The skin is like a canvas. Pigment distribution, skin appendages, skin ridges, etc. are arranged on the skin surface. The arrangement may be random but every now and then they form specific patterns. In some occasions, the specific arrangement is important for organism function or message display. In all occasions, these distinct arrays provide us with windows into the fundamental processes of pattern formation. Skin patterns can be studied by non-invasive visual observations, and dynamic changes can be recorded in vivo for analyses.
The hair distribution pattern consists of multiple hair primordia with different developmental stages, directionality, and spacing. Hair primordia on the skin can be randomly distributed, de-synchronized in hair cycling, or coordinated to give rise to a higher level pattern. When there are incremental changes of developmental stages or hair orientation from one primordium to the next, they give the impression of a wave. These waves can be parallel to each other and form stripes or radiate out from focal centers, thus forming whorls as seen in fingerprints and hair patterns on the scalp. Do these patterns result from precise genetic coding or self-organizing cellular events? Patterns such as fingerprints have similar attributes (similar width, organization plan) but they are non-identical (with enough difference to be used as individual identifiers). In fact fingerprints among monozygotic twins, while more similar than nonrelated individuals, are still different (Jain et al, 2002). Thus there is a non-genetic component in the formation of fingerprints. The hair whorls in the scalp of monozygotic twins have not been documented previously and in this issue there is a case report (Paine et al, 2004).
Whorls
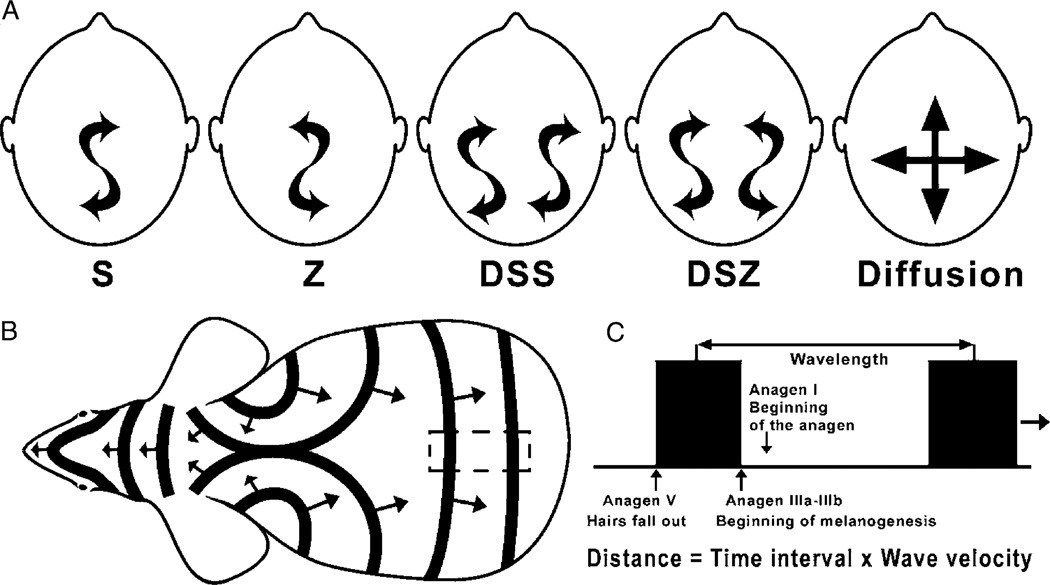
In human fetuses lanugo hairs form whorl patterns both on the scalp and trunk skin (Gworys and Domagala, 2003). On the thoracic wall there are lanugo whorls that begin bilaterally over the nipples. The whorls collide and merge along the midlines.1 In adults, whorl patterns are distinct only on the parietal scalp. Ziering and Krenitsky (2003) identified five distinct hair whorl patterns in their study from almost 500 males (Fig 1A): S (75%) and Z (11%) patterns refer to the clockwise and anti-clockwise orientation of the whorl, respectively. The DSZ (Double SZ, 3%) pattern describes the presence of two whorls: one with an S and another with a Z orientation. DSS (Double SS, 0.6%) describes a pattern with two separate S whorls. Hair in the diffusion pattern (9.8%) has no obvious orientation. The “tightness” of the whorl can also vary. When ethnic groups are analyzed, the more interesting difference is that 80% of African-Americans showed diffusive type and 29% of Asian-Americans showed counter-clockwise type whorls. However, in both cases, n is only around 25. Similarly, the number of female subjects is too small to be conclusive, although preliminary data suggest that females predominantly have a diffusion pattern.
