tool and has not been evaluated in reference to vaginal histology and serum sex hormone levels. In this study, the direct and Giemsa-stained methods of vaginal exfoliative cytology were compared in reference to vaginal fold histology and serum sex hormone levels. Both methods predicted the estrous stages similarly with mean discordance rates of 55%, 77%, 46% and 31%, for diestrus, proestrus, estrus and metestrus, respectively. From these results we conclude that vaginal exfoliative cytology may be used as a general guide to determine the desired estrous stage endpoint, and that a definitive confirmation of the estrous stage should obtained from evaluation of vaginal fold histology. Confirmation of the stage of the estrous cycle by vaginal fold histology will decrease the variability otherwise introduced by misclassification of estrous cycle stages with vaginal exfoliative cytology.
Introduction
Vaginal exfoliative cytology is a well established tool for the assessment and classification of the rat estrous cycle stages (Gupta et al., 1989, Kalra and Kalra, 1974, Marcondes et al., 2002, Martins et al., 2005, Montes and Luque, 1988, Parakkal, 1974, Pessina, 2005, Spornitz et al., 1999, Yoshinaga et al., 1969). However, in comparison to the rat, the mouse estrous cycle is more irregular and only few scientific reports link stage-dependent vaginal exfoliative cytology with serum sex hormone levels or with histology (Nelson, 1981, Rubio, 1976). The estrous cycle of the mouse is approximately 4–5 days long (Caligioni, 2001, Goldman et al., 2007) and during this period, the vaginal mucosa undergoes remarkable structural changes (Li and Davis, 2007, Rendi et al., 2011). Based on vaginal exfoliative cytology, the mouse estrous cycle is divided into 4 stages: proestrus, estrus, metestrus and diestrus. These estrous stages have been previously characterized by direct (non stained) cytology, stained cytology, histologically and by scanning electron microscopy (Li and Davis, 2007, Marcondes et al., 2002, McLean et al., 2012, Parakkal, 1974, Rendi et al., 2011, Rubio, 1976, Spornitz et al., 1999). During proestrus the vaginal smear contains many nucleated epithelial cells and few leukocytes, whereas during estrus there is marked cornification of the cells and disappearance of leukocytes. In the course of metestrus, the cornified layer is sloughed and mucosal invasion by leukocytes occurs, whereas, throughout diestrus the vaginal contents consistently lack cornified cells and leukocytes predominate (Marcondes et al., 2002, Martins et al., 2005, McLean et al., 2012, Montes and Luque, 1988).
The histologic and cytologic change that the vaginal mucosa undergoes is sex steroid hormone dependent. The main hormone that drives vaginal mucosal change is estradiol (Gupta et al., 1989). Under the influence of estradiol the vaginal mucosal epithelium stratifies and becomes cornified. On the other hand, withdrawal of estradiol leads to extensive desquamation of the mucosal epithelium to the vaginal lumen (Gupta et al., 1989). The roles of progesterone and testosterone on the vaginal mucosal epithelium are less clear but data suggest that they may have a mild antagonistic effect of estradiol effects on the vaginal mucosa (Pessina, 2005).
Mouse vaginal exfoliative cytology is used as an indicator for a stage-specific research endpoint in which mRNA or proteins are expressed in certain populations of cells, in specific tissues, or for evaluation of serum or hematological variables (Cohen et al., 2002, Spencer et al., 2008). The reliance on vaginal exfoliative cytology as an indicator of estrous stage is then absolute, and no other diagnostic measures are taken to ensure that the vaginal cytology results in correct classification of the estrous cycle (Cohen et al., 2002, Spencer et al., 2008). Therefore our goal was to test the hypothesis that vaginal cytology may not adequately predict the stages of the mouse estrous cycle. More specifically, because different methods of vaginal exfoliative cytology have been used to classify mouse estrous stages (Goldman et al., 2007, McLean et al., 2012), we compared the direct and Giemsa-stained cytological methods for their accuracy.
Consequently, the purpose of this research was to test which of the two methods of vaginal exfoliative cytology predicts more accurately the mouse estrous cycle stages. Specifically, our hypothesis was that Giemsa-stained smears will have a better predictive accuracy than direct smears because of increased recognition of cellular detail. A secondary goal of this work was to characterize the hormonal profile of the female C57BL/6 mouse estrous cycle because there are few reports of these data in the literature (Kovacic and Parlow, 1972, Nelson, 1981).
Materials and Methods
Animals
3-month-old wild-type C57BL/6 female mice were used for all experiments. Mice were housed at the University of Illinois at Urbana-Champaign (UIUC) animal-care facility under 12 hours light/dark cycles. Animals were fed commercial rodent diet and had free access to water. Animal handling and procedures were approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee.
Vaginal exfoliative cytology
Vaginal smears were performed as previously described (Caligioni, 2001) with a slight modification. Briefly, mice were smeared daily for 14 days, at 0900 by application of 100 µl of PBS into the distal aspect of the vagina followed by aspiration of the flushed fluids. Samples were immediately placed into a 96-well plate and were processed within 1 hour. A drop of PBS was added to each well of the 96-well plate to separate and stir the cells and then smears were immediately read by a single, well experienced technician (PL), at 4× magnification using an inverted microscope (Olympus CKX41, Center Valley, PA, USA). Immediately after reading the direct smears, 10 µl from each well were taken and applied on a clean plain glass slide (Thermo Scientific, Waltham, MA, USA) and allowed to dry at room temperature. Dried smears were stained in one batch with Giemsa using a commercial slide stainer (Hema-tek 1000, Miles Laboratories Inc. (Bayer), Elkhart, IN, USA) at the Clinical Pathology Section of the UIUC Veterinary Diagnostic Laboratory. Samples on the edge of slides that were not stained by the commercial slide stainer were re-stained manually by Diff-quick (Fisher diagnostics, Waltham, MA, USA) according to the manufacturer recommendations. All samples were read by a single board-certified pathologist (AG).
