Abstract
Disruption of vascular endothelial growth factor (VEGF) signaling during early development results in abnormal angiogenesis and increased vascular lesions. Embryonic exposure to 0.625 to 10 mM methyl tert butyl ether (MTBE), a highly water soluble gasoline additive, resulted in a dose dependent increase in pooled blood in the common cardinal vein (CCV), cranial hemorrhages and abnormal intersegmental vessels (ISVs). The EC50s for the lesions ranked in terms of likelihood to occur with MTBE exposure were: pooled blood in the CCV, 3.2 mM [95 % CI: 2.2 – 4.7] > cranial hemorrhage, 11 mM [5.9 – 20.5] > abnormal ISV, 14.5 mM [6.5 – 32.4]. Organ systems other than the vascular system appear to develop normally, which suggests MTBE toxicity targets developing blood vessels. Equal molar concentrations (0.625 to 10 mM) of the primary metabolites, tertiary butyl alcohol (TBA) and formaldehyde, did not result in vascular lesions, which suggested that the parent compound is responsible for the toxicity. Stage specific exposures were carried out to determine the developmental period most sensitive to MTBE vascular disruption. Embryos treated until 6-somites or treated after Prim-5 stages did not exhibit a significant increase in lesions, while embryos treated between 6-somites and Prim-5 had a significant increase in vascular lesions (p ≤ 0.05). During the critical window for MTBE-induced vascular toxicity, expression of vegfa, vegfc, and flk1/kdr were significantly decreased 50, 70 and 40%, respectively. This is the first study to characterize disruption in vascular development following embryonic exposure to MTBE. The unique specificity of MTBE to disrupt angiogenesis may be mediated by the down regulation of critical genes in the VEGF pathway.
Keywords: angiogenesis, VEGF, MTBE, development, zebrafish embryo
1. INTRODUCTION
The developing cardiovascular system is often a target of chemical toxicants, and cardiovascular defects account for a large percentage of embryo and fetal lethality in lower and higher vertebrates (Mone et al., 2004; Heideman et al., 2005). Organogenesis is closely linked to vascular development because tissue growth is restricted by hypoxia. Formation of the vertebrate closed circulatory system involves both vasculogenesis and angiogenesis. Vasculogenesis is de novo blood vessel formation by endothelial cell precursors (angioblasts) derived from progenitor cells (hemangioblasts) in the bone marrow (Choi et al., 1998; Vogeli et al., 2006). Angiogenesis is the development of blood vessels from pre-existing vessels, and is integral to organ growth and repair. Hypoxia-initiated angiogenesis in embryos is mediated by hypoxia inducible factor 1 (HIF1), a heterodimeric transcription factor complex composed of cytoplasmic HIF1-α and nuclear HIF1-β (ARNT) (Ryan et al., 1998; Ramirez-Bergeron et al., 2004). Together, they are responsible for the regulation of hypoxia compensatory mechanisms including the upregulation of a key signaling protein, vascular endothelial growth factor (VEGF) (Levy et al., 1995; Forsythe et al., 1996; Adelman et al., 1999). VEGF is essential to vasculogenesis, angiogenesis, and hematopoiesis. In mammals, deletion of one vegf allelele inhibits blood island formation, angiogenesis, lumen formation, and survival of the embryo (Carmielet et al., 1996; Ferrara et al., 1996). Multiple VEGF isoforms (VEGF a–d) and vascular endothelial growth factor receptors (VEGFR1/Flt1, VEGFR2/Flk1/KDR, and VEGFR3/Flt4) are required in various combinations for endothelial cell differentiation and migration (Habeck et al., 2002; Covassin, et al., 2006; Bahary, et al., 2007). Regulation of HIF1, VEGF, and VEGFR in the embryo is crucial to angiogenesis and, therefore, to organogenesis and survival.
Methyl tert butyl ether (MTBE) [C5H12O] is a low molecular weight volatile organic compound (MW 88.15 g/mol) used as a gasoline additive to increase the burning efficiency of fuel in combustion engines. Due to a high water solubility [476 mM, 42 g/L at 25 °C], MTBE can readily contaminate both surface and groundwater at any point in its lifecycle (Brown, 1997; Ahmed, 2001; Squillace and Moran, 2007). As of August 2007, 25 states enacted either complete or partial bans on the use of MTBE in gasoline (US EPA, 2007). Litigation continues over the reparations oil companies were required to pay to municipalities where ground water supplies were contaminated with MTBE from leaky underground gasoline storage containers. While MTBE use in the US has declined since its peak in the mid 1990s, its use as a gasoline additive continues worldwide (Rosell et al., 2006; vanWezel et al., 2009). Production of MTBE remains high, averaging just over 1 million barrels per month in 2009 and 2010 (DOE-EIA, 2010).
In humans, MTBE is readily eliminated by expiration or metabolized and excreted in urine (Amberg et al., 2001). Primary metabolism of MTBE in mammals occurs in the liver through oxidative demethylation by cytochrome P-450 2A6 or 3A4 (Hong et al., 1997; Hong et al., 1999; Le Gal et al., 2001). MTBE oxidation results in the formation of tert-butyl alcohol (TBA) and formaldehyde (Fig. 1). Circulating levels of TBA are directly correlated to the MTBE exposure because the tertiary alcohol is metabolized at a much slower rate than MTBE (Amberg, et al., 2001).
Fig. 1.
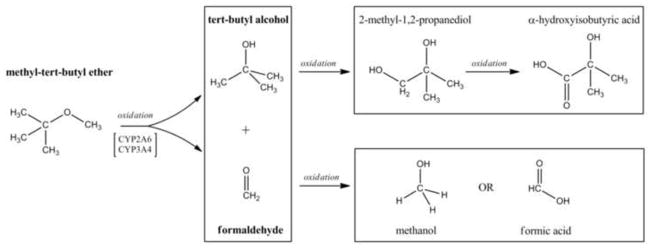
Oxidation of MTBE to known vertebrate metabolites. In mammals, MTBE is oxidized by CYP 2A6 and 3A4 to TBA and formaldehyde (Hong et al., 1999; Le Gal et al., 2001). Primary metabolites (bold faced) can be excreted or undergo further oxidation. The enzymes that oxidize TBA to 2-methyl-1,2-propanediol to α-hydroxyisobutyric acid are unknown. Formaldehyde is oxidized to methanol by alcohol dehydrogenase or to formic acid by aldehyde dehydrogenase. Based on Amberg et al. (2001) and Du et al. (2005).
Other than acute lethality, MTBE’s effects on aquatic vertebrates are largely uninvestigated. In a review of early invertebrate and vertebrate LC50 studies, Werner et al. (2001) reported that environmental levels of MTBE were unlikely to cause acute toxic effects in freshwater organisms. LC50s for adult fish were in the 6.8 – 11.3 mM (600–1000 mg/L) range (Werner et al., 2001; Moreels et al., 2006a; Naddafi et al., 2008). Embryonic exposures to sub-lethal concentrations of MTBE have thus far been limited to the African catfish (Clarias gariepinus) and the Japanese medaka (Oryzias latipes). Moreels et al. (2006b) reported that exposure to 0.7 – 1.3 mM (65–111 mg/L) MTBE reduced the number of viable African catfish embryos at 24h, decreased survival for 3 day post hatch larvae and led to an increase in craniofacial and spinal abnormalities. In the Japanese medaka embryos, exposure to 10.2 mM (900 mg/L) MTBE resulted in increased prevalence of hypopigmented blood, tail curvature and developmental stage delay, and exposure to 29.5 mM (2600 mg/L) MTBE completely inhibited vascular growth (Longo, 1995).
MTBE is acutely toxic at moderately high concentrations, but the vascular inhibition (Longo, 1995) and developmental lesions (Moreels et al., 2006b) observed in fish embryos occur at lower, sub-lethal concentrations. Here we examined the effect of MTBE on zebrafish embryos (Danio rerio) and the developing vascular system. Embryonic exposure to sub-lethal concentrations of MTBE disrupted angiogenesis and altered VEGF -VEGFR regulation by decreasing transcript levels of vegf-a, vegf-c and flk1/kdr during a critical period of vascular development. This is the first study which explores the vascular disrupting effects of MTBE in an in vivo model.
2. METHODS
2.1 Animal Handling
Transgenic zebrafish fli1-EGFPs (Fli1s) used for all experiments were obtained from the Zebrafish International Resource Center. Fli1s express enhanced green fluorescent protein driven by the fli1 promoter in all vascular endothelial cells (Lawson and Weinstein, 2002). Expression of EGFP in all endothelial cells of Fli1s allows for in vivo examination of vasculature morphology in live embryos. Adults were maintained and bred in an Aquatic Habitat recirculating system on a 14:10 light: dark cycle. Fish system water was maintained between 26 and 28°C, <0.05 ppm nitrite, <0.2 ppm ammonia, and pH between 7.2 and 7.7. The husbandry (#03-014) and embryonic exposure protocols (#08-025) were approved by the Rutgers University Animal Care and Facilities Committee. Embryos were raised in 60 μg/ml Instant Ocean in ddH2O (egg water). During exposures, embryos were incubated at 25 °C in an attempt to slow down development. The standard timeline of zebrafish development is based on development at 28.5 °C, but normal development will occurs between 25 and 33 °C (Kimmel, et al., 1995). The zebrafish stages of development corresponding to hours post fertilization (hpf) for both 25 and 28.5 °C are present in Table 1. The hpf at 25 °C were determined using the conversion equation established by Kimmel et al. (1995): HT = h/(0.055T 0.57). A concerted effort was made to ensure that all embryos were at the same stage at the beginning of each experiment.
Table 1.