Figure 1.
(A) The types of parietal hair whorls. Schematic view from the top of a human head. Frontal regions are toward the top of the diagram (after Ziering and Krenitsky, 2003). Arrows describe the orientations of hairs in whorls. (B) The traveling wave pattern that mimics patterns generated by the BZ reaction (after Suzuki et al, 2003). Arrows describe the direction of wave propagation. (C) Details of the traveling wave (from the rectangle region bordered by the broken line in B).
So, is the whorl pattern genetically controlled? Paine et al (2004) showed that one of the monozygotic twins has an S type pattern, while the other has a DSZ pattern. The monozygosity of the twins was verified by PCR analysis. Therefore, there must be an epigenetic component in the determination of the hair whorl. While conserved molecular pathways underlie all hair follicle formation, local environmental and fortuitous factors can influence the final hair pattern.
Skin generally follows inherited linear patterns (Blaschko lines). These are seen in the hairless streaks on the scalp of patients with oral–facial–digital syndrome (Happle et al, 1984), suggesting that the wave of hair follicle formation may be lineage related. There also have been attempts to associate the direction of hair whorls with right- or left-handedness (Klar, 2003). More cases will have to be analyzed in this interesting area to more fully appreciate this relationship.
Waves
Hair waves may represent patterning events in time etched in space (on the skin surface). In adult animals, since hairs go through constant molting and regeneration, the integument reflects the dynamic cell behaviors underlying hair cycles in vivo. The majority of postnatal human hair growth seems to be de-synchronized. In a rare case, a 4-y-old boy was reported to have regions of scalp hairs exhibit shortened anagen and continuous synchronized hair cycles (Thai and Sinclair, 2003). Several recent works show interesting wave-like hair growth in mice. Visualizing hair waves is facilitated by hair cycle dependent changes in integument pigmentation and hair loss in nude mice and Msx2 knockout mice.
Militzer (2001) analyzed more than 400 nude mice on albino (NMRI, foxn1nu) and pigmented (C57BL/6, foxn1nu) backgrounds for more than one year. Pink skin turns dark when hairs enter anagen and turns pink again when anagen is completed. Skin pigmentation changes progress in a wave-like fashion on the skin surface of these mice. When mice are young, all hairs cycle synchronously, but with increasing age the hair cycles over different regions desynchronize. Thus, the skin pigment pattern breaks into distinct stripes and patches. As mice age, the stripes and patches become narrower/smaller and eventually appear random.
Traveling hair waves are seen most dramatically in the Foxn1nu mice on the C57BL/6 background (Suzuki et al, 2003). This mutant mouse has a defect in the Foxn1 gene that results in the precocious termination of the hair cycle and causes the loss of hair shafts. This is quickly followed by the re-initiation of a new anagen, so the hairs cycle much faster. Thus the dynamic of the pigmentation pattern described above occurs faster than that of classical nude mice. In the young mice, the pigmentation oscillation takes place synchronously throughout the mouse skin. As the mice age, the pigmentation pattern breaks into wide stripes that progress over time to become narrower bands (Fig 1B). The width of the pigmented stripe equals the period of active melanogenesis in the hair follicle (in this case, between anagen III a, b to V) times the speed at which those hair follicles progress in anagen. The wavelength (the distance between two pigmented stripes) equals the duration of the hair cycle times the average speed of wave spreading (which may not be constant) (Fig 1C). In an ideal situation, some mice (usually 47 months) show narrow and roughly evenly spaced pigmented stripes that travel along the trunk; however, many mice show irregular, fragmented or very wide stripes. So, there are still many unknown biological (and most likely epigenetic) factors that remain to be learned.
Ma et al (2003) reported “cyclic alopecia” in the Msx2 knockout mice. The phenotype is due to the fact that hair fibers are defective and are dislodged during catagen. The skin of these mice during anagen is black and hairy, but during telogen is bald and non-pigmented. As the hair cycle waves advance, the alopecic regions re-enter anagen and regain pigmentation in a progressive order. Long-term observation of hairy and bald skin regions revealed a “cyclic alopecia” phenomenon. Hairs within one skin domain cycle in waves but not with hairs in neighboring domains (which also cycle in waves, but with an independent rhythm). In essence, the “traveling stripes” of the Foxn1nu mice are the manifestation of the same phenomenon. They represent the leading edges of the hair growth domains. Traveling stripes can also be identified in Msx2 knockout or classical nude mice by taking time-lapsed snapshots of pattern progression and lining them up; however, the Foxn1nu mice bring out the waves more acutely. Hair cycle waves are not restricted to the described mutant mice, and apparently are present in normal mice — albeit with different wavelengths.
