Abstract
We previously reported on enhanced susceptibility to death of lymphocytes from Alzheimer’s disease (AD) patients when exposed to hydrogen peroxide (H2O2)-induced oxidative stress and an increased resistance to death in those of patients with a history of skin cancer. This is consistent with our hypothesis proposing that the cellular machinery controlling cell death is deregulated in opposite directions in Alzheimer’s disease (AD) and cancer, to explain the inverse association observed in epidemiological studies. Here we investigated whether the observed increased susceptibility correlates with the degree of dementia severity. Peripheral lymphocytes from 23 AD patients, classified using the Clinical Dementia Rating (CDR) into severe dementia (CDR 3, n=10) and mild-to-moderate dementia (CDR 1–2, n=13), and 15 healthy controls (HC) (CDR 0), were exposed to H2O2 for 20 hours. Lymphocyte death was determined by flow cytometry and propidium iodide staining. The greatest susceptibility to H2O2-induced death was observed for lymphocytes from severe dementia patients, whereas those with mild-to-moderate dementia exhibited intermediate values, compared to healthy controls. A significant increase in the apoptosis/necrosis ratio was found in AD patients. Poly (ADP-ribosyl) polymerase-1 (PARP-1) inhibition significantly protected from H2O2-induced death of lymphocytes, whereby a lower degree of protection was observed in severe AD patients. Moreover, inhibition of PARP-1 abolished the differences in apoptosis/necrosis ratios observed between the three groups of patients. These results support the notion that AD is a systemic disorder, whereby enhanced susceptibility to H2O2-induced death in peripheral lymphocytes correlates with dementia severity and enhanced death in AD patients is attributable to a PARP-dependent increase in the apoptosis/necrosis ratio.
Keywords: Alzheimer, apoptosis, dementia, necrosis, PARP-dependent cell death, peripheral lymphocytes
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most frequent cause of dementia. The causes of the disease are unknown but many factors are implicated, including toxic effects of amyloid and tau [1, 2], inflammation [3] and oxidative damage [4]. Cellular aging and neurodegenerative diseases lead to increased generation of reactive oxygen species (ROS) that modify biomolecules, diminishing the normal functions of neuronal cells and eventually leading to neuronal loss in AD. In epidemiological studies we and others have found that AD and cancer are inversely associated, such that patients with a history of cancer in the past have a reduced risk of developing Alzheimer type dementia and inversely, those with AD have a reduced risk of developing cancer in the future [5–12]. In the search for molecular mechanisms to explain the inverse epidemiological relationship observed between Alzheimer Disease (AD) and cancer we have proposed that the cellular machinery controlling cell death might be deregulated in opposite directions to explain the mutual protection observed between the two disorders. Changes in the expression of key molecules involved in the regulation of cell cycle or cell death/survival, such as p53, Pin1, wnt [12–14] may explain these observations. We previously reported that lymphocytes from AD patients are more susceptible to oxidative cell death induced by H2O2 exposure than those from control subjects [15]. Using flow cytometry, electron microscopy and by measuring caspase activity, we determined that H2O2 exposition induced both necrotic and apoptotic death of lymphocytes, the latter being independent of caspase activity and dependent on poly (ADP-ribosyl) polymerase-1 (PARP-1) activity [16]. To understand the relationship between lymphocyte death and normal aging, we evaluated the susceptibility to H2O2-induced death of lymphocytes from healthy subjects of ages ranging from 24–95 years [16]. We showed that aging was not associated with an overall increase in lymphocyte susceptibility to death, but that there was a change in the pattern of cell death: necrotic death prevailed in healthy young subjects, while in cognitively normal aged subjects there was an increase in apoptotic death. As a consequence, an increase in the apoptosis/necrosis ratio was observed with age [16]. Moreover, we determined that PARP-1 inhibition with 3-Aminobenzamide (3-ABA) provided significant protection against H2O2-induced cell death, whereby protection tended to be lower in patients with AD [15, 16]. In this study we evaluated whether the severity of dementia in AD patients correlates with the susceptibility to H2O2 -induced cell death and changes in the apoptosis/necrosis ratio. In addition, we explored whether the protection provoked by PARP-1 inhibition varies with the degree of dementia severity.
MATERIALS
RPMI 1640 medium and fetal bovine serum were from Biological Industries (Kibbutz Beit-Haemek, Israel). Ficollpaque™ PREMIUM was from GE Healthcare (Little Chalfont, UK). The H2O2 was from Merck (Darmstadt, Germany). N-acetyl cysteine (NAC), sodium 4,5-dihydroxybenzene-1,3-disulfonate (Tiron) and 3-Aminobenzamide (3-ABA) were from Sigma Chemical (St Louis, MO). The antioxidant EUK-134 and TBARS assay kit were from Cayman chemical (Ann Arbor, Mi). OxiSelect™ Glutathione Reductase Assay Kit was from Cell Biolab (San Diego, CA). Fluorescence-activated cell sorting (FACS) was from Becton Dickinson (Mountain View, CA).