Evaluation of precision of vaginal exfoliative cytology and vaginal fold histology
Twenty representative direct smears, Giemsa-stained smears and vaginal fold histological sections, were randomized four times by a third party. AG blindly read Giemsa-stained smears and vaginal fold histological sections, and PL blindly read the direct smears, and results were recorded. Intra-observer coefficients of variability for each of the cytological methods were calculated from 80 vaginal exfoliative cytology smears, and the intra-observer coefficient of variability for histology was calculated from 80 vaginal fold histological sections.
Vaginal fold histology
Histologic evaluation of vaginal tissues was used as the “gold standard” for determining estrous stage and was performed as previously described (Li and Davis, 2007, Rendi et al., 2011). After 14 days of cytological evaluation of the estrous cycle, mice were euthanized immediately after cytological sample collection at predetermined assigned estrous stages. Euthanasia was performed by CO2 asphyxia and cervical dislocation. Vaginal tissue was surgically excised and fixed in 4% paraformaldehyde for a minimum period of 24 hours. Once fixed, the tissue was trimmed sagittally near the cervix so that the vaginal folds lateral to the cervix would be included in the tissue block. The tissues were then manually processed in the following manner: the tissues were incubated for 30 minutes each in 70%, 80%, 90%, 95% and twice in 100% ethanol, then 50%/50% and 70%/30% xylene/ethanol, and twice in 100% xylene. Tissues were then incubated for 90 minutes in 50%/50% xylene/paraffin at 60°C and for additional 90 minutes in pure paraffin at 60°C and were then embedded. Paraformaldehyde-fixed, paraffin-embedded tissue blocks were sectioned 4 µm thick, mounted on glass slides and manually stained with hematoxylin and eosin (Richard Allen) in the following manner: deparaffinization was achieved by drying the slides in an oven at 60°C for 20 minutes followed by incubation for 2 minutes 3 times in xylene, twice in 100% alcohol, once in 90% and 70% alcohol and once in running tap water. Slides were then incubated for 2 minutes in hematoxylin and washed under running tap water for 2 minutes. Slides were briefly dipped 4 times in acid alcohol [0.5% HCl in 70% EtOH] to remove excess hematoxylin and then washed under running water for 1 minute. Next slides were briefly dipped in ammonia water [0.1% ammonium hydroxide in H2O] and then washed under running water for 1 minute. Slides were then incubated twice in 95% ethanol for 1 minute, once in eosin for 30 seconds, twice in 95% ethanol for 1 minute, twice in 100% ethanol for 2 minutes, and 3 times in xylene for 2 minutes, followed by coverslipping.
Blood collection
Blood was collected at 0930 after collection and analysis of vaginal exfoliative cytological samples. Immediately following euthanasia, blood was collected from the abdominal vena cava into serum separator tubes (BD 365959 Plastic Capillary Blood Collection Microtainer Tube, Burlington, NC, USA) and allowed to clot for 15 min. Then, serum separated tubes containing clotted blood were centrifuged at 4°C for 15 min at maximum speed (eppendorf centrifuge 5804, Hauppauge, NY, USA). Separated sera were transferred to cryotubes (Thermo Scientific, Waltham, MA, USA), immediately frozen in liquid nitrogen and stored at −80°C pending further analysis.
Serum hormone measurement
Measurements of serum estradiol and testosterone were performed in triplicates using commercial EIA kits (Craig et al., 2010) (DRG, Marburg, Germany) according to the manufacturer recommendations with a slight modification. For estradiol, the 25 pg/ml company standard was diluted with the company 0 pg/ml standard to final concentrations of 12.5 pg/ml and 6.25 pg/ml. For the testosterone, the company 0.2 ng/ml standard was diluted with the company 0 ng/ml standard to a final concentration of 0.1 ng/ml. The assay detection limit for estradiol was 0.156 pg and for testosterone 0.0025 ng. No samples were below the detection limit of the estradiol assay and 4 samples were below the detection limit of the testosterone assay (1 in proestrus, 1 in estrus, and 2 in metestrus). The results for testosterone that were below the detection limit of the assay were recorded as the lowest detection limit of the assay (0.05 ng/ml). For both estradiol and testosterone kits, the intraassay coefficient of variation were less than 10%. Serum luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels were measured as previously reported (Brothers et al., 2010). The assay detection limit for LH was 0.0012 ng and for FSH 0.0008 ng. No samples were below the detection limit of the assay.
Statistical Analysis
Data analyses were performed using statistical software (SPSS, New York, NY, USA). All normally distributed continuous data were analyzed with a parametric test (ANOVA) and a post hoc test (Least Significant Difference test). All non-normally distributed continuous data were analyzed with a nonparametric test (Kruskal Wallis). For all analyses alpha value was set at 0.05.