Developmental Stages in the Zebrafish Embryo as Described by Kimmel et al. (1995)
| Hours Post Fertilization (hpf)
| |||
|---|---|---|---|
| 28.5 °C | 25 °C | Embryo Stage | Description |
| 10 | 12.4 | Bud | Head and tail are distinct |
| 12 | 14.9 | 6-somite | Optic primoridium notable, Neural keel formation |
| 14 | 17.4 | 10-somite | Rudimentary pronephros |
| 16 | 19.9 | 14-somite | Brain subdivisions are distinguishable |
| 19 | 23.6 | 21-somite | Angioblasts coalesce at midline to form major vessels |
| 22 | 27.3 | 26-somite | Blood island cells differentiate |
| 24 | 29.8 | Prim-5 | Heart beats, circulation begins |
| 30 | 37.3 | Prim-15 | Primary vessels are patent, CCV is broad across yolk |
| 36 | 44.7 | Prim-25 | Brain and trunk vascularization begins |
| 42 | 52.2 | High-pec | Liver primordial, Atrium/Ventricle are distinct in heart |
| 48 | 59.6 | Long-pec | Brain microvasculature is patent, CCV less prominent |
| 60 | 74.5 | Pec fin | Retina fully pigmented, hatching gland prominent |
| 72 | 89.4 | Protruding mouth | All ISVs are patent, circulation through gills begins |
| 96 | 119.3 | Hatch | Free of chorion, limited movement |
2.2 Chemicals
All chemicals were obtained from Sigma Aldrich: Methyl tert-butyl ether (MTBE) [purity 99.9 %], tert-Butanol (TBA) [≥ 99.5 %], or Fisher Scientific: Formaldehyde. All chemical solutions were made the day of treatment with aerated egg water.
2.3 LC50 Studies
Exposures were performed in triplicate at nominal concentrations of 6.25, 12.5, 25, 50, 100 and 200 mM MTBE in sealed glass scintillation vials to avoid volatilization of the chemical (N = 3 vials per concentration, 15 embryos each vial). Exposure began at 3 hpf (512 cell stage) and embryos were observed daily for mortality until 120 hpf. The study was repeated with 6.25, 12.5, 18.75, 25 and 50 mM in triplicate. LC50 and confidence intervals were calculated at 120 hpf using the Litchfield-Wilcoxon method (Litchfield and Wilcoxon, 1949).
2.4 Dose Response Vial Studies
Embryos were exposed individually (N = 20 per treatment) in sealed 4 ml glass vials to static, non-renewal concentrations of 0.625, 1.25, 2.5, 5, and 10 mM nominal concentrations of MTBE, TBA or formaldehyde, and observed under light and fluorescence microscopy. Embryos were observed daily for the 5 day developmental period characteristic of zebrafish maintained at 25 °C. After hatch, eleutheroembryos (sac-fry) were maintained under the same experimental conditions until 3 day post hatch (dph) observations were made. Three dph survival was used as a marker of sac fry viability, and is based on normal survival rates of control embryos (Wisk and Cooper, 1990a). Embryos not hatched by 3 dph were considered dead (non-viable). EC50 and confidence intervals for lesion occurrence were determined using the Litchfield-Wilcoxon method (Litchfield and Wilcoxon, 1949). The MTBE, TBA and formaldehyde dose response studies were each repeated 3 times. A follow up metabolite study used the vial study paradigm to expose embryos to 10 mM TBA, 10 mM formaldehyde, or a mixture of 10 mM TBA and 10 mM formaldehyde (N = 20 per treatment).
2.5 Histology
To characterize the lesions histologically, embryos were exposed at the 512 cell stage to 5 mM MTBE. Embryos were selected for fixation at 48, 72 or 96 hpf for histology based on the appearance of a severe lesion. Embryos were fixed over night at room temperature in a solution of 1 % formaldehyde, 2 % gluteraldehyde, and 1 % calcium acetate. Fixed embryos were rinsed with 0.1 M phosphate buffer 4 times and stored in 50 % ethanol at 4 °C. Before tissue processing, fixed embryos were stained with 1 % osmium tetraoxide. Embryos were further dehydrated with ethanol, then infiltrated with acetone: EPON, and embedded in EPON in BEEM capsules. The EPON resin blocks were trimmed, and thick sections were cut with glass knives. Sections were stained with toluidine blue-O (Gonzalez Santander et al., 1997).
2.6 Stage Specific Exposures
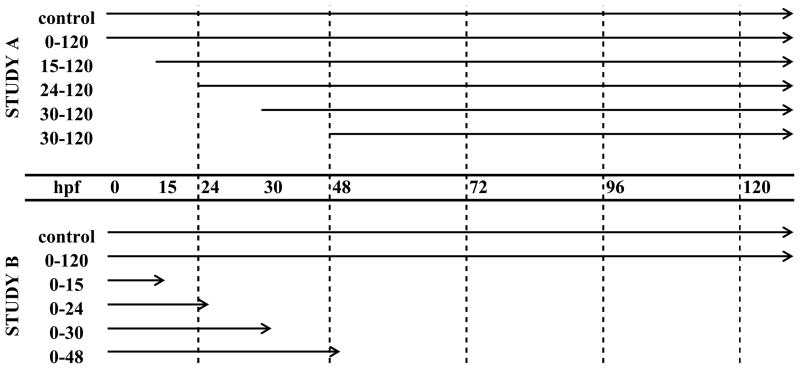
A staggered exposure regime was used to determine the beginning (Study A) and end (Study B) of the critical window for MTBE-induced specific lesions (Fig. 2). In Study A, embryos (N = 25) were exposed in individual glass vials to static nonrenewal treatments of 10 mM MTBE at an initial time point: 15, 24, 30, or 48 hpf. In Study B, embryos (N = 25) were treated in individual vials to static nonrenewal treatments of 10 mM MTBE at 3 hpf (time zero) and treatment was discontinued at 15, 24, 30, or 48 hpf. The target time points correspond approximately to the following stages at 25 °C: 6-somites (15 hpf), 21-somites (24 hpf), prim-5 (30 hpf), and prim-25 (48 hpf) (Table 1). In the third study (Study C, N = 25) embryos were exposed to 10 mM MTBE during the critical period (15 hpf to 30 hpf; 6-somites to prim-5), determined by Study A and B (N = 20 per treatment). Embryos were observed daily under light and fluorescence microscopy for 5 days. Studies A, B and C were each repeated twice. The stage specific exposures were based on previous work with Japanese medaka embryos demonstrating stage specificity following 2,3,7,8 - tetrachloro-p-dioxin exposures (2,3,7,8-TCDD) (Wisk and Cooper, 1990a).
Fig. 2.
Study design schematic for MTBE Critical Period: Studies A and B. The arrows represent the length of treatment with 10 mM MTBE. The target time points correspond approximately to the following embryonic stages at 25 °C: 6-somites (15 hpf), 21-somites (24 hpf), prim-5 (30 hpf), and prim-25 (48 hpf). In Study C, not shown here, embryos were exposed to 10 mM MTBE from only 15 to 30 hpf (6-somites to prim-5).
2.7 Real-time Polymerase Chain Reaction
Embryos were exposed to a static non-renewal treatment of 0 (control) or 5 mM MTBE until 24 hpf (21-somites) in glass scintillation vials to determine the effect of MTBE on the mRNA expression of key genes associated with angiogenesis. Treatment with 5 mM MTBE was used for these studies because it corresponded to the calculated EC90 for total lesions based on the 3 dose response studies (2.4). The 21-somite stage was chosen because it is associated with the formation of the major descending and ascending vessels (Kimmel et al., 1995; Isogai et al., 2003) and an increase in zebrafish vegfa mRNA expression (Liang et al., 1998). Approximately 50 embryos were used per treatment, each treatment was performed in triplicate, and the experiment was repeated three times. Real-time PCR sample preparation was carried out as described in Hillegass et al. (2007). Briefly, embryos were snap frozen in liquid nitrogen at 24 hpf. RNA was isolated (Invitrogen: TRIzol) and DNase treated (Ambion: DNase I [RNase-free]), and cDNA (Biorad: iScript cDNA Synthesis Kit) was made for real-time PCR (Biorad: iQ SYBR Green Supermix). A 2-step qPCR protocol was used: 95°C to 60°C for 35 cycles, and primer sets were selected to work with the same qPCR protocol. Data were normalized to 28S and quantified using standard curves generated for each primer set. The primer sets were as follows: zf vegfa (NM_131408) fwd 5′-TGC TCC TGC AAA TTC ACA CAA-3′, rev 5′-ATC TTG GCT TTC ACA TCT GCA A-3′, product size 85 bp; zf vegfc (NM_205734) fwd 5′-ATA AAC CAC CCT GCG TGT CTG TCT-3′, rev 5′-TCC TTG CTT GAC TGG AAC TGT GA - 3′, product size 132 bp; zf flk1/kdr (NM_001024653) fwd 5′TCT CCA TAT CCG GGT GTG TGC ATT-3′, rev 5′TCT GGT ATA TTT CAG GCG TGG CGT-3′, product size 103 bp; zf hif1α-like protein (NM_001012371) fwd 5′-TCT GGT TCT TGA GTG CAG GGT TCT-3′, rev 5′-AGT GGC AGT AGG TGA ACG TCA TGT-3′, product size 119 bp.
2.8 Statistical analysis
Statistical analyses were performed using the SigmaPlot version 11 computer software package. Differences in lesion occurrence were determined using Chi-Squared analysis and Fisher Exact test. The Student t-Test was used to determine significance between mRNA levels in MTBE and control groups. The probability level for statistical significance was p ≤ 0.05.
3. RESULTS
3.1. MTBE mortality and dose response studies
In the first LC50 study, embryos were exposed to 6.25, 12.5, 25, 50, 100 and 200 mM MTBE. All embryos in the 50, 100 and 200 mM treatment groups died by 24 hpf (21-somites). In 100 and 200 mM exposed embryos, mortality occurred prior to the segmental phase of development. Development of 50 mM exposed embryos ceased by the 10-somites (17 hpf), but the cranial and caudal regions, normally present at this stage, were not defined. By 120 hpf, 57% of embryos died in the 25 mM triplicates, while in both 6.25 and 12.5 mM MTBE, mortality was less than 20 %. In the second LC50 study, embryos were exposed to 6.25, 12.5, 18.75, 25, and 50 mM MTBE. Again, 50 mM exposed embryos died by 10 somites, and mortality was less than 20 % in 6.25 and 12.5 mM MTBE. Mortality for 18.75 and 25mM MTBE were 31 and 49%, respectively. The calculated LC50s and confidence intervals (CI) for both studies were similar (Table 2): 19 mM (CI = 15.9 – 22.3) and 20 mM (CI= 17.9 – 22.7).
