Abstract
Novel treatments for epilepsy are necessary because many epilepsy patients are resistant to medication. Metabotropic glutamate receptors (mGluRs), specifically mGluR 2 and 3, may serve as antiepileptic targets because of their role in controlling synaptic release. In this study, we administered a Group 2 mGluR agonist, LY379268, one of two mGluR2-specific positive allosteric modulators, BINA or CBiPES, or a cocktail of both BINA and LY379268 in a series of experiments using the pilocarpine model of SE. In one study, groups received treatments 15 minutes prior to pilocarpine, while in a second study groups received treatments after SE had been initiated to determine whether the drugs could reduce development and progression of SE. We measured bouts of stage 5 seizures, latency to the first seizure, and the maximum Racine score to characterize the seizure severity. We analyzed mouse EEG with implanted electrodes using a power analysis. We found that pretreatment and posttreatment with LY379268 was effective at reducing both behavioral correlates and power in EEG bandwidths associated with seizure, while CBiPES was less effective and BINA was ineffective. These data generally support continued development of mGluR2 pharmacology for novel antiepileptic drugs, though further study with additional drugs and concentrations will be necessary.
Keywords: Pilocarpine, metabotropic glutamate receptor, status epilepticus, mice
1.1 INTRODUCTION
Temporal lobe epilepsy (TLE) is a chronic condition characterized by recurrent seizures that involve the medial or lateral temporal lobe. Antiepileptic drugs (AEDs) can be effective, but nearly 30% of patients are refractory to AEDs, and some medications possess negative side effects that reduce patient compliance. There is also a wide range of individual responsiveness to AEDs, therefore the development of novel pharmacological targets remains an important goal.
The pilocarpine model of TLE mimics the process of epileptogenesis, and possesses many characteristics of the human disorder. Pilocarpine administration results in an acute period of status epilepticus (SE) that is defined by continuous seizure activity lasting at least 30 minutes. After the initial period of SE, there is a “latent period” during which significant neural reorganization occurs followed by chronic life-long susceptibility to spontaneous, recurrent seizures (Cavalheiro et al., 1996; Curia et al., 2008; Müller et al., 2009; Perez-Mendes et al., 2011; Turski et al., 1989, 1984, 1983). The maintenance and generalization of SE and the development of spontaneous recurrent seizures (SRS) is thought to occur through hyperglutamatergic activity via NMDA receptors in the hippocampus (Nagao et al., 1996; Priel and Albuquerque, 2002; Smolders et al., 1997). Therefore, pilocarpine administration in wild-type mice provides the opportunity to assess novel therapies that interfere with excessive glutamate signaling.
A potential target for such novel therapies are the Group 2 metabotropic glutamate receptors (mGluRs), comprising mGluR2 and mGluR3 (Alexander and Godwin, 2006a; Moldrich et al., 2003). Unlike ionotropic glutamate receptors, mGluRs do not transmit fast synaptic responses (Conn, 2003). MGluRs tend to produce longer lasting effects than ionotropic glutamate receptors due to their G-protein involvement (Conn and Pin, 1997). The Group 2 mGluRs are coupled to the Gi/o protein and may inhibit glutamate release via inhibition of high threshold calcium channels, activation of potassium channels and/or by inhibition of neurotransmitter release (Anwyl, 1999; Cochilla and Alford, 1998; Scanziani et al., 1995; Takahashi et al., 1996). In particular, mGluR2 appears to be exclusively positioned outside of the active zone of synapses where it may only be activated during high frequency neuronal activity (Alexander and Godwin, 2006b, 2005; Cartmell and Schoepp, 2000; Knöpfel and Uusisaari, 2008; Shigemoto et al., 1997), similar to that which occurs during SE (Blumenfeld et al., 2009; Chen and Wasterlain, 2006; Morimoto et al., 2004; Racine, 1972). In most systems studied to date, mGluR2 is specifically expressed presynaptically (Petralia et al., 1996; Shigemoto et al., 1997), which may allow for interrupting hyperexcitable activity before it spreads across the synapse and brain. Thus, mGluR2 exhibits a distinctive localization that may lend itself to abolishing or reducing the activity at hyperexcitable synapses.
Several Group 2 mGluR agonists, such as LY354740, LY389795 and LY379268, have been found to be anticonvulsant in limbic and generalized motor seizure models (Attwell et al., 1998a, 1998b; Kłodzińska et al., 2000; Miyamoto et al., 1997; Moldrich et al., 2001a, 2001b; Monn et al., 1997). Also, the effects of Group 2 agonists can be abolished by pretreatment with Group 2 antagonists, revealing the specificity of the drugs and receptor system against seizures (Folbergrová et al., 2001). While it is difficult to specifically target mGluR2 because of a lack of specific agonists, the positive allosteric modulators (PAM) 3’-[[(2-cyclopentyl-2,3-dihydro-6,7-dimethyl-1-oxo-1H-inden-5-yl)oxy]methyl]-[1,1’-biphenyl]-4-carboxylic acid (BINA) and N-[4’-cyano-biphenyl-3-yl)-N-(3-pyridinylmethyl)-ethanesulfonamide hydrochloride) (CBiPES) have pharmacological specificity at mGluR2 (Ahnaou et al., 2009; Benneyworth et al., 2007; Bonnefous et al., 2005; Fell et al., 2010; Galici et al., 2006; Johnson et al., 2005, 2003; Sanger et al., 2012).
In the present study, we centered our investigation on the acute effects of the Group 2 mGluR agonist LY379268 and the PAMs BINA and CBiPES in treatment of pilocarpine-induced SE. To do this, we tested the hypothesis that administration of Group 2 mGluR active compounds prior to pilocarpine administration (pretreatment) would reduce the behavioral and electroencephalographic progression of acute SE. The second hypothesis tested was that administration of these compounds after SE initiation induced by pilocarpine (posttreatment) would decrease the progression to clonic/tonic (C/T) seizure in acute SE.
Briefly, we found that mGluR2-active compounds (including LY379268 alone and a cocktail of LY379268+BINA) were capable of reducing the behavioral and electroencephalographic correlates of acute SE if given prior to pilocarpine. We also observed that these drugs could reduce the progression to C/T seizure activity of acute SE if given after pilocarpine administration, though higher doses were necessary. CBiPES alone was also capable of reducing some of the behavioral manifestations of seizure when given prior to pilocarpine.
1.2 METHODS
1.2.1 Rotarod Pilot Study
All animal procedures were approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee. We performed rotarod trials in pilot animals (n = 36) in order to assess gross motor performance after administration of mGluR2 active drugs. There were six groups, with six mice in each group: saline (0.25 mls), diazepam (5 mg/kg), LY379268 (10 mg/kg), LY379268 (20 mg/kg), BINA (100 mg/kg), and cocktail (LY379268 (20 mg/kg) + BINA (100 mg/kg)). Mice were randomly assigned to a group and given four habituation trials on the rotarod apparatus (SDI Inc., San Diego, CA) on day 1. Trials lasted up to 180 seconds or until the mouse fell from the rod.
On test day (day 2), mice were given a preinjection trial and then tested again 10 minutes, 30 minutes, 1 hour, and 5 hours after injection. Time spent on the rotarod was measured and postinjection performance was compared to the preinjection trial within each group. The diazepam group was included as a positive control for a compound that is commonly known to interfere with motor performance and ability (Savić et al., 2009; Willerslev-Olsen et al., 2011).
1.2.2 Surgery
A tethered electroencephalography/electromyography (EEG/EMG) acquisition system (Pinnacle Technologies Inc, Lawrence KS) was used for these studies. For surgical implantation of EEG electrodes, a subset of male C57Bl/6 mice (n = 48) were anesthetized using ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). Supplemental oxygen was provided and atropine (0.04 mg/kg) was given preoperatively to suppress bronchial secretions. Once areflexia was apparent, a 1 cm incision was made and the skull exposed. Four pilot holes were drilled through the skull for placement of stainless steel screws. These screws terminated in a preamplifier headmount that was affixed to the skull using dental acrylic. Silver epoxy was used to cover each screw and maintain electrical continuity with the headmount. Two EMG leads were placed into the neck musculature. The incision was sutured around the headmount and topical antibiotic was applied. Mice were moved to a recovery cage and given ketoprofen (5 mg/kg) for pain management. Mice recovered at least one week before initiating any subsequent experiment. Systemic antibiotics were not given, as post-operative infection was considered exclusionary criteria for the study according to our protocol.