Results
Histological and cytological characteristics of the stages of the mouse estrous cycle
In Figure 1 and Figure 2, the histological and cytological characteristics of the mouse estrous cycle stages are presented. The proestrus stage is characterized histologically by a 10–13 cell thick mucosa of which the stratum mucification layer stain lightly with eosin, whereas the stratum corneum layer becomes keratinized resulting in a “pink line”. Mitoses are frequent, and few leukocytes are present. During proestrus, Giemsa-stained cytology smears have a predominance of nucleated epithelial cells approximately 25–30 µm in diameter, that have lightly basophilic fibrillar cytoplasm and a single, relatively small, central round nucleus. In the direct smear, proestrus is characterized by predominance of round to polygonal cells, approximately 20–25 µm in diameter, that occasionally have a discernible small round nucleus. The estrus stage is characterized histologically by an approximately 12 cell thick mucosa of which the superficial nucleated layer (stratum mucification) is lost, and the cornified layer (stratum corneum) has become superficial. There are rare mitoses and leukocytes are absent. During estrus, Giemsa-stained cytology smears have predominance of polygonal anucleate epithelial cells approximately 35–50 µm in diameter, that have a denser basophilic fibrillar cytoplasm. In the direct smear, estrus is characterized by predominance of anucleate polygonal cells, approximately 25–40 µm in diameter. The metestrus stage is characterized histologically by delamination of the cornified mucosal layer (stratum corneum) and by exocytosis of leukocytes through the mucosa. During metestrus, Giemsa-stained cytology smears have a combination of approximately even numbers of leukocytes and polygonal anucleate epithelial cells, approximately 35–50 µm in diameter with a basophilic fibrillar cytoplasm, and fewer nucleated epithelial cells, approximately 25–30 µm in diameter, that have lightly basophilic fibrillar cytoplasm and a single, relatively small, central round nucleus. In the direct smear, metestrus is characterized by a combination of approximately even numbers of leukocytes and anucleate polygonal cells, approximately 25–40 µm in diameter, and fewer round to polygonal cells, approximately 20–25 µm in diameter, that occasionally have a discernible small round nucleus. The diestrus stage is characterized histologically by a 4–7 cell thick mucosa that contains mucified surface epithelial cells (stratum mucification), and luminal mucus, leukocytes, and desquamated cells. During diestrus Giemsa-stained cytology smears have predominance of round cells with segmented nucleus (neutrophils) that is often condensed (pyknotic). In the direct smear, diestrus is characterized by predominance of cells with a size of approximately 10 µm in diameter.
Figure 1.
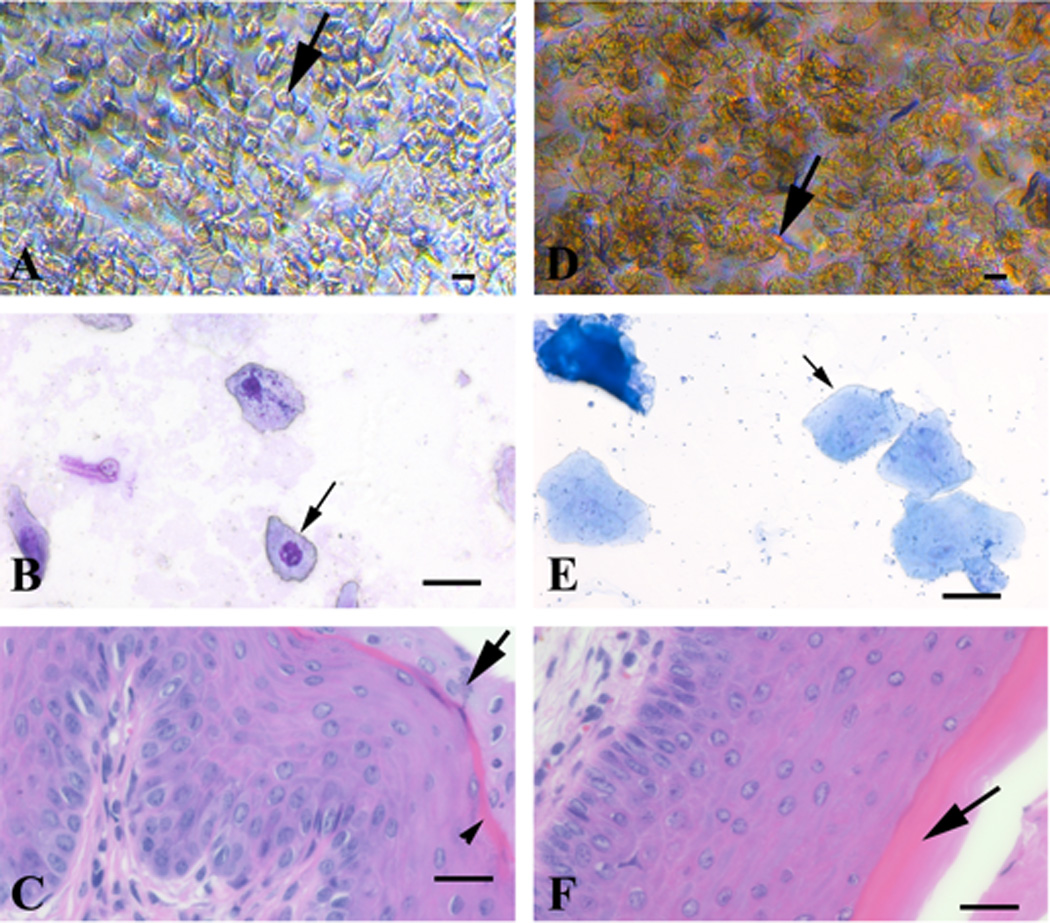
Direct and Giemsa-stained vaginal exfoliative cytology, and vaginal fold histology of proestrus and estrus stages of the estrous cycle from 3-month-old naturally cycling C57BL/6 female mice. Proestrus stage (A–C): the direct smear (A) has a predominance of round to polygonal cells (20–25 µm in diameter) that occasionally have a discernible small round nucleus (black arrow). The Giemsa-stained cytology smear (B) has a predominance of nucleated epithelial cells (25–30 µm in diameter) that have lightly basophilic fibrillar cytoplasm and a single, relatively small, central round nucleus (black arrow). Histologically (C) the mucosa is 10–13 cell thick, the stratum mucification stain lightly with eosin (black arrow), whereas the stratum corneum layer becomes keratinized resulting in a “pink line” (black arrowhead). Estrus stage (D-F): the direct smear (D) has a predominance of anucleate polygonal cells (25–40 µm in diameter) (black arrow). The Giemsa-stained cytology smear (E) has a predominance of polygonal anucleate epithelial cells (35–50 µm in diameter) (black arrow) that have a denser basophilic fibrillar cytoplasm. Histologically (F) the mucosa is 12 cell thick, the superficial nucleated layer is lost (stratum mucification), and the cornified layer (stratum corneum) has become superficial (black arrow). Leukocytes are absent. Bar = 25 µm.