Table 2.
Calculated LC50s and EC50s with embryonic MTBE exposure
| 0–120h MTBE Exposure (mM) | 95% CI | |
|---|---|---|
| Mortality (LC50) Study 1 | 19 | 15.9 – 22.3 |
| Mortality (LC50) Study 2 | 20 | 17.9 – 22.7 |
| Total Lesions (EC50)† | 1.2 | 0.8 – 1.8 |
| Individual Lesions (EC50)† | ||
| Pericardial Edema | 20 | 9.6 – 41.6 |
| Pooled Blood in CCV | 3.2 | 2.2 – 4.7 |
| Cranial Hemorrhage | 11 | 5.9 – 20.5 |
| Abnormal ISV | 14.5 | 6.5 – 32.4 |
Data determined by averaging the three individual MTBE dose response vial studies
Dose response studies using sub-lethal concentrations of MTBE (0.625 – 10 mM) were conducted to characterize the lesions associated with exposure (Table 3). Average mortality in both control and treatment groups over the 120 hrs of development was 5%. Hatching at 120 hpf was reduced in all MTBE treatment groups, but a significant decrease was observed only in the 10mM treatment group (45% hatched as compared to 80% in control). However, no significant difference in 3 dph survival was observed in any treatment group. The lesions observed with sub-lethal MTBE treatment were consistent with the disruption of vascular development. There was a dose dependent increase in the number of embryos exhibiting pooled blood in the common cardinal vein (CCV), cranial hemorrhages, abnormal intersegmental vessel (ISV) formation, pericardial edema, and reduced circulating red blood cells (RBCs) and other blood formed elements (Table 3). At the lowest concentration tested, 0.625 mM, 40% of embryos exhibited at least one vascular related lesion, but was not significantly different from control (15%). However, in both 5 mM (75%) and 10 mM (95%) MTBE treatment groups, significantly (p ≤ 0.05) more embryos exhibited lesions than in control (15%). Pooled blood in CCV occurred in 50 % of embryos exposed to 5.0 mM MTBE and in 75 % of embryos exposed to 10.0 mM, which was significantly more than 10% in control. Cranial hemorrhages in MTBE exposed embryos was significant (p ≤ 0.05) at 10.0 mM, and trended toward significance in 5.0 mM (p = 0.09). The concentrations at which the occurrence of abnormal ISVs was significantly different from control (p ≤ 0.05) were 5.0 and 10.0 mM. The incidence of pericardial edema also increased with dose, but was not significantly increased at any concentration. It is important to note that while 60 % of embryos treated with 10 mM MTBE appeared to have a reduction or lack of circulating RBCs, this lesion was often associated with severe hemorrhages, and was considered a result of blood loss into the connective tissue, not an effect on hematopoiesis. However, because the development of endothelial and blood cells are related (Choi et al., 1998; Vogeli et al., 2006), it is possible that MTBE may have an effect on the hematopoietic branch of cardiovascular development. Further, much larger studies would need to be conducted to determine if the reduction in circulating formed elements was a direct effect of treatment, or secondary to the severe hemorrhages that result from MTBE exposure.
Table 3.
Percent of embryos exhibiting lesions with MTBE, TBA or Formaldehyde exposure
| Control | 0.625mM MTBE |
1.25mM MTBE |
2.5mM MTBE |
5.0mM MTBE |
10.0mM MTBE |
10.0mM TBA |
10.0mM CH2O |
10.0mM TBA+CH2O |
|
|---|---|---|---|---|---|---|---|---|---|
| All Lesions | 15 (± 10) | 40 (± 13) | 45 (± 8) | 45 (± 12) | 75 (± 22)* | 95 (± 9)* | 10 (± 3) | 25 (± 15) | 20 (± 4) |
| Death (by 120hpf) | 5 (± 6) | 5 (± 3) | 5 (± 8) | 5 (± 3) | 5 (± 3) | 5 (± 6) | 5 (± 6) | 5 (± 3) | 5 (± 4) |
| Hatch (at 120hpf) | 80 (± 3) | 75 (± 26) | 60 (± 28) | 60 (± 20) | 65 (± 24) | 45 (± 6)* | 60 (± 13) | 55 (± 9) | 65 (± 11) |
| 3dph Survival | 90 (± 5) | 85 (± 3) | 85 (± 3) | 85 (± 0) | 85 (± 9) | 90 (± 5) | 70 (± 13) | 80 (± 14) | 85 (± 4) |
| Reduced Circulating RBCs | 5 (± 10) | 0 (± 0) | 5 (± 5) | 15 (± 12.5) | 45 (± 30)* | 60 (± 37.5)* | 5 (± 3) | 0 (± 0) | 5 (± 4) |
| Pericardial Edema | 5 (± 5) | 5 (± 5) | 5 (± 5) | 5 (± 5) | 15 (± 10) | 35 (± 15)* | 5 (± 8) | 0 (± 0) | 5 (± 7) |
| Pooled Blood in CCV | 10 (± 10) | 15 (± 5) | 15 (± 5) | 20 (± 15) | 50 (± 5)* | 75 (± 20)* | 0 (± 0) | 5 (± 5) | 5 (± 0) |
| Cranial Hemorrhage | 5 (± 5) | 10 (± 5) | 15 (± 15) | 25 (± 5) | 30 (± 5) | 45 (± 5)* | 5 (± 9) | 10 (± 8) | 5 (± 0) |
| Abnormal ISVs | 5 (± 5) | 15 (± 10) | 15 (± 10) | 20 (± 10) | 35 (± 5)* | 60 (± 5)* | 5 (± 3) | 10 (± 13) | 5 (± 7) |
Values in the table are percentages ± standard deviation based on the average number of embryos in each category from replicate studies. (N=20)
Significantly different from control (P ≤ 0.05).
The EC50 for all (total) lesions with continuous embryonic exposure to MTBE is 1.2 mM [95% CI = 0.8 – 1.8]. EC50s for the individual vascular lesions are reported in Table 2. Based on these EC50s, lesions were ranked in terms of likelihood to occur with MTBE exposure: pooled blood in the CCV, 3.2 mM [2.2 – 4.7] > cranial hemorrhage, 11 mM [5.9 – 20.5] > abnormal ISV, 14.5 mM [6.5 – 32.4] > pericardial edema, 20 mM [9.6 – 41.6].
3.2. Characterization of MTBE specific lesions
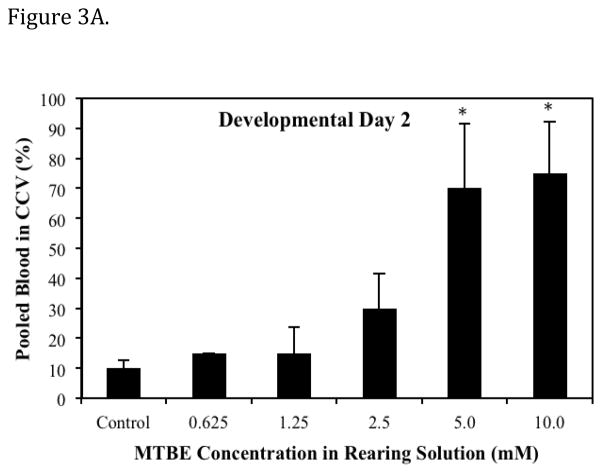
The predominant lesions observed following MTBE exposure were pooled blood in CCV (the ducts of cuvier), cranial hemorrhages, and abnormal ISVs. Lesions were first apparent at 48 hpf (Prim - 25, Table 1). Formation of the major descending and ascending vessels, which occurs prior to 48 hpf, does not appear to be disrupted by MTBE. The appearance of a lesion was directly associated with the embryonic stage and phase of cardiovascular development. The MTBE induced lesions occurred at specific developmental time periods, and were often transient, except in incidences where lesions were extremely severe. The dose dependent effect of MTBE on the percentage of embryos with pooled blood in CCV on day 2 of development is presented in Fig. 3A. At the Prim-25 stage of zebrafish development, the CCV is a broad vessel that occupies approximately half the surface area of the yolk (Kimmel et al., 1995). Normally, blood cells exit the posterior cardinal vein laterally and flow across the yolk toward the heart in the CCV. In MTBE treated embryos, blood cells pooled in the most ventral portion of the CCV, and cells were either stagnant or pulsated with heart contractions. Pulsation resulted in a limited number of blood cells entering the heart and therefore entering the circulation. In most individuals, this lesion was absent on day 3, even in the 5.0 mM and 10.0 mM MTBE treatment groups where 70–75% embryos exhibited pooled blood in the CCV on day 2.
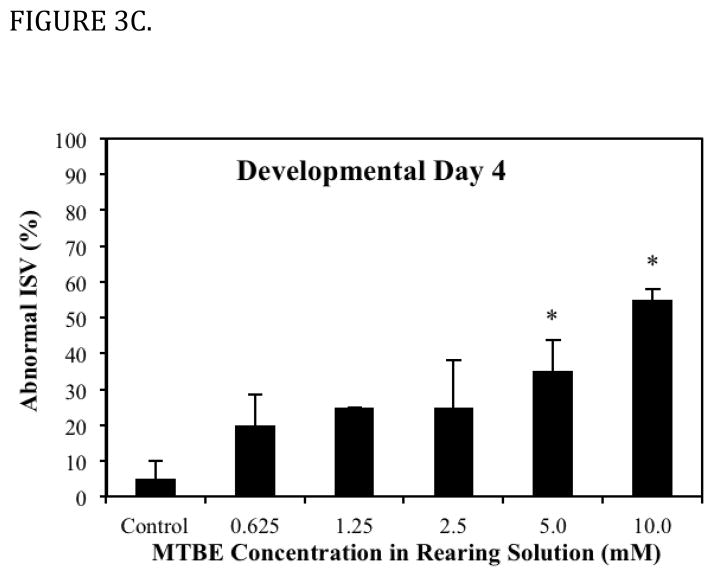
Fig. 3.