PATIENTS
A total of 38 individuals, 23 AD patients −10 severe dementia (CDR 3), 13 mild-to-moderate dementia (CDR 1–2), and 15 healthy donors (CDR 0) were recruited after providing informed consent approved by the Ethics Committee of the Hospital Clínico de la Universidad de Chile. In severe cases of dementia, the consent was provided by their caregivers. Care was taken to avoid performing the H2O2 experiments in lymphocytes from patients with signs of infection or inflammation, because this might alter lymphocyte susceptibility. Five patients from the control group, 3 from the mild-moderate group and 1 from the severe dementia group were also analyzed in the previous manuscript (Behrens et al 2012). However they donated blood samples for new experiments in this study. AD diagnosis was established following the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria [17] and dementia severity was rated with the CDR, whereby CDR 1, 2 and 3 indicates mild, moderate, and severe dementia, respectively, and CDR 0 corresponds to the absence of any cognitive impairment [18], the Mini-mental State Examination (MMSE) [19] and the Montreal Cognitive Assessment (MoCA) [20]. Maximum score for MMSE and MoCA is 30, with lower scores associated with greater cognitive deterioration (Table 1). Healthy controls were submitted to the same neurological and neuropsychological evaluations.
Table 1.
Demographic data of study participants.
| Healthy Controls CDR 0 |
AD CDR 1–2 |
AD CDR 3 |
|
|---|---|---|---|
| N=15 | N=13 | N=10 | |
| Age, y mean ± SE | 75.7 ± 1.7 | 77.1 ± 2.3 | 80.7 ± 2.3 |
| Age range, y | 63–89 | 68–93 | 68–90 |
| Female sex, (%) | 12 (80) | 10 (77) | 10 (100) |
| Education, y | 10.9 ± 1.7 | 10.0 ± 1.2 | 9.9 ± 0.9 |
| MoCA1 test | 26.1 ± 0.7 | 10.3 ± 1.8 | 1.4 ± 0.8 |
| MMSE2 | 29.0 ± 0.3 | 15.7 ± 1.4 | 3.9 ± 1.9 |
| Hypertension (%) | 4 (27) | 10 (69) | 6 (60) |
| Diabetes (%) | 3 (20) | 2 (13) | 5 (50) |
| Tobacco | 4 (27) | 4 (27) | 4 (40) |
| Cholinesterase inhibitors (%) | NA | 9 (69) | 4 (40) |
| Memantine (%) | NA | 8 (61) | 3 (30) |
Montreal Cognitive Assessment.
Mini-mental State Examination.
NA = not applicable.
METHODS
Peripheral blood lymphocytes were obtained by venopucture (15 ml) and extracted by Ficoll-Hypaque density centrifugation [16] and exposed to increasing concentrations of H2O2 for 20 hours, in the presence or absence of the PARP-1 inhibitor 3-Aminobenzamide (3-ABA) 5mM. The samples were normalized to cell count at a density of 1×106 cells per ml. In a different set of experiments the protection of lymphocytes from ROS-mediated damage conferred by PARP-1 inhibition using 3-ABA was evaluated in parallel with treatments using different antioxidants applied 1 hour before H2O2 treatment; N-acetyl cysteine (NAC), 5mM, EUK-134, 20 uM, and Sodium 4,5-dihydroxybenzene-1,3-disulfonate (Tiron) 4mM (3–5 patients per group). Cell death was evaluated by flow cytometry following propidium iodide (PI) staining, whereby viable (PI-negative), apoptotic (PI-positive, hypodiploid) and necrotic (PI-positive diploid) cells were distinguished [16]. Apoptotic death was corroborated with concomitant annexin V staining [21]. Cellular damage induced by oxidative stress was determined using thiobarbituric acid reactive substances (TBARs) assay in freshly extracted lymphocytes from AD and healthy donors following instructions provided by the manufacturer. Glutathione reductase activity was measured with the assay kit OxiSelect™. Reduced glutathione (GSH) and oxidized glutathione (GSSG) levels in lymphocytes were assayed by fluorimetry as previously described [17]. The results were expressed in nmol of GSH and GSSG per milligram of protein.
Statistical Analysis
Differences between the three experimental groups at each dose in lymphocyte survival, apoptosis, and necrosis were analyzed using a SPSS general linear model with Bonferroni correction, and the data were adjusted for age and sex. Results were expressed as means ± standard error of the mean (SEM). Differences p ≤0.05 were considered statistically significant.