1.2.3 Behavior
During the pre- (treatment given before pilocarpine) and posttreatment (treatment given after pilocarpine) studies, we measured the onset and severity of their behavioral response to pilocarpine administration. The measures taken included: the latency to the first stage 5 C/T seizure, bouts of individual C/T seizures during SE, and the maximum Racine score. When an animal failed to reach a C/T seizure in response to pilocarpine, they were automatically assigned a latency score of 180 min, which was the maximum length of observation. These animals were assumed to have not developed a C/T response to pilocarpine, but still allowed for them to be included in their group for statistical comparisons. In the posttreatment studies, treatment was given when an animal reached a stage 3 Racine score, which is a readily apparent behavioral response to pilocarpine. Seizure scoring was performed using an adapted Racine scale (Racine, 1972). The scale is as follows: 0 (lack of any apparent seizure activity), 1 (oral automatisms), 2 (head nodding), 3 (forelimb clonus), 4 (rearing), 5 (clonic/tonic seizure with rearing and falling), 6 (wild running/bouncing), to 7 (death as a consequence of pilocarpine and resulting seizures). The experimenters scoring the behavioral activity administered the treatments and were therefore not blinded to the groups. Group analyses were not conducted, however, until completion of all experiments. Electrographical recordings were consistent with the observed behavioral activity.
1.2.4 Pilocarpine Administration
Pilocarpine (330 mg/kg) was administered to mice (n = 114) 30 min after an injection of methyl-scopolamine (1 mg/kg), which was used to inhibit peripheral effects of pilocarpine and reduce mortality. This dose of pilocarpine was chosen because it has been demonstrated in the literature to be the lowest concentration of pilocarpine used that most reliably elicits seizures (Curia et al., 2008; Turski et al., 1983). Pilocarpine was given outside of the home cage while mice were individually housed in monitoring cages. Animals were monitored for three hours for behavioral scoring. In a subset of animals, EEG monitoring was also performed during the first hour. EEG sampling occurred at a rate of 200 Hz with a preamplifier applied band pass filter from 0.5 to 40 Hz. For the pretreatment studies, drugs were given 15 minutes prior to pilocarpine. The groups in that study included saline (referred to as “Pilo Only”, n = 14), LY379268 (10 mg/kg, n = 14), BINA (100 mg/kg, n = 14), and cocktail, which received both LY379268 and BINA (10 and 100 mg/kg respectively, n = 14). For the posttreatment studies, drugs were given immediately after the animal had the first stage 3 Racine seizure, which is characterized by forelimb clonus. The groups in that study were saline (“Pilo Only”, n = 12), LY379268 (20 mg/kg, n = 12), BINA (100 mg/kg, n = 10), and cocktail, which received both LY379268 and BINA (20 and 100 mg/kg respectively, n = 12). Finally, a second mGluR2-specific positive allosteric modulator (PAM), CBiPES (30 mg/kg), was tested for efficacy in both pre- and posttreatment behavioral experiments (n = 6 in both studies).
1.2.5 Drugs
LY379268 was a kind gift from Eli Lilly and Company and was dissolved in 0.9% saline. CBiPES was also kindly provided by Eli Lilly and Company and was dissolved in a vehicle containing 1% carboxymethylcellulose, 0.25% Tween 80 and 0.05% Dow Antifoam, with the pH adjusted to 7.4. BINA was a gift by Dr. Jeffrey Conn (Vanderbilt University, Nashville, TN) and was dissolved in a vehicle containing 10% Tween 80 and 10% NaOH with the pH adjusted to 7.4. Diazepam was manufactured by Hospira, Inc (Lake Forest, IL). Methyl-scopolamine and pilocarpine were purchased from Sigma (St. Louis, MO) and both dissolved in 0.9% saline. All drugs were given intraperitoneally in a volume range of 0.25 to 0.5 mls.
1.2.6 Data Analysis
Rotarod data were analyzed using a repeated-measures ANOVA with a Dunnett’s post hoc to test for significant differences within each group’s performance before and after injection. Digitized EEG signals were transformed into power spectral data using a custom-written Matlab script. Analysis of that transformed data was performed using a Kruskal-Wallis test with Dunn’s post hoc analysis. The spectral bands analyzed were defined as follows: delta (0.5 to 3 Hz), theta (4 to 7 Hz), alpha (8 to 12 Hz), and beta (13 to 25 Hz). EEG spectral power data for each mouse was normalized to its baseline. Mice for each group were then averaged together. Histograms represent a fold change in spectral power for the defined bandwidths. If normality could be assumed for data that were continuous in nature, analysis was performed using a one-way ANOVA with Tukey’s post hoc test. If behavioral data were discrete in nature, a Kruskal-Wallis test with a Dunn’s post hoc was used. If the behavioral data were categorical in nature, then a Chi-square test of homogeneity was used to test for statistically significant differences.
1.3 RESULTS
1.3.1 Rotarod Pilot Study
A repeated-measures ANOVA was performed within each experimental group with a Dunnett’s post hoc to determine if any group’s postinjection performance was significantly different from their preinjection performance on the rotarod. The diazepam group spent significantly less time on the rotarod 10 and 30 minutes after the injection (p < 0.01, n = 6, Table 1) compared to before the injection. No other group exhibited differences in gross motor ability at any time point after injection compared to before the injection. The means ± SEM for the time (in seconds) spent on the rotarod for each group at every time point are presented in Table 1. The mGluR-active drugs used in this study do not appear to significantly inhibit motor performance on the rotarod test.
Table 1. Drugs active at Group 2 mGluRs do not perturb gross motor ability.
C57Bl/6 mice were used (n = 6 per group) to determine whether performance on a rotarod apparatus would be altered by injection with either saline, diazepam (5 mg/kg), LY379268 (10 mg/kg), LY379268 (20 mg/kg), BINA (100 mg/kg), or a cocktail of both BINA and LY379268 at the highest dose. Diazepam was used to demonstrate the effect of an antiepileptic drug that is also known to inhibit motor function. Time spent on the rotarod (mean ± SEM) was measured prior to injection, and then 10 min, 30 min, 1 h, and 5 h postinjection. A repeated measures ANOVA with Dunnett’s post hoc analysis was used to determine which time points postinjection were significantly different from the preinjection performance within each particular group. The only significant differences were found in the diazepam group, at 10 and 30 min postinjection.
| Preinjection | 10 min post | 30 min post | 1 hr post | 5 hr post | |
|---|---|---|---|---|---|
| Saline | 140.50 ± 19.45 |
124.16± 20.66 |
117.72 ± 24.11 |
117.40 ± 20.84 |
112.12 ± 21.79 |
|
Diazepam (5 mg/kg) |
134.04 ± 19.12 |
49.48 ± 6.69* |
38.18 ± 5.5* |
90.62 ± 23.37 |
147.22 ± 21.07 |
|
LY379268 (10 mg/kg) |
159.94 ± 12.28 |
138.66 ± 21.55 |
146.10± 15.27 |
134.40 ± 23.94 |
135.84 ± 22.72 |
|
LY37926S (20 mg/kg) |
56.9 ± 12.88 |
42.9 ± 4.32 |
41.98 ± 4.90 |
67.98 ± 9.72 |
46.48 ± 4.48 |
|
BINA (100 mg/kg) |
119.58 ± 18.47 |
103.44 ± 14.27 |
101.98 ± 22.42 |
99.88 ± 19.20 |
100.82 ± 20.28 |
|
Cocktail (LY379268(20 mg/kg) +BINA) |
99.70 ± 21.45 |
93.34 ± 25.74 |
93.68 ± 24.20 |
103.90 ± 21.35 |
96.04 ± 18.90 |
p < 0.01.