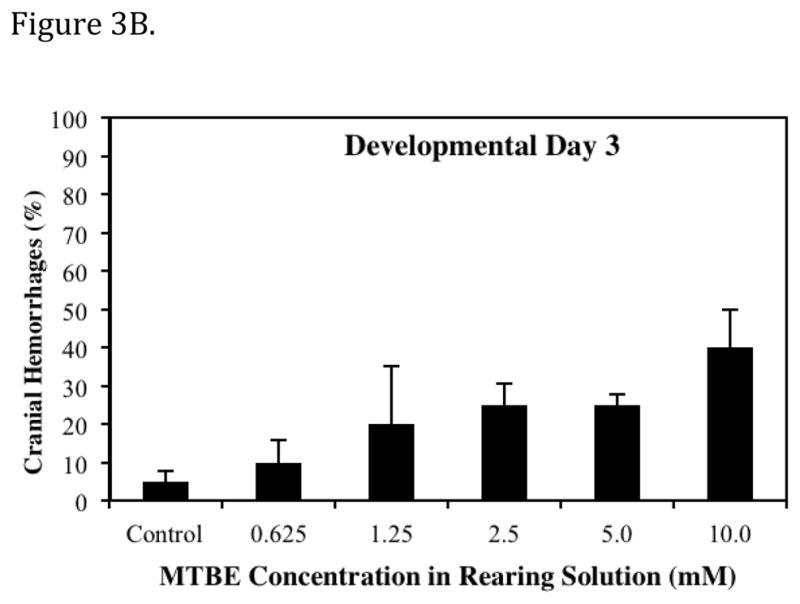
Dose dependent effects of MTBE on the percent of embryos exhibiting a specific vascular lesion. (A) Developmental Day 2 begins with the High Pec stage of embryo development. At this stage the CCV is a broad vein across the yolk. With MTBE treatment, blood cells pool in the lower portion of the CCV. The percent of embryos exhibiting this lesion is the higher on day 2 than on other days. (B) Developmental Day 3 begins with the Long Pec stage of embryo development. At this stage the microvasculature of the brain becomes patent. Embryos exposed to MTBE trend toward a dose dependent increase in cranial hemorrhages. (C) By Developmental Day 4, most of the vasculature in the embryo is formed and patent. MTBE treated embryos exhibit a dose dependent increase in abnormal development of ISVs, located between the dorsal somite muscles. *Significantly different from control (P ≤ 0.05). Data represents the average of three independent MTBE dose response vial studies.
Formation of microvasculature in the brain occurs primarily during the Pec Fin through the Long Pec stages of development (Table 1, Fig. 4A) on day 3 (Isogai et al., 2001). MTBE exposed embryos exhibited cranial hemorrhages as early as day 2, but the lesion occurrence was greatest on day 3, when most of the cranial vasculature was intact. The dose dependent effect of MTBE on occurrence of cranial hemorrhages on day 3 is presented in Fig. 3B. Cranial hemorrhages were predominantly in the forebrain/midbrain region of the head, and the ventricles (Fig. 4B). Histological examination of the lesion showed RBCs extravasated into the brain tissue (Fig. 4C). The nucleated RBCs did not appear to be undergoing hemolysis (Fig. 4D).
Fig. 4.
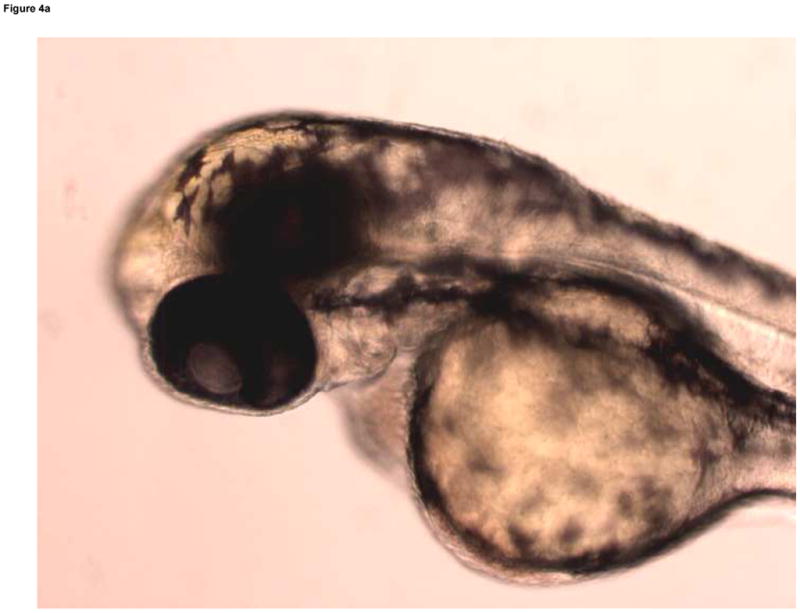
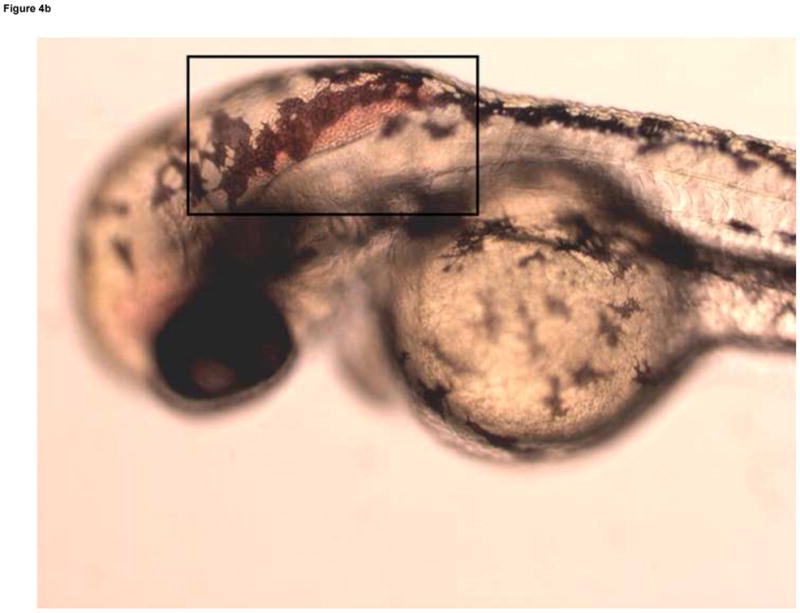
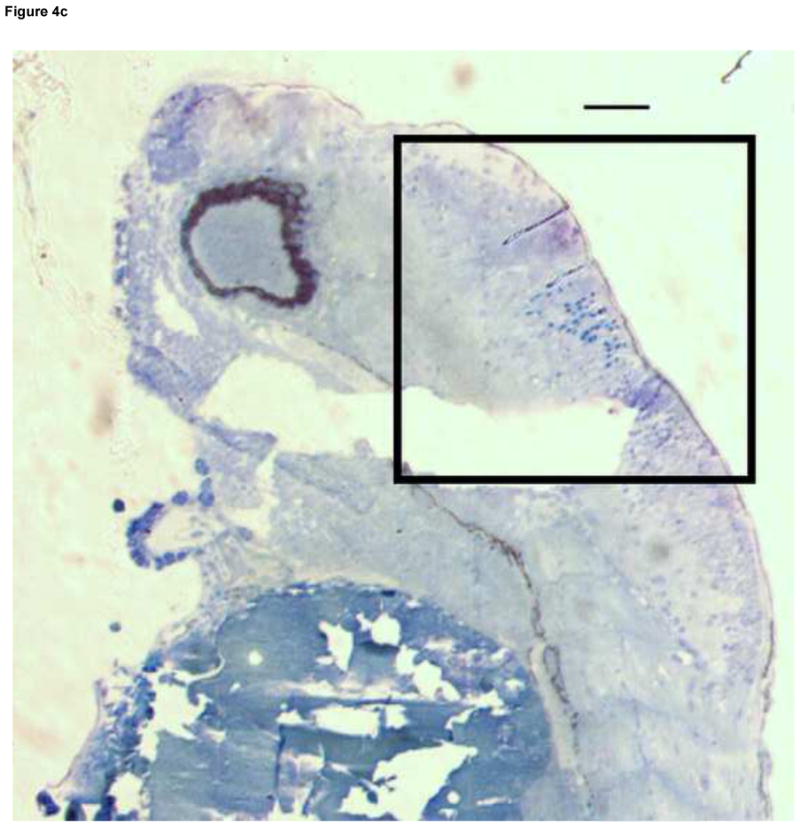
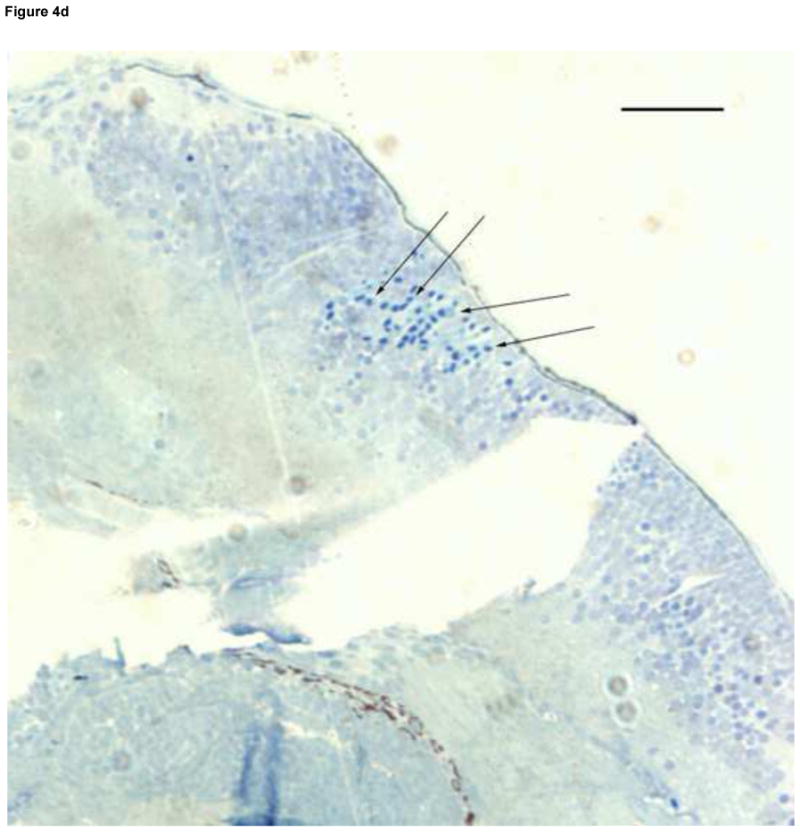
Developmental Day 3 (Long Pec) embryos with (A) normal cranial vascular flow or (B) a cranial hemorrhages in a zebrafish exposed to 10 mM MTBE. Cranial hemorrhages are predominantly present in the forebrain/midbrain region of the head, and the ventricles (10x); black box. (C) Toluiduine blue staining in a 72 hpf embryo revealed nucleated RBCs extravasated into the brain tissue (10x); black box. (D) Red blood cells did not appear to be undergoing hemolysis (20x); arrows. Scale bar = 100μm.