RESULTS
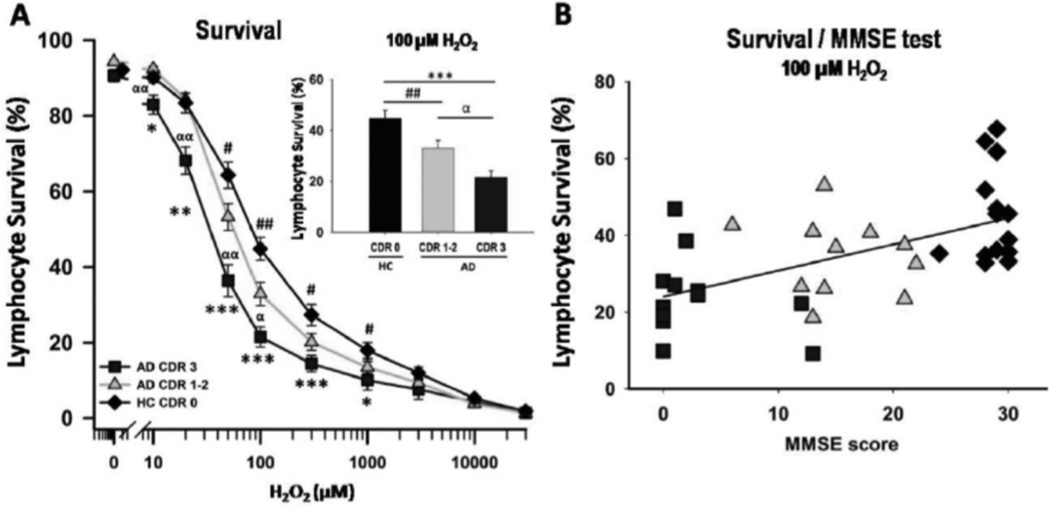
A significant interaction between H2O2 concentrations and CDR groups was found for lymphocyte survival (p <0.005). Pairwise comparisons indicated that upon exposure to H2O2, lymphocytes from AD patients showed increased susceptibility to death compared with control lymphocytes. The dose-response curves of lymphocyte viability, at concentrations of H2O2 ranging from 10 µM to 30 mM, were shifted to the left (enhanced sensitivity) for lymphocytes from AD patients compared to HC (Fig. 1A). A larger shift was observed for lymphocytes from severe AD patients and intermediate for lymphocytes from mild-to-moderate AD patients. Upon treatment with 100 µM H2O2, survival values were 20 ± 4%, 33 ± 3% and 47 ± 3% for severe dementia, mild-to-moderate dementia, and HC lymphocytes, respectively (mean ± SEM, Fig. 1Ainset). Lower MMSE scores were associated with lower lymphocyte survival values upon exposure to 100 µM H2O2, with a significant correlation (2-tailed) with Kendall’s tau-b (0.389; p<0.001) and Spearman-Rho correlation (0.566, p<0.0001) (Fig. 1B). A similar correlation was obtained for the MoCA, Kendall’s tau-b 2-tailed (0.449; p<0.0001) and Spearman-Rho 2-tailed test (0.622, p<0.0001) (supplementary material Fig. 1). Treating MMSE as a categorical variable, using Chilean published cut-offs [22] also rendered significant differences between healthy controls and mild/moderate and severe AD (but not between mild/moderate and severe AD, data not shown).
Fig. (1).
H2O2-induced death of peripheral lymphocytes from AD patients correlated with the degree of dementia severity. Lymphocytes from 10 severe dementia AD patients (CDR 3) (squares), 13 mild-to-moderate AD (CDR 1–2) (triangles), and 15 healthy controls (rhomboids) were exposed to H2O2 for 20 h and cell death was determined by flow cytometry and propidium iodide staining. A: Survival values. Means ± S.E. * =AD CDR 3 vs HC; # =AD CDR 1–2 vs HC; α =AD CDR 3 vs AD CDR1–2. One symbol: p<0.05; 2 symbols: p<0.005; 3 symbols: p<0.0001. Inset: Lymphocyte survival values (%, mean ± SEM) measured at 100 µM H2O2, in the three groups of patients. B: Survival values measured at 100 µM H2O2 in lymphocytes from individual participants plotted against their MMSE scores. Kendall’s tau-b 2-tailed correlation (0.389; p<0.001). Symbols as in (A).