1.3.2 Pretreatment Study
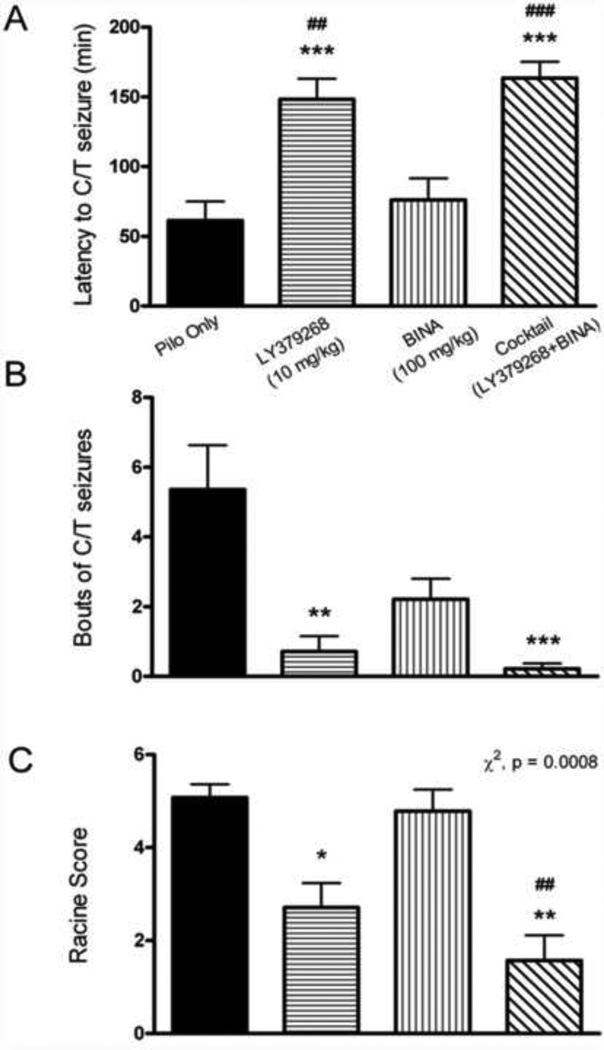
The latency to the first C/T seizure was significantly increased for the LY379268 and cocktail groups compared to the Pilo Only group (one-way ANOVA with Tukey’s post hoc, p < 0.001, n = 14 for all groups, mean ± SEM for LY379268 = 148.2 ± 14.61 min, cocktail = 163.5 ± 11.63 min, Pilo Only = 61.29 ± 13.76 min, Fig 1a). Also, the LY379268 and cocktail groups had a significantly increased latency to the first C/T seizure compared to the BINA group (one-way ANOVA with Tukey’s post hoc, p < 0.001 for the cocktail group, p < 0.01 for the LY379268 group, mean ± SEM for BINA = 76.07 ± 15.44 min, Fig 1a). Mice pretreated with LY379268 had fewer bouts of C/T seizures during SE (Kruskal-Wallis test with Dunn’s post hoc analysis; p < 0.01, mean ± SEM = 0.71 ± 0.44 bouts) than Pilo Only mice (mean ± SEM = 5.36 ± 1.27 bouts, Fig 1b). Mice pretreated with the cocktail also had significantly fewer bouts of C/T seizures compared to Pilo Only mice (p < 0.001, mean ± SEM for cocktail = 0.21 ± 0.15, Fig 1b). Lastly, using a chi-square test for homogeneity, it was found that there were significant differences between all of the groups Racine scores (p = 0.0008, mean ± SEM Racine score for Pilo Only = 5.07 ± 0.29, LY379268 = 2.70 ± 0.52, BINA = 4.79 ± 0.46, cocktail = 1.57 ± 0.54, Fig 1c). Post-hoc tests revealed that mice treated with LY379268 had a lower Racine score compared to Pilo Only mice; mice treated with the cocktail had a lower Racine score compared to Pilo Only mice and BINA mice.
Figure 1. Pretreatment with Group 2 mGluR active drugs reduces behavioral severity of pilocarpine induced SE.
A. Latency to the first C/T seizure during SE was significantly increased for the LY379368 and the cocktail groups compared to the Pilo Only group (n = 14 in all groups, one-way ANOVA with Tukey’s post hoc, p < 0.001). The LY379268 and cocktail groups also had an increased latency to the first C/T seizure compared to the BINA group (one-way ANOVA with Tukey’s post hoc, p < 0.01 for the LY379268 group, n = 14; p < 0.001 for the cocktail group). B. Average bouts of C/T seizures during SE were significantly reduced with pretreatment with LY379268 compared to the Pilo Only group (Kruskal-Wallis test with Dunn’s post hoc, p < 0.01). Pretreatment with the cocktail also significantly reduced bouts of C/T