Figure 2.
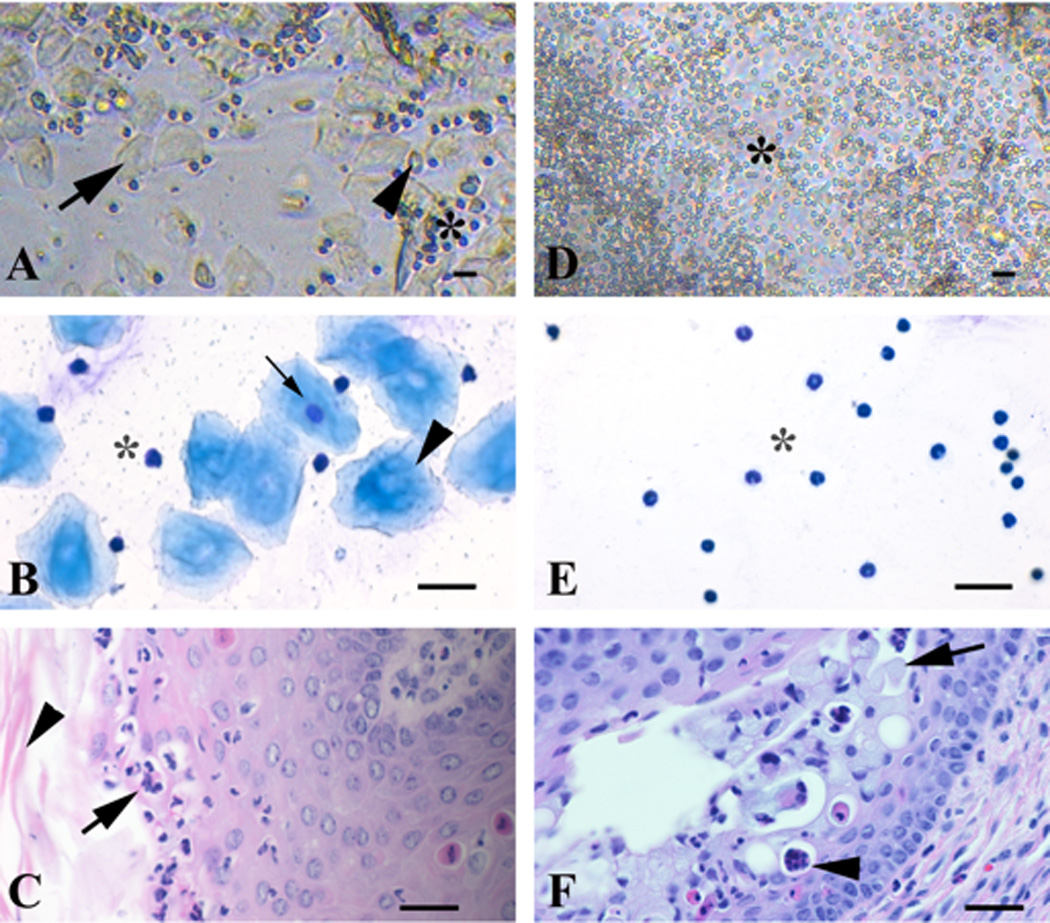
Direct and Giemsa-stained vaginal exfoliative cytology, and vaginal fold histology of metestrus and diestrus stages of the estrous cycle from 3-month-old naturally cycling C57BL/6 female mice. Metestrus stage (A–C): the direct smear (A) has a combination of approximately even numbers of leukocytes (asterisk), anucleate polygonal cells (25–40 µm in diameter) (black arrow) and fewer round to polygonal cells (20–25 µm in diameter) that occasionally have a discernible small round nucleus (black arrowhead). Giemsa-stained cytology smear (B) has a combination of approximately even numbers of leukocytes (asterisk) and anucleate polygonal epithelial cells (35–50 µm in diameter) (black arrowhead) with a basophilic fibrillar cytoplasm, and fewer nucleated epithelial cells (25–30 µm in diameter) (black arrow) that have lightly basophilic fibrillar cytoplasm and a single, relatively small, central round nucleus. Histologically (C) there is delamination of the cornified mucosal layer (stratum corneum; black arrowhead) and exocytosis of leukocytes through the mucosa (black arrow). Diestrus stage (D–F): the direct smear (D) has a predominance of leukocytes (10 µm in diameter) (asterisk). The Giemsa-stained cytology smear (E) has a predominance of round cells with segmented nucleus (neutrophils) that is often condensed (pyknotic) (asterisk). Histologically (F) the mucosa is 4–7 cell thick, and contains mucified surface epithelial cells (stratum mucification; black arrow), and luminal mucus, leukocytes (black arrowhead), and desquamated cells. Bar = 25 µm.