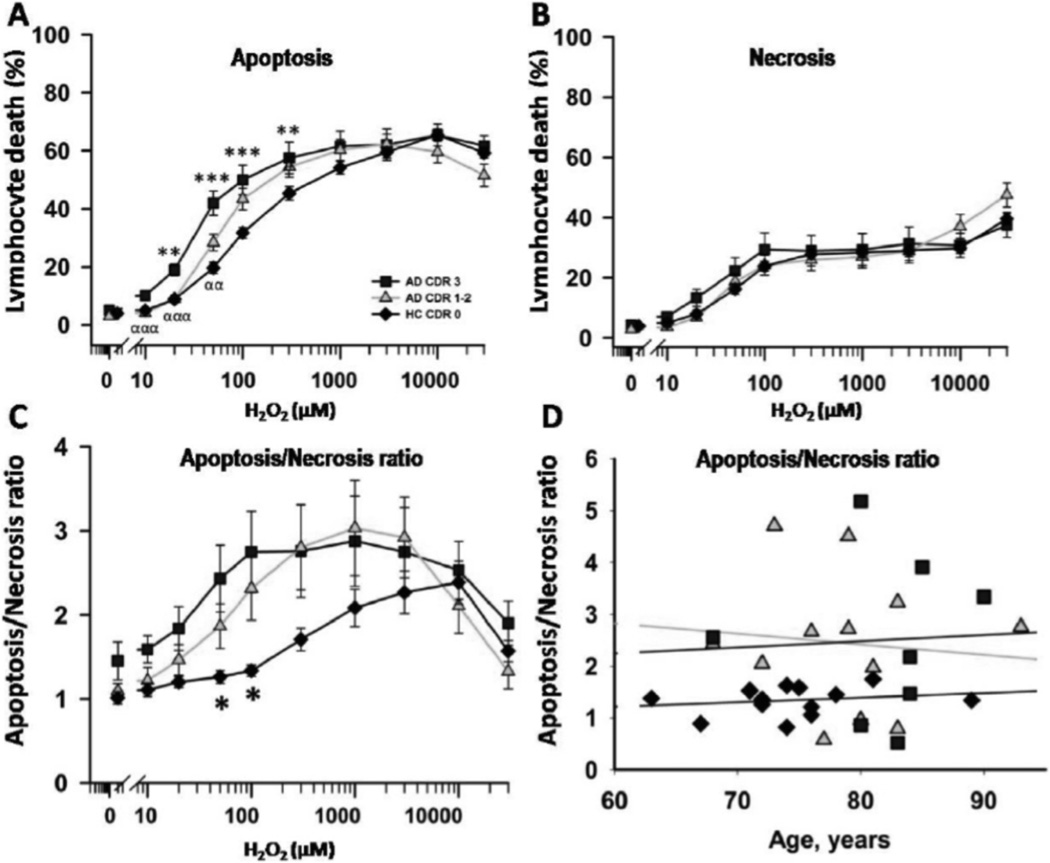
The increased cell death in AD patients was due to higher levels of apoptosis, corroborated by PI and annexin co-staining (Fig. 2A and B). The ratios of apoptotic to necrotic (A/N) death of lymphocytes calculated for each H2O2 concentration showed that A/N ratios were significantly greater in AD patients than in HC (Fig. 2C). Since we had previously reported that aging is associated with an increase in the A/N ratios of lymphocyte death in HC, we evaluated whether age affected the A/N ratio in AD patients. Results shown in Fig. 2D show that no apparent relationship exists between age and the A/N ratio measured at 100 µM H2O2, neither in AD nor HC lymphocytes (Fig. 2D).
Fig. (2).
Apoptotic/necrotic ratios after H2O2-induced death of peripheral lymphocytes. A and B: Apoptosis and necrosis values measured in the experiments in Fig. (1A). C: Apoptosis/necrosis ratios calculated at each H2O2 concentrations in the three groups of patients (mean ± SEM). D: Apoptosis/necrosis ratios of individual participants in the three groups plotted against age. Symbols as in Fig. (1A).
