Abstract
In this issue of Molecular Cell, Ye et al. (2015) demonstrate that mTORC1 globally regulates miRNA biogenesis under nutrient-rich conditions via the E3 ubiquitin ligase Mdm2, which promotes Drosha degradation.
Mechanistic target of rapamycin (mTOR) is a conserved protein kinase and a component of the mTOR complex 1 (mTORC1). mTORC1 senses multiple stimuli, such as nutrients and growth factors, to control a variety of downstream pathways involved in metabolism and cell growth (Zoncu et al., 2011). Cells and organisms grow when conditions are favorable and nutrients are plentiful. mTORC1 coordinates nutrient availability with cell growth by stimulating anabolic processes like protein synthesis and by inhibiting cellular catabolism through autophagy repression in nutrient rich conditions (Jewell et al., 2013). Recently, chronic treatment of cancer cells with the potent mTORC1 inhibitor rapamycin was shown to alter microRNA (miRNA) profiles (Sun et al., 2010; Totary-Jain et al., 2013). However, the mechanistic link between mTORC1 and miRNA biogenesis was unknown. In this issue, Ye et al. (2015) fill in the missing gap by providing evidence that nutrients, such as glucose and amino acids, regulate global miRNAs through mTORC1. Specifically, nutrient-induced mTORC1 activation increases the levels of the E3 ubiquitin ligase Mdm2, which ubiquitinates and targets the miRNA-processing enzyme Drosha for proteasomal-dependent degradation (Figure 1). Degradation of Drosha results in reduced miRNA processing and global downregulation of steady-state miRNA levels. These new findings emphasize the impact that nutrients and the cellular environment have on miRNA biogenesis and compliment results observed in mouse studies, where maternal diet was shown to alter a subset of miRNAs in the offspring through mTORC1 (Alejandro et al., 2014).