seizures compared to the Pilo Only group (Kruskal-Wallis test with Dunn’s post hoc, p < 0.001). C. The Racine scores were also significantly different between all pretreatment groups (Chi square test for homogeneity, p = 0.0008). Post hoc tests revealed that mice treated with LY379268 had a lower Racine score compared to Pilo Only mice. Cocktail treated mice had a lower Racine score compared to Pilo Only and BINA mice. Seizures were considered C/T if they reached at least a stage 5 Racine score. The Pilo Only group received saline as a control pretreatment prior to pilocarpine. Compared to Pilo Only: *, p<0.05; **, p < 0.01; ***, p < 0.001. Compared to BINA: ##, p < 0.01; ###, p < 0.001.
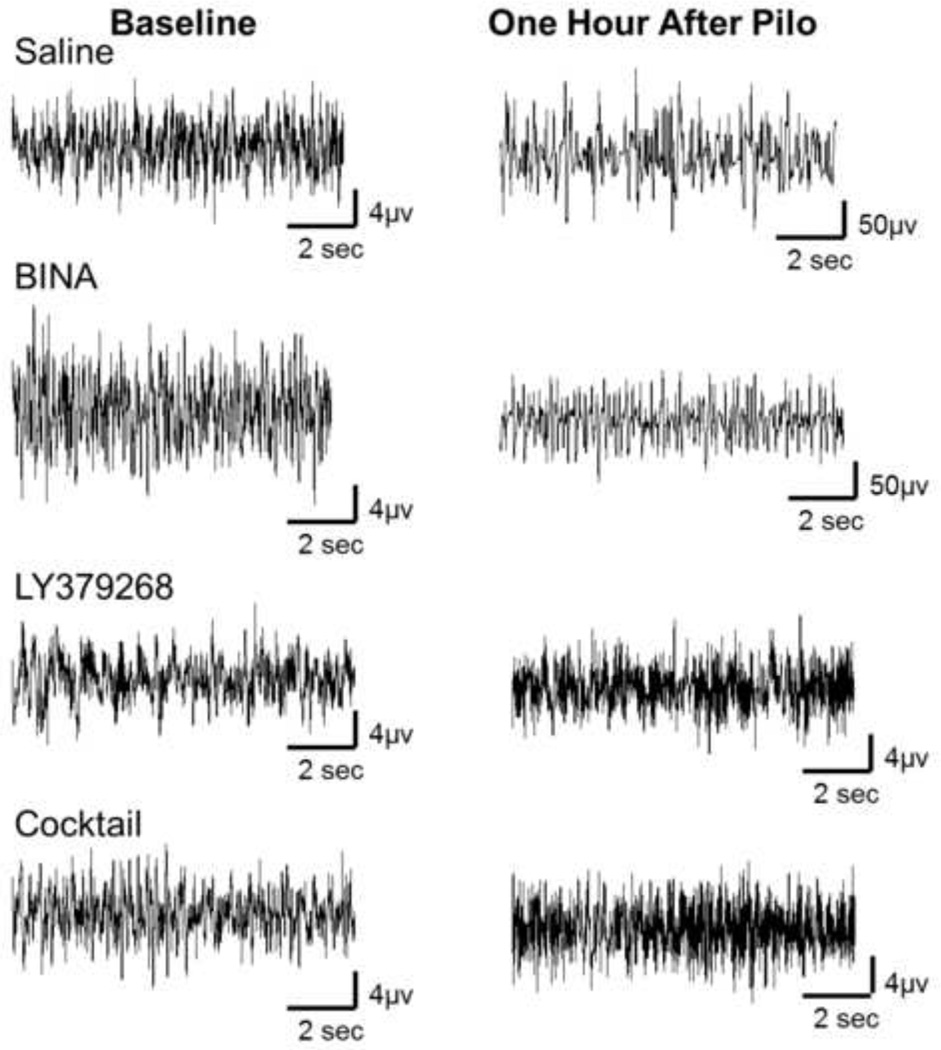
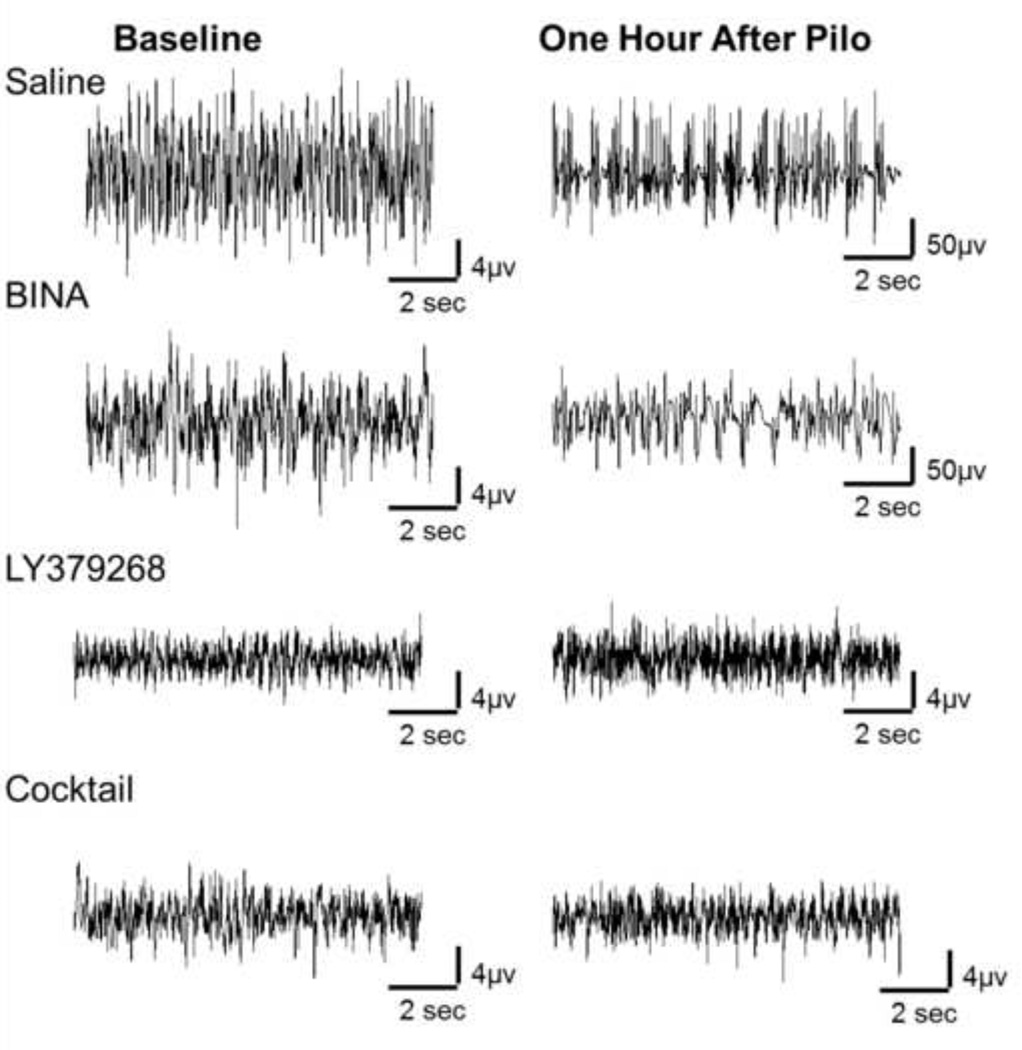
Figure 2 represents sample EEG traces from one mouse in each treatment group measured during the pretreatment study demonstrating activity at baseline prior to pilocarpine administration and activity 60 minutes after pilocarpine administration. To account for amplitude differences at baseline across EEG recording rigs, EEG spectral data for each mouse was normalized to its baseline. Power spectral data 60 minutes after pilocarpine administration when Group 2 mGluR-active drugs were given as pretreatment is shown in Figure 3. Fold change in power is represented on the Y axis. Power in each frequency bandwidth (delta- 0 to 3 Hz, theta- 4 to 7 Hz, alpha- 8 to 12 Hz, and beta- 13 to 25 Hz) was averaged, normalized to baseline activity in that frequency bandwidth, and a Kruskal-Wallis test with Dunn’s post hoc was performed across groups within each frequency band.
Figure 2. Pretreatment Study, sample traces from one mouse representing each treatment group.
Baseline, activity at baseline prior to pilocarpine administration. One hour after Pilo, activity 60 minutes after pilocarpine administration. To account for amplitude differences at baseline across EEG recording rigs, EEG spectral data for each mouse was normalized to its baseline. Column treatment groups, Saline, BINA (100mg/kg), LY379268 (10mg/kg), Cocktail (LY378268 (10mg/kg) + BINA (100mg/kg)).
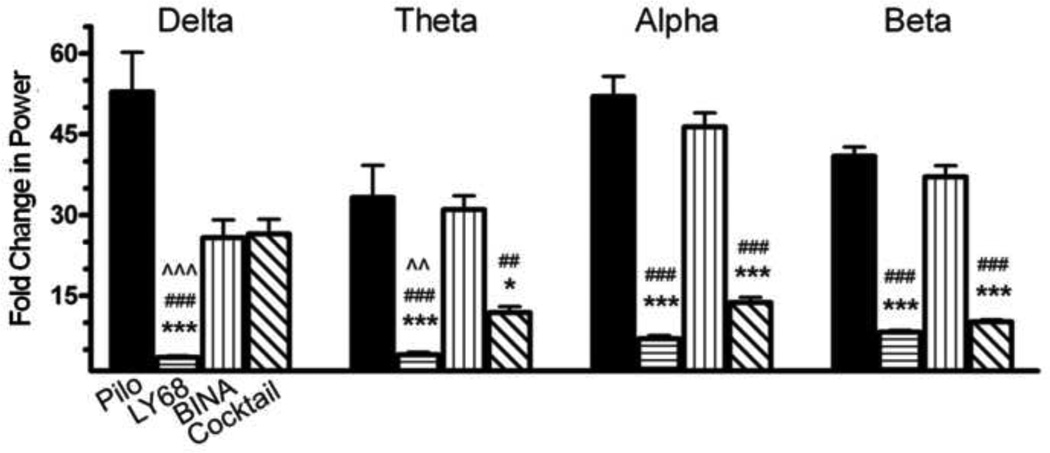
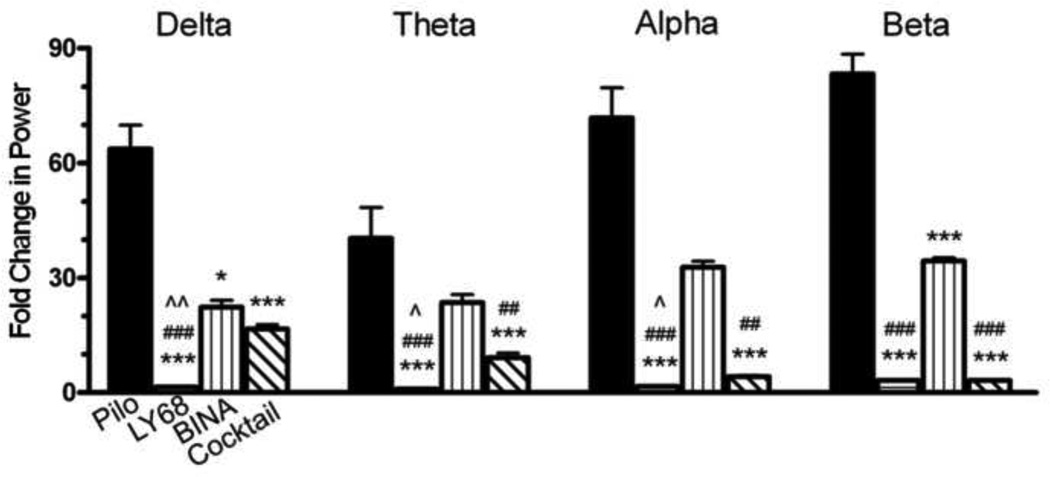
Figure 3. Pretreatment with Group 2 mGluR drugs reduces power of pilocarpine-induced SE in EEG recordings 60 minutes after pilocarpine administration.