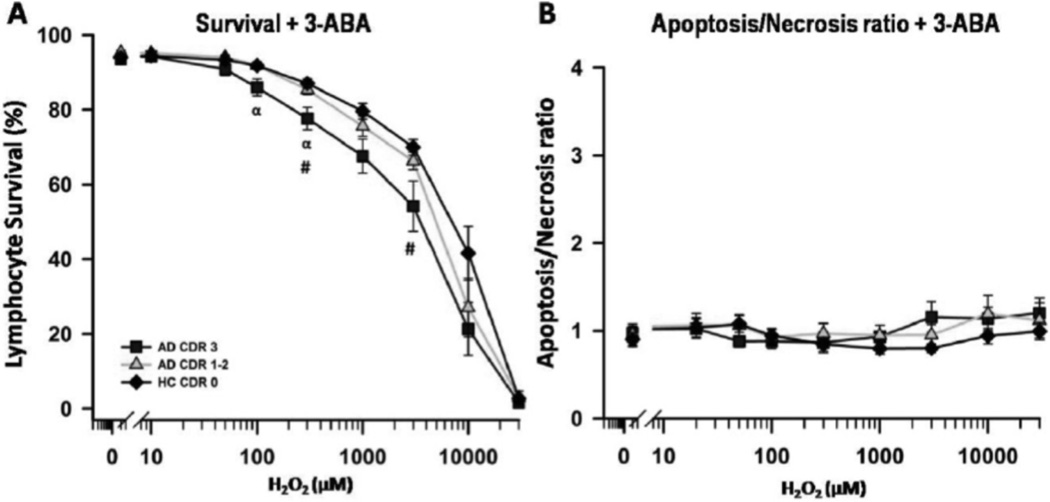
Because we have previously observed a potent protection of H2O2-induced death of lymphocytes with PARP-1 inhibition with 3-ABA, we tested the effects of this inhibitor in lymphocytes from patients with different dementia severity. PARP-1 inhibition with 3-ABA significantly protected lymphocytes from H2O2-induced cell death, and the extent of protection was significantly lower for lymphocytes from severe dementia patients (Fig. 3A). Furthermore, upon PARP-1 inhibition, the A/N ratios were equivalent to 1 in the three groups of patients tested at different H2O2 concentrations (Fig. 3B). To further explore the mechanism of H2O2-induced damage, in a different set of experiments, we tested the effect of 3-ABA in parallel with the effect of antioxidants known to act at different cellular sites; N-acetyl cysteine (NAC), a precursor in the formation of the antioxidant glutathione, EUK-134, a superoxide dismutase mimetic, and Sodium 4,5-dihydroxybenzene-1,3-disulfonate (Tiron), a free radical scavenger. The three antioxidants provided approximately 80% protection against H2O2-induced death at 100 uM concentration, but were not protective at higher H2O2 concentrations (Fig. 2 supplementary material). Instead 3-ABA conferred more than 80% protection up to 1 mM H2O2 concentration, suggesting that these effects occured downstream of antioxidant action (Fig. 2 supplementary material and Fig. 3A).
Fig. (3).
H2O2-induced death of peripheral lymphocytes in the presence of 3-ABA, a PARP-1 inhibitor. PARP-1 inhibition induced a marked protection from H2O2-induced death, with a significantly reduced degree of protection in severe AD lymphocytes (A). B: Apoptosis/necrosis ratios measured in the presence of PARP-1 inhibition in the three groups of patients. Symbols as in Fig. (1A).
In accordance with data in the literature [23], levels of lipid peroxidation as a marker of cellular oxidative damage were elevated in lymphocytes from AD patients compared to controls (Supplementary Fig. 3). The intrinsic antioxidant machinery of lymphocytes from AD patients was measured as activity of glutathione reductase (GRD) and GSH/GSSH ratios. Neither GRD activity nor the GSH/GSSG ratios were significantly different in lymphocytes from AD patients compared to controls (Supplementary Fig. 3).
DISCUSSION
Overall the data presented here show that increased susceptibility to apoptotic death of lymphocytes from AD patients after oxidative damage by exposure to H2O2 correlates with the degree of dementia severity. The augmented susceptibility was observed both as an increase in the overall percentage of death and as an increase in the A/N ratios. The increase in cell death correlated well with the cognitive impairment independent of the method of quantification used (CDR and MMSE or MoCA). These data reinforce conclusions from our previous studies which identified a higher susceptibility of lymphocytes from AD patients to cell death induced by H2O2 [15].
The increase in A/N ratios observed in AD lymphocytes is not due to differences in the age of patients with severe AD, and was greater than expected to occur by aging [16]. In our previous report we observed an increase in apoptosis/ necrosis ratio associated with age, when measured at ages ranging from 24 to 95 years; the smaller differences in age of the patients in this study (from 63 to 89 years) did not permit observing age-dependent variations.
These results also support the notion that AD is a systemic disorder [24], in which alterations in susceptibility to cell death are also observed for cells that are not part of the nervous system. Our results support the idea that lymphocytes isolated from the blood provide peripheral correlates of brain function [25, 26]. It is important to mention that these results do not allow us to distinguish whether increased susceptibility to cell death is a reflection of the progression of the brain disorder, or whether this change plays a causative role in the genesis of the disease state.
Several studies in the literature have reported on increased lipid peroxidation and decreased antioxidant defenses in plasma and blood cells of AD patients compared to controls [23, 27–29]. Accordingly, we found increased lipid peroxidation in lymphocytes from AD patients. Decreased activity of GRD has been reported in erythrocytes and in immortalized lymphocytes of AD patients [28, 30] and some reports have shown reduced GSH/GSSG ratios in AD lymphocytes [31] but not others [32]. We did not observe significant differences in GRD activity nor GSH/GSSG ratios in AD lymphocytes compared to controls.