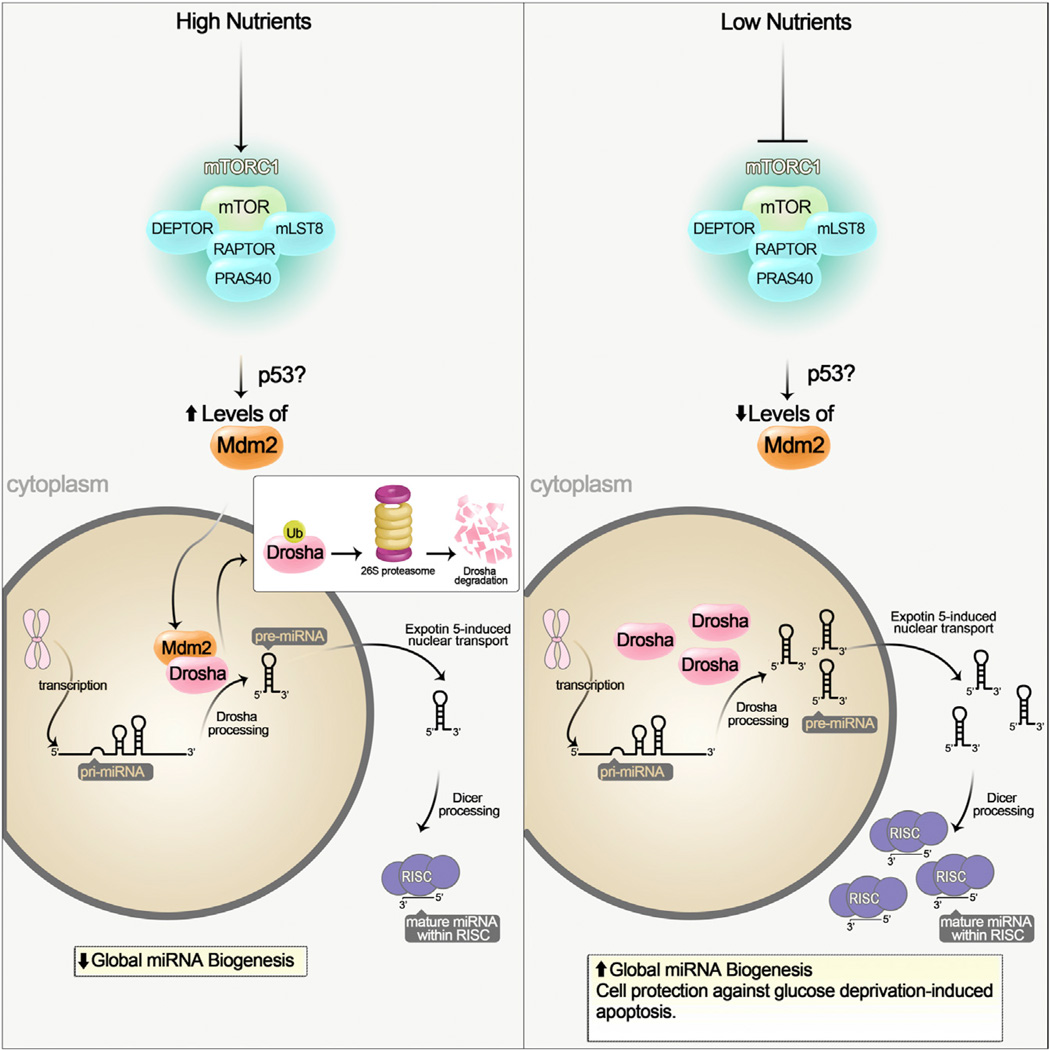
Figure 1. Nutrients Regulate Global miRNA Biogenesis through an mTORC1-Mdm2-Drosha Pathway.
(Left) Under nutrient sufficiency mTORC1 is activated, and it increases the levels of the ubiquitin E3 ligase Mdm2. mTORC1 may control Mdm2 levels through a p53-dependent and -independent pathway. Mdm2 ubiquitinates and targets the miRNA-processing enzyme Drosha for proteasomal-dependent degradation. This results in a global decrease of miRNA biogenesis. (Right) Under nutrient deficiency mTORC1 is not active and Mdm2 levels are low. Drosha levels are elevated leading to an increase in global miRNA biogenesis.
The human genome encodes some 1000 miRNAs, and dysregulation of miRNAs is often associated with many human diseases, particularly cancer (Mendell and Olson, 2012). miRNAs are a class of small non-coding regulatory RNAs that are ~21–22 nucleotides in length and function in RNA silencing and post-transcriptional regulation of gene expression. The generation of miRNAs is achieved by two RNase III-type endonucleases Drosha and Dicer. miRNA biosynthesis is under tight spatial control that starts in the nucleus with the synthesis of a long transcript known as primary miRNA (pri-mRNA). Drosha and its interacting partner DiGeorge syndrome critical region gene 8 (DGCR8) process the pri-miRNA to a precursor miRNA (pre-miRNA), and the pre-miRNA is then exported from the nucleus into the cytoplasm by exportin-5. Dicer-dependent processing converts the pre-miRNA to mature miRNA, which unites with the Argonaute (Ago) family of proteins within the RNA-induced silencing complex (RISC). RISC utilizes the miRNAs as guide to silence post-transcriptional genes (Ha and Kim, 2014). Understanding how the cellular environment, such as nutrients, controls the basic machinery involved in miRNA biogenesis is of great interest in biology research.