In the delta range, the LY379268 group shows decreased power compared to all other groups (p < 0.001, n = 6 in all groups). In the theta frequency range, the LY379268 group shows decreased power compared to the Pilo Only group (p < 0.001), the BINA group (p < 0.001) and the cocktail group (p < 0.01). The power is also decreased in the theta range for the cocktail group compared to the Pilo Only group (p < 0.05) and the BINA group (p < 0.01). In the alpha and beta range, the LY379268 and cocktail groups show decreased power compared to the Pilo Only and BINA groups (p < 0.001 for both comparisons). The Pilo Only group received saline as a control pretreatment prior to pilocarpine. The frequency ranges were defined as delta (0.5-3 Hz), theta (4–7 Hz), alpha (8–12 Hz) and beta (13–25 Hz). Power has been normalized to the baseline power for each mouse in the defined frequency range. Compared to Pilo Only: *, p < 0.05; ***, p < 0.001. Compared to BINA: ##, p < 0.01; ###, p < 0.001. Compared to Cocktail: ^^, p<0.01; ^^^, p<0.001. Column abbreviations: Pilo: Pilo Only, LY68: LY379268 (10 mg/kg), BINA: BINA (100 mg/kg), Cocktail: Cocktail (LY379268 (10 mg/kg) + BINA (100 mg/kg)).
Sixty minutes after pilocarpine administration (Fig 3), the LY379268 group had significantly less power than the Pilo Only, BINA and Cocktail groups (p < 0.001) in the delta bandwidth. In theta bandwidth, the LY379268 group continued to demonstrate less power than the Pilo Only and BINA groups (p < 0.001), as well as the cocktail group (p < 0.01). Also in the theta range, the cocktail group had significantly less power than the Pilo Only group (p< 0.05) and the BINA group (p < 0.01). Finally in the alpha and beta bandwidths, the LY379268 and cocktail groups had signficantly less power than the Pilo Only group (p < 0.001). The EEG data suggests that LY379268 and the cocktail mitigated the abnormal power increases that were seen in control mice that received only pilocarpine with saline as pretreatment.
Taken together, the behavioral and spectral data suggest that LY379268 (10 mg/kg) alone can alter the course of SE when given prior to pilocarpine. LY379268 lessened pilocarpine-induced power changes and also increased the time until initial seizure onset, decreased the number of individual C/T seizures during SE and lowered the average maximum Racine score. BINA does not appear to provide protection against pilocarpine-induced power changes in EEG or in behavioral expression of SE. BINA alone did not increase the latency to the first C/T seizure, reduce the bouts of C/T seizures, or lower the average Racine score. The cocktail of both LY379268 and BINA does not seem to provide any more protection from SE than LY379268 does alone. In general it appears there is no added benefit of administering the cocktail as opposed to the mGluR2/3 agonist alone as pretreatment against pilocarpine-induced SE.
1.3.3 Posttreatment Study
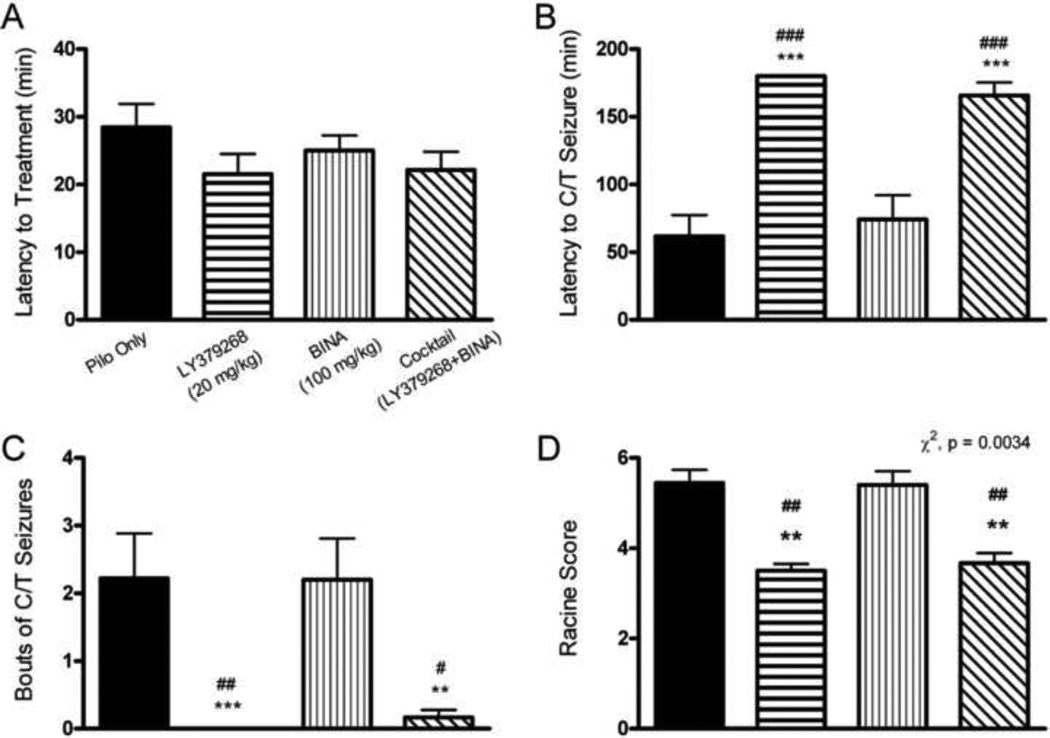
In the posttreatment study, treatment was administered when the mouse reached a stage 3 seizure after pilocarpine administration. There were no significant differences between groups in the latency to treatment administration (Fig 4a). A stage 3 seizure occurred and treatment was given on average 23.74 ± 2.69 (mean ± SEM) minutes after pilocarpine.
Figure 4. Posttreatment with Group 2 mGluR active drugs reduces severity of pilocarpine induced SE behavioral measures.
The average latency to stage 3 Racine seizures did not vary between groups (one-way ANOVA with Tukey’s post hoc, p > 0.05, n ≥ 10 in all groups, mean latency for treatment administration was 24 min). B. Latency to the first C/T seizure during SE was significantly increased for the LY379368 and the cocktail groups compared to the Pilo Only group and the BINA group (one-way ANOVA with Tukey’s post hoc, p < 0.001 for both comparisons, n = 12 in these three groups). No mouse that received LY379268 posttreatment ever reached a stage 5 C/T seizure, therefore that entire group was scored as 180 min latency (see Methods), as that was the maximum length of observation time for this study. C. Average bouts of C/T seizures during SE were significantly reduced with posttreatment with LY379268 and the cocktail compared to the Pilo Only group that received saline posttreatment (Kruskal-Wallis test with Dunn’s post hoc, p < 0.01 for the cocktail group, p < 0.001 for the LY379268 group, n ≥ 10 in all groups). The LY379268 and cocktail groups also experienced fewer bouts of seizures than the BINA group (p < 0.05 for the cocktail group, p < 0.01 for the LY379268 group). D. The Racine scores were also significantly different between posttreatment groups (Chi square test of homogeneity, p = 0.0034, n ≥ 10 in all groups). Post hoc tests revealed a significant difference in Racine score between the Pilo only group compared to the LY379268 and cocktail groups. These groups also had a significantly lower Racine score compared the the BINA group. Seizures were considered C/T if they reached at least a stage 5 Racine score. The Pilo Only group received saline as a control posttreatment after animals reached a stage 3 Racine seizure. Compared to Pilo Only: **, p < 0.01; ***, p < 0.001. Compared to BINA: #, p < 0.05; ##, p < 0.01; ###, p < 0.001.