Models
What are the fundamental principles underlying these phenomena (Ball, 1999)? Do hair arrangement patterns represent temporal events that were etched in the embryonic past? How do the dynamic waves play actively upon the background of cycling hair follicles? Two major models for pattern formation in reaction–diffusion systems (biological or non-biological) have been proposed: the Turing structure for stationary, periodic patterns, and the Belousov–Zhabotinskii (BZ) reaction for propagating waves (Maini, 2003; Vanag and Epstein, 2003). The BZ reaction (Zhabotinsky and Zaikin, 1973) may lead to waves observed here. The BZ reaction has two components: (1) chemical reaction A in which one of the products serves as an auto-catalyst, resulting in a positive feedback for reaction A and (2) a competing non-autocatalytic reaction B that will take over when the concentration of reactants required for reaction A drops. Thus the reaction can oscillate back and forth. When the products of reactions A and B have different colors, the dynamics of these chemical reactions can be visualized. However, the most remarkable feature of the BZ reaction is the spatial patterns it can generate. By deliberately not homogenizing the chemical reaction mixture, there will be small spatial variations of reactant concentrations from region to region. The auto-catalytic nature of reaction A allows small variations to be amplified into big differences. Thus some regions can participate in reaction A, while adjacent regions participate in reaction B. The color will vary from one place to another, and the oscillating chemical reactions become spatial–temporal traveling waves and spirals, forming spectacular patterns in vitro (Ball, 1999).
The BZ reaction was proposed to explain the traveling hair stripes (Suzuki et al, 2003). The authors saw a resemblance between the hair growth pattern and the concentric waves generated by the BZ reaction. It is conceivable that the excitable media (here, the skin) oscillates between two states (products) in time and space. The two states being pigmented (or anagen IIIa, b to V, in this case) and non-pigmented (hair dislodged, telogen). Such an oscillation can indeed produce dynamic patterns composed of alternating traveling stripes of pigmented and non-pigmented skin. The reactants of reaction A may be some molecules that promote the re-entry to anagen (Suzuki et al, 2003 speculate that Shh or FGF 7 may be involved), and can also laterally activate adjacent responsive hair follicles. The inevitable progression toward telogen can be considered as reaction B. In addition, the authors suggested that there may be two starting points of hair growth (so called pacemakers) located at the dorso-ventral border of the armpit regions (Fig 1B). These pacemakers continuously initiate new anagen waves that spread in the form of stripes. As stripes from both the left and right sides reach the midline, they do not transverse each other but rather annihilate one another: one stripe cannot pass through skin that has just had a stripe generated from the opposite side travel through it. The similarity of the wave pattern makes this assumption very compelling.
Hair whorls on the scalp also strongly suggest that propagating chemical waves may take place when the hair follicles that comprise them were generated. All types of hair whorls (Ziering and Krenitsky, 2003) can be formed by the BZ reaction. One can speculate that during hair induction, the diffusion and reaction of activator and inhibitor molecules required for new hair placode formation establish the successive waves of hair induction, following the simple BZ reaction algorithm. Since hair follicles are periodically arranged, it is true that hair whorls in fact represent segmented, rather than continuous spiral lines. In a recent work, the BZ reaction was shown to be able to generate segmented spirals in a non-biological system (Vanag and Epstein, 2003). This further strengthens the idea that basic mechanisms of pattern formation may be applied to various biological systems, even though they appear to be highly complex.