Evaluation of precision of vaginal exfoliative cytology and vaginal fold histology readings
The ability of the authors to consistently reproduce the same cytological and histological results (precision) was determined. The authors read twenty direct (PL) and Giemsa-stained (AG) vaginal exfoliative cytological smears, and twenty vaginal fold histological sections (AG), 4 times following randomization and blinding. The calculated intra-observer coefficients of variation for direct and Giemsa-stained cytology, and vaginal fold histology were 9.5%, 5.6% and 0%, respectively.
Comparison of direct and Giemsa-stained cytology to vaginal fold histology
First we sought to determine the accuracy of the cytological analysis of vaginal smears in comparison to histology of the vaginal folds (Li and Davis, 2007, Rendi et al., 2011, Walmer et al., 1992) (Table 1). To achieve that, the respective direct and Giemsa-stained vaginal exfoliative cytologies were compared to the vaginal fold histology that was obtained from each animal at the day of euthanasia (n=50). There were 60% (6/10) and 50% (5/10) agreement between direct and Giemsa-stained cytologies and histology in the classification of the diestrus stage. In addition, there were 73% (8/11) and 82% (9/11) agreement between direct and Giemsa-stained cytologies and histology in the classification of proestrus, and 46% (6/13) agreement between direct and Giemsa-stained cytologies and histology in the classification of estrus. Finally, there were 25% (4/16) and 38% (6/16) agreement between direct and Giemsa-stained cytologies and histology in the classification of metestrus. The pattern of misclassification of the correct estrous stage by cytology was as follows: diestrus was misclassified as proestrus and metestrus, and proestrus was misclassified as metestrus (by both cytological methods), as diestrus (by direct cytology) or as estrus (by Giemsa-stained cytology). Estrus was misclassified as either metestrus or proestrus (by both cytological methods) or as diestrus (by direct smear). Finally, metestrus was misclassified as diestrus (by both cytological methods) or proestrus (by Giemsa-stained smear).
Table 1.
Direct and Giemsa-stained vaginal exfoliative cytology classification of estrous cycle stages relative to vaginal fold histology in 3-month-old, naturally cycling female C57BL/6 mice.
| Target stages | Diestrus | Proestrus | Estrus | Metestrus |
|---|---|---|---|---|
| n | 10 | 11 | 13 | 16 |
| Direct smear — accuracy of classification | 60% (6/10) | 73% (8/11) | 46% (6/13) | 25% (4/16) |
| Target stage misclassified as | Proestrus (3/10) Metestrus (1/10) |
Metestrus (1/11) Diestrus (2/11) |
Metestrus (3/13) Diestrus (2/13) Proestrus (2/13) |
Diestrus (12/16) |
| Giemsa-stained smear — accuracy of classification | 50% (5/10) | 82% (9/11) | 46% (6/13) | 38% (6/16) |
| Target stage misclassified as | Proestrus (3/10) Estrus (1/10) Metestrus (1/10) |
Estrus (1/11) Metestrus (1/11) |
Metestrus (6/13) Proestrus (1/13) |
Diestrus (9/16) Proestrus (1/16) |
Multiple comparisons between direct and Giemsa-stained vaginal exfoliative cytology
We then determined how different the two methods of cytological evaluation of vaginal smears are from each other (Table 2). To attain that goal, daily direct and Giemsa-stained vaginal exfoliative cytologies (n=12) collected over a period of 16 days, were compared to one another. We found that out of 186 smears, the classification of estrous stage was different between direct and Giemsa-stained smears 49 times (26%). The highest disagreements were between classification of diestrus and metestrus (18/49; 36.7%), and proestrus and estrus (16/49; 32.7%). The two lowest disagreements were between classification of diestrus and estrus (1/49; 2.0%) and proestrus and metestrus (3/49; 6.1%). Moderate levels of disagreements were present between classification of diestrus and proestrus (7/49; 14.3%) and estrus and metestrus (4/49; 8.2%).
Table 2.