A one-way ANOVA with Tukey’s post hoc analysis determined there was a significantly increased latency to the onset of the first C/T seizure for the LY379268 (n = 12) and the cocktail (n = 12) posttreatment groups as compared to the Pilo Only group (n = 12, p < 0.001, latency mean ± SEM for LY379268 = 180 ± 0 min, cocktail = 165.7 ± 9.68 min, Pilo Only = 61.78 ± 15.65 min, Fig 4b). Also, the cocktail and LY379268 groups had significantly increased latencies to the first C/T seizure compared to the BINA group (n = 10, p < 0.001, latency mean ± SEM for BINA = 74.2 ± 17.93 min, Fig 4b). LY379268 posttreated mice had fewer bouts of C/T seizures during SE than Pilo Only mice (p < 0.001, Kruskal-Wallis test with Dunn’s post hoc analysis, bouts mean ± SEM for LY379268 = 0 ± 0, bouts mean ± SEM for Pilo Only = 2.22 ± 0.66, Fig 4c). The cocktail posttreated mice had significantly fewer bouts of seizures than the Pilo Only group (p < 0.01) and the BINA group (p < 0.05, bouts mean ± SEM for cocktail = 0.17 ± 0.11, bouts mean ± SEM for BINA = 2.2 ± 0.61). Lastly, using a chi square test for homogeneity, the posttreatment groups had significantly different Racine scores (p = 0.0034, Racine score mean ± SEM for Pilo Only = 5.44 ± 0.29, LY379268 = 3.5 ± 0.15, BINA = 5.4 ± 0.3, cocktail = 3.67 ± 0.22, Fig 4d). Post-hoc tests revealed that mice treated with LY379268 had a lower Racine score compared to Pilo Only and BINA mice; mice treated with the cocktail also had a lower Racine score compared to Pilo Only and BINA mice.
Figure 5 represents sample EEG traces from one mouse in each posttreatment group demonstrating activity at baseline prior to pilocarpine administration and activity 60 minutes after pilocarpine administration. To account for amplitude differences at baseline across EEG recording rigs, EEG spectral data for each mouse was normalized to its baseline. Sixty minutes after pilocarpine (Fig 6), the LY379268, BINA and cocktail groups all exhibited significantly less power compared to the Pilo Only group (p < 0.001 for the LY379268 and cocktail groups, p < 0.05 for the BINA group) in the delta frequency range. Also in the delta range, the LY379268 group had significantly less power than both the BINA group (p < 0.001) and the cocktail group (p < 0.01). In the theta and alpha ranges, the LY379268 group showed less power compared to the Pilo Only and the BINA groups (p < 0.001), as well as the cocktail group (p < 0.05). Also in the theta and alpha ranges, the cocktail group showed significantly less power than the Pilo Only group (p < 0.001) and the BINA group (p < 0.01). Finally, in the beta range, all groups exhibited less power than the Pilo Only group (p < 0.001), and the LY379268 and cocktail groups showed less power than the BINA group (p < 0.001). Spectral power remained around baseline levels in the different frequency bandwidths in these groups whereas there was a significant increase in mice treated with saline (Pilo Only group).
Figure 5. Pretreatment Study, sample traces from one mouse representing each treatment group.
Baseline, activity at baseline prior to pilocarpine administration. One hour after Pilo, activity 60 minutes after pilocarpine administration. To account for amplitude differences at baseline across EEG recording rigs, EEG spectral data for each mouse was normalized to its baseline. Column treatment groups, Saline, BINA (100mg/kg), LY379268 (20mg/kg), Cocktail (LY378268 20mg/kg) + BINA (100mg/kg)).
Figure 6. Posttreatment with Group 2 mGluR drugs reduces power of pilocarpine induced SE in EEG recordings 60 minutes after pilocarpine administration.
In the delta frequency range, the LY379268 and cocktail groups show decreased power compared to the Pilo Only group (p < 0.001, n = 6 in all groups), as does the BINA group (p < 0.05). Also in the delta range, the LY379268 group shows decreased power compared to the BINA group (p < 0.001) and the cocktail group (p < 0.01). In the theta and alpha ranges, the LY379268 group demonstrated decreased power compared to the Pilo Only group (p < 0.001), the BINA group (p < 0.001) and the cocktail group (p < 0.05). Also in the theta and alpha bandwidths, the cocktail group had significantly decreased power compared to the Pilo Only group (p < 0.001) and the BINA group (p < 0.01). Finally in the beta range, all other groups had less power than the Pilo Only group (p < 0.001), and the LY379268 and cocktail groups also had less power than the BINA group (p < 0.001). The Pilo Only group received saline as a control posttreatment after animals reached a stage 3 Racine seizure. The frequency ranges were defined as delta (0.5-3 Hz), theta (4–7 Hz), alpha (8–12 Hz) and beta (13–25 Hz). Power has been normalized to the average power at baseline in each frequency range. Compared to Pilo Only: **, p < 0.01; ***, p < 0.001. Compared to BINA: ##, p < 0.01; ###, p < 0.001. Compared to cocktail: ^, p < 0.05; ^^, p < 0.01. Column abbreviations: Pilo: Pilo Only, LY68: LY379268 (20 mg/kg), BINA: BINA (100 mg/kg), Cocktail: Cocktail (LY379268 (20 mg/kg) + BINA (100 mg/kg)).
The posttreatment data suggest that Group 2 mGluR activation with LY379268 reduces behavioral seizures, as evidenced by increased latency to a C/T seizure, reduced bouts of C/T seizures and decreased Racine scores compared to the Pilo Only group. The BINA group was never significantly different from the Pilo Only group, and the LY379268 group was never significantly different from the cocktail group on any behavioral measure. The EEG data generally demonstrated that after treatment was given, any activation of mGluR2/3 with an agonist or in combination with a PAM, some protection would be provided in the form of reduced power compared to Pilo Only animals. Since LY379268 was not found to be different from the cocktail group, there may be no added benefit of a PAM in treating C/T seizures with mGluR2 active drugs.
1.3.4 CBiPES Study
We performed an additional round of behavioral studies using CBiPES (30 mg/kg, n = 6 in both the pre- and posttreatment groups), a recently created PAM that has been shown to potentiate the effect of LY379268 (Johnson et al., 2005) as well as mimic the antipsychotic effects of LY379268 (Fell et al., 2010). The same behavioral measures were recorded as in the previous experiments, including latency to the first C/T seizure, bouts of C/T seizures, and the maximum Racine score.
CBiPES (30 mg/kg, n = 6) given as pretreatment before pilocarpine resulted in significantly longer latency to the first C/T seizure compared to Pilo Only pretreatment (one-way ANOVA with Tukey’s post hoc , p < 0.05, latency mean ± SEM for CBiPES = 132.3 ± 23.84 min, Table 2). When given as posttreatment, CBiPES still significantly increased the latency to the first C/T seizure compared to the Pilo Only posttreatment group (one-way ANOVA with Tukey’s post hoc , p < 0.05, latency mean ± SEM for CBiPES = 135.3 ± 28.33 min, Table 2). CBiPES also significantly reduced the bouts of C/T seizures during SE compared to Pilo Only when given as pretreatment (Kruskal-Wallis with Dunn’s post hoc, p < 0.01, Table 2). When given as posttreatment, CBiPES did not significantly reduce the bouts of C/T seizures during SE compared to the Pilo Only posttreatment group (Kruskal-Wallis with Dunn’s post hoc, p > 0.05, Table 2). Finally, although differences were found in the latency to the first seizure and bouts of seizures during SE, no significant difference in Racine scores were found between these groups in either the pre- or posttreatment studies (chi-square test for homogeneity, p > 0 .05, Table 2).
Table 2. Pre and posttreatment behavioral differences between the CBiPES and Pilo Only groups (Mean ± SEM).