The skin is an excitable medium. It conducts reactions among signaling molecules that determine the formation of skin appendages or the distribution of active melanocytes. In development, the pattern is a snapshot of activity that took place during the embryonic past. For hair cycling, the rapid shedding and renewal of skin appendages in Foxn1 and Msx2 mutant mice help us visualize the waves of change that sweep across the skin surface. Thus the integument is one of the best models for analyzing biological pattern formation. For all the complex patterns we observe, is there a genetic program that dictates specific patterns during hair induction? There are many levels of skin/skin appendage morphogenesis. We think that there are genetic and epigenetic controls that operate at different levels (Jiang et al, 2004). We believe that while signaling molecular pathways are basic requirements for hair induction and cycling, there are epigenetic reactions (e.g., Turing reaction–diffusion, BZ reaction that govern the subsequent self- organization and propagation. The fact that monozygotic twins have different hair whorl types/ fingerprints is evidence that patterning has a major epigenetic component. The similarity of hair whorls and traveling waves with patterns generated by the BZ reaction suggests that basic physical–chemical laws may be applied to explain biological patterns. The Turing model is another example. It operates at the mesenchymal cell condensation/ tissue interaction level and can explain the self-organizing, stochastic behaviors in the induction of hair and feather placodes (Nagorcka and Mooney, 1985; Jiang et al, 1999; Meinhardt and Gierer, 2000). Beyond molecular genetics, we need to decipher physical–chemical rules of cell/tissue interactions and their molecular basis to truly understand morphogenesis.
Footnotes
Domagala, personal communication.
References
- Ball P. The Self-made Tapestry: Pattern Formation in Nature. Oxford: Oxford University Press; 1999. [Google Scholar]
- Gworys B, Domagala Z. The typology of the human fetal lanugo on the thorax. Ann Anat. 2003;185:383–386. doi: 10.1016/S0940-9602(03)80066-3. [DOI] [PubMed] [Google Scholar]
- Happle R, Fuhrmann-Rieger A, Fuhrmann W. How are the Blaschko lines arranged on the scalp? Hautarzt. 1984;35:366–369. (in German) [PubMed] [Google Scholar]
- Jain AK, Prabhakar S, Pankanti S. On the similarity of identical twin fingerprints. Pattern Recognition. 2002;35:2653–2663. [Google Scholar]
- Jiang TX, Jung H-S, Widelitz RB, Chuong CM. Self organization is the initial event in periodic feather patterning: Roles of signaling molecules and adhesion molecules. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Widelitz RB, Shen W-M, et al. Integument pattern formation involves genetic and epigenetic controls operated at different levels: Feather arrays simulated by a digital hormone model. Int J Dev Biology. 2004 doi: 10.1387/ijdb.041788tj. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ. Human handedness and scalp hair-whorl direction develop from a common genetic mechanism. Genetics. 2003;165:269–276. doi: 10.1093/genetics/165.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Liu J, Wu T, et al. ‘‘Cyclic alopecia’’ in Msx2 mutants: Defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini PK. How the mouse got its stripes. Proc Natl Acad Sci USA. 2003;100:9656–9657. doi: 10.1073/pnas.1734061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. Bioessays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Militzer K. Hair growth pattern in nude mice. Cells Tissues Organs. 2001;168:285–294. doi: 10.1159/000047845. [DOI] [PubMed] [Google Scholar]
- Nagorcka BN, Mooney JR. The role of a reaction–diffusion system in the initiation of primary hair follicles. Theor Biol. 1985;114:243–272. doi: 10.1016/s0022-5193(85)80106-5. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hirata M, Kondo S. Traveling stripes on the skin of a mutant mouse. Proc Natl Acad Sci USA. 2003;100:9680–9685. doi: 10.1073/pnas.1731184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine ML, Paine CT, Machin GA. Hair whorls and monozygosity. J Invest Dermatol. 2004;122:1057–1058. doi: 10.1111/j.0022-202X.2004.22420.x. [DOI] [PubMed] [Google Scholar]
- Thai KE, Sinclair RD. Short anagen hair with persistent synchronized pattern of scalp hair growth. J Am Acad Dermatol. 2003;49:949–951. doi: 10.1016/s0190-9622(03)00453-5. [DOI] [PubMed] [Google Scholar]
- Vanag VK, Epstein IR. From the Cover: Segmented spiral waves in a reaction–diffusion system. Proc Natl Acad Sci USA. 2003;100:14635–14638. doi: 10.1073/pnas.2534816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhabotinsky AM, Zaikin AN. Autowave processes in a distributed chemical system. J Theor Biol. 1973;40:45–61. doi: 10.1016/0022-5193(73)90164-1. [DOI] [PubMed] [Google Scholar]
- Ziering C, Krenitsky G. The Ziering whorl classification of scalp hair. Dermatol Surg. 2003;29:817–821. doi: 10.1046/j.1524-4725.2003.29214.x. [DOI] [PubMed] [Google Scholar]