The dorsal axial vessels between somites, ISVs, begin as sprouts off the major descending and ascending vessels early in vascular development, but these vessels are not all fully open, or patent, until the Protruding Mouth stage (Table 1) on day 4 (Isogai et al., 2001). Circulation was present through all ISVs in controls on day 4, and blood cells were visible passing through the vessel lumens with light microscopy. Under fluorescence microscopy, patent ISVs had a dim green lumenal area framed by bright green edges (Fig. 5A). Areas with poor or absent circulation through somites (observed under light microscopy) were associated with bright green ISVs that lacked the dim green lumen (under fluorescence microscopy). In MTBE treated embryos, circulation is not present in all ISVs on day 4, and these embryos exhibited ISVs that appeared bright green under fluorescence (Fig. 5B). Furthermore, decreased or lack of circulation through the trunk resulted in abnormal formation of the somite muscles in those regions, observed in the day 4 histology of 5 mM MTBE treated embryos. Toluidine blue staining in day 4 controls reveals the characteristic chevron shaped somite, with few gaps in the muscle fibers (Fig. 5C). Somites of MTBE treated embryos, which exhibited decreased circulation through ISVs prior to collection on day 4, appear broader than control somites, and the muscle fibers contain regions of vacuolization (Fig. 5D,E).
Fig. 5.
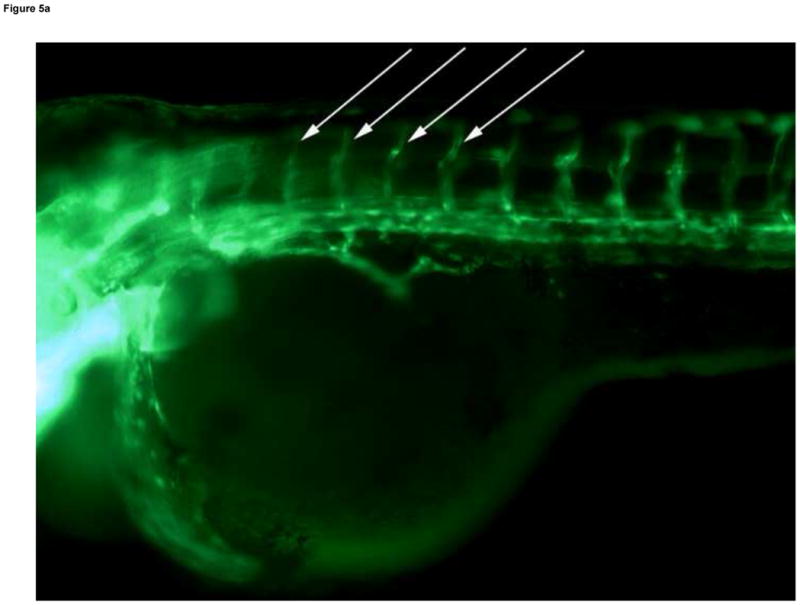
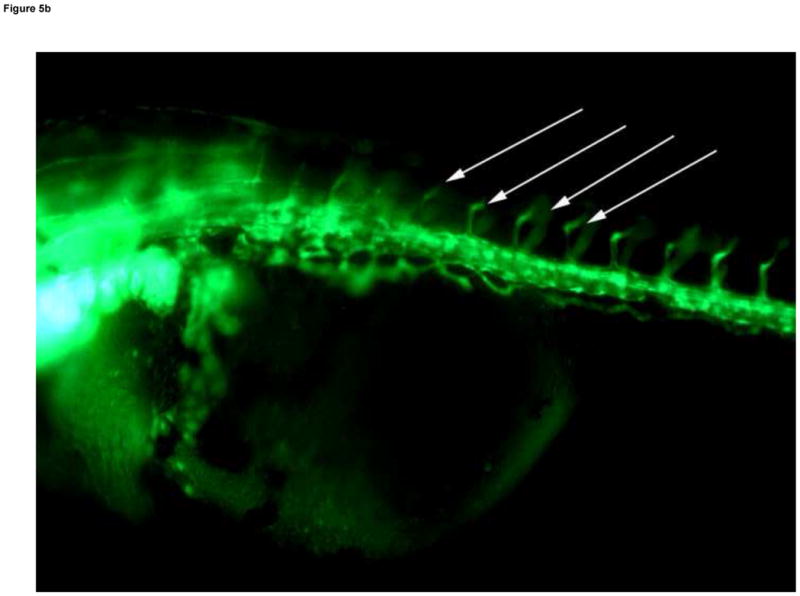



Developmental Day 4 (Protruding Mouth) embryos with (A) fully patent ISVs that appear as bright green edged tubes with a dim green lumenal area in controls (10x) - white arrows; or (B) abnormal ISV which lack proper circulation and appear as bright green vessels lacking a dim lumen in 10 mM MTBE treated embryos (10x) - white arrows. (C) Toluidine blue staining of day 4 control embryos reveals chevron shaped somite muscles; light blue stained tissue above dark blue yolk (20x). (D) Somites in MTBE treated embryos appear rounder and broader than in controls (20x) and (E) muscle fibers appear more disorganized (40x) – black arrows. Scale bar = 100μm.
3.3. Embryonic Exposure to MTBE Primary Metabolites: TBA and Formaldehyde
The vial study paradigm was repeated with equal molar concentrations of TBA or formaldehyde, the primary metabolites of MTBE (Hong et al., 1997; Hong et al., 1999; Le Gal et al., 2001), in order to assess whether the metabolites play a role in the appearance of MTBE specific lesions. No significant lesions were observed at any concentration tested of either metabolite. A follow up study was conducted to test whether or not concomitant exposure to both primary metabolites would induce the vascular lesions observed with MTBE exposure. Embryos were exposed to either 10mM TBA alone, 10 mM formaldehyde alone, 10 mM TBA and 10 mM formaldehyde together, 10 mM MTBE, or control (no treatment), and observed for daily as previously described. No significant lesions were observed in the TBA alone, formaldehyde alone, or the TBA plus formaldehyde treatments as compared to control. Embryonic exposure to the primary metabolites, alone or concomitantly, did not result in the increased vascular lesions and vascular disruptions that were observed with exposure to the parent compound MTBE (Table 3).
3.5. Critical Periods for Embryonic MTBE Exposure
Since MTBE associated lesions were not observed prior to 48 hpf and MTBE did not appear to affect the development of the major vasculature structures, the periods of cardiovascular development used for the critical period studies included 4 stages: (1) prior to angioblast migration to midline (15 hpf, 6-somites), (2) major descending/ascending blood vessel formation (24 hpf, 21-somites), (3) the commencement of circulation (30 hpf, prim-5), and (4) after all major vascular structures are in place and patent (48 hpf, prim-25). The percent of embryos in Study A or B (Fig. 2) that exhibited MTBE specific lesions on developmental days 2, 3 and 4 are presented in Table 4 and 5, respectively. Similar to the dose response study (section 3.1), the vascular lesions occurred on specific days of treatment: pooled blood in CCV on day 2, cranial hemorrhages on day 3, and abnormal ISVs on day 4. In Study A, the embryos exposed to MTBE between 15–120 hpf or 24–120 hpf exhibited significantly more lesions (p ≤ 0.05) than control embryos, while the number of lesions observed in embryos exposed to MTBE between 30–120 hpf or 48–120 hpf were not significantly different from controls (Table 4). In Study B, all embryos exposed from 0–30 hpf and 0–48 hpf had significantly more (p ≤ 0.05) lesions than control embryos or than the embryos exposed from 0–15 hpf and 0–24 hpf (Table 5). Together, these studies suggest that the critical period for MTBE induced vascular disruption is between 15 and 30 hpf (the 6 somites and Prim-5 stages).
Table 4.
Percent of embryos exhibiting specific lesions on different developmental days in Critical Period: Study A
| Duration of Exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 2 Lesions | Control | 0–120hpf | 15–120hpf | 24–120hpf | 30–120hpf | 48–120hpf |
| Reduced Circulating RBCs | - | 16 | - | - | - | - |
| Pericardial Edema | - | - | - | - | - | - |
| Pooled Blood in CCV | - | 44* | 36* | 32* | 16 | 16 |
| Cranial Hemorrhage | - | - | - | - | - | - |
| Abnormal ISVs | - | - | - | - | - | - |
| Duration of Exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 3 Lesions | Control | 0–120hpf | 15–120hpf | 24–120hpf | 30–120hpf | 48–120hpf |
| Reduced Circulating RBCs | - | - | 4 | - | - | - |
| Pericardial Edema | - | 4 | - | 4 | 4 | - |
| Pooled Blood in CCV | - | 4 | - | - | - | - |
| Cranial Hemorrhage | - | 36* | 44* | 32 | 28 | 16 |
| Abnormal ISVs | - | - | - | - | - | - |
| Duration of Exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 4 Lesions | Control | 0–120hpf | 15–120hpf | 24–120hpf | 30–120hpf | 48–120hpf |
| Reduced Circulating RBCs | - | 4 | 4 | 12 | 8 | 4 |
| Pericardial Edema | - | - | - | 4 | 16 | - |
| Pooled Blood in CCV | - | - | - | - | - | - |
| Cranial Hemorrhage | - | 12 | 12 | 20 | 20 | 12 |
| Abnormal ISVs | - | 44* | 48* | 44* | 24 | 12 |
Values in table are percentages (N = 25)
Significantly different from control (P ≤ 0.05)
Table 5.