Latency to the first C/T seizure, bouts of C/T seizures and Racine score are presented for the experimental groups receiving pilocarpine only or CBiPES as pre- or posttreatment. Asterisks indicate significant differences between those groups.
| Pretreatment | |||
|---|---|---|---|
| Latency to C/T seizure | Bouts of C/T seizures | Racine Score | |
| Pilo Only | 61.29 ±13.76 | 5.36 ±1.27 | 5.07 ± 0.29 |
|
CBiPES (30 mg/kg) |
132.3 ± 23.84 min* | 0.56 ± 0.29* | 3.67 ± 0.57 |
| Posttreatment | |||
| Pilo Only | 61.78 ± 15.65 | 2.22 ± 0.66 | 5.44 ± 0.29 |
|
CBiPES (30 mq/kq) |
135.3 ± 28.33 min* | 0.67 ±0.49 | 4.5±0.56 |
1.4 DISCUSSION
Our study indicates that activation of Group 2 mGluRs reduces the behavioral and electrographic course of acute SE induced by pilocarpine, as evidenced by reduced behavioral seizure and EEG spectral power in animals treated with Group 2 mGluR compounds either before or after pilocarpine administration.
Pilo Only animals in both the pre- and posttreatment experiments that received only saline as an intervention demonstrated a typical progression through acute SE induced by pilocarpine. The SE inhibiting effects of the compounds were not due to any gross motor disturbances as evidenced by consistent performance on the rotarod (Table 1). Our positive control diazepam, a commonly used AED, significantly inhibited motor performance. We did not observe similar effects with the mGluR active drugs, which suggests that inhibition of seizure activity by these compounds is not due to inhibition of motor behavior. Some studies have noted the motor inhibiting quality of higher doses of LY379268 (Cartmell et al., 1999; Feinberg et al., 2002), however, 20mg/kg (highest dose used in our study) did not significantly reduce motor ability from observed preinjection performance.
In both pre- and posttreatment experiments, we observed a reduction in behavioral seizure in mice treated with LY379268 compared to the Pilo Only group (Figures 1 and 4). LY379268 increased latency to C/T seizure, reduced C/T seizure bouts, and decreased Racine seizure score. Complimentary to these behavioral findings, we observed no increase in EEG spectral power during status in mice treated with LY379268 (Figures 3 and 6). There was at least a 30-fold increase in spectral power in the different frequency bandwidths during status in control animals treated with saline prior to or after pilocarpine administration. The spectral power in the defined frequency bandwidths of mice treated with LY379268 stayed relatively the same as to what was observed in their baseline activity. In the posttreatment study, the mice had reached a stage 3 Racine seizure prior to treatment, which required a higher dose of LY379268 compared to the pretreatment investigation. A pilot group of posttreatment mice given the same dose of LY379268 used in the pretreatment study was not significantly different from the Pilo Only mice on any behavioral measure (data not shown).
In both pre- and posttreatment studies, the cocktail of LY379268 and BINA did not provide greater protection against pilocarpine-induced SE than when the LY379268 was given alone. The mice in the cocktail group exhibited similar behavioral measures as those in the LY279268 group (Figures 1 and 4). This suggests that the anti-convulsive effect of the cocktail is most likely due to LY379268 and not synergy between the BINA and the agonist. It has been previously hypothesized that simultaneous orthosteric and allosteric ligand binding at mGluR2 may increase receptor sensitivity to the endogenous and/or orthosteric ligand (Johnson et al., 2003). Mechanistically, allosteric modulation of mGluRs may increase receptor sensitivity, receptor availability, and/or efficacy of receptor homodimerization (Johnson et al., 2005; Kew and Kemp, 2005), however in our study, it does not appear that any of these allosteric modulations could overcome the changes produced by pilocarpine without the orthosteric ligand on board as well.
More studies are needed, but one plausible explanation of this effect may be that LY379268 activation of mGluR3 on glial cells tonically reduces pilocarpine-induced glutamate release, and the addition of BINA does not potentiate the effect as it is not pharmacologically active at mGluR3. Other explanations for BINA not adding any benefit when used in combination with LY379268 are that the dose used was not in the effective range and that BINA may not have crossed the blood brain barrier. Previous studies examining BINA in models of psychosis and anxiety found significant effects on their measure of interest using lower doses and the same administration technique (Benneyworth et al., 2007; Galici et al., 2006). Therefore, we believe an appropriate dose of BINA was used in our seizure model, and although we did not confirm with cortical measures of BINA concentration, the significant effects in other studies would suggest that BINA is crossing the blood brain barrier at the administered concentrations.
In contrast, with CBiPES alone pre- and posttreatment, we did find a significant increase in the latency to the first C/T seizure compared to the Pilo Only group. CBiPES also reduced the bouts of C/T seizures in the pretreatment study. This supports previous work in anxiety models, such as stress-induced hyperthermia, in which CBiPES was found to mimic the effect of LY379268 when administered alone (Fell et al., 2010; Johnson et al., 2005; Sanger et. al., 2012). However, in both pre- and posttreatment studies, CBiPES did not decrease the overall Racine score. Interestingly, CBiPES was more easily dissolved than BINA under our conditions, and while both drugs were entirely dissolved for injection, this cannot be excluded as a possible explanation of the difference in efficacy between these two PAMS. Both of these compounds have been characterized as selective mGluR2 PAMS (Ahnaou et al., 2009; Benneyworth et al., 2007; Bonnefous et al., 2005; Fell et al., 2010; Galici et al., 2006; Johnson et al., 2005, 2003; Sanger et al., 2012). However, off-target effects during seizure activity cannot be excluded. A ceiling effect may have occurred with the dose of LY379268 used in the cocktail groups. We did not test lower doses of LY379268 in combination with either modulator. Further evaluation of the two modulators is necessary to parse out differences in efficacy and to determine if a different combination of doses in the cocktail treatment could provide more of a synergistic effect.
Studies evaluating mGluR2/3 expression have demonstrated a reduction 24 hours after pilocarpine-induced SE with reports of long-term loss (Ermolinsky et al. 2008; Garrido-Sanabria et al., 2008), which consequently could lead to increased excitability in the brains of these animals. The results of the two studies suggest that delaying mGluR2/3 drug administration by 24 hours or more after pilocarpine would yield little to no effect on hyperexcitability in epileptic rodents. Contrary to this, a recent study evaluating hippocampi of patients with TLE reported an increase in mGluR2/3 expression (Das et al., 2012). More specifically, studies have also demonstrated an increase in mGluR2/3 expression on reactive astrocytes in both animal models and patients with epilepsy (Aronica et al., 2003; Seifert et al., 2006; Steinhäuser and Seifert, 2002; Tang and Lee, 2001). The effect of our treatment schedule on the development (or lack therof) of subsequent SRS in the chronic period of pilocarpine treated mice remains to be determined.
Overall we demonstrate that treatment with mGluR2 active compounds reduces acute SE behavior, and the observed decrease in EEG spectral power suggests that there is less synchronized activity typically associated with SE. Certainly, treating acute SE is most critical in a clinical setting, but preventing future seizures precipitated by an episode of SE should also be of consideration. Future studies are needed to determine if these drugs inhibit the development of SRS after pilocarpine, as well as the resulting neuropathological changes that occur with epileptogenesis. If it could be demonstrated that these drugs also prevent or reduce the incidence of SRS, it would further support the development of drugs that target Group 2 mGluRs for the treatment of epilepsy.
Highlights.
We test the effects of group 2 mGluR active compounds on pilocarpine-induced SE.
Group 2 mGluR agonist, LY379268, reduced behavioral and EEG correlates of SE.
BINA, a group 2 mGluR PAM, had no effect on SE.
CBiPES, a group 2 mGluR PAM, modestly reduced behavioral correlates of acute SE.
Acknowledgements
The authors wish to express our gratitude to P. Jeffrey Conn at Vanderbilt University for providing BINA. We would also like to thank Eli Lilly and Company for kindly providing the LY379268 and CBiPES. Also, we would like to express our gratitude to John Day-Brown, Hong Qu Shan, David Klorig and Walter Wiggins for their helpful comments and suggestions during the writing phase of this manuscript.
This work was supported by NIH grants F31AA017048, T32AA7565, R21EY018159, R01AA016852, and R01AA015568, Citizens United for Research on Epilepsy, and the Tab Williams Family Fund.
Abbreviations
- AED
antiepileptic drug
- C/T
clonic/tonic
- EEG
electroencephalography
- EMG
electromyography
- mGluR
metabotropic glutamate receptor
- PAM
positive allosteric modulator
- SE
status epilepticus
- SRS
spontaneous recurrent seizures
- TLE
temporal lobe epilepsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erin H. Caulder, Email: ecaulder@wakehealth.edu.