Rate of disagreement between direct and Giemsa-stained exfoliative cytology in classification of estrous cycle stages in 3-month-old female C57BL/6 mice
| Disagreements between | Rate (n=49) |
|---|---|
| Diestrus - Metestrus | 36.7% (18/49) |
| Proestrus - Estrus | 32.7% (16/49) |
| Diestrus - Proestrus | 14.3% (7/49) |
| Estrus - Metestrus | 8.2% (4/49) |
| Proestrus - Metestrus | 6.1% (3/49) |
| Diestrus - Estrus | 2.0% (1/49) |
| Total disagreement rate | 26% (n=186) |
Serum hormone levels of estradiol, testosterone, LH and FSH
Finally, we sought to correlate the estrous stages as classified by vaginal histology with the serum hormone levels of estradiol, testosterone, LH and FSH (Figure 3). Individual sex hormones levels, other than testosterone, have been previously correlated with the mouse estrous cycle (Achiraman et al., 2011, Nelson, 1981). However, to the best of our knowledge, a complete sex hormone profile that is correlated to the C57BL/6 mouse estrous cycle has not been published. To accomplish that, serum was obtained at the time of euthanasia. The results of serum hormone analyses were crossed against the corresponding estrous stage as determined by vaginal fold histology and then hormones levels were compared between the different estrous stages. Serum estradiol progressively increased from metestrus through proestrus (ANOVA p-value < 0.001). Proestrus estradiol serum concentration was higher than all other estrus stages (p-value < 0.001). During estrus, estradiol serum concentration was significantly lower than proestrus (p-value < 0.001) and higher than metestrus (p-value = 0.005). During metestrus, estradiol serum concentration was significantly lower than proestrus (p-value < 0.001), estrus (p-value = 0.005) and diestrus (p-value = 0.034). During diestrus, estradiol serum concentration was lower than proestrus (p-value < 0.001) and higher than metestrus (p-value = 0.034). Serum testosterone was low during metestrus and estrus, peaked during diestrus, then started to decrease during proestrus. However, it was not statistically different between the estrous stages (ANOVA p-value = 0.253). Serum LH and FSH concentrations were not statistically different across the entire estrous cycle (Kruskal-Wallis Test p-values = 0.333 and 0.643, respectively). LH concentration tended to be higher during proestrus and metestrus and FSH concentration tended to be higher during proestrus.
Figure 3.
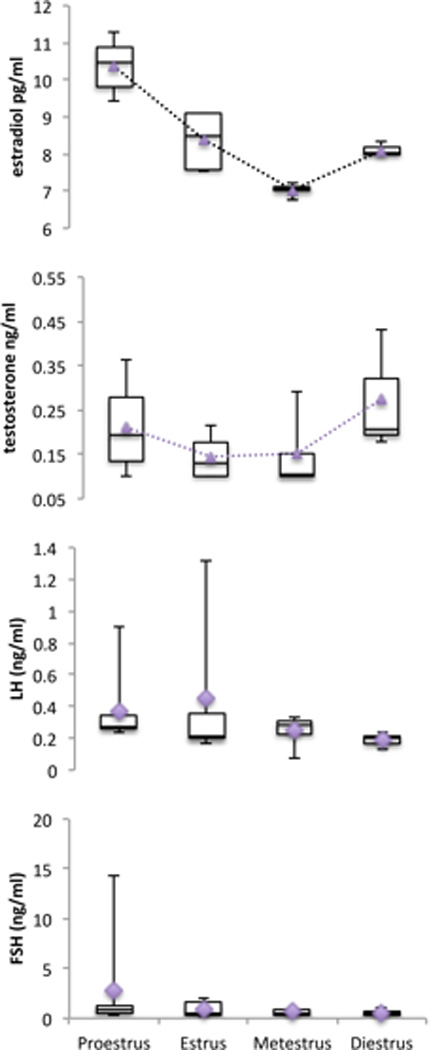
Box plot graphic data of serum estradiol, testosterone, LH, and FSH concentrations during proestrus (n=7), estrus (n=5), metestrus (n=4), and diestrus (n=3) from 3-month-old C57BL/6 naturally cycling female mice. The bottom and top of the boxes represent the first and third quartiles, and the band inside the box represents the median. Whiskers represent the minimum and maximum points in the data. Diamonds and triangles represent the mean. Serum estradiol progressively increased from metestrus through proestrus, being lowest at metestrus and highest at proestrus (p<0.001). Serum testosterone was not statistically different between the estrus stages (p=0.253), was low during metestrus and estrus, and peaked during diestrus, starting again to decrease during proestrus. Serum LH and FSH levels were not statistically different across the entire estrous cycle (p=0.333 and p=0.643, respectively).
Discussion
The aim of this study was to determine if Giemsa-stained vaginal exfoliative cytology is superior to direct vaginal exfoliative cytology in predicting the correct estrous stage. Therefore, the estrous stage predicted by each cytological method was compared with estrous stage classified by vaginal fold histology. This work is novel because in previous publications (Brothers et al., 2010, Caligioni, 2001, Nelson, 1981, Nelson et al., 1982, Spencer et al., 2008), either direct smears or stained smears, but not both, have been utilized for the assessment of mouse estrous stages. Therefore, our comparison between direct and Giemsa-stained vaginal exfoliative cytology cannot be directly contrasted with any previous work. Our results indicate that both methods have similar inconsistencies in predicting the correct estrous stage (Table 1). Therefore, when there is a need to follow the estrous cycle in mice, either method may be used according to the preference of the user, as long as the investigator is comfortable and acquainted with performing the technique and interpreting the results.