Considering the importance of both mTORC1 and miRNAs in cancer development, it is perhaps not surprising that some crosstalk between them exists. The results by Ye et al. (2015) reveal the intricate molecular details involved in this crosstalk by uncovering an mTORC1-Mdm2-Drosha pathway that regulates global miRNA biogenesis. Nutrient-induced mTORC1 activation appears to increase Mdm2 mRNA and protein levels. However, the precise mechanism by which mTORC1 controls Mdm2 levels is not clear. The increase in Mdm2 mRNA suggests that mTORC1 regulates Mdm2 at the transcriptional level. Therefore, it seems likely that mTORC1-dependent phosphorylation of a transcriptional regulator of Mdm2 may be involved. Furthermore, Mdm2 has not been reported to be a substrate for mTORC1. Is Mdm2 phosphorylated by mTORC1? Does mTORC1 shuttle into the nucleus to modulate Mdm2 levels? Does mTORC1 regulate Mdm2 protein levels in the cytoplasm, or maybe at the lysosome, where mTORC1 is activated? Interestingly, Mdm2 was identified as a binding partner and an E3 ubiquitin ligase for Drosha. Mdm2-dependent ubiquitination of Drosha targeted Drosha to the proteasome for subsequent degradation. The tumor suppressor p53 is a well-established transcriptional regulator of Mdm2 and has been implicated down-stream of mTORC1 regulation (Lee et al., 2007). Thus, the authors investigated if p53 was involved in this signaling cascade. Elevated mTORC1 activity increased Mdm2 mRNA ~10-fold, which was abolished in the absence of p53. However, despite unchanged Mdm2 mRNA levels with high mTORC1 activity in p53 null cells, Mdm2 protein levels were still significantly high when compared with p53 null cells where mTORC1 activity was low. Taken together, the authors conclude that nutrient-induced mTORC1 activation regulates Mdm2 by a p53-dependent transcriptional route and an alternative p53-independent post-transcriptional route. Two distinct pathways downstream of mTORC1 may control Mdm2 levels and global miRNA biogenesis.
In further exploring glucose deprivation through mTORC1, Drosha appeared to be critical for cell sensitivity to apoptosis. Because Drosha levels were significantly elevated under glucose starvation, the authors speculated that it may upregulate miRNAs crucial for cell survival under such conditions. In fact, silencing Drosha under glucose deprivation increased cell apoptosis, suggesting that miRNA biogenesis may play an essential role in cellular resistance to energy depletion. Performing a high-throughput screen utilizing a miRNA mimic library, which contains double-stranded RNA molecules that mimic native miRNAs, the authors identified four miRNA mimics that could rescue low glucose-induced cell apoptosis when Drosha was silenced. miR-297, miR-376b-3p, miR-567, and miR-627-5p increased resistance of the Drosha-silenced cells to glucose deprivation. Two of the four miRNAs, miR-297 and miR-567, significantly increased Drosha protein levels, suggesting that these two miRNAs may protect cells from apoptosis directly through Drosha levels. Thus, the mTORC1-Mdm2-Drosha pathway appears to play an important role in cellular adaptation to glucose deprivation.
Ye et al. (2015) describe a pathway where nutrients regulate global miRNAs through an mTORC1-Mdm2-Drosha signaling cascade. This study reveals how miRNAs may be regulated or sense environmental signals, such as nutrients. It would be interesting to know if other stimuli, like growth factors or stress, signal through the mTORC1-Mdm2-Drosha pathway to control global miRNA biogenesis. Is mTORC1 activity in general important, or do certain cues that filter through mTORC1 matter? Does Drosha deficiency affect cell survival under serum starvation conditions or in the absence of growth factors? In any event, the results of this study pave the way for new research on the crosstalk between mTORC1 and miRNA biogenesis.
REFERENCES
- Alejandro EU, Gregg B, Wallen T, Kumusoglu D, Meister D, Chen A, Merrins MJ, Satin LS, Liu M, Arvan P, Bernal-Mizrachi E. J. Clin. Invest. 2014;124:4395–4410. doi: 10.1172/JCI74237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Kim VN. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Nat. Rev. Mol. Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Inoki K, Karbowniczek M, Petroulakis E, Sonenberg N, Henske EP, Guan KL. EMBO J. 2007;26:4812–4823. doi: 10.1038/sj.emboj.7601900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JT, Olson EN. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ge Y, Drnevich J, Zhao Y, Band M, Chen J. J. Cell Biol. 2010;189:1157–1169. doi: 10.1083/jcb.200912093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totary-Jain H, Sanoudou D, Ben-Dov IZ, Dautriche CN, Guarnieri P, Marx SO, Tuschl T, Marks AR. J. Biol. Chem. 2013;288:6034–6044. doi: 10.1074/jbc.M112.416446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Liu Y, Chen C, Tang F, Wu Q, Wang X, Liu CG, Liu X, Liu R, Liu Y, Zheng P. Mol. Cell. 2015;57:708–720. doi: 10.1016/j.molcel.2014.12.034. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]