Melissa A. Riegle, Email: mriegle@wakehealth.edu.
References
- Ahnaou A, Dautzenberg FM, Geys H, Imogai H, Gibelin A, Moechars D, Steckler T, Drinkenburg WHIM. Modulation of group II metabotropic glutamate receptor (mGlu2) elicits common changes in rat and mice sleep-wake architecture. Eur. J. Pharmacol. 2009;603:62–72. doi: 10.1016/j.ejphar.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Presynaptic inhibition of corticothalamic feedback by metabotropic glutamate receptors. J. Neurophysiol. 2005;94:163–175. doi: 10.1152/jn.01198.2004. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006a;71:1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW. Unique presynaptic and postsynaptic roles of Group II metabotropic glutamate receptors in the modulation of thalamic network activity. Neuroscience. 2006b;141:501–513. doi: 10.1016/j.neuroscience.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Koumentaki A, Abdul-Ghani AS, Croucher MJ, Bradford HF. Specific group II metabotropic glutamate receptor activation inhibits the development of kindled epilepsy in rats. Brain Res. 1998a;787:286–291. doi: 10.1016/s0006-8993(97)01500-x. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Singh Kent N, Jane DE, Croucher MJ, Bradford HF. Anticonvulsant and glutamate release-inhibiting properties of the highly potent metabotropic glutamate receptor agonist (2S,2’R, 3’R)-2-(2’,3’-dicarboxycyclopropyl)glycine (DCG-IV) Brain Res. 1998b;805:138–143. doi: 10.1016/s0006-8993(98)00698-2. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D. Expression and cell distribution of group I and group II metabotropic glutamate receptor subtypes in taylor-type focal cortical dysplasia. Epilepsia. 2003;44:785–795. doi: 10.1046/j.1528-1157.2003.54802.x. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefous C, Vernier J-M, Hutchinson JH, Gardner MF, Cramer M, James JK, Rowe BA, Daggett LP, Schaffhauser H, Kamenecka TM. Biphenyl-indanones: allosteric potentiators of the metabotropic glutamate subtype 2 receptor. Bioorg. Med. Chem. Lett. 2005;15:4354–4358. doi: 10.1016/j.bmcl.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J. Pharmacol. Exp. Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–1019. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Chen JWY, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Alford S. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann. N. Y. Acad. Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RSG, Avoli M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Wallace IVGC, Holmes C, McDowell ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray SK, Banik NL. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–246. doi: 10.1016/j.neuroscience.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolinsky B, Pacheco Otalora LF, Arshadmansab MF, Zarei MM, Garrido-Sanabria ER. Differential changes in mGlu2 and mGlu3 gene expression following pilocarpine-induced status epilepticus: a comparative real-time PCR analysis. Brain Res. 2008;1226:173–180. doi: 10.1016/j.brainres.2008.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG, Schoepp DD, Anderson K. The selective group mGlu2/3 receptor agonist LY379268 suppresses REM sleep and fast EEG in the rat. Pharmacol. Biochem. Behav. 2002;73:467–474. doi: 10.1016/s0091-3057(02)00843-2. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Katner JS, Johnson BG, Khilevich A, Schkeryantz JM, Perry KW, Svensson KA. Activation of metabotropic glutamate (mGlu)2 receptors suppresses histamine release in limbic brain regions following acute ketamine challenge. Neuropharmacology. 2010;58:632–639. doi: 10.1016/j.neuropharm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Folbergrová J, Haugvicová R, Mares P. Attenuation of seizures induced by homocysteic acid in immature rats by metabotropic glutamate group II and group III receptor agonists. Brain Res. 2001;908:120–129. doi: 10.1016/s0006-8993(01)02620-8. [DOI] [PubMed] [Google Scholar]
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, De Paulis T, Conn PJ. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J. Pharmacol. Exp. Ther. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- Garrido-Sanabria ER, Otalora LFP, Arshadmansab MF, Herrera B, Francisco S, Ermolinsky BS. Impaired expression and function of group II metabotropic glutamate receptors in pilocarpine-treated chronically epileptic rats. Brain Res. 2008;1240:165–176. doi: 10.1016/j.brainres.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Baez M, Jagdmann GE, Britton TC, Large TH, Callagaro DO, Tizzano JP, Monn JA, Schoepp DD. Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J. Med. Chem. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology (Berl.) 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Kew JNC, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl.) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kłodzińska A, Bijak M, Chojnacka-Wójcik E, Kroczka B, Swiader M, Czuczwar SJ, Pilc A. Roles of group II metabotropic glutamate receptors in modulation of seizure activity. Naunyn Schmiedebergs Arch. Pharmacol. 2000;361:283–288. doi: 10.1007/s002109900197. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Uusisaari M. Modulation of excitation by metabotropic glutamate receptors. Results Probl Cell Differ. 2008;44:163–175. doi: 10.1007/400_2007_035. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Ishida M, Shinozaki H. Anticonvulsive and neuroprotective actions of a potent agonist (DCG-IV) for group II metabotropic glutamate receptors against intraventricular kainate in the rat. Neuroscience. 1997;77:131–140. doi: 10.1016/s0306-4522(96)00442-3. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Chapman AG, De Sarro G, Meldrum BS. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur. J. Pharmacol. 2003;476:3–16. doi: 10.1016/s0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS. Anti-epileptic activity of group II metabotropic glutamate receptor agonists (--)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268) and (--)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY389795) Neuropharmacology. 2001a;41:8–18. doi: 10.1016/s0028-3908(01)00044-2. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Talebi A, Beart PM, Chapman AG, Meldrum BS. The mGlu(2/3) agonist 2R,4R-4-aminopyrrolidine-2,4-dicarboxylate, is anti- and proconvulsant in DBA/2 mice. Neurosci. Lett. 2001b;299:125–129. doi: 10.1016/s0304-3940(00)01732-8. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J. Med. Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Bankstahl M, Löscher W. Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice. Exp. Neurol. 2009;219:284–297. doi: 10.1016/j.expneurol.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Perez-Mendes P, Blanco MM, Calcagnotto ME, Cinini SM, Bachiega J, Papoti D, Covolan L, Tannus A, Mello LE. Modeling epileptogenesis and temporal lobe epilepsy in a non-human primate. Epilepsy Res. 2011 doi: 10.1016/j.eplepsyres.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Priel MR, Albuquerque EX. Short-term effects of pilocarpine on rat hippocampal neurons in culture. Epilepsia 43 Suppl. 2002;5:40–46. doi: 10.1046/j.1528-1157.43.s.5.18.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Sanger H, Hanna L, Colvin EM, Grubisha O, Ursu D, Heinz BA, Findlay JD, Vivier RG, Sher E, Lodge D, Monn JA, Broad LM. Pharmacological profiling of native group II metabotropic glutamate receptors in primary cortical neuronal cultures using a FLIPR. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Savić MM, Milinković MM, Rallapalli S, Clayton T, Sr Joksimović S, Van Linn M, Cook JM. The differential role of alpha1- and alpha5-containing GABA(A) receptors in mediating diazepam effects on spontaneous locomotor activity and water-maze learning and memory in rats. Int. J. Neuropsychopharmacol. 2009;12:1179–1193. doi: 10.1017/S1461145709000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Thompson SM. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampus: are Ca2+ channels involved? Neuropharmacology. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhäuser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br. J. Pharmacol. 1997;121:1171–1179. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhäuser C, Seifert G. Glial membrane channels and receptors in epilepsy: impact for generation and spread of seizure activity. Eur J Pharmacol. 2002;447:227–237. doi: 10.1016/s0014-2999(02)01846-0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Tang FR, Lee WL. Expression of the group II and III metabotropic glutamate receptors in the hippocampus of patients with mesial temporal lobe epilepsy. J Neurocytol. 2001;30:137–143. doi: 10.1023/a:1011939223872. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:37–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lundbye-Jensen J, Petersen TH, Nielsen JB. The effect of baclofen and diazepam on motor skill acquisition in healthy subjects. Exp Brain Res. 2011;213:465–474. doi: 10.1007/s00221-011-2798-5. [DOI] [PubMed] [Google Scholar]