Other reports in the literature have observed increased death of peripheral lymphocytes of AD patients [33–36], and defects in G1/S cell cycle checkpoint regulation [37, 38]. The increase in apoptosis may reflect a change in the activity or expression of key molecules belonging to the cellular machinery of protection against oxidative damage, such as molecules involved in cell cycle or cell survival/death mechanisms; p53, PARP, PIN1, and wnt [12–14]. For example, increased levels of p53 have been reported in peripheral lymphocytes of AD patients [39]. A recent report found several genes and pathways, among them p53, wnt signaling and Pin1, that were deregulated in opposite directions in cancer and AD [40]. It is intriguing to speculate that the increases in apoptotic death of lymphocytes detected as the disease progresses in AD patients may be due to increased p53 levels in more advanced stages of dementia. In this perspective, it is interesting to mention a recent report demonstrating that p53 is involved not only in triggering apoptosis, but also in the regulation of necrotic, PARP-dependent cell death [39, 41].
PARP-1 is a nuclear enzyme that polymerizes multiple adenosine diphosphate (ADP)-ribose molecules to nuclear proteins consuming large amounts of nicotinamide adenine dinucleotide (NAD+) that are also essential for processes like DNA repair. Under conditions where severe DNA damage is inflicted, such as under conditions of exacerbated oxidative stress, PARP-1 activation becomes excessive, depleting the cell of NAD+ and ATP, and promoting a form of caspase-independent cell death, coined parthanatos [42]. In our previous studies we observed that PARP inhibition protected the cells from H2O2-induced cell death, whereby a tendency (not statistically significant) towards a lower degree of protection was detectable in AD lymphocytes [15]. In that study, results were obtained using samples from AD patients with mild, moderate and severe disease that were considered as a single group. In the studies presented here, we found that by separating dementia cases according to severity, significant differences became detectable and that lymphocytes obtained from the severely demented group of AD patients were significantly more susceptible to oxidative stress induced damage (and death), further strengthening the working hypothesis that lymphocytes from AD patients are more susceptible to this atypical PARP-dependent type of death. The presence of antioxidants such as Tiron, EUK-134 and NAC protected against H2O2-induced death at concentrations of 100 µM, but not at higher H2O2 concentrations. This suggests that PARP-1 action, at the DNA level, is downstream of the effect of the antioxidants. Excessive PARP-1 activation has been linked to the progression of several chronic diseases and neurodegenerative disorders [42, 43], and more recently PARP was implicated in AD pathogenesis [44, 45]. Inhibition of PARP has been extensively explored in the perspective of developing effective treatments for cancer [46], and our observations suggest this target may deserve further attention in the treatment of Alzheimer’s disease.
Finally, Alzheimer’s disease (AD), the most frequent cause of dementia, is characterized by the lack of adequate biomarkers for disease diagnosis and objective stratification of disease severity. Bearing in mind our results, we propose that measurement of lymphocyte death induced by H2O2 may be exploited as a biological marker of AD severity, although further research is needed to corroborate this potential.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients and their families for participating in the study. We thank also Cecilia Zúñiga and Carolina Aranguiz for technical support, and Karina Elmes for coordinating patient visits.
FUNDING
This work was supported by Fondo de Ciencia y Tecnología (Fondecyt) Chile (grant Nº1080569, 1110189 to [MIB]); Fondecyt1130250, ACT1111 and CONICYTFONDAP 15130011 to [AFGQ]); National Institute of Aging P50 AG005681, P01 AG003991, and P01 AG026276, salaries for [CMR] and [CX]; CMR also supported by the Farrell Family Research Fund, the generous support of F. Simmons and O. Mohan, and the Charles F. and Joanne Knight Alzheimer’s Research Initiative of the Washington University Alzheimer’s Disease Research Center.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
DISCLOSURE STATEMENT
None of the authors have conflicts of interest related to this study.
AUTHORS’ CONTRIBUTIONS
Daniela P Ponce: Performed research, designed experiments, collected data, analyzed data, revised paper.
Felipe Salech: Performed research, designed experiments, collected data, analyzed data, revised paper.
Mónica Silva: Performed research, collected data.
Chengjie Xiong: Analyzed data, revised paper.
Catherine M Roe: Analyzed data, revised paper.
Mauricio Henriquez: Designed experiments, analyzed data, revised paper.
Andrew FG Quest: Designed experiments, analyzed data, contributed important reagents, and revised paper.
María Isabel Behrens: Directed the project, designed experiments, contributed important reagents, analyzed data, wrote paper.