Percent of embryos exhibiting specific lesions on different developmental days in Critical Period: Study B
| Duration of Exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 2 Lesions | Control | 0–120hpf | 0–15hpf | 0–24hpf | 0–30hpf | 0–48hpf |
| Reduced Circulating RBCs | - | 8 | - | - | 4 | 8 |
| Pericardial Edema | - | - | - | - | - | - |
| Pooled Blood in CCV | - | 32* | - | 16 | 8 | 24* |
| Cranial Hemorrhage | - | - | - | - | - | - |
| Abnormal ISVs | - | - | - | - | - | - |
| Duration of Exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 3 Lesions | Control | 0–120hpf | 0–15hpf | 0–24hpf | 0–30hpf | 0–48hpf |
| Reduced Circulating RBCs | - | 4 | - | - | 4 | 4 |
| Pericardial Edema | - | 8 | - | - | - | - |
| Pooled Blood in CCV | - | 4 | - | - | 4 | 4 |
| Cranial Hemorrhage | - | 36* | 12 | 12 | 16 | 28* |
| Abnormal ISVs | - | - | - | - | - | - |
| Duration of Exposure | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 4 Lesions | Control | 0–120hpf | 0–15hpf | 0–24hpf | 0–30hpf | 0–48hpf |
| Reduced Circulating RBCs | - | 8 | - | - | 4 | 8 |
| Pericardial Edema | - | 4 | - | - | - | - |
| Pooled Blood in CCV | - | - | - | - | - | - |
| Cranial Hemorrhage | - | 8 | 12 | 8 | 16 | 4 |
| Abnormal ISVs | - | 48* | 4 | 20 | 44* | 60* |
Values in table are percentages (N = 25)
Significantly different from control (P ≤ 0.05)
In order to directly test the proposed critical period, embryos were exposed to 10 mM MTBE from only 15 to 30 hpf (the 6 somites to Prim-5 stages). Embryos exposure during the critical period, exhibited a significant increased in vascular lesions compared to control (Table 6). On developmental days 2, 3 and 4, embryos were no longer in 10 mM MTBE, but 36%, 40%, and 38% of embryos exhibited the MTBE-specific vascular lesions that were present in the embryos treated for the entire developmental period. The critical period for MTBE induced vascular disruption was determined to be between 15 and 30 hpf (the 6 somites and Prim-5 stages).
Table 6.
Percent of embryos exhibiting lesions in Critical Period: Study C.
| Day 2 Lesions | Day 3 Lesions | Day 4 Lesions | Total Lesions | |
|---|---|---|---|---|
| Control | 6 (± 2) | 10 (± 6) | 6 (± 6) | 12 (± 6) |
| 0–120hpf | 68 (± 16)* | 54 (± 6)* | 50 (± 2)* | 74 (± 20)* |
| 15–30hpf | 36 (± 4)* | 40 (± 12)* | 38 (±62)* | 60 (± 11)* |
Embryos were exposed to 10 mM MTBE during the time period indicated by the hpf.
Values in table are percentages ± range (N = 25). Study repeated twice.
Significantly different from control (P ≤ 0.05)
3.6. Expression of vascular specific genes
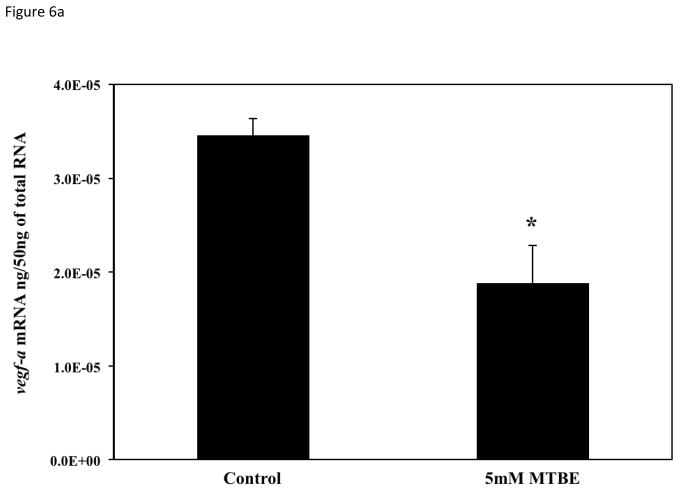
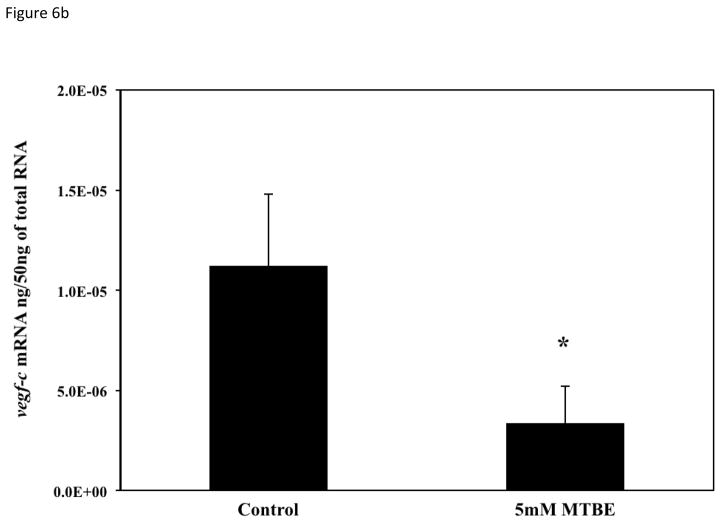
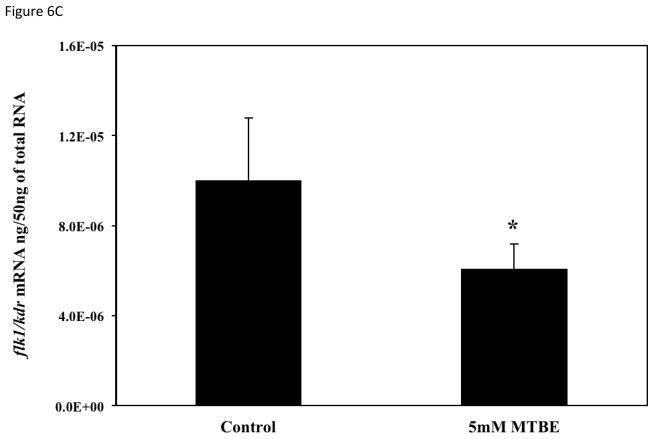
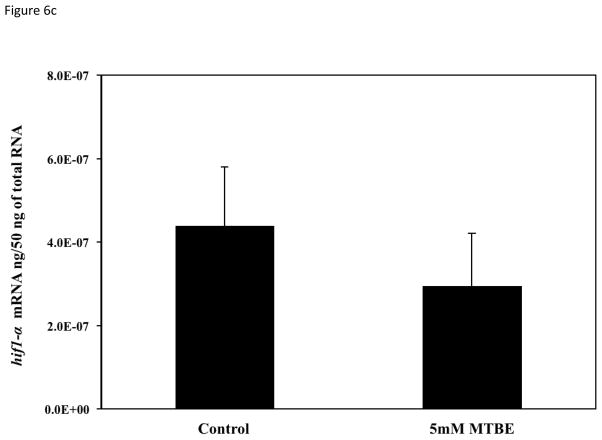
To determine if the vascular lesions associated with MTBE treatment were related to a disruption in the molecular regulation of angiogenesis, mRNA expression of vegfa, vegfc, flk1/kdr, and hif1α-like at 21-somites was quantified with real-time PCR. The 21-somite stage was chosen because it is associated with the formation of the major descending and ascending vessels, which while not affected by MTBE, are required for microvascular angiogenesis (Kimmel et al., 1995; Isogai et al., 2003; Herbert et al., 2009) and an increase in zebrafish vegfa mRNA expression (Liang et al., 1998). The 21-somite stage also corresponds to the middle of the critical period determined in the critical period study (section 3.5). Expression of the primary VEGF isoform, vegfa, as well as the vegfc isoform, was significantly decreased (p ≤ 0.05) in embryos treated with 5 mM MTBE when compared to controls (Fig. 6a and b). Expression of the primary VEGFR, flk1/kdr, was also significantly reduced (Fig. 6c). Vegfa, vegfc and flk1/kdr were reduced by 50 %, 70 % and 40%, respectively. MTBE exposure did not affect hif1α-like mRNA transcript levels at 21-somites (Fig. 6d).
Fig. 6.
Average mRNA expression of (A) vegfa (B) vegfc (C) flk1/vegfr2 and (D) hif1-α-like were determined for zebrafish embryos exposed to 5 mM MTBE at the 512-cell stage and collected for mRNA at 21-somites (N = 3). The graphs are representative of 3 individual experiments. Error bars indicate standard deviation. *Significantly different from control (p ≤ 0.05).
4. DISCUSSION
This study is the first to characterize MTBE toxicity in the developing zebrafish, and the first to investigate the microvascular disrupting effects. All MTBE-induced lesions were related to a disruption in angiogenesis: reduced circulation of RBCs, pericardial edema, pooled blood in the CCV, cranial hemorrhages, and abnormal ISV development (Table 3). Chemical-induced cardiovascular toxicity is frequently observed in developing teleosts from a wide variety of compounds (Wisk and Cooper, 1990b; Spitzbergen et al., 1991; Henry et al., 1997; Dong et al., 2004; Incardona et al., 2004; Antkiewicz et al., 2005; Carney et al., 2007; Hillegass et al., 2007; Zhou et al., 2009). However, these effects involve multiple organ systems and are not attributed to a specific targeted cell or organ type. For example, three-ring PAHs caused cardiac malformation and vascular aberrations, and in addition, disrupted kidney, neural tube and cranium development in zebrafish embryos (Incardona et al., 2004). Similarly, 2,3,7,8-tetrachlorodibenzo-p-dioxin caused pericardial and yolk-sac edema, necrosis-associated vascular leakage in the cranium, disrupted blood flow in the trunk, decreased heart rate and cranial facial abnormalities (Henry et al., 1997, Dong et al., 2004; Antkiewicz et al., 2005). While vascular aberrations and pericardial edema occurred with MTBE exposure, the alterations in heart rate, cardiac morphology, and cranial facial structure did not. Yolk-sac edema and secondary lesions in the kidney and neural tube were also not present in MTBE treated zebrafish. Nor did the cranial histology (Fig. 4b–c) reveal necrosis near the site of hemorrhage. In zebrafish, MTBE specifically targeted developing microvasculature, and is therefore unique among environmental contaminants.