However, an important observation from this study is that cytological evaluation of vaginal smears in mice may only roughly approximate the estrous stage. If a research endpoint is estrous stage dependent, for example, temporal expression of a protein (Cohen et al., 2002, Spencer et al., 2008), than at the time of animal euthanasia it is essential that vaginal tissue samples are collected and histologically classified for the correct estrous stage. Therefore we suggest utilization of vaginal fold histology as the final method that would classify the appropriate estrous stage. The advantages of vaginal fold histology are that each estrous cycle stage has its own unique characteristics (Li and Davis, 2007, Rendi et al., 2011, Rubio, 1976, Walmer et al., 1992) that are different from the other stages, therefore it is easy to determine the estrous stage without confusion, and multiple tissue sections can be easily mounted on a single slide thus increasing the accuracy of classification.
When evaluating the mismatches in classification of the estrous stages between the two cytological methods (Table 2) it is evident that the highest prevalence of mismatches is between two consecutive estrous stages (for example estrus and metestrus) and the lowest prevalence of mismatches is between two stages that do not occur one after the other (for example, proestrus and metestrus or estrus and diestrus). Because the cytological classification of the estrous stages relies on differences in the proportions of anucleate and nucleate vaginal epithelium, and neutrophils (Goldman et al., 2007), and because the transition between two consecutive estrous stages is continuous and not abrupt (Nelson et al., 1982), we expected to find higher mismatches between successive estrous cycle stages. In view of this inherent methodological bias, when planning a study design that involves comparisons of an outcome variable between stages, it would be better to choose two opposing stages as study endpoints (e.g. estrus and diestrus) than two consecutive stages. By doing so, the variability in the outcome variable will decrease because of reduced probability of misclassification of the estrous cycle stage.
Our secondary goal was to correlate the levels of estradiol, testosterone and serum gonadotropins with the estrous cycle stages through vaginal fold histology. We found that a single morning blood sample was sufficient to demonstrate changes in serum estradiol and testosterone levels across the estrous cycle. The pattern of serum estradiol levels was similar to a previous publication (Nelson, 1981) although the absolute values differed between the studies, most likely because of different assay methodology. Despite a significant difference in estradiol levels at different estrous stages, daily sampling for serum estradiol levels by phlebotomy cannot be considered as a good tool for monitoring the estrous stage in mice, because it may lead to unwarranted morbidity and mortality (Forbes et al., 2010). To the best of our knowledge, this is the first study to report serum testosterone levels across the estrous cycle in adult C57BL/6 mice. Albeit the change in serum testosterone levels was similar in pattern to that of serum estradiol, it did not reach a statistically significant difference, possibly because of a small sample size. A plausible explanation for the similarity in the patterns of serum estradiol and testosterone levels is that testosterone, an aromatizable source of estradiol in the ovary, is derived from growing follicles (Tsonis et al., 1984). In addition, testosterone may have a role in hypothalamic regulation of the estrous cycle, similarly to the well-established role that estradiol has (Berga and Naftolin, 2012, Caraty and Franceschini, 2008, Caraty et al., 2010, Christian and Moenter, 2010). For example, in the hypothalamus, testosterone action may be mediated by androgen receptors that are expressed by kisspeptin neurons (Clarkson et al., 2012), the key regulators of GnRH release (Caraty and Franceschini, 2008, Clarkson et al., 2010, Colledge and d'Anglemont de Tassigny, 2010). Hence, the potential role of testosterone in regulation of the estrous cycle is yet to be determined. In contrast to serum estradiol and testosterone, there was no change in serum gonadotropins across the estrous cycle. This is consistent with previous studies that indicated that the timing of sampling has a pronounced impact on serum gonadotropin hormone levels (Kovacic and Parlow, 1972). Moreover, the release of serum gonadotropins from the pituitary is pulsatile (Lumpkin et al., 1984, Steyn et al., 2013) therefore repeated sampling over time should be more appropriate for monitoring the mouse estrous cycle than once daily sampling.
A limitation in our study involved the assessment of serum estradiol and testosterone in mouse sera by DRG EIA kits. These kits have not been previously validated for use with mouse serum. Nevertheless, in a preliminary analysis, we found that after extending the assays detection limits to 0.156 pg and 0.0025 ng for estradiol and testosterone, respectively, the recovery rates were within an acceptable range (± 15%). Therefore, we think that the observed trends in serum estradiol and testosterone are representative of the actual changes during the mouse estrous cycle.
In conclusion, when a research endpoint is dependent upon a correct classification of the estrous cycle stage, we suggest monitoring the estrous cycle by vaginal exfoliative cytology. Then, when the desired endpoint is met, vaginal tissue should be collected for routine histology. The evaluation of vaginal fold histology will enable accurate classification of the desired estrous stage. Otherwise, using cytology alone as a basis for attributing treatment-related effects may increase the variability in the results.