SUPPLEMENTARY MATERIALS
Supplementary Fig. (1). Lymphocyte survival under oxidative stress plotted against cognitive status measured with MoCA test.
Supplementary Fig. (2). Effect of antioxidants on H2O2-induced death of lymphocytes from AD patients.
Supplementary Fig. (3). Oxidative stress and antioxidant defenses in lymphocytes from AD patients.
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Hardy J, Bogdanovic N, Winblad B, Portelius E, Andreasen N, Cedazo-Minguez A, et al. Pathways to Alzheimer's disease. J Intern Med. 2014;275(3):296–303. doi: 10.1111/joim.12192. [DOI] [PubMed] [Google Scholar]
- 2.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 3.Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4(2):185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- 4.Pohanka M. Alzheimer´s disease and oxidative stress: a review. Curr Med Chem. 2013;21(3):356–364. doi: 10.2174/09298673113206660258. [DOI] [PubMed] [Google Scholar]
- 5.Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64(5):895–898. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- 6.Roe CM, Fitzpatrick AL, Xiong C, Sieh W, Kuller L, Miller JP, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74(2):106–112. doi: 10.1212/WNL.0b013e3181c91873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, et al. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frain L, Swanson D, Betensky R, Cho K, Gagnon D, Kowall N, et al. In: AAIC, editor. A reduced risk of Alzheimer's disease is associated with the majority of cancers in a national cohort of veterans; Alzheimer's Association International Conference; Boston. 2013. p. 617. [Google Scholar]
- 9.Attner B, Lithman T, Noreen D, Olsson H. Low cancer rates among patients with dementia in a population-based register study in Sweden. Dement Geriatr Cogn Disord. 2010;30(1):39–42. doi: 10.1159/000315509. [DOI] [PubMed] [Google Scholar]
- 10.White RS, Lipton RB, Hall CB, Steinerman JR. Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology. 2013;80(21):1966–1972. doi: 10.1212/WNL.0b013e3182941990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology. 2013;81(4):322–328. doi: 10.1212/WNL.0b013e31829c5ec1. [DOI] [PubMed] [Google Scholar]
- 12.Tabarés-Seisdedos R, Rubenstein JL. Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat Rev Neurosci. 2013;14(4):293–304. doi: 10.1038/nrn3464. [DOI] [PubMed] [Google Scholar]
- 13.Behrens MI, Lendon C, Roe CM. A common biological mechanism in cancer and Alzheimer's disease? Curr Alzheimer Res. 2009;6(3):196–204. doi: 10.2174/156720509788486608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabarés-Seisdedos R, Dumont N, Baudot A, Valderas JM, Climent J, Valencia A, et al. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12(6):604–608. doi: 10.1016/S1470-2045(11)70041-9. [DOI] [PubMed] [Google Scholar]
- 15.Behrens MI, Silva M, Salech F, Ponce DP, Merino D, Sinning M, et al. Inverse susceptibility to oxidative death of lymphocytes obtained from Alzheimer's patients and skin cancer survivors: increased apoptosis in Alzheimer's and reduced necrosis in cancer. J Gerontol A Biol Sci Med Sci. 2012;67(10):1036–1040. doi: 10.1093/gerona/glr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrens MI, Silva M, Schmied A, Salech F, Manzur H, Rebolledo R, et al. Age-dependent increases in apoptosis/necrosis ratios in human lymphocytes exposed to oxidative stress. J Gerontol A Biol Sci Med Sci. 2011;66(7):732–740. doi: 10.1093/gerona/glr039. [DOI] [PubMed] [Google Scholar]
- 17.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 21.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31(1):1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Quiroga P, Albala C, Klaasen G. Validation of a screening test for age associated cognitive impairment, in Chile. Rev Med Chil. 2004;132(4):467–478. doi: 10.4067/s0034-98872004000400009. [DOI] [PubMed] [Google Scholar]
- 23.Kadioglu E, Sardas S, Aslan S, Isik E, Esat Karakaya A. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer's disease. Biomarkers. 2004;9(2):203–209. doi: 10.1080/13547500410001728390. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010;6(7):405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 25.Tomassoni D, Catalani A, Cinque C, Di Tullio MA, Tayebati SK, Cadoni A, et al. Effects of cholinergic enhancing drugs on cholinergic transporters in the brain and peripheral blood lymphocytes of spontaneously hypertensive rats. Curr Alzheimer Res. 2012;9(1):120–127. doi: 10.2174/156720512799015118. [DOI] [PubMed] [Google Scholar]