Environmental contamination by MTBE is well documented (Brown, 1997; Irwin, 1997; Ahmed, 2001; Post, 2001; Squillace and Moran, 2007). The LC50s previously reported for adult zebrafish and adult rainbow trout are 7.7 mM (679 mg/L) and 8.7 mM (769 mg/L) MTBE, respectively (Moreels et al., 2006a; Naddafi et al., 2008). In our study, the LC50 for MTBE at 120 hpf in the zebrafish embryo was determined to be 19 – 20 mM (1675 – 1763 mg/L) (Table 2). The EC50 of MTBE for total lesion occurrence in zebrafish embryos was determined to be 1.2 mM (105 mg/L) (Table 2), which is greater than reported MTBE surface water contamination (Irwin, 1997; Post, 2001). Due to the high solubility of MTBE (476 mM, 42 g/L), in certain spill scenarios, much higher concentrations could be obtained in surface waters, which would impact aquatic species at the site of the spill and downstream.
In our studies, neither of the putative primary metabolites, TBA and formaldehyde, resulted in vascular lesions at the same molar concentrations of MTBE that resulted in vascular lesions. MTBE metabolism in zebrafish and other aquatic organisms has not been carried out; however in mammalian systems (Fig. 1), TBA and formaldehyde are the primary metabolites (Hong et al., 1997; Hong et al., 1999; Le Gal et al., 2001). Moreels et al. (2006b) reported that exposure to 12.88 mM (955 mg/L) TBA led to an increase in catfish embryo and larval mortality, but not to the craniofacial and spinal abnormalities that were observed in catfish exposed to MTBE. In Japanese medaka, TBA was 3 to 4 times less toxic than MTBE, and did not inhibit vascular growth (Longo, 1995). In mammals, TBA metabolism to minor metabolites 2-methyl-1,2-propanediol and 2-hydroxyisobutyric acid occurs slowly (Fig. 1) (Amberg et al., 2001; Phillips et al., 2008). Formaldehyde is biotransformed to either methanol or formic acid by alcohol dehydrogenase or aldehyde dehydrogenase, respectively (Du et al., 2005). The presence of these minor metabolites in zebrafish exposed to MTBE, TBA or formaldehyde is unknown. However, the minor metabolites are unlikely to be the cause of the vascular lesions, as they would occur in low concentrations relative to MTBE, and no toxicity was observed following treatment with TBA or formaldehyde. Therefore, the toxic effects that resulted from embryonic exposure to MTBE were likely mediated by the parent compound, and not the primary or secondary metabolites.
The MTBE specific lesions occurred when embryos were exposed between 15 and 30 hpf (6-somites to Prim-5), which correlates to the beginning of angiogenesis (Table 1). In a similar stage stage specific exposure study with Japanese medaka, toxicity of 2,3,7,8-TCDD only occurs after the development of the liver (Wisk and Cooper, 1990). The manifestation of lesions at different developmental periods is explained by ongoing organogenesis (Kimmel et al., 1995). In the zebrafish, the formation of the major descending and ascending blood vessels occurs around 21-somites (24 hpf) and circulation follows the initial heart contractions by Prim-5 (30 hpf). Much of the primary vascularization in the embryo is patent by Prim-25 (48 hpf), the microvasculature of the brain develops by Pec Fin (72 hpf), and the ISVs are patent by the Protruding Mouth stage (96 hpf) (Kimmel et al., 1995; Isogai et al., 2003). The disappearance of the lesions, except in severe cases, may be explained by the ability of the embryo to repair minor to moderate lesions. Hemorrhages observed at 72 hpf (Fig. 4) could be a result of poorly developed or delayed cell junctions in the cerebral microvaculature. Loss of the distinct chevron shape of the somite muscle at 96 hpf (Fig. 5) was likely a result of poor nutrient and oxygen levels, due to abnormal ISVs, and could partially explain the delayed hatch observed in 10 mM exposed MTBE embryos. The long term ramifications of the appearance and repair of these lesions at the biochemical and functional tissue level are not known. However, it is likely that alterations in the vascularization of the brain and the muscle could be manifested in focal CNS damage and/or decreased swimming ability. In a preliminary study examining juvenile survival following embryonic exposure to 1.25 mM and 10 mM MTBE, 45 and 75% of juveniles died by 30 dph (Bonventre et al., 2008). Additionally, embryonic MTBE exposure significantly diminished larval touch response at 3 dph (5mM) and the number of larvae swimming at 5 dph (1.25mM and 5mM) (Bonventre et al., 2008). Disruption of angiogenesis at an early embryonic stage impaired organ development and affected post hatch survival.
The vascular lesions resulting from embryonic exposure to MTBE parallel those observed in the vegf morpholino antisense studies. The vegfa-morphant phenotypically exhibits pericardial edema, no or reduced circulating red blood cells, and aberrant or absent vasculature (Nasevicius et al., 2000a). Bahary et al. (2007) evaluated the differences between duplicate vegfa genes in the zebrafish (vegfaa, vegfab) and found that knockdown of either gene results in a similar disruption of ISVs and increased cranial hemorrhages. Knockdown of either vegfr2 duplicated genes also results in the inhibition of ISVs (Bahary et al., 2007), a lesion that had been previously reported in a VEGFR2 mutant zebrafish (Habeck et al., 2002). Carmielet et al. (1996) showed that the VEGFa knockout in mice was embryonic lethal, however zebrafish morphants survive. Similarly, VEGFR2 knockout mice are embryonic lethal, lacking differentiated angioblasts and any vascular growth (Shalaby et al., 1995). There are several possible reasons for the species differences. First, zebrafish embryos are smaller and able to diffuse oxygen into tissues from their rearing solutions. Secondly, morpholinos are transient, and their ability to knockdown the gene diminishes overtime (Nasevicius et al., 2000b). Finally, the duplication of the zebrafish genome means that knocking down one VEGF leaves another functional gene that may compensate for the loss. However, knockdown of both vegfaa and vegfab in zebrafish embryos was still not lethal (Bahary et al., 2007), which may suggest that other VEGF isoforms, such as VEGFc, play a greater role in compensating for the loss of VEGFa in the zebrafish than they do in the mouse model.
The MTBE-induced phenocopy of a vegfa-morphant, along with the decreased mRNA of vegfa, vegfc and flk1/vegfr2 (Fig. 6a–c) during the critical period (15 – 30 hpf), suggests that MTBE vascular toxicity is mediated by dysregulation of the VEGF pathway. The mechanism by which MTBE disrupts vegf expression is unknown. Several growth factors, oncogenes, and cytokines are known to regulate vegf expression (reviewed in Ferrara et al., 2003). Developmental pathways that promote transcription of genes involved in the regulation of embryogenesis may be involved in MTBE toxicity. For example, the Wnt canonical pathway, critical to cell growth and tumor development, up-regulates VEGF indirectly by stabilizing β-catenin, which is able to bind the VEGF promoter (Eswaran et al., 2003). β-catenin also anchors endothelial cell junction protein VE-Cadherin to the cytoskeleton, which is important to the integrity and maturation of a vessel (Lampugnani et al., 1995). HIF1 mediation of hypoxia, however, is still the most significant factor governing angiogenesis in the embryo (Ryan et al. 1998, Ramirez-Bergeron et al., 2004). Deletion of Hif1-α in knockout mice is lethal; examination of the knockouts on embryonic day 8.5 revealed a reduced vascular network in the yolk sac, lack of cephalic vascular development, and reduced numbers of somites (Ryan et al., 1998). The fact that MTBE did not decrease hif1α-like mRNA transcript levels at 21-somites, does not exclude HIF1 from a role in the mechanism of MTBE vascular toxicity. Stability of the HIF1α cytoplasmic component under low oxygen conditions, or the ability of the HIF1 α /HIF1β heterodimer to bind to the VEGF promoter are alternative endpoints that may be disrupted by MTBE and would result in decreased vegf expression (Ivan et al., 2001; Wang and Semenza, 1993). Further work is required to understand the underlying mechanism of MTBE disruption of vascular development in the zebrafish embryo.
5. CONCLUSIONS
MTBE exposure during embryonic development resulted in a unique targeting of the microvasculature angiogenesis, while other organ systems were spared. However, exposure to primary metabolites, TBA and formaldehyde, did not cause the specific vascular lesions observed with exposure to the parent compound: pooled blood in the CCV, cranial hemorrhages, and abnormal ISV development. This suggests that the sub-lethal vascular lesions were a result of MTBE and not its metabolites. Staggered exposure periods during early development demonstrated that exposure between 15–30 hpf (the 6-somites to Prim-5 stages) was necessary to produce the vascular lesions that were first visible at 48 hpf (Long Pec stage). Within the critical window, transcript levels for two isoforms of the primary regulator of angiogenesis, vegfa and vegfc, and the primary receptor flk1/kdr, were significantly reduced. MTBE exposure phenocopied the effects observed following morpholino knockdown of vegfa-morphants (Nasevicius et al., 2000; Bahary et al., 2007). Together, these data suggest that MTBE-induced vascular disruption of newly formed blood vessels is mediated, at least in part, by the alteration of VEGF - VEGFR regulation in the embryo.