References
- Achiraman S, Archunan G, Sankarganesh D, Rajagopal T, Rengarajan RL, Kokilavani P, Kamalakkannan S, Kannan S. Biochemical analysis of female mice urine with reference to endocrine function: a key tool for estrus detection. Zoological science. 2011;28:600–605. doi: 10.2108/zsj.28.600. [DOI] [PubMed] [Google Scholar]
- Berga S, Naftolin F. Neuroendocrine control of ovulation. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2012;28(Suppl 1):9–13. doi: 10.3109/09513590.2012.651929. [DOI] [PubMed] [Google Scholar]
- Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell metabolism. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing Reproductive Status/Stages in Mice. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2001. [Google Scholar]
- Caraty A, Franceschini I. Basic aspects of the control of GnRH and LH secretions by kisspeptin: potential applications for better control of fertility in females. Reproduction in domestic animals = Zuchthygiene. 2008;43(Suppl 2):172–178. doi: 10.1111/j.1439-0531.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Caraty A, Franceschini I, Hoffman GE. Kisspeptin and the preovulatory gonadotrophin-releasing hormone/luteinising hormone surge in the ewe: basic aspects and potential applications in the control of ovulation. Journal of neuroendocrinology. 2010;22:710–715. doi: 10.1111/j.1365-2826.2010.02022.x. [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocrine reviews. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Han S-K, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Molecular and cellular endocrinology. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Shamas S, Mallinson S, Herbison AE. Gonadal steroid induction of kisspeptin peptide expression in the rostral periventricular area of the third ventricle during postnatal development in the male mouse. Journal of neuroendocrinology. 2012;24:907–915. doi: 10.1111/j.1365-2826.2012.02294.x. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Zhu L, Nishimura K, Pollard JW. Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology. 2002;143:1413–1422. doi: 10.1210/endo.143.4.8754. [DOI] [PubMed] [Google Scholar]
- Colledge WH, d'Anglemont de Tassigny X. The role of kisspeptin signalling in the regulation of the GnRH-gonadotrophin ovarian axis in mice. Annales d'endocrinologie. 2010;71:198–200. doi: 10.1016/j.ando.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Craig ZR, Leslie TC, Hatfield KP, Gupta RK, Flaws JA. Mono-hydroxy methoxychlor alters levels of key sex steroids and steroidogenic enzymes in cultured mouse antral follicles. Toxicology and applied pharmacology. 2010;249:107–113. doi: 10.1016/j.taap.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes N, Brayton C, Grindle S, Shepherd S, Tyler B, Guarnieri M. Morbidity and mortality rates associated with serial bleeding from the superficial temporal vein in mice. Lab animal. 2010;39:236–240. doi: 10.1038/laban0810-236. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth defects research. Part B, Developmental and reproductive toxicology. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Gupta PD, Vijayasaradhi S, Reddy AG. Keratinization of rat vaginal epithelium. III. Effect of estradiol on keratinization. Biology of the cell / under the auspices of the European Cell Biology Organization. 1989;65:281–289. [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Temporal Interrelationships Among Circulating Levels of Estradiol, Progesterone and LH During the Rat Estrous Cycle: Effects of Exogenous Progesterone. Endocrinology. 1974;95:1711–1718. doi: 10.1210/endo-95-6-1711. [DOI] [PubMed] [Google Scholar]
- Kovacic N, Parlow AF. Alterations in serum FSH-LH ratios in relation to the estrous cycle, pseudopregnancy, and gonadectomy in the mouse. Endocrinology. 1972;91:910–915. doi: 10.1210/endo-91-4-910. [DOI] [PubMed] [Google Scholar]
- Li S, Davis B. Evaluating rodent vaginal and uterine histology in toxicity studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:246–252. doi: 10.1002/bdrb.20120. [DOI] [PubMed] [Google Scholar]
- Lumpkin MD, DePaolo LV, Negro-Vilar A. Pulsatile release of follicle-stimulating hormone in ovariectomized rats is inhibited by porcine follicular fluid (inhibin) Endocrinology. 1984;114:201–206. doi: 10.1210/endo-114-1-201. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian Journal of Biology. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Martins RR, Pereira NML, Silva TMA. Liquid-base cytology: a new method for oestral cycle study in Wistar's rats. Acta cirúrgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2005;20(Suppl 1):78–81. doi: 10.1590/s0102-86502005000700009. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SAL. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of visualized experiments : JoVE. 2012 doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta anatomica. 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Nelson JF. Altered Profiles of Estradiol and Progesterone Associated with Prolonged Estrous Cycles and Persistent Vaginal Cornification in Aging C57BL/6J Mice. Biology of reproduction. 1981;24:784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biology of reproduction. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Parakkal PF. Cyclical changes in the vaginal epithelium of the rat seen by scanning electron microscopy. The Anatomical record. 1974;178:529–537. doi: 10.1002/ar.1091780302. [DOI] [PubMed] [Google Scholar]
- Pessina MA. Differential Effects of Estradiol, Progesterone, and Testosterone on Vaginal Structural Integrity. Endocrinology. 2005;147:61–69. doi: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- Rendi MH, Muehlenbachs A, Garcia RL, Boyd KL. 17. Female Reproductive System. Elsevier Inc; 2011. [Google Scholar]
- Rubio CA. The exfoliating cervico-vaginal surface. II. Scanning electron microscopical studies during the estrous cycle in mice. The Anatomical record. 1976;185:359–372. doi: 10.1002/ar.1091850308. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spornitz UM, Socin CD, Dravid AA. Estrous stage determination in rats by means of scanning electron microscopic images of uterine surface epithelium. The Anatomical record. 1999;254:116–126. doi: 10.1002/(SICI)1097-0185(19990101)254:1<116::AID-AR15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154:4939–4945. doi: 10.1210/en.2013-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis CG, Carson RS, Findlay JK. Relationships between aromatase activity, follicular fluid oestradiol-17 beta and testosterone concentrations, and diameter and atresia of individual ovine follicles. J Reprod Fertil. 1984;72:153–163. doi: 10.1530/jrf.0.0720153. [DOI] [PubMed] [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992;131:1458–1466. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Hawkins RA, Stocker JF. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969;85:103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]