- 26.Esteras N, Muñoz Ú, Alquézar C, Bartolomé F, Bermejo-Pareja F, Martín-Requero Á. Altered calmodulin degradation and signaling in non-neuronal cells from Alzheimer's disease patients. Curr Alzheimer Res. 2012;9(3):267–277. doi: 10.2174/156720512800107564. [DOI] [PubMed] [Google Scholar]
- 27.Puertas MC, Martínez-Martos JM, Cobo MP, Carrera MP, Mayas MD, Ramírez-Expósito MJ. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol. 2012;47(8):625–630. doi: 10.1016/j.exger.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Torres LL, Quaglio NB, de Souza GT, Garcia RT, Dati LM, Moreira WL, et al. Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2011;26(1):59–68. doi: 10.3233/JAD-2011-110284. [DOI] [PubMed] [Google Scholar]
- 29.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2010;469(1):6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Buizza L, Cenini G, Lanni C, Ferrari-Toninelli G, Prandelli C, Govoni S, et al. Conformational altered p53 as an early marker of oxidative stress in Alzheimer's disease. PLoS One. 2012;7(1):e29789. doi: 10.1371/journal.pone.0029789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal. 2006;8(11–12):1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- 32.Cecchi C, Latorraca S, Sorbi S, Iantomasi T, Favilli F, Vincenzini MT, et al. Gluthatione level is altered in lymphoblasts from patients with familial Alzheimer's disease. Neurosci Lett. 1999;275(2):152–154. doi: 10.1016/s0304-3940(99)00751-x. [DOI] [PubMed] [Google Scholar]
- 33.Bartolomé F, de Las Cuevas N, Muñoz U, Bermejo F, Martín-Requero A. Impaired apoptosis in lymphoblasts from Alzheimer's disease patients: cross-talk of Ca2+/calmodulin and ERK1/2 signaling pathways. Cell Mol Life Sci. 2007;64(11):1437–1448. doi: 10.1007/s00018-007-7081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindowski K, Peters J, Gorriz C, Schramm U, Weinandi T, Leutner S, et al. Apoptosis of CD4+ T and natural killer cells in Alzheimer's disease. Pharmacopsychiatry. 2006;39(6):220–228. doi: 10.1055/s-2006-954591. [DOI] [PubMed] [Google Scholar]
- 35.Yoon SC, Kwon YA, Kim H, Kim S, Ahn Jo S, Kim DK. Altered cell viability and proliferation activity of peripheral lymphocytes in patients with Alzheimer's disease. Psychiatry Investig. 2010;7(1):68–71. doi: 10.4306/pi.2010.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160(4):1627–1637. [PubMed] [Google Scholar]
- 37.Song J, Wang S, Tan M, Jia J. G1/S checkpoint proteins in peripheral blood lymphocytes are potentially diagnostic biomarkers for Alzheimer's disease. Neurosci Lett. 2012;526(2):144–149. doi: 10.1016/j.neulet.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Moh C, Kubiak JZ, Bajic VP, Zhu X, Smith MA, Lee HG. Cell cycle deregulation in the neurons of Alzheimer's disease. Results Probl Cell Differ. 2011;53:565–576. doi: 10.1007/978-3-642-19065-0_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M, Wang S, Song J, Jia J. Combination of p53(ser15) and p21/p21(thr145) in peripheral blood lymphocytes as potential Alzheimer's disease biomarkers. Neurosci Lett. 2012;516(2):226–231. doi: 10.1016/j.neulet.2012.03.093. [DOI] [PubMed] [Google Scholar]
- 40.Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A. Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses. PLoS Genet. 2014;10(2):e1004173. doi: 10.1371/journal.pgen.1004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montero J, Dutta C, van Bodegom D, Weinstock D, Letai A. p53 regulates a non-apoptotic death induced by ROS. Cell Death Differ. 2013;20(11):1465–1474. doi: 10.1038/cdd.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci (Landmark Ed) 2009;14:1116–1128. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virág L. 50Years of poly(ADP-ribosyl)ation. Mol Aspects Med. 2013;34(6):1043–1045. doi: 10.1016/j.mam.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Strosznajder JB, Czapski GA, Adamczyk A, Strosznajder RP. Poly(ADP-ribose) polymerase-1 in amyloid beta toxicity and Alzheimer's disease. Mol Neurobiol. 2012;46(1):78–84. doi: 10.1007/s12035-012-8258-9. [DOI] [PubMed] [Google Scholar]
- 45.Abeti R, Duchen MR. Activation of PARP by oxidative stress induced by β-amyloid: implications for Alzheimer's disease. Neurochem Res. 2012;37(11):2589–2596. doi: 10.1007/s11064-012-0895-x. [DOI] [PubMed] [Google Scholar]
- 46.Hilton JF, Hadfield MJ, Tran MT, Shapiro GI. Poly(ADP-ribose) polymerase inhibitors as cancer therapy. Front Biosci. 2013;8:1392–1406. doi: 10.2741/4188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.