Acknowledgments
This research was carried out at the New Jersey Agricultural Experiment Station (NJAES) with funding from NJAES (01201) through Cooperative State Research, Education and Extension Services, the National Institute of Environmental Health Sciences (ES07148), the Environmental and Occupational Health Sciences Institute (ES05022), the New Jersey Water Resources Research Institute (2010NJ198B), New Jersey Department of Environmental Protection, Division of Science, Research and Technology (SR09-019).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish, Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed FE. Toxicology and human health effects following exposure to oxygenated or reformulated gasoline. Tox Let. 2001;123:89–113. doi: 10.1016/s0378-4274(01)00375-7. [DOI] [PubMed] [Google Scholar]
- Amberg A, Rosner E, Dekant W. Toxicokinetics of methyl tert butyl ether and its metabolites in humans after oral exposure. Toxicol Sci. 2001;61:62–67. doi: 10.1093/toxsci/61.1.62. [DOI] [PubMed] [Google Scholar]
- Bahary N, Goishi K, Stuckenholz C, Weber G, Leblanc J, Schafer CA, Berman SS, Klagsbrun M, Zon LI. Duplicate VegfA genes and orthologues of the KDR receptor tyrosine kinase family mediate vascular development in the zebrafish. Blood. 2007;110 (10):3627–36. doi: 10.1182/blood-2006-04-016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. Atmospheric and potable water exposure to methyl tert-butyl ether. Reg Tox Pharm. 1997;25:256–276. doi: 10.1006/rtph.1997.1104. [DOI] [PubMed] [Google Scholar]
- Bonventre JA, Bugel SM, White LA, Cooper KR. Decreased VEGF expression in zebrafish (Danio rerio) embryos exposed to MTBE correlates with disrupted angiogenesis and decreased post hatch survival. SOT 48th Annual Meeting.2009. [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoek A, Harpal K, Eberhardt C, Declercg C, Pawling J, Moons L, Collen D, Risau W, Naggy A. Abrnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:436–438. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carney MW, Erwin K, Hardman R, Yuen B, Volz DC, Hinton DE, Kullman SW. Differential developmental toxicity of naphthoic acid isomers in medaka (Oryzias latipes) embryos. Mar Pollut Bullet. 2008;57:255–266. doi: 10.1016/j.marpolbul.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci USA. 2006;103 (17):6554–9. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin, Toxicol. Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Dranitsaris G, Edwards S, Edwards J, Leblanc M, Abbott R. Bevacizumab in combination with folfiri chemotherapy in patients with metastatic colorectal cancer: an assessment of safety and efficacy in the province of Newfoundland and Labrador. Curr Oncol. 2010;17 (5):12–6. doi: 10.3747/co.v17i5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du HF, Xu LH, Wang HF, Liu YF, Tang XY, Liu KX, Peng SX. Formation of MTBE-DNA adducts in mice measured with accelerator mass spectroscopy. Environ Toxicol. 2005;20:397–401. doi: 10.1002/tox.20124. [DOI] [PubMed] [Google Scholar]
- Eswaran W, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, Randazzo F, Gundel R, Warren RS, Escobedo J, Aukerman SL, Taylor RN, Fantl WJ. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63(12):3145–53. [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powel-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of VEGF gene. Nature. 1996;308:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Medicine. 2003:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza G. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16 (9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S. Analysis of zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12 (16):1405–1412. doi: 10.1016/s0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- Heidman W, Antkiewicz DS, Carney SA, Peterson RE. Zebrafish and cardiac Toxicology. Cardiovas Tox. 2005;5:203–214. doi: 10.1385/ct:5:2:203. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (danio rerio) Toxicol Appl Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DYR. Arterial-Venous Segregation by Selective Cell Sprouting: An Alternative Mode of Blood Vessel Formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, White LA. Matrix metalloproteinase-13 is required for zebrafish (Danio rerio) development and is a target for glucocorticoids. Toxicol Sci. 2007;100 (1):168–179. doi: 10.1093/toxsci/kfm192. [DOI] [PubMed] [Google Scholar]
- Hong JY, Yang CS, Lee M, Wang YY, Huang W, Tan Y, Patten CJ, Bondoc FY. Role of cytochromes P450 in the metabolism of methyl tert-butyl ether in human livers. Arch Toxicol. 1997;71:266–269. doi: 10.1007/s002040050386. [DOI] [PubMed] [Google Scholar]
- Hong JY, Wang YY, Bondoc FY, Lee M, Yang CS, Hu WY, Pan J. Metabolism of Methyl tert-butyl ether and other gasoline ethers by human liver microsomes and heterologously expressed human cytochromes P450: Identification of CYP2A6 as a major catalyst. Toxicol Appl Pharma. 1999;160:43–48. doi: 10.1006/taap.1999.8750. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev Biol. 2001;230 (2):278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholtz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbon Toxicol. Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Irwin RJ, editor. Environmental Contaminants Encyclopedia: MTBE entry. United Stages National Park Service, Water Resources Div, Water Operations Branch; 1997. [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203 (3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Gonzalez Santander R, Martinez Cuadrado G, Gonzalez-Santander Marinez M, Monteagudo M, Martinez Alonso FJ, Toledo Lobo MV. The Use of Different Fixatives and Hydrophilic Embedding Media (Historesiny and Unicryly) for the Study of Embryonic Tissues. Microsc Res Tech. 1997;36(3):151–8. doi: 10.1002/(SICI)1097-0029(19970201)36:3<151::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174(4):593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biology. 2002;240:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Le Gal A, Dreano Y, Gervasi PG, Berthou F. Hyman cytochrome P450 2A6 is the major enzyme involved in the metabolism of three alkoxyethers used in oxyfuels. Toxicol Let. 2001;124:47–58. doi: 10.1016/s0378-4274(00)00286-1. [DOI] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270 (22):13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Liang D, Xu X, Chin AJ, Balasubramaniyam NV, Teo MA, Lam TJ, Weinberg ES, Ge R. Cloning and characterization of vascular endothelial growth factor (VEGF) from zebrafish, Danio rerio. Biochim Biophys Acta. 1998;1397 (1):14–20. doi: 10.1016/s0167-4781(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96 (2):99–113. [PubMed] [Google Scholar]
- Longo SE. MS Thesis. Rutgers University, Graduate School; New Brunswick: 1995. Effects of methyl tert-butyl ether and napthaline on the embryo of the Japanese medaka. [Google Scholar]
- Mone SM, Gillman MW, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113:1058–1069. [PubMed] [Google Scholar]
- Moreels D, Van Cauwenberghe K, Debaere B, Rurangwa E, Vromant N, Bastiaens L, Diels L, Springael D, Merckx R, Ollevier F. Long-term exposure to environmentally relevant doses of methyl-tert-butyl ether causes significant reproductive dysfunction in the zebrafish (Danio rerio) Environ Toxicol Chem. 2006a;25 (9):2388–93. doi: 10.1897/05-054r1.1. [DOI] [PubMed] [Google Scholar]
- Moreels D, Lodewijks P, Zegers H, Rurangwa E, Vromant N, Bastiaens L, Diels L, Springael D, Merckx R, Ollevier F. Effect of short-term exposure to methyl-tert-butyl ether and tert-butyl alcohol on the hatch rate and development of the African catfish, Clarias gariepinus. Environ Toxicol Chem. 2006b;25 (2):514–9. doi: 10.1897/04-641r.1. [DOI] [PubMed] [Google Scholar]
- Naddafi K, Nabizadeh R, Baiggi A. Bioassay of methyl tertiary-butyl ether (MTBE) toxicity on rainbow trout fish. J Hazard Mater. 2008;154 (1–3):403–6. doi: 10.1016/j.jhazmat.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000a;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene knockdown in zebrafish. Nat Genet. 2000b;26(2):216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Phillips S, Palmer RB, Brody A. Epidemiology, toxicology, and healthy effects of methyl tert-butyl ether (MTBE) J Med Toxicol. 2008;4 (2):115–126. doi: 10.1007/BF03160966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post G, editor. MTBE in New Jersey’s Environment. New Jersey Department of Environmental Protection; 2001. [Google Scholar]
- Ramirez-Bergeron DL, Runge A, Cowden Dahl KD, Fehling HJ, Keller G, Simon C. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–4634. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- Rosell M, Lacorte S, Barcelo D. Analysis, occurrence and fate of MTBE in the aquatic environment over the past decade. Trends Anal Chem. 2006;25:1016–1029. [Google Scholar]
- Ryan HE, Lo J, Johnson RS. Hif-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17 (11):3005–15. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of like trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat Toxicol. 1991;19:41–72. [Google Scholar]
- Squillace PJ, Moran MJ. Factors associated with sources, transport, and fate of volatile organic compounds and their mixtures in aquifers of the United States. Environ Sci Technol. 2007;41:2123–2130. doi: 10.1021/es061079w. [DOI] [PubMed] [Google Scholar]
- United States DOE-EAI. [Accessed Dec, 2010];Monthly MTBE production, updated 12/30/10. 2009 http://tonto.eia.doe.gov/dnav/pet/pet_pnp_oxy_dc_nus_mbbl_m.htm.
- United States EPA. [Accessed Oct, 2009];State actions banning MTBE (Statewide) 2007 EPA420-B-07-013: http://www.epa.gov/MTBE/420b07013.pdf.
- Van Wezel A, Puijker L, Vink C, Versteegh A, de Voogt P. Odour and flavor thresholds of gasoline additives (MTBE, ETBE and TAME) and their occurrence in Dutch drinking water collection areas. Chemosphere. 2009;76 (5):672–676. doi: 10.1016/j.chemosphere.2009.03.073. [DOI] [PubMed] [Google Scholar]
- Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268(29):21513–21518. [PubMed] [Google Scholar]
- Werner I, Koger CS, Deanovic LA, Hinton DE. Toxicity of methy-tert-butyl ether to freshwater organisms. Environ Pollution. 2001;111:83–88. doi: 10.1016/s0269-7491(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Wisk JD, Cooper KR. The stage specific toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in embryos of the japanese medaka (Oryzias latipes) Environ Toxicol Chem. 1990a;9(9):1159–1169. [Google Scholar]
- Wisk JD, Cooper KR. Comparison of the toxicity of several polychlorinated dibenzo- p-dioxins and 2,3,7,8-tetrochlorobenzofuran in embryos of the Japanese medaka (Oryzias latipes) Chemosphere. 1990b;20:361–377. [Google Scholar]
- Zhou S, Dong Q, Li S, Guo J, Wang X, Zhu G. Developmental toxicity of cartap on zebrafish embryos. Aquat Toxicol. 2009;95:339–346. doi: 10.1016/j.aquatox.2009.10.006. [DOI] [PubMed] [Google Scholar]